Abstract
Research on cardiac hypertrophy and heart failure is frequently based on pressure overload mouse models induced by TAC. The standard procedure is to perform a partial thoracotomy to visualize the transverse aortic arch. However, the surgical trauma caused by the thoracotomy in open-chest models changes the respiratory physiology as the ribs are dissected and left unattached after chest closure. To prevent this, we established a minimally invasive, closed chest approach via lateral thoracotomy. Herein we approach the aortic arch via the 2nd intercostal space without entering the chest cavities, leaving the mouse with a less traumatic injury to recover from. We perform this operation using standard laboratory settings for open chest TAC procedures with equal survival rates. Apart from maintaining physiological breathing patterns due to the closed chest approach, the mice seem to benefit by showing rapid recovery, as the less invasive technique appears to facilitate a fast healing process and to reduce immune response after trauma.
Keywords: Medicine, Issue 134, TAC, closed-chest, mouse, transverse aortic constriction, pressure overload, thoracotomy
Introduction
Mouse models are often used to mimic human diseases1. Transverse aortic constriction (TAC) is used to induce pressure overload and left ventricular hypertrophy2. The open-chest TAC model in mice was validated by Rockman et al.3 and the surgical procedure is described in detail by DeAlmeida et al.4. Banding of the transverse aorta is more favorable in comparison to abdominal aortic constriction because a larger portion of the circulation can compensate negative effects of this latter procedure2.
The banding of the transverse aorta leads to an increased arterial pressure in the ascending aorta and brachiocephalic artery but leaves sufficient perfusion of the organs via the distal vessels (i.e. the left common carotid artery, the left subclavian artery, and descending aorta). This leads to an increased cardiac afterload and an elevated cardiac wall stress. The wall stress subsequently decreases due to fiber thickening5. The chronic change in cardiac hemodynamics results in maladaptation and dilatation of the left ventricle. This way the TAC creates a reproducible model of cardiac hypertrophy eventually leading to heart failure.
The standard procedure for TAC as described by DeAlmeide et al.4 approaches the aortic arch via a partial upper thoracotomy via dissection of the ribs or the sternum and entering the mediastinum as well as the pleural cavity. This allows for a good view of the aortic arch and its side branches. Unfortunately, the dissected ribs cannot be reattached, which leaves them floating freely and thereby altering the breathing dynamics.
We, therefore, established a minimally invasive closed-chest approach to the aortic arch using a lateral surgical approach via the 2nd intercostal space. The greatest advantage of this model is the ability to perform TAC without even cutting through the ribs. The surgical trauma is limited to the incision of the skin and the dissection of the intercostal muscles. This procedure minimizes the trauma itself and helps to maintain adequate chest stability.
Here we describe a detailed step-by-step procedure to perform TAC surgery in mice without performing the total or the upper thoracotomy. High frequency Doppler was used to ensure the success of TAC as previously described 6,7.
Protocol
This protocol was approved by the Ethics Committee for Animal Experimentation LANUV Recklinghausen (#84-02.04.2016.A374). Generally, this procedure is performed on adult mice >10 weeks of age. However, it is possible to perform this surgery on younger animals as well. Surgical tools must be sterilized before use and all steps are to be performed under aseptic conditions.
1. Induction of Anesthesia and Intubation
Inject buprenorphine 0.1 µg/g body weight intraperitoneally for pain relief. Repeat the intraperitoneal injections of 0.1 mg/kg buprenorphine every 8 h for the next three days after surgery.
For induction, place the mouse into an anesthesia induction box which is connected to the vaporizer set to 3.0 Vol% of isoflurane with an oxygen flow of 1 L/min.
Ensure deep narcosis by inducing a tactile stimulus. NOTE: Increase Vol% of isoflurane up to 5%, if anesthesia induction fails or narcosis is not deep enough.
Pinch the tail of the mouse to ensure reflex absence. In case of complete absence of reflexes, weigh the mouse for optimal ventilator setup (see 1.12).
Move the mouse to a temperature-controlled operating table to maintain a body temperature of 37 °C throughout the procedure.
Place the nose of the mouse in a plastic cone which is connected to the anesthesia induction box to maintain narcosis.
Fix the upper incisors of the mouse with a nylon suture. Fixate the extremities with an adhesive tape.
Apply pressure on the hind paw with the tip of the forceps to ensure an adequate narcosis again. In the absence of a withdrawal reflex proceed with the following steps. NOTE: Increase volume % of isoflurane if anesthesia induction fails or narcosis is not deep enough and wait for the absence of the withdrawal reflex.
Place sterile ophthalmic lubricant on the corneas to prevent desiccation under anesthesia.
Lubricate the rectal probe to avoid rectal trauma. Insert a rectal temperature probe to ensure a core temperature of 37 °C.
Depilate the throat and the upper chest with depilatory cream according to the manufacturer's instructions. Wipe off the cream after 1 min. If necessary, repeat this step until successful. NOTE: Use cotton-tipped swabs in case of bleeding.
Clean the depilated area with 70% ethanol. Then apply povidone-iodine for local skin disinfection 3 times and for at least 3 min.
Adjust ventilator settings to physiological parameters. Set the respiratory rate to 150/min and tidal volumes to 8-10 µL/g body weight (BW).
Put on a new pair of sterile gloves. Place the mouse under a surgical microscope and place a sterile fenestrated drape over the mouse.
Incise the skin at the midline about 3 mm under the mandibular down to the 2nd rib. Identify midline and connective tissues of the submandibular gland. Then use angled intracapsular forceps to gently divide the gland at the midline bluntly with two forceps and explore the tracheal muscle.
Prepare the trachea gently by pulling the para-tracheal muscles apart bluntly with intracapsular forceps.
Pull on the tongue with forceps to straighten the throat for easier intubation conditions and gently insert an intubation cannula (OD 1.2 mm) inside the trachea. Confirm the intubation by direct visualization of the tube inside the trachea and by checking for proper chest movement.
Adjust the isoflurane concentration after intubation to 2% isoflurane with a flow of 1.0 L/min and 100% O2. NOTE: If breathing movements do not stop or mice start moving, first increase the respiratory rate up to 180/min. If necessary, increase the isoflurane concentration up to 3.5% secondarily until the mouse stops breathing on its own. Evaluate leakages or inadequate filling of the vaporizer as the most common problems.
- Alternatively, perform intubation as suggested in the following sub steps.
- Position the mouse on a table at a 60° angle.
- Fixate the extremities of the mouse with adhesive tape and recline the head.
- Place a cold-light source directly on the skin above the larynx.
- Pull the tongue gently with forceps to visualize the vocal chords.
- Insert a plastic tube of an IV-cannula (24 G) through the vocal chords and connect the plastic tube to the ventilator settings.
- Connect the ventilator to the cannula to confirm intubation by synchrone chest movements. NOTE: If breathing movements do not stop or mice start moving, first increase the respiratory rate up to 180 per min. If necessary, increase secondarily the isoflurane concentration up to 3.5% until the mouse stops breathing on its own. Evaluate leakages or inadequate filling of the vaporizer as the most likely problems.
2. Preoperative Doppler Measurement
Prepare both carotid arteries which lay adjunct to the trachea by gently pulling the connective tissue apart with forceps.
Place the tip of the 20 MHz Doppler probe with some sterile ultrasound gel on the right and the left carotid artery at an angle less than 45°.
Slowly rotate the probe to move it lateral and medial to find a Doppler signal and tilt the probe to optimize the signal.
Use a Doppler software to display and store flow velocities in the right and left common carotid arteries on a computer.
3. Thoracotomy
Use a set of sterile gloves for each individual mouse to prevent surgical site infections.
Expand the skin incision down to the 2nd intercostal space with a scissor.
Identify the 2nd intercostal space visually by counting the ribs and then bluntly penetrate this space with intracapsular forceps. NOTE: The 1st rib is located under the clavicle and is therefore not visible because the 2nd intercostal space is found between first visible rib (i.e. 2nd rib) and the 3rd rib.
Open the 2nd intercostal space with the help of forceps tips and insert the retractors.
Adjust the retractors with a rubber band attached to the operating table to have a clear view of the thymus.
In case of bleeding use a cotton-tip and press on the superficial vessels for 2 min.
4. Banding of the Transverse Aorta
Adjust the magnification to 200% to identify midline and connective tissues. Then use angled forceps to gently divide the thymus. Remove fatty tissue until the aortic arch can be seen clearly.
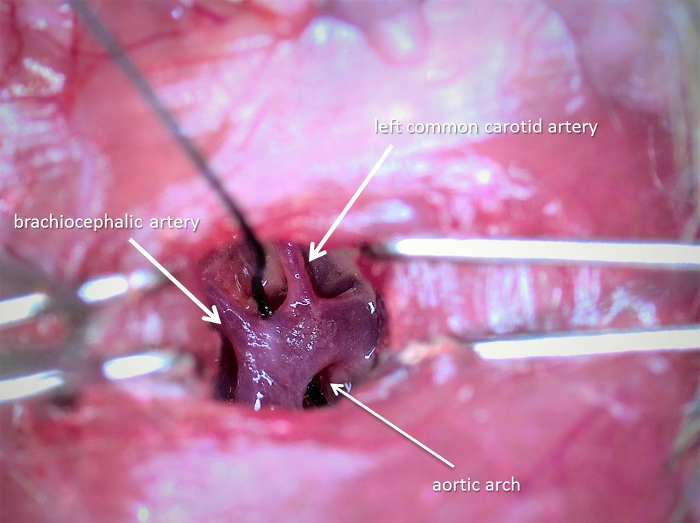
Prepare a tunnel with angled tying forceps under the transverse aorta between the brachiocephalic artery and left common carotid artery (see Figure 1). Hold the end of a 6.0 suture with the help of fine forceps and pass the thread under the aortic arch.
Take the thread with the second forceps from the other side of the arch.
Cut off a 3-mm long piece length of a 27 G to use the needle as a the spacer for TAC-ligation for mice weighing between 19 - 25 g body weight and a 26 G needle as a spacer for mice >25 g BW.
Carefully place the spacer parallel to the transverse aorta.
Prepare a loose double knot on the spacer and ensure optimal placement of the spacer in parallel to the aorta. Then tie the first throw and quickly perform a second contrary throw. Remove the spacer promptly.
To perform sham mice for control, follow the same protocol omitting the ligation of the aorta.
Close the 2nd intercostal space with a 6.0 polypropylene suture. Pay special attention to the subclavian vessels when ligating.
Suture the skin using a 6.0 polypropylene suture in a continuous suture pattern.
5. Confirmation of Successful Ligation of the Transverse Aorta
Place a 20 MHz Doppler probe on both sides of the neck at a 45° angle as in section 2.
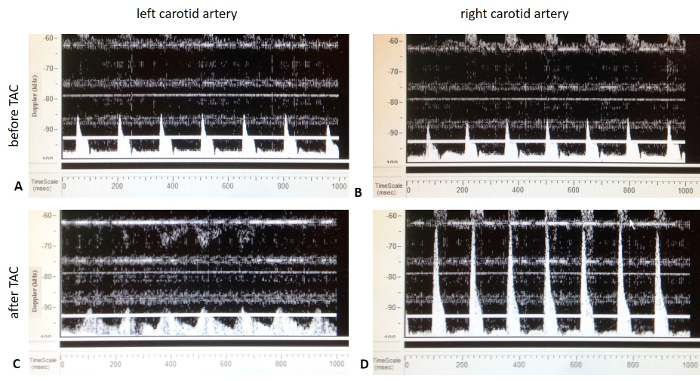
Document the flow velocities on each side. NOTE: A successful TAC can be validated by Doppler flow velocity as shown in Figure 2. A flow velocity ratio of 4 - 10 between the right and left carotid artery usually guarantees adequate TAC (see Figure 2).
6. Heart harvest
Induce narcosis according to steps 1.2. and 1.3.
Place the mouse in an euthanasia chamber and adjust the carbon dioxide flow rate to displace 10 - 30% of the volume/minute.
Fixate the mouse on a surgery table. Open the abdomen with scissors and harvest blood from the inferior vena cava with a cannula for further analysis.
Cut the diaphragm and the sternal bone with strong scissors and remove the heart.
Remove all arterial and connective tissue before weighing the heart.
Separate the right ventricle and the septum from the left ventricle and weigh both samples.
Freeze both tissue samples in liquid nitrogen.
Representative Results
A successful TAC guarantees the induction of pressure overload and left ventricular hypertrophy. An ad hoc validation of pressure overload can be achieved using Doppler flow velocity measurement as shown in Figure 2. While preoperative blood flow velocity is equal in both carotid arteries, TAC causes an augmented blood velocity in the right carotid artery due to elevated pressure in the left ventricle and aorta while causing post-stenotic attenuated blood flow velocity in the left carotid artery.
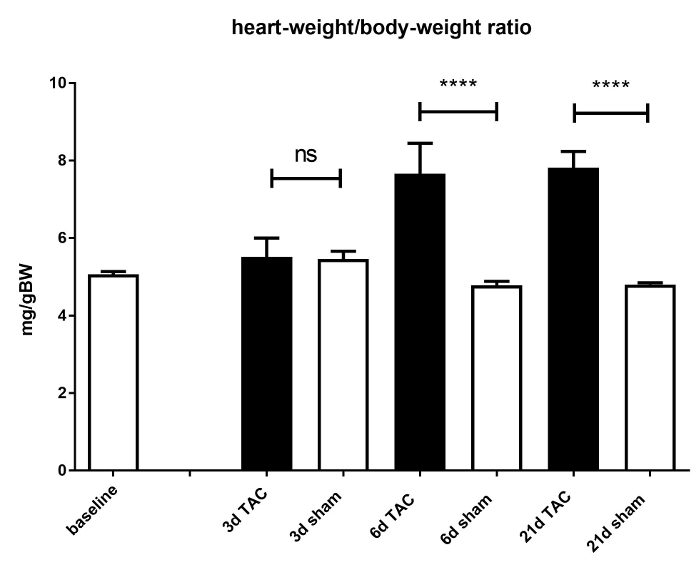
The efficacy of TAC and its resulting hypertrophy was validated by calculation of heart weight/body weight ratios (HW/BW; mg/g) of C57BL/6J male mice at day 3, 6, and 21 days post-surgery. The HW/BW ratios significantly increased in TAC mice compared to non-banded mice 6 days after surgery (4.78 ± 0.18 vs7.66±1.43 mg/g, p <0.0001). This ratio was nearly constant after 21 days (4.8 ± 0.11 vs7.81 ± 0.65 mg/g, p <0.0001) (see Figure 3). The survival rate is mainly dependent on intra-operative bleeding: it can be reduced to under 5% through regular practice. The survival rate after 21 days depends mainly on the genotype. For mice not suffering from functional heart diseases the survival rate amounts to >85%. The survival rate in the presented C57BL/6J mice after 21 days amounted to 88%.
Systolic blood pressure and cardiac function was measured in intubation anesthesia and performed with a 1.4 French pressure conductance catheter8 as described by others.9 Heart rate (HR) has a significant effect on left ventricular (LV) contractility. There were no differences in the heart rates (HR) of aortic banded and non-aortic banded mice (p = 0.1456) after 21 days (see Figure 4A). A constant banding of the aorta (p =<0.0001) was proven by an increased systolic blood pressure measured after 21 days (see Figure 4B).
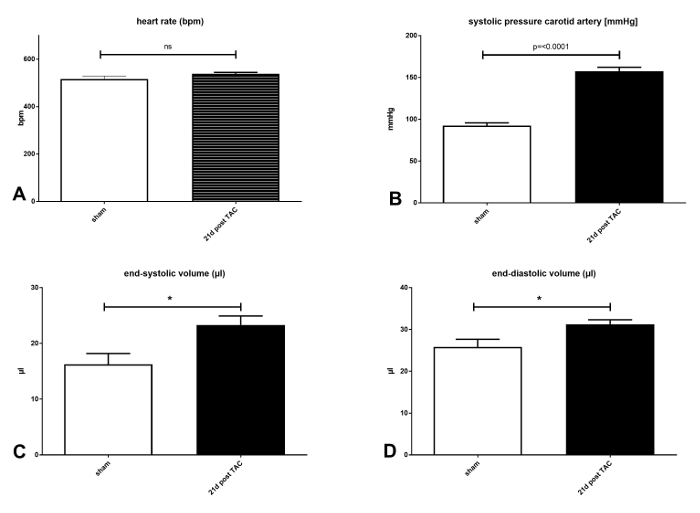
As has been discussed in the literature, C57BL/6J mice are commonly known to develop eccentric hypertrophy with systolic dysfunction10 after TAC. An increase of left ventricular diameter was found, which also appears significant in pressure volume measurements. End-systolic volume increased from 16.25 µL (± 1.935 µL) to 23.31 µL (± 1.617µL). This change was significant (p = 0.0131) (see Figure 4C). End-diastolic volume increased from 25.81 µl (± 1.852 µL) to 31.24 µl ± (1.093 µL). This change was significant (p = 0.0268) (see Figure 4D).
One-way ANOVA followed by Bonferroni's posthoc testing was performed to compare TAC and sham groups. In case of pressure volume measurements, groups were compared using an unpaired t-test with Welch's correction. All data has been presented as mean ± SEM (error bars).
Figure 1: The surgical approach via the 2nd intercostal space at 200% magnification. This picture was taken with the surgical microscope and displays the aortic arch with a thread between the brachiocephalic artery and left common carotid artery. Please click here to view a larger version of this figure.
Figure 2: Representative pulsed-wave Doppler imaging from both carotid arteries (sham vs. TAC mice). A) Pulsed-wave Doppler imaging of the left carotid artery before TAC. B) Pulsed-wave Doppler imaging of the right carotid artery before TAC. C) Image ofPulsed-wave Doppler of the left carotid artery after TAC.The blood flow velocity is reduced compared to figure 2A. D) Pulsed-wave Doppler of the right carotid artery after TAC. The blood flow velocity is increased in comparison to figure 2B. Please click here to view a larger version of this figure.
Figure 3: Heart weight / body weight ratio. Cardiac hypertrophy is induced due to TAC. This is demonstrated by a significant increase in heart weight/body weight ratio. Mice without aortic banding (i.e. sham mice; white bars) were compared to TAC operated mice (black bars) after 3, 6, and 21 days. 6 days after TAC the heart weight /body weight ratio increased significantly in TAC mice. This effect is only slightly pronounced after 21 days. Significance was set to p =<0.05. ns = not significant; ****p <0.0001. Data are presented as mean ± SEM (error bars). n = 6 - 9 per group. Please click here to view a larger version of this figure.
Figure 4: Hemodynamic parameters measured via pressure–volume catheter in mice (C57BL/6J) with and without TAC 21 days after surgery: A) Heart rate (HR) in beats per minute (bpm). There was no difference in HR in both groups indicating a comparable narcosis during the invasive measurements. B) Systolic blood pressure in the right common carotid artery (sBP). The significant increase of sBP after 21 days indicates a constant constriction of the aortic arch. C) End-systolic volumes (ESV) are significantly increased (p = 0.0131) after 21 days and show an increased afterload due to the TAC induced dilatation of the ventricle. D) End-diastolic volume (ESV) is increased (p =0 .0268). Significance was set to p =<0.05. ns = not significant; *p <0.05; ****p <0.0001. Data are presented as mean ± SEM (error bars); n = 8 - 13 per group. Please click here to view a larger version of this figure.
Discussion
The rapid onset of hypertension due to TAC differs from clinically relevant hypertrophy caused by aortic stenosis or hypertension. Nevertheless, the use of small animal models to induce heart failure has many advantages and is, therefore, chosen by many investigators11. This closed chest-model improves the already existing models of the surgical technique to induce transverse aortic constriction in mice4.
The most critical step is the passage under the aortic arch. A too tight suture around the aorta may cause a fatal reduction of blood flow to important organs such as the kidneys. According to the law of Hagen-Poiseuille, flow is mainly dependent on the radius. Therefore, some weight-adapted spacers were used in our protocol. This procedure makes this model more universally applicable, particularly in regard to very young or old mice, depending on the individual experimental setup.
Surgical trauma itself induces an immune response and should be reduced to an absolute minimum to prevent distorting effects. Fast recovery and high survival rates are mandatory, especially in complex animal models. Historically, unlike thoracotomy in human patients, the rib cage in mice is not restored after TAC surgery. Therefore, restitution to physiological breathing movements is limited due to the free floating ribs, which are not reconnected to the sternum.
Minimally invasive techniques for TAC are also used by others12,13. In both models, the aortic arch is reached through a midline incision and an upper partial sternotomy. Although both models are less invasive than open chest models, surgeons have to remove ribs or parts of the sternum to reach the aorta. We believe that maintaining the physiology of the whole rib cage aids faster recovery. Therefore, this protocol improves already existing protocols and helps minimize the surgical trauma itself.
Due to the more apical surgical access, a post-surgical hyperinflation of the lungs for prevention of atelectasis or pneumothoraces, as has been sometimes described4,14, is not required. This access prevents a barotrauma of the lungs, which can be induced by clamping the expiratory tube to open up atelectasis in existing models. This protocol also includes an individualized physiological ventilation strategy. It is tempting to speculate that an individually adapted ventilation aids in reducing ventilator-associated complications such as barotrauma. A weight adapted ventilation strategy was used to avoid effects on the systemic cytokine production by the ventilation itself15.
In conclusion, these techniques represent an alternative and improved model for inducing cardiac hypertrophy in mice.
Although trauma is minimized by avoiding thoracotomy, the superior effect regarding the reduction of inflammation is not shown in this publication. Unfortunately, limitations set by animal protection laws did not allow us to perform open chest TAC in parallel with minimal invasive TAC for comparison because this minimally invasive model has been established for years already. Therefore, these statements are based on the previous experiences of our group.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We thank Stilla Frede and Susanne Schulz for their technical assistance. This study received no funding.
References
- Tarnavski O. Mouse surgical models in cardiovascular research. Methods Mol Biol Clifton NJ. 2009;573:115–137. doi: 10.1007/978-1-60761-247-6_7. [DOI] [PubMed] [Google Scholar]
- Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics. 2004;16(3):349–360. doi: 10.1152/physiolgenomics.00041.2003. [DOI] [PubMed] [Google Scholar]
- Rockman HA, et al. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci U S A. 1991;88(18):8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deAlmeida AC, van Oort RJ, Wehrens XHT. Transverse Aortic Constriction in Mice. J Vis Exp JoVE. 2010. [DOI] [PMC free article] [PubMed]
- Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56(1):56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CJ, Reddy AK, Madala S, Michael LH, Entman ML, Taffet GE. Doppler estimation of reduced coronary flow reserve in mice with pressure overload cardiac hypertrophy. Ultrasound Med Biol. 2008;34(6):892–901. doi: 10.1016/j.ultrasmedbio.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AK, et al. Pulsed Doppler signal processing for use in mice: applications. IEEE Trans Biomed Eng. 2005;52(10):1771–1783. doi: 10.1109/TBME.2005.855709. [DOI] [PubMed] [Google Scholar]
- Shioura KM, Geenen DL, Goldspink PH. Assessment of cardiac function with the pressure-volume conductance system following myocardial infarction in mice. Am J Physiol - Heart Circ Physiol. 2007;293(5):H2870–H2877. doi: 10.1152/ajpheart.00585.2007. [DOI] [PubMed] [Google Scholar]
- Zhang B, Davis JP, Ziolo MT. Cardiac Catheterization in Mice to Measure the Pressure Volume Relationship: Investigating the Bowditch Effect. JoVE J Vis Exp. 2015. p. e52618. [DOI] [PMC free article] [PubMed]
- Barrick CJ, Rojas M, Schoonhoven R, Smyth SS, Threadgill DW. Cardiac response to pressure overload in 129S1/SvImJ and C57BL/6J mice: temporal- and background-dependent development of concentric left ventricular hypertrophy. Am J Physiol Heart Circ Physiol. 2007;292(5):H2119–H2130. doi: 10.1152/ajpheart.00816.2006. [DOI] [PubMed] [Google Scholar]
- Patten RD, Hall-Porter MR. Small animal models of heart failure: development of novel therapies, past and present. Circ Heart Fail. 2009;2(2):138–144. doi: 10.1161/CIRCHEARTFAILURE.108.839761. [DOI] [PubMed] [Google Scholar]
- Zaw AM, Williams CM, Law HKW, Chow BKC. Minimally Invasive Transverse Aortic Constriction in Mice. J Vis Exp JoVE. 2017. [DOI] [PMC free article] [PubMed]
- Tavakoli R, Nemska S, Jamshidi P, Gassmann M, Frossard N. Technique of Minimally Invasive Transverse Aortic Constriction in Mice for Induction of Left Ventricular Hypertrophy. J Vis Exp. 2017. [DOI] [PMC free article] [PubMed]
- Kim S-C, Boehm O, Meyer R, Hoeft A, Knüfermann P, Baumgarten G. A Murine Closed-chest Model of Myocardial Ischemia and Reperfusion. J Vis Exp JoVE. 2012. [DOI] [PMC free article] [PubMed]
- Veldhuizen RA, Slutsky AS, Joseph M, McCaig L. Effects of mechanical ventilation of isolated mouse lungs on surfactant and inflammatory cytokines. Eur Respir J. 2001;17(3):488–494. doi: 10.1183/09031936.01.17304880. [DOI] [PubMed] [Google Scholar]


