Key Points
Question
Among patients with lower-extremity peripheral artery disease (PAD), does a home-based exercise intervention that consists of a wearable activity monitor and telephone coaching improve walking performance?
Findings
In this randomized clinical trial of 200 participants with PAD, a wearable activity monitor combined with telephone coaching did not significantly improve 6-minute walk distance at 9 months (5.5 m in the intervention group vs 14.4 m in the usual care group).
Meaning
These results do not support home-based exercise interventions of wearable devices combined with telephone coaching to improve walking performance in patients with PAD.
Abstract
Importance
Clinical practice guidelines support home-based exercise for patients with peripheral artery disease (PAD), but no randomized trials have tested whether an exercise intervention without periodic medical center visits improves walking performance.
Objective
To determine whether a home-based exercise intervention consisting of a wearable activity monitor and telephone coaching improves walking ability over 9 months in patients with PAD.
Design, Setting, and Participants
Randomized clinical trial conducted at 3 US medical centers. Patients with PAD were randomized between June 18, 2015, and April 4, 2017, to home-based exercise vs usual care for 9 months. Final follow-up was on December 5, 2017.
Interventions
The exercise intervention group (n = 99) received 4 weekly medical center visits during the first month followed by 8 months of a wearable activity monitor and telephone coaching. The usual care group (n = 101) received no onsite sessions, active exercise, or coaching intervention.
Main Outcomes and Measures
The primary outcome was change in 6-minute walk distance at 9-month follow-up (minimal clinically important difference [MCID], 20 m). Secondary outcomes included 9-month change in subcomponents of the Walking Impairment Questionnaire (WIQ) (0-100 score; 100, best), SF-36 physical functioning score, Patient-Reported Outcomes Measurement Information System (PROMIS) mobility questionnaire (higher = better; MCID, 2 points), PROMIS satisfaction with social roles questionnaire, PROMIS pain interference questionnaire (lower = better; MCID range, 3.5-4.5 points), and objectively measured physical activity.
Results
Among 200 randomized participants (mean [SD] age, 70.2 [10.4] years; 105 [52.5%] women), 182 (91%) completed 9-month follow-up. The mean change from baseline to 9-month follow-up in the 6-minute walk distance was 5.5 m in the intervention group vs 14.4 m in the usual care group (difference, −8.9 m; 95% CI, −26.0 to 8.2 m; P = .31). The exercise intervention worsened the PROMIS pain interference score, mean change from baseline to 9 months was 0.7 in the intervention group vs −2.8 in the usual care group (difference, 3.5; 95% CI, 1.3 to 5.8; P = .002). There were no significant between-group differences in the WIQ score, the SF-36 physical functioning score, or the PROMIS mobility or satisfaction with social roles scores.
Conclusions and Relevance
Among patients with PAD, a home-based exercise intervention consisting of a wearable activity monitor and telephone coaching, compared with usual care, did not improve walking performance at 9-month follow-up. These results do not support home-based exercise interventions of wearable devices and telephone counseling without periodic onsite visits to improve walking performance in patients with PAD.
Trial Registration
clinicaltrials.gov Identifier: NCT02462824
This randomized controlled trial compares the effect of a 9-month home-based exercise intervention comprising a wearable activity monitor and telephone coaching vs usual care on 6-minute walk distance among patients with peripheral artery disease.
Introduction
Supervised treadmill exercise significantly improves walking performance in patients with lower-extremity peripheral artery disease (PAD) and is recommended by clinical practice guidelines as a first-line therapy (class IA recommendation: supervised treadmill exercise is recommended or should be performed based on high-quality evidence from >1 randomized clinical trial).1,2,3,4,5 However, traveling to an exercise center 3 times per week for supervised treadmill exercise can be burdensome and many patients with PAD decline participation.2,6
Practice guidelines also recommend home-based walking exercise for patients with PAD (class IIA recommendation: treatment is reasonable and can be useful based on high-quality evidence from >1 randomized clinical trial).1 This recommendation is based on 3 randomized trials in which the home-based exercise interventions required periodic visits to the medical center every 1 to 4 weeks throughout the intervention.1,2,7,8,9
Functional impairment is common in patients with PAD, including in those without classic claudication symptoms.10,11 Effective interventions that do not require regular medical center visits are likely to be more accessible and acceptable to patients with PAD than exercise interventions requiring periodic medical center visits.
In the Home-Based Monitored Exercise for PAD (HONOR) randomized clinical trial, feedback from patients with PAD was used to design an appealing home-based exercise intervention. The trial tested whether an intervention that combined telephone coaching and a wearable activity monitor to promote home-based walking exercise (but did not require periodic medical center visits throughout the intervention) significantly improved walking endurance and patient-reported outcomes at 9-month follow-up compared with usual care.
Methods
The institutional review board at Northwestern University and all recruitment sites approved the protocol. Participants provided written, informed consent. Participants were randomized between June 18, 2015, and April 4, 2017. Randomization ended when the target sample size was achieved. The final follow-up visit was on December 5, 2017. The trial protocol appears in Supplement 1.
Patient Contributions to the Study Design
Focus groups and pilot studies of patients with PAD were held to solicit feedback for designing an appealing trial. For example, because of participant enthusiasm for a wearable activity monitor, a wearable activity monitor was incorporated into the exercise intervention. Patients with PAD also were asked to discuss meaningful outcomes and rank questionnaires according to the degree to which each questionnaire measured how PAD limits their daily life. The ranking of the patient questionnaires was used to select questionnaire outcomes.
Identification of Potential Participants
Three medical centers (University of Minnesota, New York University, and Northwestern University) participated. Potential participants were identified from physician referrals, lists of patients with PAD, and response to advertisements and mailings to patients aged 50 years or older. In addition, patients with PAD who previously participated in research at each center and expressed interest in future research were contacted.
Inclusion Criteria
Inclusion criteria included patients with an ankle brachial index (ABI) of 0.90 or less at baseline.12 Patients with an ABI greater than 0.90 at baseline were eligible if a hospital-affiliated vascular laboratory report demonstrated PAD, angiography of lower extremities showed stenosis of 70% or greater, or medical records documented prior revascularization of lower extremities. In addition, patients with an ABI from 0.90 to 1.00 were eligible if the ABI decreased by 20% after a heel-rise test.13
Exclusion Criteria
A patient was excluded if he or she had an amputation below or above the knee; was confined to a wheelchair; required use of a walking aid other than a cane; had a walking impairment for a reason other than PAD; or had a foot ulcer, critical limb ischemia, or significant visual or hearing impairment. A patient was excluded if he or she had a major surgery or revascularization during the previous 3 months or a planned surgery or revascularization during the next 9 months; was currently or recently (within the past 3 months) participating in a clinical trial or cardiac rehabilitation; or had Parkinson disease or required oxygen with activity. A patient was excluded if he or she had New York Heart Association class III or IV heart failure or angina, an increase in angina pectoris during the prior 6 months, or an abnormal baseline stress test. A patient was excluded if he or she was already exercising at a level similar to the intervention, did not speak English, or if his or her walking was primarily limited by symptoms from something other than PAD.
A patient who had been treated for cancer during the past 2 years was excluded unless it was early-stage cancer, the prognosis was excellent, and the cancer was unlikely to return during study enrollment. For these patients, eligibility was determined either by medical record review or by direct consultation between the principal investigator (M.M.M.) and the treating physician. Patients with a Mini-Mental State Examination score of less than 23 were excluded.14
Patient Characteristics and Randomization
A handheld Doppler instrument (Pocket-Dop II, Nicolet Vascular) was used to measure systolic pressures in the right brachial, dorsalis pedis, and posterior tibial arteries and left dorsalis pedis, posterior tibial, and brachial arteries. Each pressure was measured twice. The ABI was calculated by dividing mean pressures in each leg by the mean of the 4 brachial pressures.15
Medical history and race/ethnicity were obtained using an open-ended question by trained staff.3,4 Race/ethnicity data were required by the funding agency and allowed assessment of the generalizability of the results. Height and weight were measured. Body mass index was calculated as weight in kilograms divided by height in meters squared.
Leg symptoms were characterized with the San Diego Claudication Questionnaire.16 Intermittent claudication was defined as exertional calf pain that did not begin at rest, caused the patient to stop walking, and resolved within 10 minutes of rest. Participants without intermittent claudication were either asymptomatic (ie, reported no exertional leg symptoms) or had exertional leg symptoms other than intermittent claudication.11,16
Participants were randomized to 1 of 2 groups using a computer-generated randomization list. Randomization was stratified by study site and used block randomization and randomly selected block sizes of 4, 6, or 8.
Intervention
Home-Based Exercise
The home-based exercise intervention was designed based on patient feedback and incorporated social cognitive theory and self-regulation concepts that are important for behavioral change.17,18 During the first month of the intervention, participants were asked to attend 4 weekly sessions with the coach (weeks 1 and 2) or with other participants and the coach (weeks 3 and 4). During these sessions, participants walked for exercise and were assisted in setting goals for walking exercise and shown how to enter their walking exercise activity on the study website.
After the first month, participant contact with the coach was primarily by telephone and there were no scheduled onsite medical center visits. The coach called participants once per week during months 1 and 2, once every other week during months 3, 4, and the first half of month 5, and once per month during the latter half of month 5 through month 9. Telephone calls were structured and included discussion of progress toward exercise goals, review of the wearable activity monitor data, challenges encountered, strategies to overcome challenges, setting of new walking exercise goals, and a summary of the telephone call content.
The intervention was individualized. Participants were typically advised to exercise 5 days per week either indoors or outdoors and beginning with 10 to 15 minutes of exercise per session and working up to 50 minutes per session. Participants were asked to walk until they experienced severe leg discomfort and then rest until they were able to resume walking.
If participants could walk for 10 minutes without stopping due to ischemic leg symptoms, they were asked to increase their walking exercise speed (intensity). Participants without exertional leg discomfort were asked to walk with an intensity rating of 12/20 to 14/20 on the Borg Rating of Perceived Exertion scale.19 These methods were used successfully to improve 6-minute walk distance during a previous randomized trial of home exercise that included weekly medical center visits.9
Participants received a wearable activity monitor (FitBit Zip, FitBit Inc) to monitor their physical activity. This wearable device has been shown to be a valid and reliable measure of walking activity with less than 1% error compared with other validated activity monitors.20 Data from the wearable activity monitor were uploaded via Bluetooth on the study website and were visible to both the participant and coach.
Participants without a home computer or tablet were provided with a tablet for the study duration. During telephone contact with the coach, exercise goals (exercise frequency and duration) were entered by the coach on the website. Participants were asked to enter the minutes they walked for exercise on the website and this information was visible to the coach. Twice per month, group telephone calls for intervention participants were led by the coach and included a topic of the month such as managing pain during exercise and exercising in cold weather. Participants were encouraged to share their successes and challenges with other participants.
Usual Care
Participants randomized to usual care received no study intervention. However, participants in both study groups were telephoned monthly by a study coordinator other than the coach to obtain data on adverse events. Information on physical activity and walking exercise frequency was collected during these telephone calls every 3 months.
Outcomes
All prespecified outcomes were obtained by staff blinded to group assignment at baseline and follow-up. Investigators were blinded to outcome data until the end of the trial. The primary outcome was change in 6-minute walk distance between baseline and 9-month follow-up.
Secondary outcomes were changes between baseline and 9-month follow-up in Walking Impairment Questionnaire (WIQ) distance, speed, and stair climbing scores; the Medical Outcomes Study Short Form 36 (SF-36) physical functioning score, the Patient-Reported Outcomes Measurement Information System (PROMIS) measures of mobility, pain interference, and satisfaction with social roles and activities,21,22,23,24,25,26,27,28,29 and change in objectively measured physical activity.30 In prespecified exploratory analyses, these outcomes also were measured at 4½-month follow-up.
6-Minute Walk Distance Test
Change in 6-minute walk distance is a well-validated, objective measure of walking endurance among patients with PAD.3,4,9,31 The 6-minute walk distance test was administered by a trained and certified research assistant who followed a standardized protocol. Participants walked up and down a 100-ft hallway for 6 minutes after being instructed to cover as much distance as possible.3,4,9,31 The distance completed after 6 minutes was recorded. Twenty meters was defined as a minimal clinically important difference.32
Questionnaire Outcomes
The questionnaires were self-administered. The WIQ is a PAD-specific measure of self-reported limitations with 3 domains: walking distance, walking speed, and stair climbing.21 Each domain is scored on a scale from 0 to 100 (100 indicating the best possible score).21 A small minimal clinically important difference has not been established for WIQ scores. The SF-36 was used to assess functional status in the physical functioning domain (scale from 0-100; 100 indicating the best possible score).22 The minimal clinically important difference for the SF-36 physical functioning score ranges from 5 to 7 points.22,33
The PROMIS questionnaires measured perceived change in mobility (mobility short form), ability to engage in social roles and activities (satisfaction with social roles short form), and the degree to which pain interfered with activities (pain interference short form).23,24,25,26,27,28,29 The PROMIS measures use a T-score metric with a mean (SD) score of 50 (10) compared with the general population.23 Higher scores are better for the questionnaires on mobility and satisfaction with social roles and worse for the questionnaire on pain interference.24,25,26,27,28,29 The minimal clinically important difference is 2.0 points for mobility26 and ranges from 3.5 to 4.5 points for pain interference.24 The minimal clinically important difference has not been established for satisfaction with social roles.
Objectively Measured Physical Activity
Free-living physical activity (measured as total count per day) was acquired over 7 days with an accelerometer (ActiGraph). The accelerometer was worn on the right hip and removed only for bathing or sleeping.30
Power Calculations
Statistical power was calculated for the primary outcome of 6-minute walk distance and for the secondary questionnaire outcomes. We anticipated a 10% dropout rate at 9-month follow-up.3,4,9,34 In previous trials of exercise among patients with PAD,4,9 the SD range for change in 6-minute walk distance was 51 to 69 m. Therefore, 200 participants provided 80% power for detecting a between-group difference for change in 6-minute walk distance of 21 to 29 m using a 2-sided 2-sample t test with a significance level of .05.
Twenty meters is the minimal clinically important difference for the 6-minute walk distance.32 For the secondary questionnaire outcomes and based on SDs from prior studies,9,28,29 the trial had 80% power to detect a difference of 10.1 in the WIQ distance score, 9.2 in the WIQ speed score, and 3.3 in the PROMIS pain interference score.
Statistical Analysis
Analyses were performed according to the intention-to-treat principle. Baseline characteristics were summarized as mean and SD for continuous measures and number and percentage in each group. Changes in each outcome between baseline and 9-month follow-up and between baseline and 4½-month follow-up were compared between the groups using a 2-sample t test stratified by site. For the between-group differences, 95% CIs were constructed. Analyses for changes in outcomes between baseline and 4½-month follow-up and between baseline and 9-month follow-up were performed using multiple imputation for missing data and SAS Proc MI (SAS Institute Inc) with 80 imputed data sets. Variables used for imputation were age, ABI, body mass index, sex, race, smoking status, outcome values, leg symptoms, and comorbidities. The final statistical inference accounted for imputation variabilities.
To estimate whether the intervention effects differed in subgroups of participants, the primary outcome analyses were repeated within subsets of participants grouped by baseline characteristics using tests for interaction to determine statistical significance in post hoc exploratory analyses. The analyses were stratified by site to adjust for potential differences among the 3 sites. The a priori level for statistical significance was a 2-sided P < .05. For all of the analyses, SAS version 9.4 was used. Because there were multiple secondary outcomes and no adjustment for multiple comparisons, results for the secondary outcomes should be interpreted as exploratory.
Results
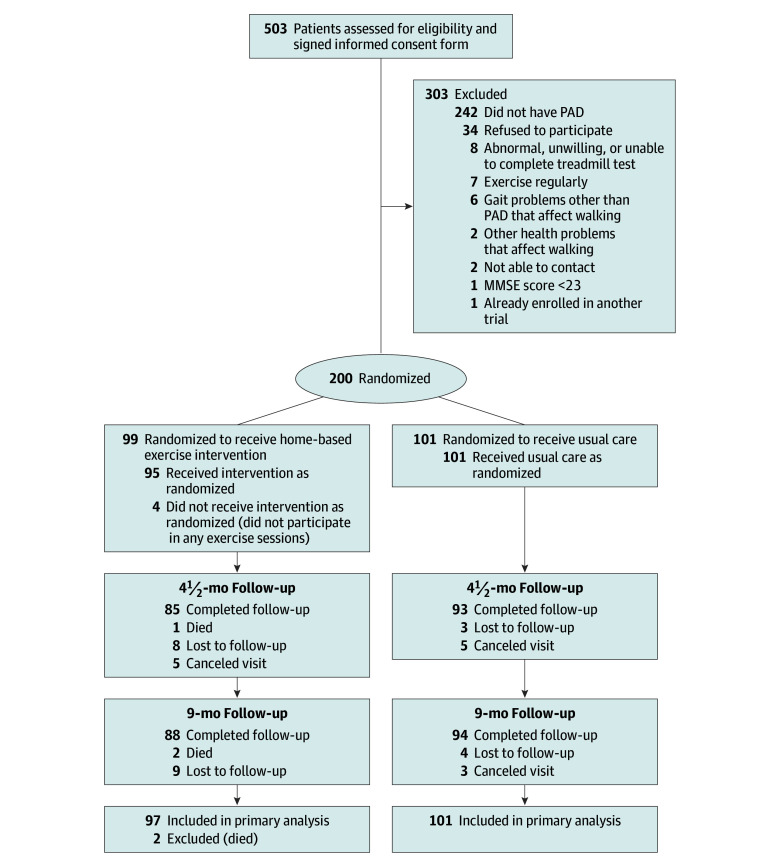
Of 503 participants who provided written informed consent, 200 were randomized (Figure 1). The mean (SD) age was 70.2 (10.4) years, the mean (SD) ABI was 0.66 (0.15), 105 (52.5%) were women, 100 (50%) were black, and 182 (91%) completed 9-month follow-up. The usual care group had a higher prevalence of current or former cigarette smoking; however, baseline characteristics were otherwise balanced between the groups (Table 1).
Figure 1. Study Participation and Follow-up Rates.
MMSE indicates Mini-Mental State Examination; PAD, peripheral artery disease. The reasons for exclusion were not obtained for patients who did not provide informed consent because hundreds of potential participants without PAD were excluded by telephone prior to attending a study visit and there were insufficient resources to track the reasons for exclusion. The MMSE score range is 0 to 30 (patients with a score of <23 were excluded).
Table 1. Characteristics of Participants Randomized to Home-Based Exercise vs Usual Care.
| Home-Based Exercise (n = 99)a |
Usual Care (n = 101)a |
|
|---|---|---|
| Age, mean (SD), y | 70.1 (10.6) | 70.4 (10.1) |
| Female sex | 54 (54.5) | 51 (50.5) |
| Black race | 49 (49.5) | 51 (50.5) |
| Ankle brachial index, mean (SD)b | 0.65 (0.15) | 0.67 (0.14) |
| Body mass index, mean (SD)c | 29.6 (5.3) | 29.9 (5.3) |
| Current or former smoker | 79 (79.8) | 91 (90.1) |
| Myocardial infarction | 16 (16.2) | 21 (20.8) |
| Heart failure | 8 (8.1) | 11 (11.0) |
| Stroke | 10 (10.1) | 16 (15.8) |
| Angina | 18 (18.2) | 24 (24.0) |
| Cancer | 18 (18.2) | 19 (18.8) |
| Diabetes | 35 (35.4) | 32 (31.7) |
| Classic claudication symptoms | 17 (17.2) | 22 (21.8) |
| Exertional leg pain other than claudication | 68 (68.7) | 67 (66.3) |
| No exertional leg symptoms | 14 (14.1) | 12 (11.9) |
| History of lower extremity revascularization | 36 (36.4) | 44 (43.6) |
| 6-min walk distance, mean (SD), m | 331 (100) | 336 (97) |
| Medical Outcomes Study Short Form 36 physical component summary score, mean (SD)d | 35.4 (9.4) | 36.9 (9.0) |
| Walking Impairment Questionnaire score, mean (SD)d | ||
| Distance | 37.7 (29.5) | 37.9 (27.3) |
| Speed | 36.4 (19.4) | 40.0 (24.5) |
| Stair climbing | 48.7 (27.2) | 47.4 (27.8) |
| PROMIS score, mean (SD)e | ||
| Pain interferencef | 56.5 (8.4) | 56.7 (7.5) |
| Role satisfactiong | 48.2 (10.0) | 48.8 (10.5) |
| Mobilityg | 33.3 (5.3) | 33.8 (5.4) |
Abbreviation: PROMIS, Patient-Reported Outcomes Measurement Information System.
Data are expressed as No. (%) unless otherwise indicated. Two participants were missing data for myocardial infarction, 1 was missing data for heart failure, 1 was missing data for the Short Form 36 physical component score, and 2 were missing data for the Walking Impairment Questionnaire distance score at baseline.
This comparison excluded 34 participants who were eligible based on prior revascularization or other criteria and who had a baseline ankle brachial index greater than 0.90.
Calculated as weight in kilograms divided by height in meters squared.
Range: 0 to 100; a score of 100 indicates the best possible score.
Based on item response theory; therefore, these subcomponents have no defined minimum or maximum value. The lowest and highest scores observed have been 20 and 80, respectively.
A higher score indicates a worse outcome.
A higher score indicates the best outcome.
Intervention Adherence and Rates of Walking for Exercise
Of the 99 participants randomized to the exercise intervention group, 92 (93%) attended all 4 onsite visits. The completion rate was 73.9% (1295 of 1753 participants) for scheduled intervention telephone calls. After excluding participants who died or dropped out, the completion rate was 79.2% (1204 of 1521 participants) for scheduled intervention telephone calls. The follow-up rates were 89% (178 of 200 participants) at 4½ months and 91% (182 of 200 participants) at 9 months.
Thirteen participants (including 1 death) (6.5%) had no accelerometer data at baseline or 4½-month follow-up. An additional participant who died before 9-month follow-up had no accelerometer data at baseline or at 9-month follow-up. Of the remainder, data were imputed for at least 1 visit for 91 of 187 participants (49%) contributing to the 4½-month change in accelerometer physical activity outcome and for 93 of 186 participants (50%) contributing to the 9-month change in accelerometer physical activity outcome.
At 3-month follow-up, participants in the exercise intervention group reported greater increases in walking exercise frequency (from 1.7 episodes/week at baseline to 3.7 episodes/week; within-group difference, 2.0 episodes/week) vs participants in the usual care group (from 1.5 episodes/week at baseline to 2.2 episodes/week; within-group difference, 0.7 episodes/week) (between-group difference for the mean change from baseline to follow-up, 1.3 episodes/week [95% CI, 0.4 to 2.3 episodes/week], P = .005) and at 6-month follow-up (from 1.7 to 4.3 episodes/week [within-group difference, 2.8 episodes/week] vs from 1.5 to 2.4 episodes/week [within-group difference, 0.9 episodes/week], respectively; between-group difference for the mean change from baseline to follow-up, 1.9 episodes/week [95% CI, 0 to 3.8 episodes/week], P = .045).
At 9-month follow-up, there was no statistically significant difference in reported walking exercise frequency for the exercise intervention group (from 1.7 episodes/week at baseline to 3.5 episodes/week; within-group difference, 1.9 episodes/week) vs the usual care group (from 1.5 episodes/week at baseline to 2.3 episodes/week; within-group difference, 0.8 episodes/week) (between-group difference, 1.1 episodes/week [95% CI, −0.2 to 2.4 episodes/week], P = .09) (Table 2).
Table 2. Changes in Number of Blocks Walked and Walking Exercise Frequency During Past Week.
| Home-Based Exercise, Mean (SD) (n = 97) | Usual Care, Mean (SD) (n = 101) | Between-Group Difference for Mean Change From Baseline (95% CI) | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Within-Group Differencea | Baseline | Follow-up | Within-Group Differencea | |||
| Distance Walked During Past Week, No. of Blocks | ||||||||
| 3-mo follow-up | 17.3 (26.3) | 28.8 (51.3) | 11.7 (52.3) | 17.5 (30.8) | 22.4 (39.1) | 4.9 (29.6) | 6.7 (−6.3 to 19.7) | .31 |
| 6-mo follow-up | 17.3 (26.3) | 45.6 (103.6) | 28.0 (100.0) | 17.5 (30.8) | 21.0 (45.3) | 3.6 (33.3) | 24.4 (−1.2 to 49.9) | .06 |
| 9-mo follow-up | 17.3 (26.3) | 28.7 (53.7) | 11.4 (51.1) | 17.5 (30.8) | 24.2 (37.8) | 6.7 (36.9) | 4.6 (−9.2 to 18.4) | .51 |
| Walking Exercise Frequency During Past Week, No. of Episodes | ||||||||
| 3-mo follow-up | 1.7 (2.9) | 3.7 (2.5) | 2.0 (3.7) | 1.5 (2.1) | 2.2 (3.0) | 0.7 (2.5) | 1.3 (0.4 to 2.3) | .005 |
| 6-mo follow-up | 1.7 (2.9) | 4.3 (6.8) | 2.8 (7.3) | 1.5 (2.1) | 2.4 (3.5) | 0.9 (3.3) | 1.9 (0 to 3.8) | .045 |
| 9-mo follow-up | 1.7 (2.9) | 3.5 (4.2) | 1.9 (5.0) | 1.5 (2.1) | 2.3 (2.9) | 0.8 (2.9) | 1.1 (−0.2 to 2.4) | .09 |
Indicates mean change between baseline and follow-up.
Among participants in the exercise intervention group with valid data from a wearable device throughout the intervention period, there was no significant change in mean steps per day (3945 steps/d [SD, 2689 steps/d] for days 1-60; 3744 steps/d [SD, 2698 steps/d] for days 66-126; and 3551 steps/d [SD, 2933 steps/d] for days 192-252). Between baseline and 4½ months, the mean change was −200 steps/d (95% CI, −594 to 194 steps/d; P = .31) and between baseline and 9 months was −394 steps/d (95% CI, −972 to 185 steps/d; P = .18).
Primary Outcome
At 9-month follow-up, there was no significant difference for the mean change in 6-minute walk distance for the exercise intervention group (from 330.5 m [SD, 100.2 m] at baseline to 333.4 m [SD, 115.1 m]; within-group difference, 5.5 m) vs the usual care group (from 336.2 m [SD, 96.6 m] at baseline to 348.2 m [SD, 98.1 m]; within-group difference, 14.4 m) (between-group difference for the mean change from baseline to follow-up, −8.9 m [95% CI, −26.0 to 8.2 m], P = .31; Table 3).
Table 3. Effects of Home-Based Walking Exercise Intervention on Primary and Secondary Outcomesa.
| Home-Based Exercise (n = 97) | Usual Care (n = 101) | Between-Group Difference for Mean Change From Baseline (95% CI) |
P Value | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline, Mean (SD) |
9-mo Follow-up, Mean (SD) |
Within-Group Difference (95% CI)b |
Baseline, Mean (SD) |
9-mo Follow-up, Mean (SD) |
Within-Group Difference (95% CI)b |
|||
| 6-minute walk distance, m | 330.5 (100.2) | 333.4 (115.1) | 5.5 (−8.7 to 19.7) | 336.2 (96.6) | 348.2 (98.1) | 14.4 (0.5 to 28.3) | −8.9 (−26.0 to 8.2) | .31 |
| WIQ scorec | ||||||||
| Distance | 38.0 (29.4) | 52.2 (33.5) | 10.6 (3.3 to 17.9) | 37.7 (27.1) | 46.1 (30.0) | 4.8 (−2.4 to 12.0) | 5.8 (−3.0 to 14.7) | .20 |
| Speed | 36.7 (19.3) | 41.3 (26.4) | 4.1 (−1.1 to 9.2) | 40.0 (24.4) | 43.4 (24.4) | 2.8 (−2.3 to 7.9) | 1.3 (−5.0 to 7.5) | .69 |
| Stair climbing | 49.2 (27.1) | 52.2 (32.4) | 2.5 (−3.7 to 8.7) | 47.4 (27.6) | 50.5 (29.4) | 2.6 (−3.4 to 8.7) | −0.2 (−7.6 to 7.3) | .97 |
| SF-36 physical functioning scorec | 35.8 (9.4) | 36.5 (10.8) | 0.4 (−1.5 to 2.4) | 36.9 (9.0) | 39.0 (9.6) | 1.8 (0 to 3.7) | −1.4 (−3.7 to 1.0) | .24 |
| PROMIS scored | ||||||||
| Pain interferencee | 56.4 (8.4) | 56.6 (9.0) | 0.7 (−1.1 to 2.6) | 56.7 (7.5) | 53.4 (8.8) | −2.8 (−4.6 to −1.0) | 3.5 (1.3 to 5.8) | .002 |
| Role satisfactionf | 48.5 (9.9) | 48.2 (9.7) | −0.1 (−2.4 to 2.1) | 48.8 (10.4) | 50.4 (10.3) | 1.7 (−0.6 to 4.0) | −1.8 (−4.6 to 1.0) | .20 |
| Mobilityf | 33.3 (5.3) | 33.0 (6.3) | −0.3 (−1.3 to 0.7) | 33.8 (5.3) | 33.1 (5.9) | −0.7 (−1.7 to 0.3) | 0.4 (−0.8 to 1.7) | .47 |
| Accelerometer | ||||||||
| No. of participants | 91 | 91 | 95 | 95 | ||||
| Physical activity outcome | 112 445 (67 129) | 111 767 (72 263) | −494 (−13 110 to 12 123) | 115 069 (71 988) | 112 669 (70 318) | −2427 (−13 439 to 8585) | 1934 (−14 872 to 18 740) | .82 |
Abbreviations: PROMIS, Patient-Reported Outcomes Measurement Information System; SF-36, Medical Outcomes Study Short Form 36; WIQ, Walking Impairment Questionnaire.
Results exclude 2 participants who died prior to completing 9-month follow-up. Missing data were imputed; however, data for the 2 participants who died before completing 9-month follow-up were not included in the imputed analyses. The data were imputed as follows: 6-minute walk distance: 21 participants (10.6%); WIQ distance score: 21 participants (10.6%); WIQ speed score: 17 (8.6%); WIQ stair climbing score: 17 (8.6%); SF-36 physical functioning score: 19 (9.6%); PROMIS pain interference score: 17 (8.6%); PROMIS role satisfaction score: 17 (8.6%); PROMIS mobility score: 17 (8.6%); accelerometer measured physical activity: 93 (50%).
Indicates mean change between baseline and 9-month follow-up.
Range from 0 to 100; a score of 100 indicates the best possible score.
Based on item response theory; therefore, these subcomponents have no defined minimum or maximum value. The lowest and highest scores observed for PROMIS have been 20 and 80, respectively.
A higher score indicates a worse outcome.
A higher score indicates the best outcome.
Secondary Outcomes
At 9-month follow-up, there was no difference in change in the mean score for WIQ distance in the exercise intervention group (from 38.0 [SD, 29.4] at baseline to 52.2 [SD, 33.5]; within-group difference, 10.6) compared with the usual care group (from 37.7 [SD, 27.1] at baseline to 46.1 [SD, 30.0]; within-group difference, 4.8) (between-group difference for the mean change from baseline to follow-up, 5.8 [95% CI, −3.0 to 14.7], P = .20; Table 3).
At 9-month follow-up, the mean score for PROMIS pain interference changed in the exercise intervention group from 56.4 (SD, 8.4) at baseline to 56.6 (SD, 9.0) (within-group difference, 0.7) vs from 56.7 (SD, 7.5) at baseline to 53.4 (SD, 8.8) (within-group difference, −2.8) in the usual care group (between-group difference for the mean change from baseline to follow-up, 3.5 [95% CI, 1.3 to 5.8], P = .002), indicating a more favorable change in the pain interference score in the usual care group. There were no other statistically significant differences for the secondary outcomes between the exercise intervention group and the usual care group (Table 3).
Exploratory Analyses
At 4½-month follow-up, the mean 6-minute walk distance in the exercise intervention group changed from 330.0 m (SD, 99.8 m) at baseline to 339.0 m (SD, 113.7 m) (within-group difference, 9.8 m) vs from 336.2 m (SD, 96.6 m) at baseline to 335.7 m (SD, 104.2 m) (within-group difference, 0 m; 95% CI, −12.6 to 12.7 m) in the usual care group (between-group difference for the mean change from baseline to follow-up, 9.8 m [95% CI, −6.2 to 25.7 m], P = .23). There were no statistically significant between-group differences in the mean change from baseline to follow-up between the exercise intervention and usual care groups for any other outcomes at 4½-month follow-up (eTable 1 in Supplement 2).
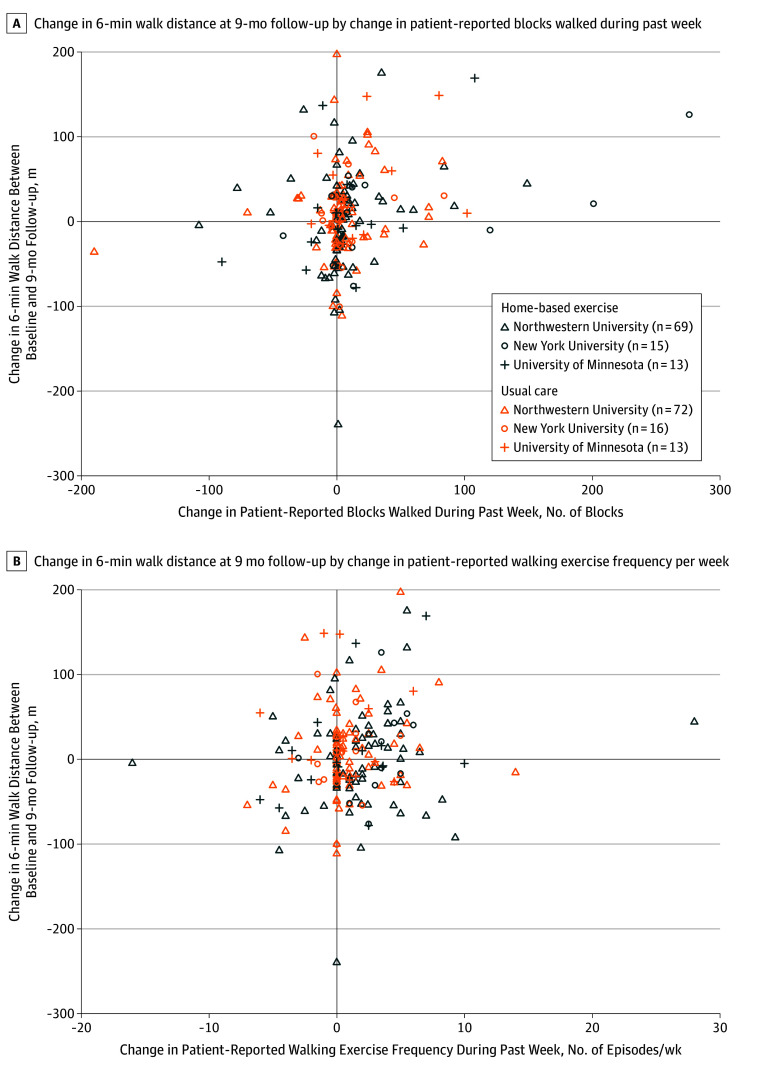
There were no statistically significant interactions in the post hoc exploratory analyses for response to the exercise intervention according to age, sex, race, severity of PAD, baseline performance in 6-minute walk distance, study site, diabetes, current smoking, or intermittent claudication symptoms (Figure 2). Changes in the numbers of blocks walked during the past week and patient-reported walking exercise frequency per week in the post hoc exploratory analyses were plotted by change in 6-minute walk distance (Figure 3). There was a weak positive correlation between change in 6-minute walk distance at 9-month follow-up and change in patient-reported blocks walked during the past week (Figure 3A) and change in patient-reported walking exercise frequency per week (Figure 3B).
Figure 2. Change in Response to the Home-Based Walking Exercise Intervention by Participant Characteristics.
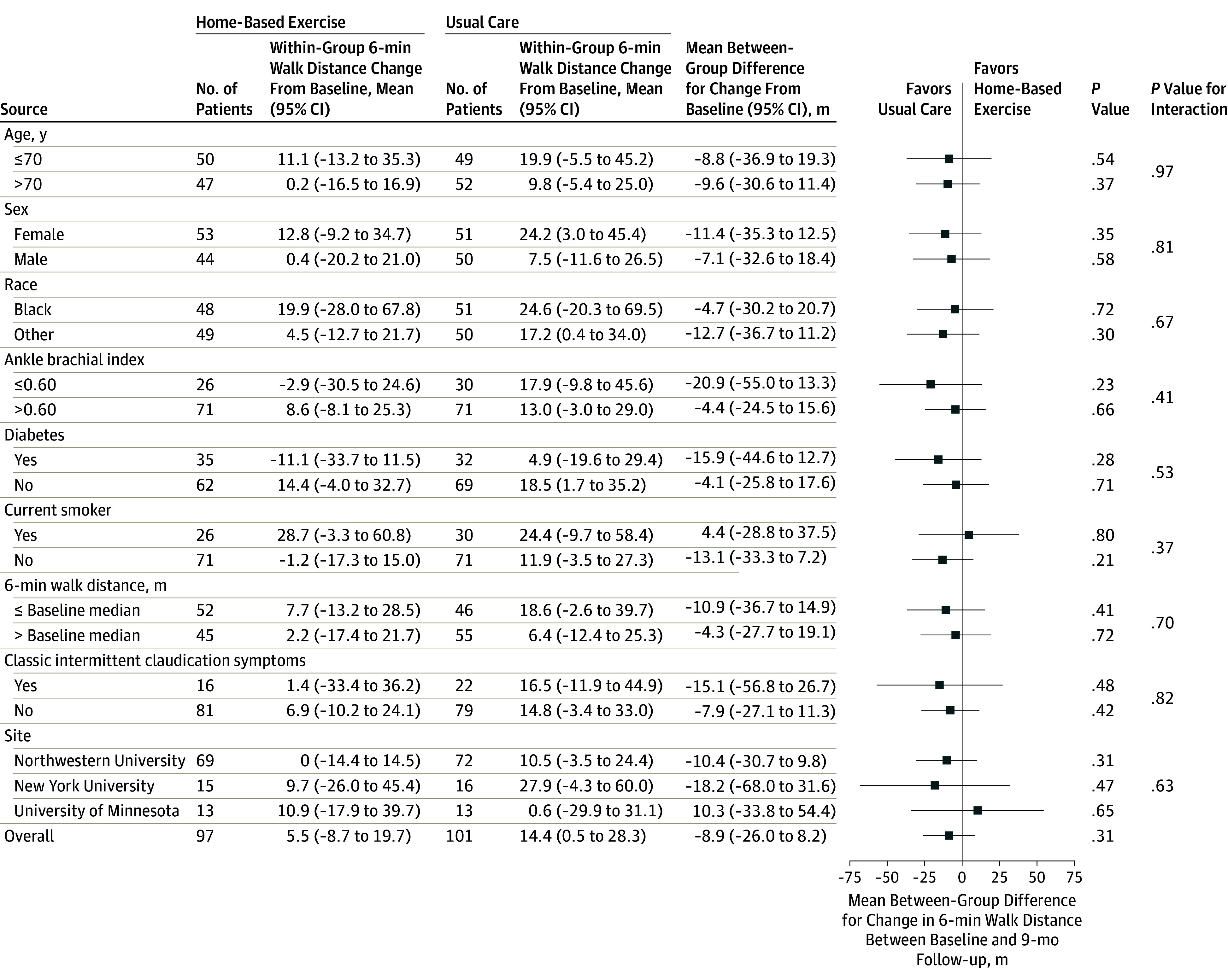
Figure 3. Change in 6-Minute Walk Distance Between Baseline and 9-Month Follow-up According to Patient-Reported Change in Physical Activity and Frequency of Walking Exercise.
Adverse Events
Forty-three participants (45%) in the exercise intervention group vs 51 (51%) in the usual care group reported more difficulty than usual with exercise or activity during the trial and 45 (47%) vs 48 (48%), respectively, reported new or increasing shortness of breath during activity. Fifty-five serious adverse events occurred among 23 participants in the exercise intervention group and 23 events occurred among 15 participants in the usual care group (eTable 2 in Supplement 2).
Two deaths occurred and both were participants in the exercise intervention group. One participant died due to critical limb ischemia complicated by sepsis and the other due to metastatic cancer. Neither was considered related to study participation. Adverse event rates are reported in eTable 3 in Supplement 2.
Discussion
Among patients with PAD, a home-based exercise intervention of telephone coaching combined with a wearable activity monitor did not improve 6-minute walk distance at 9-month follow-up compared with usual care. The usual care group reported greater improvement in PROMIS-measured pain interference score for daily activities compared with the exercise intervention group at 9-month follow-up. There were no significant differences in other secondary outcomes, including the PROMIS mobility score, the PROMIS satisfaction with social roles score, WIQ scores, quality of life, or objectively measured physical activity levels.
Clinical practice guidelines recommend supervised treadmill exercise for patients with PAD, and clinical trials have shown supervised treadmill exercise significantly improves treadmill walking performance and 6-minute walk distance.1,3,4,5 However, supervised treadmill exercise requires frequent medical center visits and such attendance is difficult for many patients with PAD.6 Three randomized trials demonstrated that interventions promoting walking exercise at home, which included scheduled medical center visits every 1 to 4 weeks throughout the intervention, significantly improved the 6-minute walk distance by approximately 45 to 53 m, which is consistent with a large meaningful change.7,8,9
In comparison, supervised treadmill exercise interventions improved the 6-minute walk distance by only 15 to 33 m.3,4,8 Home-based exercise interventions are convenient and accessible, and because of their large effect on 6-minute walk distance, these types of interventions are a potentially important treatment for patients with PAD. To our knowledge, no prior randomized trials have tested a behavioral intervention of home-based exercise among patients with PAD in which most of the contact with the coach occurred during telephone calls and was enhanced by a wearable activity monitor without periodic visits to the medical center throughout the intervention.
There are several potential explanations for the lack of benefit from the exercise intervention in this trial. First, for behavioral interventions, remote coaching is less potent than in-person visits.35 Second, based on feedback from patients, this trial used a wearable activity monitor. However, the wearable monitor measured activity throughout the day, whereas the intervention was designed to increase discrete episodes of walking exercise each day. This mismatch between the wearable device measurement and the exercise recommendations may have encouraged participants to increase overall activity level (which has not been shown to improve walking endurance among patients with PAD) rather than increase walking exercise (which has been shown to improve walking endurance among patients with PAD).3,4,7,8,9
Third, the monthly counseling via telephone calls during the final 4½ months of the intervention may have been too infrequent. The frequency of walking exercise was significantly greater among participants in the exercise intervention group than in the usual care group at 3- and 6-month follow-up; however, there was no significant difference at 9-month follow-up. Fourth, patients with PAD who are interested in home-based exercise without medical center visits throughout the intervention may be less committed than patients who agree to participate in supervised exercise.
Additional characteristics of this trial should be noted. First, the findings are consistent with a recent study of 431 healthy people in which a wearable physical activity monitor did not increase physical activity levels.36 In the current study, the exercise intervention did not increase the frequency or amount of exercise at 9-month follow-up. These results are important given the increasing number of individuals using wearable devices to improve health.36
Second, the observed improvement in PROMIS-measured pain interference among participants in the usual care group compared with those in the exercise intervention group may reflect greater ischemic leg symptoms during attempts to exercise in the exercise intervention group. Observational studies suggested that some patients with PAD limit their activity to avoid ischemic leg symptoms, which may have reduced PROMIS-measured pain interference with daily activities in the usual care group.10,11,37
Third, the increases in the frequency of walking exercise per day reported by participants in the exercise intervention group were not consistent with the activity data recorded by the wearable device or the objective physical activity monitor, suggesting that participants in the exercise intervention group may have perceived they were exercising more than their actual behavior. Fourth, consistent with prior observational studies and randomized trials,3,4,9,11,37 most participants with PAD in this trial did not have classic claudication symptoms. The results did not differ according to the presence vs absence of intermittent claudication symptoms.
Fifth, the results reported herein should not be construed as evidence that home-based exercise is ineffective for patients with PAD. Behavioral interventions in randomized clinical trials that included periodic visits to a medical center throughout the intervention achieved large meaningful improvements in the 6-minute walk distance among patients with PAD.7,8,9 Furthermore, these prior home-based exercise interventions achieved greater improvement in the 6-minute walk distance than supervised exercise interventions.3,4,9
Sixth, in previous randomized trials, 6-minute walk distance declined in the control group.3,4,9 In this trial, the usual care group reported increasing their physical activity and both walking exercise frequency and 6-minute walk distance did not decline. Patients randomized to usual care may have decided to increase exercise on their own because they were not receiving any active intervention such as in an attention-control intervention.
Limitations
This study has several limitations. First, the results may not be generalizable to participants who did not meet eligibility criteria or who were not interested in increasing their exercise activity level. Second, data on location of PAD (aorto-iliac vs superficial femoral) were not collected.
Third, there were multiple secondary outcome measures and no adjustment for multiple comparisons. Fourth, the absence of immediate coach review and feedback of uploaded activity and exercise data may have resulted in an insufficiently potent exercise intervention. Fifth, participants in the exercise intervention group adhered to 79% of scheduled intervention telephone calls with a coach, which may have been insufficient.
Sixth, the minimal clinically important difference for the 6-minute walk distance has not been defined specifically for patients with PAD. Seventh, the telephone calls every 3 months used to measure walking exercise behavior in the usual care group may have been too infrequent to measure uptake of exercise activity. Eighth, there were substantial data missing for the objective measure of physical activity.
Conclusions
Among patients with PAD, a home-based exercise intervention consisting of a wearable activity monitor and telephone coaching, compared with usual care, did not improve walking performance at 9-month follow-up. These results do not support home-based exercise interventions of wearable devices and telephone counseling without periodic onsite visits to improve walking performance in patients with PAD.
Trial protocol
eTable 1. Change in study outcomes at 4.5 month follow-up by group
eTable 2. Serious adverse events by group in the HONOR trial
eTable 3. Adverse event rates by group in the HONOR trial
References
- 1.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in Circulation. 2017;135(12):e791-e792]. Circulation. 2017;135(12):e726-e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDermott MM, Kibbe MR. Improving lower extremity functioning in peripheral artery disease: exercise, endovascular revascularization, or both? JAMA. 2017;317(7):689-690. [DOI] [PubMed] [Google Scholar]
- 3.McDermott MM, Ferrucci L, Tian L, et al. Effect of granulocyte-macrophage colony-stimulating factor with or without supervised exercise on walking performance in patients with peripheral artery disease: the PROPEL randomized clinical trial. JAMA. 2017;318(21):2089-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301(2):165-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conte MS, Pomposelli FB, Clair DG, et al. ; Society for Vascular Surgery Lower Extremity Guidelines Writing Group; Society for Vascular Surgery . Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg. 2015;61(3)(suppl):2S-41S. [DOI] [PubMed] [Google Scholar]
- 6.Harwood AE, Smith GE, Cayton T, Broadbent E, Chetter IC. A systematic review of the uptake and adherence rates to supervised exercise programs in patients with intermittent claudication. Ann Vasc Surg. 2016;34:280-289. [DOI] [PubMed] [Google Scholar]
- 7.Gardner AW, Parker DE, Montgomery PS, Scott KJ, Blevins SM. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation. 2011;123(5):491-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner AW, Parker DE, Montgomery PS, Blevins SM. Step-monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: a randomized controlled trial. J Am Heart Assoc. 2014;3(5):e001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA. 2013;310(1):57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDermott MM. Lower extremity manifestations of peripheral artery disease: the pathophysiologic and functional implications of leg ischemia. Circ Res. 2015;116(9):1540-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286(13):1599-1606. [DOI] [PubMed] [Google Scholar]
- 12.Aboyans V, Criqui MH, Abraham P, et al. ; American Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia . Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890-2909. [DOI] [PubMed] [Google Scholar]
- 13.Amirhamzeh MM, Chant HJ, Rees JL, Hands LJ, Powell RJ, Campbell WB. A comparative study of treadmill tests and heel raising exercise for peripheral arterial disease. Eur J Vasc Endovasc Surg. 1997;13(3):301-305. [DOI] [PubMed] [Google Scholar]
- 14.Heun R, Papassotiropoulos A, Jennssen F. The validity of psychometric instruments for detection of dementia in the elderly general population. Int J Geriatr Psychiatry. 1998;13(6):368-380. [DOI] [PubMed] [Google Scholar]
- 15.McDermott MM, Criqui MH, Liu K, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32(6):1164-1171. [DOI] [PubMed] [Google Scholar]
- 16.Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1(1):65-71. [DOI] [PubMed] [Google Scholar]
- 17.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31(2):143-164. [DOI] [PubMed] [Google Scholar]
- 18.Baumeister RF, Heatherton TF, Tice DM. Losing Control: How and Why People Fail at Self-Regulation. San Diego, CA: Academic Press; 1994. [Google Scholar]
- 19.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-381. [PubMed] [Google Scholar]
- 20.Kooiman TJM, Dontje ML, Sprenger SR, Krijnen WP, van der Schans CP, de Groot M. Reliability and validity of ten consumer activity trackers. BMC Sports Sci Med Rehabil. 2015;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regensteiner JG, Steiner JF, Panzer RJ, et al. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol. 1990;2:142-152. [Google Scholar]
- 22.Ware JE, Snow KK, Kosinski M, et al. SF-36 Health Survey: Manual and Interpretation Guide. Boston, MA: Health Institute at New England Medical Center; 1993. [Google Scholar]
- 23.Cella D, Riley W, Stone A, et al. ; PROMIS Cooperative Group . The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CX, Kroenke K, Stump TE, et al. Estimating minimally important differences for the PROMIS pain interference scales: results from 3 randomized clinical trials. Pain. 2018;159(4):775-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahn EA, Beaumont JL, Pilkonis PA, et al. The PROMIS satisfaction with social participation measures demonstrated responsiveness in diverse clinical populations. J Clin Epidemiol. 2016;73:135-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahn EA, DeWalt DA, Bode RK, et al. ; PROMIS Cooperative Group . New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychol. 2014;33(5):490-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hays RD, Spritzer KL, Fries JF, Krishnan E. Responsiveness and minimally important difference for the Patient-Reported Outcomes Measurement Information System (PROMIS) 20-item physical functioning short form in a prospective observational study of rheumatoid arthritis. Ann Rheum Dis. 2015;74(1):104-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schalet BD, Hays RD, Jensen SE, Beaumont JL, Fries JF, Cella D. Validity of PROMIS physical function measured in diverse clinical samples. J Clin Epidemiol. 2016;73:112-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Askew RL, Cook KF, Revicki DA, Cella D, Amtmann D. Evidence from diverse clinical populations supported clinical validity of PROMIS pain interference and pain behavior. J Clin Epidemiol. 2016;73:103-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly LA, McMillan DG, Anderson A, Fippinger M, Fillerup G, Rider J. Validity of ActiGraphs uniaxial and triaxial accelerometers for assessment of physical activity in adults in laboratory conditions. BMC Med Phys. 2013;13(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDermott MM, Guralnik JM, Criqui MH, Liu K, Kibbe MR, Ferrucci L. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation. 2014;130(1):61-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743-749. [DOI] [PubMed] [Google Scholar]
- 33.Ward MM, Guthrie LC, Alba MI. Clinically important changes in short form 36 health survey scales for use in rheumatoid arthritis clinical trials: the impact of low responsiveness. Arthritis Care Res (Hoboken). 2014;66(12):1783-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDermott MM, Guralnik JM, Criqui MH, et al. Home-based walking exercise in peripheral artery disease: 12-month follow-up of the GOALS randomized trial. J Am Heart Assoc. 2014;3(3):e000711. doi: 10.1161/JAHA.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jakicic JM, Davis KK, Rogers RJ, et al. Effect of wearable technology combined with a lifestyle intervention on long-term weight loss: the IDEA randomized clinical trial. JAMA. 2016;316(11):1161-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thosar SS, Niederhausen M, Lapidus J, et al. Self-regulated use of a wearable activity sensor is not associated with improvements in physical activity, cardiometabolic risk or subjective health status. Br J Sports Med. 2017;0:1-2. [DOI] [PubMed] [Google Scholar]
- 37.McDermott MM, Guralnik JM, Ferrucci L, et al. Asymptomatic peripheral arterial disease is associated with more adverse lower extremity characteristics than intermittent claudication. Circulation. 2008;117(19):2484-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable 1. Change in study outcomes at 4.5 month follow-up by group
eTable 2. Serious adverse events by group in the HONOR trial
eTable 3. Adverse event rates by group in the HONOR trial