Abstract
Background:
Several per- and polyfluoroalkyl substances (PFAS) are ubiquitous anthropogenic pollutants almost universally detected in humans. Experimental evidence indicates that PFAS alter glucose metabolism and insulin secretion. However, epidemiological studies have yielded inconsistent results.
Objective:
We sought to examine associations between plasma PFAS concentrations, glycemic indicators, and diabetes incidence among high-risk adults.
Methods:
Within the Diabetes Prevention Program (DPP), a trial for the prevention of type 2 diabetes among high-risk individuals, we quantified baseline plasma concentrations of nine PFAS among 957 participants randomized to a lifestyle intervention or placebo. We evaluated adjusted associations for plasma PFAS concentrations with diabetes incidence and key glycemic indicators measured at baseline and annually over up to 4.6 y.
Results:
Plasma PFAS concentrations were similar to those reported in the U.S. population in 1999–2000. At baseline, in cross-sectional analysis, a doubling in plasma perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) concentrations was associated with higher homeostatic model assessment of insulin resistance (HOMA-IR) [; 95% confidence interval (CI): 0.13, 0.66; ; 95% CI: 0.34, 0.94], function () (; 95% CI: 1.55, 17.70; ; 95% CI: 6.78, 25.08), fasting proinsulin (; 95% CI: 0.50, 2.25; ; 95% CI: 0.72, 2.71), and glycated hemoglobin () (; 95% CI: 0.002, 0.07; ; 95% CI: 0.001, 0.07). There was no strong evidence of associations between plasma PFAS concentrations and diabetes incidence or prospective changes in glycemic indicators during the follow-up period.
Conclusions:
At baseline, several PFAS were cross-sectionally associated with small differences in markers of insulin secretion and function. However, there was limited evidence suggesting that PFAS concentrations are associated with diabetes incidence or changes in glycemic indicators during the follow-up period. https://doi.org/10.1289/EHP1612
Introduction
Per- and polyfluoroalkyl substances (PFAS) are a group of fluorinated synthetic chemicals used in consumer products and industrial applications for their stain, grease, and water-repellent properties. In the 1950s, widescale production and use of PFAS started in many industrialized countries with a total estimated production of 3,200 to by 2006 (Prevedouros et al. 2006). Given their widespread use and chemical stability, several PFAS are ubiquitous and persistent in the environment, even found in remote areas including the Arctic (Butt et al. 2010). PFAS also have long elimination half-lives in humans, estimated to range from 3.5 to 8.5 y, depending on the compound (Olsen et al. 2007). Human exposure to PFAS occurs mainly through the consumption of contaminated food and drinking water, or exposure to dust and commercial products such as food packaging containing PFAS (D’eon and Mabury 2011; Fraser et al. 2011). Biomonitoring results from the National Health and Nutrition Examination Surveys (NHANES) indicate that nearly the entire U.S. general population () has detectable serum concentrations of several PFAS, including perfluorooctanesulfonic acid (PFOS), perfluorooctanoic acid (PFOA), perfluorohexane sulfonic acid (PFHxS), and perfluorononanoic acid (PFNA) (CDC 2017).
By 2002, the leading manufacturer of PFOS and perfluorooctanesulfonyl fluoride–related materials, the 3M corporation, had voluntarily phased out production of its perfluorooctanyl chemistry, which was used to produce PFOS and related compounds (Butenhoff et al. 2006). In 2006, eight other PFAS manufacturers joined the U.S. Environmental Protection Agency PFOA stewardship program to reduce production and emission of PFOA and its precursors by 2015 (U.S. EPA 2015). However, the production of PFOS, PFOA, and other PFAS precursors continues to increase in China and continental Asia (Jin et al. 2007; Land et al. 2015; Xie et al. 2013).
Health concerns related to PFAS exposure include endocrine disruption, altered lipid metabolism, and immunotoxicity (Khalil et al. 2015). In addition, several epidemiological studies have investigated associations of PFAS exposure with diabetes and markers of insulin secretion and insulin resistance (Conway et al. 2016; Lind et al. 2014; MacNeil et al. 2009; Steenland et al. 2010; Su et al. 2016). However, these studies have yielded inconsistent results, with some studies reporting positive associations, no associations, or even protective associations of some PFAS. These studies are limited due to their cross-sectional nature, quantification of a few PFAS, relatively small sample sizes, and limited outcomes measured. Given that cross-sectional studies have important limitations, such as the potential for reverse causation whereby altered metabolic health may affect PFAS elimination, there is the need for prospective epidemiological studies (Weisskopf and Webster 2017).
In the present study, we examined the association of plasma PFAS concentrations with diabetes incidence within the Diabetes Prevention Program (DPP) study, an adult population at high risk of type 2 diabetes. Furthermore, we evaluated the association of PFAS concentrations quantified at baseline with key baseline metabolic and glycemic measurements, such as homeostatic model assessments for insulin resistance (HOMA-IR), function (), fasting proinsulin, corrected insulin response, insulinogenic index, glycated hemoglobin (), and adiponectin, as well as glucose and insulin levels following oral glucose tolerance testing (OGTT). We also examined whether baseline PFAS concentrations were associated with longitudinal changes among these glycemic measurements over 2 to 5 y of follow-up. We hypothesized that higher plasma PFAS concentrations at baseline would be associated with diabetes incidence, as well as higher measures of insulin resistance at the same time point. Furthermore, we hypothesized that higher plasma PFAS concentrations at baseline would be associated with an increase in insulin resistance markers over the follow-up study period. Lastly, we evaluated effect modification by treatment assignment for diabetes incidence and changes in glycemic indicators during follow-up.
Methods
Study Population
Study subjects were participants in the DPP, a multicenter randomized clinical trial of individuals at high risk of developing type 2 diabetes, recruited between July of 1996 and May 1999. Inclusion criteria included at least 25 y of age, a body mass index (BMI) of or greater (22 or higher for Asians), and a fasting glucose concentration of 95 to and 140 to 2 h after a oral glucose load. The DPP trial recruited participants from 27 clinical centers across the United States and randomly assigned them to three study arms: a lifestyle intervention group receiving intensive training in diet, physical activity, and behavior modification; a pharmacological intervention group receiving of metformin (Glucophage); or a placebo-treated control group (Knowler et al. 2002). Participants assigned to the placebo and metformin arms of the trial received standard information about diet and exercise, but no intensive or motivational counseling. The original aims and design of the study have been extensively described elsewhere (Diabetes Prevention Program Research Group 1999). The study was terminated in May of 2001, 1 y early, due to efficacy on type 2 diabetes prevention of both the lifestyle intervention and pharmacological intervention arms of the trial.
The current study was restricted to individuals in the intensive lifestyle intervention and the placebo-treated control arm of the trial with available stored plasma samples collected at baseline. We did not include the metformin-treated group due to the potential interaction between PFAS and the pharmacological intervention on health outcomes and lack of experimental data on this issue. Furthermore, metformin’s influence on PFAS kinetics is unknown. A total of 957 participants were eligible for quantification of plasma PFAS. The institutional review board at each clinical center approved the protocol, and all participants provided written informed consent for DPP. For the current analyses, the Institutional Review Board of Harvard Pilgrim Health Care reviewed and approved all study protocols. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory did not constitute engagement in human subjects research.
Per- and Polyfluoroalkyl Substances Plasma Concentrations
Blood was collected at baseline and plasma was stored at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) central repository. Plasma samples were shipped from the NIDDK central repository to the CDC for analyses. Briefly, a modification of the online solid-phase extraction–high-performance liquid chromatography coupled to isotope dilution–tandem mass spectrometry approach described previously (Kato et al. 2011) was used to measure: linear PFOS (n-PFOS), sum of perfluoromethylheptane sulfonic acid isomers (Sm-PFOS), sum of perfluorodimethylhexane sulfonic acid isomers (Sm2-PFOS); linear PFOA (n-PFOA), sum of perfluoromethylheptanoic and perfluorodimethylhexanoic acids (Sb-PFOA); PFHxS, N-ethyl-perfluorooctane sulfonamido acetic acid (Et-PFOSA-AcOH; also known as EtFOSAA), N-methyl-perfluorooctane sulfonamido acetic acid (Me-PFOSA-AcOH; also known as MeFOSAA), and perfluorononanoic acid (PFNA). The limit of detection (LOD) was for all PFAS examined.
We summed branched and linear isomers of PFOS (n-PFOS, Sm-PFOS, Sm2-PFOS) and PFOA (n-PFOA, Sb-PFOA) to calculate total concentrations of PFOS and PFOA, respectively. Summing linear and branched isomers has been implemented in previous epidemiological studies as well as in the U.S. national report on human exposure to environmental chemicals, allowing for comparability with past and future studies (CDC 2017; Fleisch et al. 2017; Harris et al. 2017). The sum of isomers for PFOS and PFOA was performed prior to accounting for concentrations below the LOD. At least one of the isomers was always detectable for any given sample, and therefore, the sum of PFOS and PFOA isomers was 100% detectable across samples. All other PFAS concentrations below the LOD were replaced by the (Hornung and Reed 1990).
Outcome Measures
The primary outcome of the trial, as well as for the present study, was diabetes incidence by the end of the DPP follow-up period. Diabetes was prospectively diagnosed using either fasting plasma glucose levels at semiannual visits or an OGTT at the scheduled annual visits. Diabetes was defined as fasting glucose or 2-h postchallenge glucose for visits through 23 June 1997, and fasting glucose or 2-h postchallenge glucose for visits after 23 June 1997. If a participant had elevated glucose levels at the annual visit (fasting or 2-h glucose post-OGTT) or at midyear (fasting glucose only), diabetes was confirmed in a subsequent follow-up visit within 6 wk using the same method as the trigger visit (i.e., either OGTT or fasting glucose for annual and semiannual visits, respectively). A few participants had elevated glucose levels at a scheduled study visit, but diabetes was not confirmed at the subsequent follow-up visit; we referred to this event as first-fasting hyperglycemia.
The following laboratory measures related to metabolic function and glycemia were included in baseline and longitudinal analyses: fasting proinsulin (pM), fasting insulin (), 30-min post-OGTT insulin (), fasting plasma glucose (mg/dL), 30-min post-OGTT glucose (mg/dL), 2-h post-OGTT glucose (mg/dL), % glycated hemoglobin (), adiponectin (), , , , , and BMI measured at baseline. Laboratory measurements were evaluated annually from baseline, with the exception of adiponectin, which was only measured at baseline and at the first annual visit; fasting plasma glucose, which was measured semiannually; and , which was measured at baseline, 6 mo after baseline, and annually thereafter. Laboratory measures were taken prospectively at the scheduled visits until a participant received a diagnosis of diabetes or until the study was terminated in May of 2001. Analytical glycemic measurements were performed at a central laboratory (University of Washington, Seattle, WA) as previously described (Diabetes Prevention Program Research Group 2000).
Covariates
Data were obtained from the NIDDK central database repository and linked to plasma concentrations using unique identifiers provided by the NIDDK repository. We extracted demographic characteristics such as participant sex, race/ethnicity, age, BMI, education, smoking history, marital status, and treatment assignment. To protect participants’ identities, age was only available in 5-y age groups with truncation of those and y of age. We calculated BMI in for every subjects using the average of two or three weight measures reported in the initial data release and subsequently linking it to the average of two or three height measures from the NIDDK repository. We report educational attainment into , high school/GED, college graduate, and graduate school and greater. Self-reported smoking was obtained from baseline questionnaires and classified as never, former, and current smoker. Covariates were selected for adjustment a priori, and follow-up time was recorded as the number of days from randomization to the scheduled visit and transformed to years to ease interpretability.
Statistical Analyses
We calculated geometric means (GMs) and interquartile ranges for all PFAS. For two of the most prevalent PFAS, PFOS and PFOA, we report their distributions across participant characteristics. We used a nonparametric Wilcoxon rank sum test and a Kruskal-Wallis test a to evaluate unadjusted exposure differences across participants’ characteristics. We calculated Spearman correlation coefficients among PFAS plasma concentrations.
The distribution of all PFAS plasma concentrations were right skewed, and therefore, we them for statistical analyses (log base 2). We used adjusted linear regression models to estimate associations of individual baseline PFAS plasma concentrations with baseline metabolic and glycemic clinical measurements adjusting for sex, race/ethnicity, BMI (continuous), age (categorical), marital status, education, and smoking history. Additionally, we used longitudinal mixed-effects regression models with random intercepts and slopes to estimate the association of baseline plasma PFAS concentrations with prospective metabolic and glycemic clinical measurements to test if concentrations at baseline influenced glycemic or metabolic changes over the study period. Longitudinal mixed models included the fixed effects of plasma PFAS independently, sex, race/ethnicity, BMI (continuous), age (categorical), marital status, education, and smoking history as well as treatment arm and follow-up time in years. Visual inspection of scatterplots for the glycemic outcomes over the study period suggested nonlinear time trends. Therefore, we also included the fixed effects of follow-up time squared as well as the interaction between treatment and follow-up time and treatment and follow-up time squared as necessary. We report the longitudinal association for each PFAS separately with glycemic outcomes using a two-way interaction between follow-up time in years and plasma concentrations measured at baseline, using repeated measures models. A second longitudinal model tested the two-way interaction between PFAS at baseline and treatment assignment to evaluate effect modification by treatment over time. Lastly, a third longitudinal regression model tested the prospective association between baseline plasma PFAS concentrations over time by treatment using a three-way interaction of each individual PFAS, follow-up time, and treatment assignment whenever there was a significant interaction between treatment and follow-up time. Additionally, longitudinal models for HOMA-IR, , fasting insulin, fasting glucose, and included two-way interactions between follow-up time and treatment as well as follow-up time squared and treatment. The fasting proinsulin model only required an interaction between follow-up time and treatment. The corrected insulin response model did not require any interactions between follow-up time and treatment. Changes in adiponectin from baseline to the first-year follow-up were modeled using linear regression adjusted for baseline covariates. Longitudinal regression model equations used are specified in Appendix A of the Supplemental Material.
We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (95% CI) for the risk of developing diabetes relative to plasma baseline concentrations adjusted for participant’s sex, race/ethnicity, BMI (continuous), age (categorical), marital status, education, smoking history, and treatment assignment. We evaluated effect modification by treatment assignment by stratifying on treatment group as well as testing for statistical interaction of each PFAS and treatment. Kaplan-Meier survival plots were generated for PFAS observed to be significantly associated with diabetes incidence. We also conducted a secondary analysis for time to first-fasting hyperglycemia using Cox proportional hazards models for participants for which a trigger visit for elevated glucose was not confirmed during the subsequent follow-up visit (). The proportional hazards assumption was evaluated by inspecting the Schoenfeld residuals and using a global test of the proportional hazards assumption.
We evaluated effect modification between baseline PFAS plasma concentrations and treatment assignment by testing the interaction term in longitudinal models. We also considered three-way interactions between each individual PFAS, time squared, and treatment arm in longitudinal models (results not shown). Data management and analyses were performed using R (version 3.3.0; R Development Core Team).
Results
Participant Characteristics and Plasma Per- and Polyfluoroalkyl Substances at Baseline
From the 2,054 total participants randomized to the lifestyle intervention () or the placebo-control group () in the DPP, 957 (46.6%) had sufficient stored plasma for PFAS quantification and thus were included in this study. Most participants included in our study were female (65.3%), Caucasian (57.7%), obese or overweight (97.7%), with a college or graduate education (74.4%), nonsmokers (56.8%), between 40 and 64 y of age (76.5%), and who reported being married or cohabitating (67.6%). In this sample, participants were equally assigned to the intensive lifestyle intervention arm of the trial (50.3%) or the placebo-treated control group (49.7%), and a total of 204 (21.3%) developed diabetes by the end of the DPP trial period. These include 47 participants (4.9%) who had a hyperglycemic glucose level that was not confirmed during the immediate follow-up visit, although all were eventually diagnosed as diabetic and included in the time-to-diabetes analysis. DPP participants excluded from this analysis had similar incidence of diabetes (21.5%) and did not differ by sex, age, or BMI distribution. However, our sample included a lower proportion of individuals from other races (4.4%) compared to excluded DPP participants (7.3%).
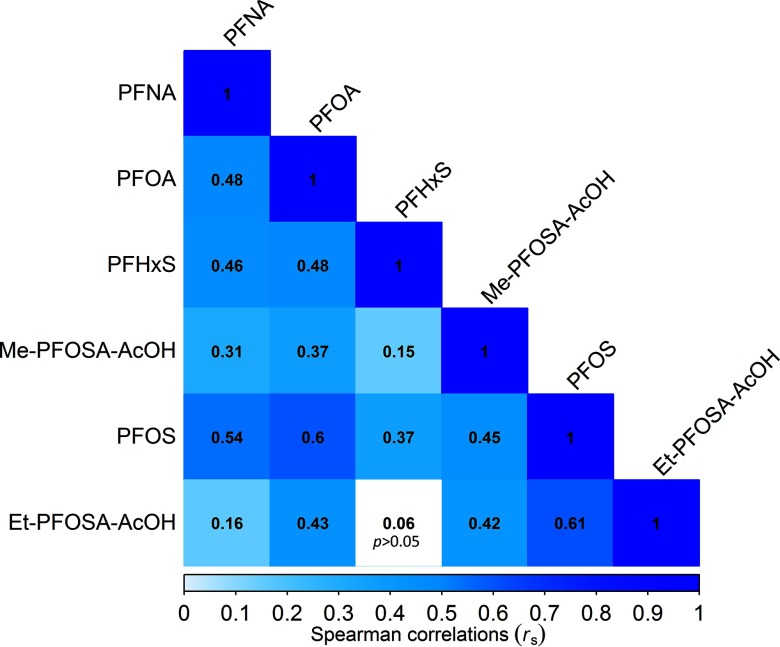
Plasma PFOS and PFOA concentrations differed by sex, race/ethnicity, and education, but were similar across BMI classification, smoking history, and treatment assignment. The distribution of PFOA but not PFOS concentration was significantly different across age groups and marital status (Table 1). At least one PFOS and PFOA isomer was detected in 100% of the samples, and both PFOS and PFOA had the highest total concentrations of all compounds measured. Among branched isomers, Sm2-PFOS was undetectable in 58.9% of the samples and Sb-PFOA in 16.6% of samples. PFHxS was below the LOD for one participant (0.1%), while Et-PFOSA-AcOH, Me-PFOSA-AcOH, and PFNA were undetectable in 3.3%, 2.6%, and 6.8% of participants, respectively (Table 2). All plasma PFAS concentrations were positively correlated (Figure 1). GMs of plasma PFAS concentrations measured at baseline in DPP (1996–1999) were similar to those measured in NHANES in 1999–2000, but higher compared to more recent serum PFAS concentrations as reported by NHANES in 2013–2014 (CDC 2017), except for PFNA concentrations that were higher compared to DPP (Table S1 and Figure S1).
Table 1.
Participant characteristics, geometric means (GMs), and interquartile ranges (IQR) for perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) in plasma (ng/mL).
| Characteristics () | (%) | PFOS GM (IQR) | PFOA GM (IQR) |
|---|---|---|---|
| Participant sex | |||
| Male | 332 (34.7%) | 28.3 (22.1) | 5.2 (3.3) |
| Female | 625 (65.3%) | 25.4 (22.4) | 4.6 (3.3) |
| Race/ethnicity | |||
| Caucasian | 552 (57.7%) | 25.7 (20.7) | 5.2 (3.2) |
| African American | 184 (19.2%) | 31.2 (28.6) | 4.6 (3.4) |
| Hispanic of any race | 179 (18.7%) | 23.6 (20.4) | 4.1 (2.7) |
| All other | 42 (4.4%) | 29.3 (25.4) | 4.5 (2.1) |
| Age (years) | |||
| 112 (11.7%) | 27.5 (22.2) | 4.7 (3.0) | |
| 40–44 | 107 (11.2%) | 24.6 (20.7) | 4.3 (3.8) |
| 45–49 | 213 (22.3%) | 26.2 (25.0) | 4.5 (2.9) |
| 50–54 | 167 (17.5%) | 28.8 (23.1) | 4.9 (3.2) |
| 55–59 | 137 (14.3%) | 28.3 (22.9) | 5.5 (3.2) |
| 60–64 | 107 (11.2%) | 23.3 (21.6) | 4.8 (2.8) |
| 114 (11.9%) | 24.8 (19.6) | 5.3 (3.6) | |
| BMI classification () | |||
| Normal (18.5–24.9) | 22 (2.3%) | 21.4 (22.6) | 4.0 (2.2) |
| Overweight (25.0–29.9) | 287 (30.0%) | 25.5 (22.2) | 4.9 (3.1) |
| Obese () | 648 (67.7%) | 27.0 (22.9) | 4.8 (3.2) |
| Education | |||
| 45 (4.7%) | 19.0 (20.4) | 3.2 (2.4) | |
| High school/GED | 200 (20.9%) | 28.6 (22.4) | 4.9 (3.1) |
| College | 469 (49.0%) | 26.8 (24.4) | 5.0 (3.1) |
| Graduate school | 243 (25.4%) | 25.5 (19.3) | 4.8 (3.1) |
| Smoking history | |||
| Nonsmoker | 544 (56.8%) | 26.3 (22.2) | 4.7 (3.5) |
| Former smoker | 356 (37.2%) | 28.9 (20.5) | 5.5 (2.9) |
| Current smoker | 57 (6.0%) | 26.1 (22.9) | 5.0 (2.9) |
| Marital status | |||
| Married/cohabitating | 647 (67.6%) | 26.5 (21.6) | 4.9 (3.0) |
| Single | 114 (11.9%) | 27.7 (22.8) | 5.1 (3.8) |
| Divorced/separated | 152 (15.9%) | 24.8 (24.6) | 4.2 (3.3) |
| Widowed | 44 (4.6%) | 29.0 (34.5) | 5.7 (4.4) |
| Treatment arm | |||
| Lifestyle intervention | 481 (50.3%) | 27.2 (23.5) | 4.9 (3.1) |
| Placebo | 476 (49.7%) | 25.6 (20.9) | 4.7 (3.1) |
Table 2.
Distribution of per- and polyfluoroalkyl substances (PFAS) plasma concentrations measured at baseline in the Diabetes Prevention Program trial.
| PFAS analyte | Chemical name | Below LODa (%) | Geometric mean (ng/mL) (IQR) |
|---|---|---|---|
| PFOS | Perfluorooctane sulfonic acid | 0 | 26.38 (22.80) |
| n-PFOS | Linear perfluorooctane sulfonic acid | 0 | 18.42 (16.90) |
| Sm-PFOS | Perfluoromethylheptane sulfonic acids | 0 | 7.32 (6.50) |
| Sm2-PFOS | Perfluorodimethylhexane sulfonic acids | 564 (58.93%) | 0.13 (0.23) |
| PFOA | Perfluorooctanoic acid | 0 | 4.82 (3.20) |
| n-PFOA | Linear perfluorooctanoic acid | 0 | 4.29 (2.90) |
| Sb-PFOA | Branched perfluorooctanoic acids | 159 (16.61%) | 0.44 (0.50) |
| PFHxS | Perfluorohexane sulfonic acid | 1 (0.10%) | 2.41 (2.40) |
| Et-PFOSA-AcOH | N-ethyl-perfluorooctane sulfonamido acetic acid | 32 (3.34%) | 1.13 (1.50) |
| Me-PFOSA-AcOH | N-methyl-perfluorooctane sulfonamido acetic acid | 29 (2.59%) | 0.94 (1.10) |
| PFNA | Perfluorononanoic acid | 65 (6.79%) | 0.53 (0.40) |
for all PFAS.
Figure 1.
Spearman correlation coefficients for plasma per- and polyfluoroalkyl substances (PFAS) concentrations measured at baseline in the Diabetes Prevention Program. Note: Et-PFOSA-AcOH, N-ethyl-perfluorooctane sulfonamido acetic acid; Me-PFOSA-AcOH, N-methyl-perfluorooctane sulfonamido acetic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid.
Baseline Cross-Sectional Associations
We observed cross-sectional associations between several PFAS and glycemia measurements (Table 3). In adjusted linear regression models, a doubling in plasma PFOS concentration was associated with higher fasting insulin (95% CI: 0.41, 2.34), higher 30-min post-OGTT insulin levels (95% CI: 0.89, 8.36), higher fasting proinsulin (95% CI: 0.50, 2.25), higher fasting glucose (95% CI: 0.03, 1.06), and 0.03% higher (95% CI: 0.002, 0.07). Consistent with the insulin and glucose results, a doubling in plasma PFOS concentration was associated with 0.39 higher HOMA-IR (95% CI: 0.13, 0.66) and 9.62 higher (95% CI: 1.55, 17.70). No associations were observed for plasma PFOS and post-OGTT glucose levels.
Table 3.
Adjusted cross-sectional associations between baseline plasma per- and polyfluoroalkyl substances (PFAS) concentrations and glycemic outcomes in the Diabetes Prevention Program trial.
| Outcome | Adjusted estimated change in the outcome per doubling in PFASs concentration (ng/mL) (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| PFOS | PFOA | PFHxS | Et-PFOSA-AcOH | Me-PFOSA-AcOH | PFNA | |||
| HOMA-IR | 956 | 0.39 | 0.64 | 0.34 | 0.25 | 0.23 | 0.20 | |
| (0.13, 0.66) | (0.34, 0.94) | (0.12, 0.55) | (0.08, 0.41) | (0.03, 0.43) | (, 0.42) | |||
| Fast insulin () | 956 | 1.37 | 2.26 | 1.17 | 0.87 | 0.80 | 0.59 | |
| (0.41, 2.34) | (1.16, 3.35) | (0.39, 1.95) | (0.26, 1.48) | (0.06, 1.54) | (, 1.38) | |||
| 30-min insulin () | 945 | 4.63 | 7.85 | 6.07 | 1.42 | 1.07 | 1.30 | |
| (0.89, 8.36) | (3.63, 12.07) | (3.09, 9.06) | (, 3.78) | (, 3.92) | (, 4.37) | |||
| Fast proinsulin (pM) | 954 | 1.37 | 1.71 | 1.31 | 0.45 | 0.41 | 0.89 | |
| (0.50, 2.25) | (0.72, 2.71) | (0.61, 2.01) | (, 1.00) | (, 1.08) | (0.18, 1.61) | |||
| 956 | 9.62 | 15.93 | 7.96 | 6.28 | 5.40 | 3.56 | ||
| (1.55, 17.70) | (6.78, 25.08) | (1.46, 14.47) | (1.21, 11.36) | (, 11.56) | (, 10.2) | |||
| Corrected insulin response | 945 | 0.03 | 0.04 | 0.03 | 0.01 | 0.001 | 0.005 | |
| (, 0.05) | (0.01, 0.07) | (0.01, 0.05) | (, 0.02) | (, 0.02) | (, 0.03) | |||
| Insulinogenic index | 945 | 0.05 | 0.08 | 0.06 | 0.01 | 0.01 | ||
| (, 0.11) | (0.01, 0.15) | (0.02, 0.11) | (, 0.05) | (, 0.04) | (, 0.06) | |||
| Fast glucose (mg/dL) | 957 | 0.55 | 0.66 | 0.29 | 0.30 | 0.19 | 0.45 | |
| (0.03, 1.06) | (0.07, 1.24) | (, 0.70) | (, 0.63) | (, 0.58) | (0.03, 0.87) | |||
| 30-min glucose (mg/dL) | 947 | 0.64 | 1.69 | 0.64 | 0.31 | 0.49 | 0.46 | |
| (, 2.35) | (, 3.63) | (0.05, 2.79) | (, 1.39) | (, 1.79) | (, 1.86) | |||
| 2-h glucose (mg/dL) | 940 | 0.02 | 0.57 | 0.58 | ||||
| (, 0.95) | (, 0.95) | (, ) | (, 1.30) | (, 1.50) | (, 0.66) | |||
| (%) | 954 | 0.03 | 0.04 | 0.02 | 0.004 | 0.01 | 0.01 | |
| (0.002, 0.07) | (0.001, 0.07) | (, 0.05) | (, 0.03) | (, 0.03) | (, 0.04) | |||
| Adiponectin () | 956 | |||||||
| (, 0.11) | (, ) | (, 0.17) | (, ) | (, ) | (, 0.05) | |||
| BMIa | 957 | 0.25 | 0.15 | 0.16 | 0.06 | 0.26 | 0.25 | |
| (, 0.70) | (, 0.65) | (, 0.52) | (, 0.33) | (, 0.60) | (, 0.61) | |||
Note: Adjusted for participant sex, race/ethnicity, BMI (body mass index; continuous), age (categorical), marital status (categorical), education (categorical), and smoking history (categorical). Et-PFOSA-AcOH, N-ethyl-perfluorooctane sulfonamido acetic acid; , glycated hemoglobin; , function; HOMA-IR, homeostatic model assessment of insulin resistance; Me-PFOSA-AcOH, N-methyl-perfluorooctane sulfonamido acetic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid; SD, standard deviation.
Not adjusted for BMI.
Similarly, a doubling in plasma PFOA concentration was associated with higher fasting proinsulin (95% CI: 0.72, 2.71), higher fasting insulin (95% CI: 1.16, 3.35), higher 30-min post-OGTT insulin (95% CI: 3.63, 12.07), higher fasting glucose (95% CI: 0.07, 1.24), 0.04 higher corrected insulin response (95% CI: 0.001, 0.07), 0.08 higher insulinogenic index (95% CI: 0.01, 0.15), 0.04% higher (95% CI: 0.001, 0.07), and lower adiponectin levels (95% CI: , ). Consistent with the insulin and glucose results, a doubling in plasma PFOA was associated with 0.64 higher HOMA-IR (95% CI: 0.34, 0.94) and 15.93 higher (95% CI: 6.78, 25.08). No associations were observed between PFOA and post-OGTT glucose levels. Results for all glycemia endpoints for PFOA and PFOS are shown in Table 3. Associations among individual isomers of PFOS (n-PFOS, Sm-PFOS, Sm2-PFOS) and PFOA (n-PFOA and Sb-PFOA) were similar (Table S2).
A doubling in plasma PFHxS concentration was associated with 0.34 higher HOMA-IR (95% CI: 0.12, 0.55) and 7.96 higher (95% CI: 1.46, 14.47). Plasma PFHxS concentrations were also positively associated with fasting insulin, 30-min post-OGTT insulin levels, fasting proinsulin, corrected insulin response, and insulinogenic index. Interestingly, PFHxS concentrations were not associated with fasting glucose, but a doubling in exposure was associated with higher 30-min post-OGTT glucose (95% CI: 0.05, 2.79) and lower 2-h post-OGTT glucose (95% CI: , ).
Both Et-PFOSA-AcOH and Me-PFOSA-AcOH were associated with higher HOMA-IR and fasting insulin and lower adiponectin levels. Only Et-PFOSA-AcOH was associated with higher . Plasma PFNA concentrations were associated with higher fasting proinsulin and fasting glucose levels.
Baseline Per- and Polyfluoroalkyl Substances Concentrations and Longitudinal Glycemic Measurements
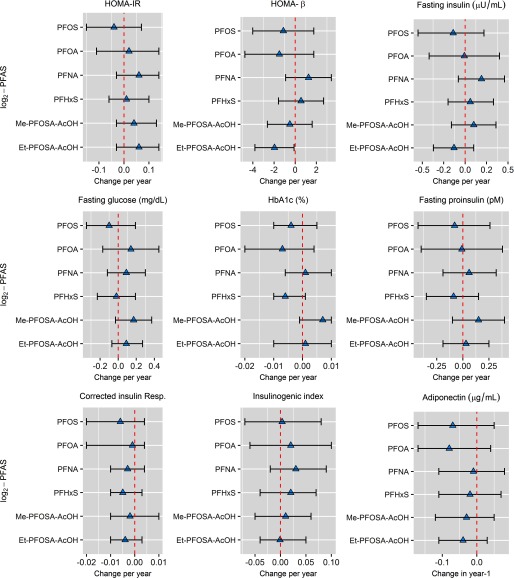
There was no evidence that baseline plasma PFAS concentrations were associated with changes in HOMA-IR, fasting insulin, fasting glucose, , fasting proinsulin, corrected insulin response, insulinogenic index, or adiponectin over time or by treatment group (Figure 2 and Table S3).
Figure 2.
Adjusted estimated change in glycemic outcomes per year for each doubling in per- and polyfluoroalkyl substances (PFAS) plasma concentrations measured at baseline in the Diabetes Prevention Program. Note: Longitudinal models adjusted for participant sex, race/ethnicity, baseline body mass index (BMI) (continuous), age (categorical), marital status (categorical), education (categorical), smoking history (categorical), time to follow-up in years, and treatment assignment (placebo/lifestyle). Et-PFOSA-AcOH, N-ethyl-perfluorooctane sulfonamido acetic acid; Me-PFOSA-AcOH, N-methyl-perfluorooctane sulfonamido acetic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid.
A doubling in plasma Et-PFOSA-AcOH concentration was associated with a 1.96 decrease in over the study period (95% CI: , ) after adjusting for sex, race/ethnicity, BMI, age, marital status, education, smoking history, treatment assignment, and the fixed effect of the interaction of treatment over time. For fasting-glucose levels, there was a significant statistical interaction between treatment assignment and plasma PFHxS concentrations over the study period (). Adjusted analyses stratified by treatment were marginal for the association of PFHxS with longitudinal changes in fasting glucose among the placebo (; 95% CI: , 0.08; ) and the intensive lifestyle intervention (; 95% CI: , 0.41; ) arm. Lastly, there was a significant statistical interaction between Me-PFOSA-AcOH plasma concentrations and treatment arm for the change in adiponectin levels from baseline to the first-year study visit (). Namely, in stratified analyses, a doubling in plasma Me-PFOSA-AcOH concentration was associated with a marginal decrease for the difference in adiponectin levels from baseline to year 1 among the placebo group (; 95% CI: , 0.01; ), but not in the intensive lifestyle intervention arm (; 95% CI: , 0.17; ).
Per- and Polyfluoroalkyl Substances Plasma Concentrations and Diabetes Incidence
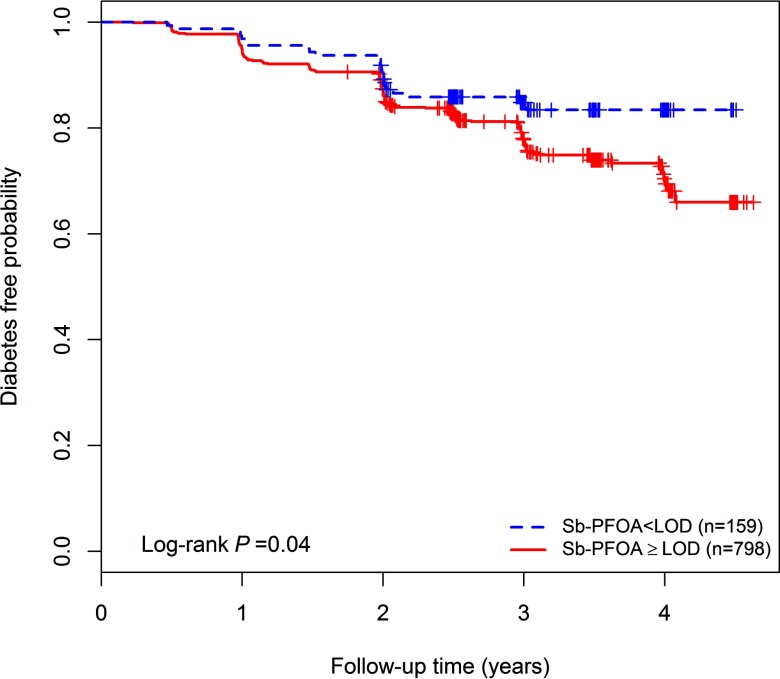
Median follow-up time was 2.9 y (range 0.2 to 4.6 y), and 204 participants (21.3%) developed diabetes by the end of the study. Baseline plasma concentrations of Sb-PFOA were associated with diabetes risk after adjusting for sex, race/ethnicity, BMI, age, marital status, education, smoking history, and treatment assignment; for every doubling in Sb-PFOA plasma concentration, the hazard of developing diabetes increased by 11% (; 95% CI: 1.00, 1.23). In stratified analyses, the incidence of diabetes among participants with detectable concentrations of Sb-PFOA was 22.6% (180/798) compared to 15.1% (24/159) for participants with undetectable Sb-PFOA concentrations (log-rank ), Figure 3. All other PFAS were not associated with diabetes incidence (Table 4). In a secondary analysis of time to first-fasting hyperglycemia (i.e., measured elevated glucose levels at the scheduled visit, but diabetes was not confirmed at the immediate follow-up visit), a doubling in plasma Sb-PFOA concentration was associated with a 29% increase in the hazard of developing hyperglycemia during the study period (; 95% CI: 1.03, 1.63) (Table S4).
Figure 3.
Kaplan-Meier plot of diabetes-free incidence in the study sample by plasma concentrations of Sb-PFOA categorized as undetectable () and detectable plasma Sb. PFOA concentrations (). Note: . Sb-PFOA, sum of perfluoromethylheptanoic and perfluorodimethylhexanoic acids.
Table 4.
Adjusted hazards ratios (HRs) for the risk of developing diabetes during study period relative to -per- and polyfluoroalkyl substances (PDFAS) plasma concentrations in the Diabetes Prevention Program (; ).
| PFAS (-scale) | Hazard ratio (HR) | |
|---|---|---|
| HR (95% CI) | -Value | |
| PFOS | 0.87 (0.74, 1.02) | 0.08 |
| n-PFOS | 0.87 (0.75, 1.02) | 0.08 |
| Sm-PFOS | 0.91 (0.78, 1.06) | 0.21 |
| Sm2-PFOS | 1.00 (0.89, 1.12) | 0.95 |
| PFOA | 1.06 (0.89, 1.28) | 0.50 |
| n-PFOA | 1.03 (0.85, 1.24) | 0.77 |
| Sb-PFOA | 1.11 (1.00, 1.23) | 0.04 |
| PFHxS | 0.98 (0.86, 1.12) | 0.79 |
| Et-PFOSA-AcOH | 0.97 (0.88, 1.08) | 0.58 |
| Me-PFOSA-AcOH | 0.96 (0.84, 1.08) | 0.49 |
| PFNA | 0.99 (0.87, 1.12) | 0.82 |
Note: Adjusted for participant sex, race/ethnicity, BMI (continuous), age (categorical), marital status (categorical), education (categorical), smoking history (categorical), and treatment assignment (placebo/lifestyle). CI, confidence interval; Et-PFOSA-AcOH, N-ethyl-perfluorooctane sulfonamido acetic acid; Me-PFOSA-AcOH, N-methyl-perfluorooctane sulfonamido acetic acid; n-PFOA, linear perfluorooctanoic acid; n-PFOS, linear perfluorooctanesulfonic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid; Sb-PFOA, sum of perfluoromethylheptanoic and perfluorodimethylhexanoic acids; Sm-PFOS, sum of perfluoromethylheptane sulfonic acid isomers; Sm2-PFOS, sum of perfluorodimethylhexane sulfonic acid isomers.
We observed a significant statistical interaction () between Me-PFOSA-AcOH concentration and treatment assignment. Stratified analyses suggested that higher plasma Me-PFOSA-AcOH concentration might be inversely associated with diabetes incidence among participants assigned to the placebo group (, 95% CI: 0.77, 1.03), but positively associated with diabetes for individuals in the intensive lifestyle intervention group (, 95% CI: 0.91, 1.42). All analyses stratified by treatment assignment were not statistically significant within either the placebo or lifestyle intervention arms of the trial, and therefore, these results should be cautiously interpreted (Table S5).
Lastly, in sensitivity analyses, we evaluated effect modification by both sex and BMI at baseline. Neither sex nor BMI at baseline modified the association between baseline PFAS plasma concentrations and diabetes incidence (Tables S6 and S7). All final adjusted Cox models met the proportional hazards assumption (global test ), and visual inspection of the Schoenfeld residuals also supported appropriate model fit.
Discussion
In this study of adults at high risk of type 2 diabetes, plasma concentrations of several PFAS measured at baseline were cross-sectionally associated with higher markers of insulin resistance and function at baseline. These associations were largely consistent across similar key glycemic indicators across multiple PFAS biomarkers. Although the magnitude of effect estimates were relatively small and thus of uncertain clinical significance, at the population level, they may be an important modifiable risk factor of metabolic risk. However, there was no strong evidence that baseline plasma PFAS influenced trajectories of insulin resistance and function during up to 4.6 y of follow-up. Concentrations of the branched isomers of PFOA (Sb-PFOA) were associated with incident diabetes, but this association should be interpreted with caution, as this was only one of several associations examined, and the concentrations of these isomers were relatively low and carry higher measurement error due to the moderate proportion of nondetectable values among participants.
PFAS are structural homologs of fatty acids, and scientific evidence suggests PFAS can disrupt metabolism and endocrine function (Guruge et al. 2006; Pedersen et al. 2016). Experimental studies and animal models have shown that PFAS can act as agonists of the peroxisome proliferator–activated receptor-alpha (), which might lead to liver damage (Intrasuksri et al. 1998; Lau et al. 2007; Sohlenius et al. 1993). Specifically, activation of can regulate peroxisome proliferation, lipid metabolism, and cell growth, and this receptor is experimentally shown to be activated by PFAS treatment (Shipley et al. 2004). In mice, PFOA exposure altered normal glucose metabolism and increased insulin sensitivity (Yan et al. 2015). Also in mice, prenatal and lactational exposure to PFOS resulted in impaired glucose tolerance and higher levels of insulin later in life (Lv et al. 2013). Although such experimental evidence suggests that PFAS can disrupt the endocrine system, epidemiologic evidence for effects on human health at environmentally relevant concentrations remains conflicted.
Several epidemiological studies have investigated the association between exposure to PFAS, diabetes incidence, and markers of both metabolism and glycemia with inconsistent results. For example, DuPont Washington Works workers occupationally exposed to some PFAS had an increased risk in mortality from diabetes compared to other workers (Leonard et al. 2008). Similarly, another occupationally exposed population had a higher than expected risk of diabetes-related deaths compared to nonexposed workers (Lundin et al. 2009). However, these two occupational studies lacked objective measurements of exposure and might be subject to exposure misclassification and bias. In a cohort study among elderly Swedish, serum PFNA and PFOA concentrations showed a nonlinear association with diabetes prevalence (Lind et al. 2014). Another study conducted in Taiwanese adults found a positive association between serum PFOS concentrations and diabetes prevalence. However, in this same study, a protective association was observed between PFOA, PFNA, and perfluoroundecanoic acid (PFUA) and prevalence of diabetes (Su et al. 2016). A large case-control study of individuals living in areas of West Virginia and Ohio where drinking water had been contaminated with high levels of PFOA, the C8 Health Project reported a protective association for PFOA serum concentrations and type 2 diabetes (MacNeil et al. 2009). Within this same population, implementing a retrospective longitudinal study with estimated exposure measures of PFOA, no association was observed for incident diabetes or fasting glucose levels (Karnes et al. 2014). Additionally, in a cross-sectional survey of the C8 Health Project, protective associations for serum concentration of PFOS, PFOA, PFHxS, and PFNA and diabetes prevalence were observed (Conway et al. 2016). However, studies from the C8 Health Project may not be generalizable to other populations, including ours, as PFOA exposures were much higher as a result of drinking water contamination in this region (Frisbee et al. 2009).
Associations for markers of insulin resistance and metabolism are also conflicting across epidemiological studies. For example, in a cross-sectional analysis of a U.S. representative sample from NHANES 1999–2000 and 2003–2004, higher serum PFNA was associated with hyperglycemia but with lower risk of metabolic syndrome, while PFOA was associated with higher function, and PFOS was associated with higher insulin, HOMA-IR, and function (Lin et al. 2009). However, an analysis limited to the 2003–2004 NHANES cycle found no evidence of an association of PFAS with HOMA-IR (Nelson et al. 2010). Similarly in the 2007–2008 Canadian Health Measures Survey, PFOS, PFOA, and PFHxS serum concentrations were not associated with plasma insulin, HOMA-IR, or metabolic syndrome (Fisher et al. 2013). In a study of Taiwanese adults, only PFOS was associated with higher glucose levels post-OGTT, but PFOA, PFNA, and PFUA were inversely associated with post glucose-load measurements, suggesting a protective effect (Su et al. 2016). In the elderly Swedish cohort, no associations were observed for seven of the PFAS measured with HOMA-IR, but PFOA was positively associated with the ratio of proinsulin to insulin (Lind et al. 2014). In a U.S.-based birth cohort (Project Viva), prenatal PFAS plasma concentrations were not associated with metabolic or glycemic measurements in in midchildhood ( of age). However, in Project Viva, higher midchildhood PFAS concentrations were cross-sectionally associated with lower HOMA-IR at the same time point, suggesting a protective cross-sectional association (Fleisch et al. 2017). Conversely, in a Danish cohort of children, higher PFOS and PFOA plasma concentrations were cross-sectionally associated with higher HOMA-IR and among overweight children (Timmermann et al. 2014). It is important to highlight that the majority of these studies were cross-sectional, and some lacked accurate biomarkers of exposure, had limited information on metabolic or glycemic biomarkers, and/or relied on self-reported diabetes prevalence not confirmed with OGTT or fasting glucose levels. Although we observed robust cross-sectional associations between higher PFOS, PFOA, PFHxS, and Et-PFOSA-AcOH and higher , only PFOA and PFHxS remained associated with the corrected measure of insulin response as well as the insulinogenic index. These two measures reflect insulin secretion and function more accurately (Hanson et al. 2000; Herzberg-Schäfer et al. 2010). A prospective study found that higher prenatal plasma PFOA concentrations were associated with increased overweight prevalence and greater waist circumference in females but not male offspring at age 20 y (Halldorsson et al. 2012). This study also showed that prenatal concentrations of PFOA, but not PFOS, PFNA, or perfluorooctane sulfonamide were associated with elevated insulin and leptin and a decrease in adiponectin levels among female offspring at 20 y of age. Another prospective study among women planning to become pregnant found a significant association between serum PFOA concentrations and risk of gestational diabetes, but not for other PFAS (Zhang et al. 2015). A recent prospective cohort of European children reported prospective associations between childhood PFOS concentration and increase adiposity in adolescence, as well as childhood PFOA concentration and a large decrease in function in adolescence (Domazet et al. 2016). Furthermore, a computational modeling approach combining genomic information, disease similarities, and high-quality scientific literature significantly associated PFOA, among other chemicals, such as arsenic, hexachlorobenzene, and 2,3,7,8-tetrachlorodibenzo-p-dioxin, with type 2 diabetes (Audouze et al. 2013). This is consistent with our results in which we observed robust positive associations of plasma PFOA concentrations at baseline with higher glycemic measurements and lower adiponectin levels measured at the same time point. Furthermore, the effect sizes for the estimated associations with the metabolic or glycemic measurements were greater for PFOA compared to any other PFAS measured. Likewise, only branched isomers of PFOA (Sb-PFOA) were associated with diabetes incidence in our study, suggesting that PFOA might be more tightly related to glycemic control compared to other PFAS. However, this result should be interpreted with caution, as concentrations of Sb-PFOA were relatively low and nondetectable in approximately 17% of participants. Our findings highlight the importance of distinguishing branched and linear isomers of PFOA and PFOS in experimental and epidemiological studies along with the ascertainment of potential sources of exposure. Telomerization primarily produces linear PFAS, and electrochemical fluorination (ECF) yields a mixture of both linear and branched isomers (Buck et al. 2011). Human exposure to PFAS from ECF is hypothesized to be declining due to changes in manufacturing processes, including phase out (Beesoon et al. 2011). However, in animal models, excretion of branched PFOA isomers is favored, and this could lead to quantification of exposure biomarkers that are predominately linear in population studies (Benskin et al. 2010), making it difficult to distinguish the exposure source(s) without collecting other information such as home environment, drinking water contamination, dietary patterns, and proximity to industrial facilities, among other factors. Furthermore, source characterization is also complicated by exposure to precursors of PFAS found in serum (D’eon and Mabury 2011).
Despite their cross-sectional consistent associations with key glycemic indicators, most baseline PFAS concentrations were not prospectively associated with glycemic indicators in this analysis. Cross-sectional associations observed in our study might be prone to reverse causation. For example, previous studies have argued that physiological factors such as glomerular filtration rate (GFR), which affects PFAS concentration in blood, might confound associations between PFAS concentration and health outcomes such as birthweight (Verner et al. 2015) and age at menopause (Dhingra et al. 2017). Even though there is no direct evidence of potential confounding for diabetes, glycemic indicators, and PFAS, there is evidence that GFR is associated with both diabetes progression and risk as well as metabolic dysfunction (Hu et al. 2017; Naderpoor et al. 2017). It is possible that in this prediabetic cohort, altered GFR might confound cross-sectional associations between baseline glycemic indicators and measured PFAS serum concentrations. Therefore, cross-sectional results must be interpreted with caution, as reverse causation cannot be ruled out.
As previously demonstrated in the DPP, the effect of the lifestyle intervention was strongly associated with diabetes incidence and had a large influence on metabolic and glycemic markers over time (Kitabchi et al. 2005). Although we controlled for treatment effect in all longitudinal models, the treatment effect could have explained almost all of the variation observed in the longitudinal data. Stratified analyses by treatment and interaction models might be underpowered to detect these associations for diabetes incidence or glycemic and metabolic markers in the placebo-control group. It is also possible that the length of follow-up time could have been too short to capture the effects of PFAS prospectively, and this could explain the effects observed at baseline, but not during the follow-up period. Correspondingly, the moderate associations at baseline may reflect metabolic damage already caused by past PFAS exposure, and no further alterations over time could have been observed during this short study period. Additionally, timing of exposure could also play a role in the association of PFAS and metabolic markers, as some prospective studies of prenatal and childhood exposure have reported prospective associations not observed in this obese and overweight adult prediabetic population. It has been suggested that developmental periods may present heightened susceptibility windows for endocrine disruption during fetal and infant development that could explain the lack of prospective associations in our study (Landrigan et al. 2003). Alternatively, our baseline cross-sectional results could have been also due to chance, but we consider this to be highly unlikely, as we observed consistent robust associations among many glycemic and metabolic markers in the direction hypothesized a priori, consistent with experimental studies.
This study has many strengths that include the objective ascertainment of plasma PFAS coupled with a strong prospective study design with stringent clinical diagnosis of diabetes that was confirmed during a secondary follow-up clinical visit. Furthermore, we have high-quality information on multiple metabolic and glycemic clinical markers during approximately 3 y of follow-up. Detailed covariate information was also available and adjusted for in multivariate models, reducing the chance for confounding. However, we lacked important information from hypothesized sources of exposures such as drinking water concentrations, diet, home environment, or other information on potential sources of PFAS exposure. The sample size available was moderate, and the number of participants that developed diabetes during the study period was substantial due to the high-risk preselection criteria. About two-thirds of participants were women, and outreach for the inclusion of minorities was also performed by DPP, making our results more generalizable. However, all individuals in this study had some level of abnormal endocrine function at enrollment as abnormal glucose levels, and being overweight or obese were among the selection criteria used. Therefore, our results may not be generalizable to healthy individuals with normal endocrine function or children. Finally, although we adjusted for many important demographic confounders, residual confounding may remain.
Conclusion
In summary, our findings show that plasma PFAS concentrations in prediabetic individuals are consistently but modestly associated cross-sectionally with higher markers of metabolic dysfunction. However, in this population of individuals at high risk for type 2 diabetes, there was limited evidence suggesting that plasma PFAS concentrations at baseline influenced glycemic measurements over a median follow-up time of approximately 3 y. Furthermore, there was limited evidence for an association of plasma PFAS concentrations and incidence of diabetes, as only branched isomers of PFOA in serum were associated with incident diabetes.
Thus, our study supports a cross-sectional association between plasma PFAS concentrations and several markers of metabolic damage measured among individuals with prediabetes, but their ability to influence metabolic markers or diabetes incidence during the follow-up time was not found in this population.
Supplemental Material
Acknowledgments
The authors wish to thank K. Kato, J. Ma, A. Kalathil, and T. Jia, who performed the quantification of PFAS biomarkers at the CDC, and D. Simon in the Department of Population Medicine for providing valuable logistical support for this project.
This work was supported by the National Institutes of Health grants R01ES024765 and K24HD069408. The DPP was conducted by the DPP Research Group and supported by the NIDDK, the General Clinical Research Center Program, the National Institute of Child Health and Human Development (NICHD), the National Institute on Aging (NIA), the Office of Research on Women's Health, the Office of Research on Minority Health, the CDC, and the American Diabetes Association. The data (and samples) from the DPP were supplied by the NIDDK Central Repositories (project number 1X01DK104234).
References
- Audouze K, Brunak S, Grandjean P. 2013. A computational approach to chemical etiologies of diabetes. Sci Rep 3:2712, PMID: 24048418, 10.1038/srep02712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesoon S, Webster GM, Shoeib M, Harner T, Benskin JP, Martin JW. 2011. Isomer profiles of perfluorochemicals in matched maternal, cord, and house dust samples: manufacturing sources and transplacental transfer. Environ Health Perspect 119(11):1659–1664, PMID: 21757419, 10.1289/ehp.1003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benskin JP, De Silva AO, Martin JW. 2010. Isomer profiling of perfluorinated substances as a tool for source tracking: A review of early findings and future applications. In: Reviews of Environmental Contamination and Toxicology, Vol. 208. de Voogt P, ed. New York:Springer, 111–160. [DOI] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, De Voogt P, et al. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7(4):513–541, PMID: 21793199, 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenhoff JL, Olsen GW, Pfahles-Hutchens A. 2006. The applicability of biomonitoring data for perfluorooctanesulfonate to the environmental public health continuum. Environ Health Perspect 114(11):1776–1782, PMID: 17107867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt CM, Berger U, Bossi R, Tomy GT. 2010. Levels and trends of poly-and perfluorinated compounds in the arctic environment. Sci Total Environ 408(15):2936–2965, PMID: 20493516, 10.1016/j.scitotenv.2010.03.015. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2017. “Fourth National Report on Human Exposure to Environmental Chemicals.” https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2017.pdf [accessed 28 July 2017].
- Conway B, Innes KE, Long D. 2016. Perfluoroalkyl substances and beta cell deficient diabetes. J Diabetes Complications 30(6):993–998, PMID: 27311784, 10.1016/j.jdiacomp.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’eon JC, Mabury SA. 2011. Is indirect exposure a significant contributor to the burden of perfluorinated acids observed in humans? Environ Sci Technol 45(19):7974–7984, PMID: 21630688, 10.1021/es200171y. [DOI] [PubMed] [Google Scholar]
- Dhingra R, Winquist A, Darrow LA, Klein M, Steenland K. 2017. A study of reverse causation: Examining the associations of perfluorooctanoic acid serum levels with two outcomes. Environ Health Perspect 125(3):416–421, PMID: 27529882, 10.1289/EHP273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group, 1999. The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 22(4):623–634, PMID: 10189543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group, 2000. The Diabetes Prevention Program: Baseline characteristics of the randomized cohort. Diabetes Care 23(11):1619–1629, PMID: 11092283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domazet SL, Grøntved A, Timmermann AG, Nielsen F, Jensen TK. 2016. Longitudinal associations of exposure to perfluoroalkylated substances in childhood and adolescence and indicators of adiposity and glucose metabolism 6 and 12 years later: The European youth heart study. Diabetes Care 39(10):1745–1751, PMID: 27489335, 10.2337/dc16-0269. [DOI] [PubMed] [Google Scholar]
- Fisher M, Arbuckle TE, Wade M, Haines DA. 2013. Do perfluoroalkyl substances affect metabolic function and plasma lipids?–Analysis of the 2007-2009, Canadian Health Measures Survey (CHMS) Cycle 1. Environ Res 121:95–103, PMID: 23266098, 10.1016/j.envres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Fleisch AF, Rifas-Shiman SL, Mora AM, Calafat AM, Ye X, Luttmann-Gibson H, et al. 2017. Early-life exposure to perfluoroalkyl substances and childhood metabolic function. Environ Health Perspect 125(3):481–487, PMID: 27586368, 10.1289/EHP303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AJ, Webster TF, Watkins DJ, Nelson JW, Stapleton HM, Calafat AM, et al. 2011. Polyfluorinated compounds in serum linked to indoor air in office environments. Environ Sci Technol 46(2):1209–1215, PMID: 22148395, 10.1021/es2038257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee SJ, Brooks AP Jr, Maher A, Flensborg P, Arnold S, Fletcher T, et al. 2009. The C8 health project: Design, methods, and participants. Environ Health Perspect 117(12):1873–1882, PMID: 20049206, 10.1289/ehp.0800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruge KS, Yeung LW, Yamanaka N, Miyazaki S, Lam PK, Giesy JP, et al. 2006. Gene expression profiles in rat liver treated with perfluorooctanoic acid (PFOA). Toxicol Sci 89(1):93–107, PMID: 16221955, 10.1093/toxsci/kfj011. [DOI] [PubMed] [Google Scholar]
- Halldorsson TI, Rytter D, Haug LS, Bech BH, Danielsen I, Becher G, et al. 2012. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: A prospective cohort study. Environ Health Perspect 120(5):668–673, PMID: 22306490, 10.1289/ehp.1104034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson RL, Pratley RE, Bogardus C, Narayan KMV, Roumain JML, Imperatore G, et al. 2000. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemioiogic studies. Am J Epidemiol 151(2):190–198, PMID: 10645822, 10.1093/oxfordjournals.aje.a010187. [DOI] [PubMed] [Google Scholar]
- Harris MH, Rifas-Shiman SL, Calafat AM, Ye X, Mora AM, Webster TF, et al. 2017. Predictors of per-and polyfluoroalkyl substance (PFAS) plasma concentrations in 6–10 year old American children. Environ Sci Technol 51(9):5193–5204, PMID: 28325044, 10.1021/acs.est.6b05811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg-Schäfer SA, Staiger H, Heni M, Ketterer C, Guthoff M, Kantartzis K, et al. 2010. Evaluation of fasting state-/oral glucose tolerance test-derived measures of insulin release for the detection of genetically impaired β-cell function. PloS One 5(12):e14194, PMID: 21152029, 10.1371/journal.pone.0014194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. J Occup Environ Hyg 5(1):46–51, 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- Hu W, Wu XJ, Ni YJ, Hao HR, Yu WN, Zhou HW. 2017. Metabolic syndrome is independently associated with a mildly reduced estimated glomerular filtration rate: A cross-sectional study. BMC Nephrol 18(1):192, PMID: 28610620, 10.1186/s12882-017-0597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intrasuksri U, Rangwala SM, O'Brien M, Noonan DJ, Feller DR. 1998. Mechanisms of peroxisome proliferation by perfluorooctanoic acid and endogenous fatty acids. Gen Pharmacol 31(2):187–197, PMID: 9688458, 10.1016/S0306-3623(98)00029-9. [DOI] [PubMed] [Google Scholar]
- Jin Y, Saito N, Harada KH, Inoue K, Koizumi A. 2007. Historical trends in human serum levels of perfluorooctanoate and perfluorooctane sulfonate in Shenyang, China. Tohoku J Exp Med 212(1):63–70, PMID: 17464105, 10.1620/tjem.212.63. [DOI] [PubMed] [Google Scholar]
- Karnes C, Winquist A, Steenland K. 2014. Incidence of type II diabetes in a cohort with substantial exposure to perfluorooctanoic acid. Environ Res 128:78–83, PMID: 24299613, 10.1016/j.envres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Kato K, Basden BJ, Needham LL, Calafat AM. 2011. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A 1218(15):2133–2137, PMID: 21084089, 10.1016/j.chroma.2010.10.051. [DOI] [PubMed] [Google Scholar]
- Khalil N, Miryoung L, Steenland K. 2015. Epidemiological findings. In: Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances. DeWitt JC, ed., New York, NY:Springer, 305–335. [Google Scholar]
- Kitabchi AE, Temprosa M, Knowler WC, Kahn SE, Fowler SE, Haffner SM, et al. 2005. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: Effects of lifestyle intervention and metformin. Diabetes 54(8):2404–2414, PMID: 16046308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. 2002. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346(6):393–403, PMID: 11832527, 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land M, de Wit CA, Cousins IT, Herzke D, Johansson J, Martin JW. 2015. What is the effect of phasing out long-chain per-and polyfluoroalkyl substances on the concentrations of perfluoroalkyl acids and their precursors in the environment? A systematic review protocol. Environ Evid 4(1):3, 10.1186/2047-2382-4-3. [DOI] [Google Scholar]
- Landrigan P, Garg A, Droller DB. 2003. Assessing the effects of endocrine disruptors in the national children's study. Environ Health Perspect 111(13):1678–1682, PMID: 14527850, 10.1289/ehp.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. 2007. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99(2):366–394, PMID: 17519394, 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Leonard RC, Kreckmann KH, Sakr CJ, Symons JM. 2008. Retrospective cohort mortality study of workers in a polymer production plant including a reference population of regional workers. Ann Epidemiol 18(1):15–22, PMID: 17900928, 10.1016/j.annepidem.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Lin CY, Chen PC, Lin YC, Lin LY. 2009. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes Care 32(4):702–707, PMID: 19114613, 10.2337/dc08-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind L, Zethelius B, Salihovic S, van Bavel B, Lind PM. 2014. Circulating levels of perfluoroalkyl substances and prevalent diabetes in the elderly. Diabetologia 57(3):473–479, PMID: 24337155, 10.1007/s00125-013-3126-3. [DOI] [PubMed] [Google Scholar]
- Lundin JI, Alexander BH, Olsen GW, Church TR. 2009. Ammonium perfluorooctanoate production and occupational mortality. Epidemiology 20(6):921–928, PMID: 19797969, 10.1097/EDE.0b013e3181b5f395. [DOI] [PubMed] [Google Scholar]
- Lv Z, Li G, Li Y, Ying C, Chen J, Chen T, et al. 2013. Glucose and lipid homeostasis in adult rat is impaired by early‐life exposure to perfluorooctane sulfonate. Environ Toxicol 28(9):532–542, PMID: 23983163, 10.1002/tox.20747. [DOI] [PubMed] [Google Scholar]
- MacNeil J, Steenland NK, Shankar A, Ducatman A. 2009. A cross-sectional analysis of type II diabetes in a community with exposure to perfluorooctanoic acid (PFOA). Environ Res 109(8):997–1003, PMID: 19740462, 10.1016/j.envres.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Naderpoor N, Lyons JG, Mousa A, Ranasinha S, De Courten MP, Soldatos G, et al. 2017. Higher glomerular filtration rate is related to insulin resistance but not to obesity in a predominantly obese non-diabetic cohort. Sci Rep 7:45522, PMID: 28368024, 10.1038/srep45522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JW, Hatch EE, Webster TF. 2010. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect 118(2):197–202, PMID: 20123614, 10.1289/ehp.0901165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305, PMID: 17805419, 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen KE, Letcher RJ, Sonne C, Dietz R, Styrishave B. 2016. Per-and polyfluoroalkyl substances (PFASs)–new endocrine disruptors in polar bears (Ursus maritimus)? Environ Int 96:180–189, PMID: 27692342, 10.1016/j.envint.2016.07.015. [DOI] [PubMed] [Google Scholar]
- Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. 2006. Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol 40(1):32–44, PMID: 16433330, 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- Shipley JM, Hurst CH, Tanaka SS, DeRoos FL, Butenhoff JL, Seacat AM, et al. 2004. Trans-activation of PPARα and induction of PPARα target genes by perfluorooctane-based chemicals. Toxicol Sci 80(1):151–160, PMID: 15071170, 10.1093/toxsci/kfh130. [DOI] [PubMed] [Google Scholar]
- Sohlenius AK, Eriksson AM, Högström C, Kimland M, DePierre JW. 1993. Perfluorooctane sulfonic acid is a potent inducer of peroxisomal fatty acid β‐oxidation and other activities known to be affected by peroxisome proliferators in mouse liver. Pharmacol Toxicol 72(2):90–93, PMID: 8386358. [DOI] [PubMed] [Google Scholar]
- Steenland K, Fletcher T, Savitz DA. 2010. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ Health Perspect 118(8):1100–1108, PMID: 20423814, 10.1289/ehp.0901827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TC, Kuo CC, Hwang JJ, Lien GW, Chen MF, Chen PC. 2016. Serum perfluorinated chemicals, glucose homeostasis and the risk of diabetes in working-aged Taiwanese adults. Environ Int 88:15–22, PMID: 26700417, 10.1016/j.envint.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Timmermann CA, Rossing LI, Grøntved A, Ried-Larsen M, Dalgård C, Andersen LB, et al. 2014. Adiposity and glycemic control in children exposed to perfluorinated compounds. J Clin Endocrinol Metab 99(4):E608–E614, PMID: 24606078, 10.1210/jc.2013-3460. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (Environmental Protection Agency). 2015. Per- and polyfluoroalkyl substances (PFASs) under TSCA. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-management-and-polyfluoroalkyl-substances-pfass [accessed 22 June 2017].
- Verner MA, Loccisano AE, Morken NH, Yoon M, Wu H, McDougall R, et al. 2015. Associations of perfluoroalkyl substances (PFAS) with lower birth weight: An evaluation of potential confounding by glomerular filtration rate using a physiologically based pharmacokinetic model (PBPK). Environ Health Perspect 123(12):1317–1324, PMID: 26008903, 10.1289/ehp.1408837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Webster TF. 2017. Trade-offs of personal versus more proxy exposure measures in environmental epidemiology. Epidemiology 28(5):635–643, PMID: 28520644, 10.1097/EDE.0000000000000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Wang T, Liu S, Jones KC, Sweetman AJ, Lu Y. 2013. Industrial source identification and emission estimation of perfluorooctane sulfonate in China. Environ Int 52:1–8, PMID: 23266910, 10.1016/j.envint.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Yan S, Zhang H, Zheng F, Sheng N, Guo X, Dai J. 2015. Perfluorooctanoic acid exposure for 28 days affects glucose homeostasis and induces insulin hypersensitivity in mice. Sci Rep 5:11029, PMID: 26066376, 10.1038/srep11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Sundaram R, Maisog J, Calafat AM, Barr DB, Buck Louis GM. 2015. A prospective study of prepregnancy serum concentrations of perfluorochemicals and the risk of gestational diabetes. Fertil Steril 103(1):184–189, PMID: 25450302, 10.1016/j.fertnstert.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.