Abstract
Background:
Observational studies have reported associations between maternal phthalate levels and adverse outcomes at birth and in the health of the child. Effects on placental function have been suggested as a biologic basis for these findings.
Objective:
We evaluated the effects of phthalates on placental function in vitro by measuring relevant candidate genes and proteins.
Materials and Methods:
Human trophoblast progenitor cells were isolated at 7–14 wk of pregnancy (two female and three male concepti), and villous cytotrophoblast cells (vCTBs) were isolated at 15–20 wk (three female and four male concepti). Cells were cultured in vitro with four phthalate metabolites and their combination at concentrations based on levels found previously in the urine of pregnant women: mono-n-butyl (MnBP, ), monobenzyl (MBzP, ), mono-2-ethylhexyl (MEHP, ), and monoethyl (MEP, ) phthalates. mRNA levels of CGA, CGB, PPARG, CYP19A1, CYP11A1, PTGS2, EREG, and the intracellular subunit of human chorionic gonadotropin () and peroxisome proliferator activated receptor () were measured in the cellular extracts, and protein levels for four forms of secreted hCG were measured in the conditioned media.
Results:
Previously reported associations between maternal phthalates and placental gene expression were reproduced experimentally: MnBP with CGA, MBzP with CYP11A1, and MEHP with PTGS2. CGB and were up-regulated by MBzP. In some cases, there were marked, even opposite, differences in response by sex of the cells. There was evidence of agonism in female cells and antagonism in male cells of by simultaneous exposure to multiple phthalates.
Conclusions:
Concentrations of MnBP, MBzP and MEHP similar to those found in the urine of pregnant women consistently altered hCG and expression in primary placental cells. These findings provide evidence for the molecular basis by which phthalates may alter placental function, and they provide a preliminary mechanistic hypothesis for opposite responses by sex. https://doi.org/10.1289/EHP1539
Introduction
Phthalates are a class of synthetic, endocrine-disrupting chemicals that are detected in all pregnant women in the United States (Woodruff et al. 2011) Prenatal maternal exposure to phthalates has been associated with short-term outcomes in pregnancy, such as the duration of labor (Adibi et al. 2009; Ferguson et al. 2014; Latini et al. 2003), the risk of preeclampsia (Cantonwine et al. 2016), and fetal sex differentiation as determined at birth (Swan et al. 2015). Long-term outcomes in the children may include negative trends in behavior, IQ, attention, and social communication [11 studies of children 0 to 12 y old reviewed by Ejaredar et al. (2015)].
The role of the placenta in these associations has been partially identified. Placental prostaglandin production, which might be a target of phthalates, is highest at the end of pregnancy and is required for the induction of labor (Tetz et al. 2015). Phthalates may alter circulating levels of antiangiogenic molecules that are produced by the placenta and are indicators of preeclampsia risk (Ferguson et al. 2015). We have identified a potential role of sex-specific placental hormone production in contributing to the effect of phthalate exposures on a neonatal marker predicting the future reproductive health of the child (Adibi et al. 2015a). Clarifying the role of the placenta in these associations will improve temporal and spatial precision in estimating the short- and long-term health consequences of prenatal exposure.
It is not possible to directly observe the critical time points in early pregnancy when mRNA and protein expression in the placenta and in the embryo/fetus are vulnerable to perturbation by tissue and circulating levels of maternal phthalates. Owing to genetic, anatomic, and physiologic differences in placental–fetal biology between species (Maltepe et al. 2010; Rawn and Cross 2008), animal models alone are insufficient to identify causal relationships in human pregnancy. As such, we believe a combination of experimental and observational models that are human-specific and informative of early pregnancy relationships is best suited to deliver these necessary insights.
Another motivation to work simultaneously in experimental and observational systems is the need to overcome a source of intractable confounding: between-person differences in placental metabolism. This is a special problem in phthalate epidemiology because the urinary biomarkers used to assess exposure are also partially products of placental metabolism (Hakkola et al. 1998). At the present time, we have no methods to assess or control for this type of bias. Experimental methods (and statistical methods applied to observational data) may drastically reduce this type of bias and give greater confidence and reproducibility regarding the unconfounded effects of the chemicals on gene and protein expression.
We selected a list of candidate genes to determine if the types of phthalate effects on gene expression that have been identified in other species and in other tissue types were also targets in the human placenta (Adibi et al. 2010). In a companion to this manuscript, we have reported sex-specific associations of eight of these candidate mRNAs measured in term placental tissue biopsies with concentrations of six maternal urinary phthalates (Adibi et al. 2017). Here, we report the experimental follow-up study to those associations using models of undifferentiated and differentiated trophoblasts (Tbs) isolated from first- and second-trimester placentas. In the placental tissue, chorionic gonadotropin (CGA) was the most strongly associated with the highest number of phthalate metabolites (Adibi et al. 2017). CGA encodes an subunit of glycoprotein hormones including placenta-specific gonadotropin, called human chorionic gonadotropin (hCG), which is a primary end point in the present study. Here, we follow up on this finding by reporting phthalate effects on mRNAs of both and subunits of hCG (encoded by CGA and by CGB and its homologues, respectively), on protein levels of intracellular and secreted hCG (dimer of and -subunits), and on the mRNA and intracellular levels of a regulatory factor of hCG, peroxisome proliferation activated receptor (). In this analysis, we compared the molecular response of human Tbs to phthalate exposure according to fetal sex differences, application of single phthalate metabolites versus mixtures, cell type, and consistency between mRNA and protein effects.
Materials and Methods
Study Subjects
Women undergoing elective pregnancy terminations between 7 and 20 wk gestation at the Women’s Options Center at the University of California, San Francisco between 2012 and 2013 donated tissue anonymously. Donation was restricted to women without fetal anomalies. Written informed consents were obtained from all subjects. The University of California, San Francisco Committee on Human Research approved the tissue collection.
Cell Culture
The trophoblast progenitor cell (TBPC) represents an undifferentiated, multipotent population derived from the mesoderm of the chorion at gestational weeks 7.0–14.9. Derivation and maintenance conditions are described elsewhere (Genbacev et al. 2011; Genbacev et al. 2016). TBPCs were cultured in six-well plates that had been incubated with 0.5% gelatin for 30 min. They were plated at 250,000 cells/well. Details on the cell-culture medium are described elsewhere (Genbacev et al. 2016). The villous cytotrophoblasts (vCTBs) were isolated and purified from placentas obtained at 15 wk 4 d to 20 wk 1 d of gestation using microdissection, enzymatic digestion, and cell culture techniques described elsewhere (Hunkapiller and Fisher 2008). Cells were plated on Matrigel® in 24-well plates at 500,000 cells/well, dosed in duplicate, and cultured in serum-free medium (Hunkapiller and Fisher 2008). The vCTBs were sampled from placentas obtained from three female and four male placentas, and the TBPCs were sampled from two female and three male placentas (these cells are referred to hereafter as female and male cells, respectively). Details on experiments and replicates are in the Supplemental Material (see Tables S5 and S6). Single phthalate metabolite doses were designed to mimic maternal urinary concentrations measured in a birth cohort study (Adibi et al. 2017): mono-n-butyl phthalate (MnBP; TCI America), monobenzyl phthalate (MBzP; TCI), mono-2-ethylhexyl phthalate (MEHP; Wako), and monoethyl phthalate (MEP; Sigma-Aldrich). Specifically, we chose urinary concentrations that in our previous study were associated with CGA mRNA levels and where the association differed in male versus female placentas [Figures 1A, B, D in Adibi et al. (2017)]. In addition, we combined the four abovementioned concentrations of single phthalates into a mixture dose. Vehicle control cells for the single metabolite doses received 0.1% dimethyl sulfoxide (DMSO), and controls for the phthalate mixture received 0.4% DMSO; both of these doses are below the DMSO concentration that affects Tb differentiation (Thirkill and Douglas 1997). The self-renewing TBPC cultures were grown for 72 h, which is the point at which they reach 90% confluency. The vCTB cultures were maintained for 40 h from the time of dosing. vCTBs are nonrenewing; therefore, 40 h is optimal to evaluate functional changes in hCG secretion while cells are metabolically active. In all experiments, cell morphology and cell density were assessed and documented by phase contrast microscopy.
Gene Expression in Cultured Cells
The genes selected for this study were measured previously in placental tissue biopsies and were found to be correlated with maternal urinary phthalates (Adibi et al. 2010, 2017), with the exception of CGB and EREG. CGB was selected because it encodes a subunit of human chorionic gonadotropin (hCG), a placental hormone that we hypothesize to be a target of phthalate exposure. The CGB primer set amplifies CGB, CGB3, CGB5, CGB7, and CGB8 (TaqMan™, ThermoFisher Scientific). EREG is an ovarian target of luteinizing hormone (LH), a gonadotropin that binds to the same receptor as hCG (Park et al. 2004). In vivo, hCG has been found to stimulate EREG, which is why we also selected EREG for this study (Huber et al. 2007). RNA was isolated using an RNeasy Plus Mini Kit (Qiagen) and measured on a NanoDrop™ spectrophotometer (ThermoFisher Scientific). Reverse transcription was carried out using an iScript™ cDNA Synthesis Kit (BioRad). A TaqMan™ assay for RPS4Y1 was used to assign sex to all control cells. The Ct (amplification cycle at which the mRNA concentration was detectable) for female cells was either cycles, or amplification was nondetectable. For males, amplification of RPS4Y1 was detected at cycles. RPS4Y1, a Y-linked gene, was selected for this purpose after reviewing three independently generated transcriptome data sets of placental cells in culture and placental biopsies to determine which of the Y-linked genes showed the greatest discrimination in males and females (J. Adibi, unpublished data, 2013). TaqMan™ gene expression assays (see Table S4) were used for mRNA quantitation, and of cDNA was loaded in a reaction volume and run in triplicate on a 7900HT quantitative polymerase chain reaction (PCR) instrument (Applied Biosystems). RN18S1 RNA was used as an internal control based on the validated convention of the laboratory where the experiments were designed and conducted (Winn et al. 2007).
hCG in Conditioned Media
Conditioned media from each well from both cell types were aliquoted and stored at . Aliquots were shipped to the University of Helsinki (Finland), where they were analyzed for a panel of hCG subunits and variants including intact hCG, , , and the hyperglycosylated form of hCG (hCG-h). Intact hCG was measured using time-resolved immunofluorometric assays (IFMAs) (DELFIA®, Perkin-Elmer Wallace), and the other forms were measured using in-house assays with monoclonal antibodies (Alfthan et al. 1992; Lee et al. 2013). The lower limit of quantitation was for intact hCG, for , for , and for hCG-h. The intact hCG assay measures only the dimer, irrespective of whether it is hyperglycosylated or not. The assay measures only free subunit, including hyperglycosylated . The assay measures the free subunit. The hyperglycosylated hCG assay (hCG-h) measures hyperglycosylated intact hCG and hyperglycosylated . The subunit is a gonadotropin subunit that is shared by all glycoprotein hormones, that is to say, hCG, TSH, LH, and FSH. lacks hCG activity but may play a role in Tb invasion (Lee et al. 2013).
Quantitative Western Blots
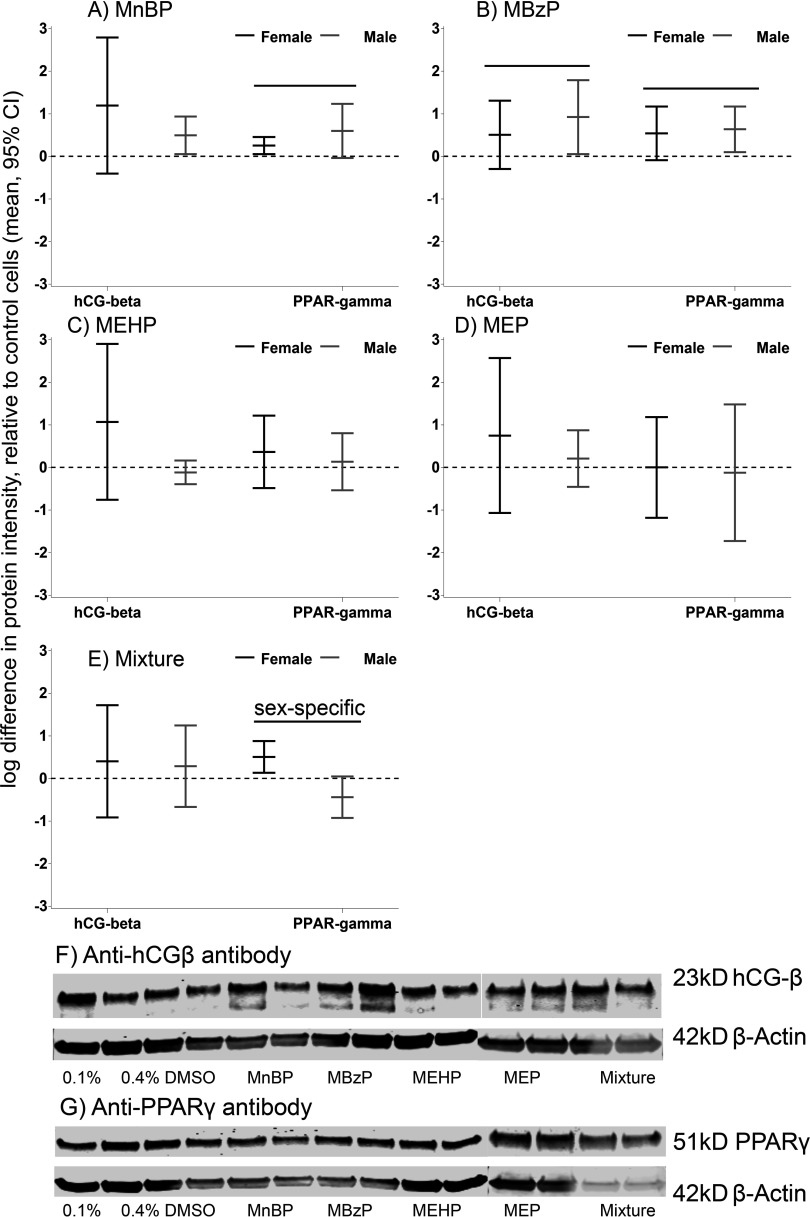
Protein was purified from the same vCTB lysate used for RNA isolation according to the manufacturer’s instructions (AllPrep® RNA/Protein Kit and RNeasy Plus, Qiagen) and was additionally cleaned up by acetone precipitation. Protein lysate was mixed with four volumes of ice-cold acetone and incubated at for 30 min. After centrifuging at for 10 min, the supernatant was discarded, and the pellet was air-dried. The pellet was then resuspended in 5% sodium dodecyl sulfate (SDS). The protein concentration was measured using a Direct Detect® Spectrometer (EMD Millipore). The protein was denatured at for 5 min in loading buffer (Li-Cor) supplemented with 5% (Bio-Rad) and separated using Any kD™ Precast Protein Gels (Bio-Rad). The proteins were transferred to nitrocellulose membrane using a Trans-Blot® Turbo™ Transfer System (Bio-Rad). After blocking in phosphate-buffered saline (PBS) blocking buffer (Li-Cor) at room temperature () for 1 h, the membrane was incubated with primary antibodies in blocking buffer at overnight. The membrane was then washed and incubated with secondary antibodies at room temperature () for 50 min. The reactive proteins were detected using an Odyssey CLx (Li-Cor) imaging system, a method that has been optimized and validated for quantitation (Wang et al. 2007). The Western blot bands were quantified using Image Studio (version 5.0; LI-COR). The primary antibodies were anti-hCG (1:2,000, Dako, A0231), (1:1,000, Cell Signaling, 24,355), and (1:5,000, Santa Cruz Biotechnology, sc4778). The secondary antibodies were IRDye 800CW donkey anti-rabbit IgG () (Li-Cor) and IRDye 680LT donkey anti-mouse IgG () (Li-Cor). The antibody yielded four bands in the Western blot, of which we quantified three: 42 kDa, 23 kDa, and 21 kDa. The 42-kDa band gave the strongest signal, and the 23- and 21-kDa bands were closest in size to the predicted band. The goal was to determine which band had the highest correlation with CGB mRNA and secreted hCG protein as validation of the Dako antibody and as further insight into possible sex differences in hCG synthetic machinery. The 42-kDa band was 4 log units higher on average than the 23-kDa band and strongly correlated with in the same sample (, ). The 21-kDa band was only detected in 57% of female cells and 43% of male cells, and it was not analyzed further ( ). Results for the 23-kDa band are presented (Figure 3F). The 23-kDa band has been used for hCG quantitation in previous studies (Racca et al. 2011). The antibody gave a single band at 51 kDa, consistent with previous studies (Senol-Cosar et al. 2016) (Figure 3G).
Figure 3.
Phthalate effects on intracellular and levels in differentiated cytotrophoblasts (vCTBs). The effects are expressed as the mean natural log difference in protein intensity and 95% confidence intervals (CIs) compared with DMSO-treated control cells. (A) MnBP; (B) MBzP; (C) MEHP; (D) MEP; (E) mixture of all four metabolites. The black lines indicate female-specific effects, and the gray lines indicate male-specific effects. Overall effects that were significant () are indicated by a line and marked “sex-specific” if the phthalate effect differed in male and female cells. Examples of (F) and (G) Western blots. Each dose group was assayed in duplicate (two lanes). This represents a single experiment conducted on cells isolated from a female placenta at 15.6 wk gestation. DMSO, dimethyl sulfoxide; , human chorionic gonadotropin ; MBzP, monobenzyl phthalate; MEHP, mono-2-ethylhexyl phthalate; MEP, monoethyl phthalate; MnBP, mono-n-butyl phthalate; , peroxisome proliferator activated receptor gamma.
Statistical Analysis
For the RNA analysis, mean Ct values (amplification cycle at which the mRNA in the sample was detected; i.e., higher Ct values indicate lower mRNA concentration in the sample) of the technical replicates () were calculated for each sample. Because of the significant variability in baseline gene and protein expression between biologic replicates and because it is established that hCG also differs significantly by day of gestation (Wald et al. 2003), we chose to analyze the data using multivariate mixed effects models. This method allows us to quantitate differences in gene and protein expression between the treated and control cells after adjustment for the clustering of values within each biologic replicate (random effect) and adjustment for fixed effects (sex, gestational age, etc.). In this case, the random effect or experiment number is a proxy for unmeasured biologic variability at baseline (i.e., genetic and epigenetic variability, maternal health, preprocedure exposures). We included as model covariates gestational age at the time of pregnancy termination, RN18S mRNA levels in the sample, sex of the cells, phthalate dose, and institution at which the experiment was conducted. We report the population marginal means and their 95% confidence intervals (CIs) for all treatment groups by sex. For plotting the changes, we used the coefficient (equivalent to the ) and its 95% CIs after transformation to the linear scale (). Sex-specific parameters were calculated, using the Estimate statement in SAS (SAS Institute Inc.), from a model that included a term for phthalate dose by sex. We used two models to estimate all dose effects: the first included the 0.1% DMSO vehicle control samples and the single-metabolite dose groups (also with 0.1% DMSO), and the second included 0.4% DMSO and the phthalate-mixture dose group (also with 0.4% DMSO). Dose group was treated as a categorical variable and interacted on sex.
We applied the same strategy in the analysis of intracellular protein and secreted hCG levels. We report all results for protein on a log scale; a one unit difference is equivalent to a 2.7-fold change on a linear scale. For ease of comparison and interpretation, we report log unit differences as fold changes (the difference between the 2 log values transformed to the linear scale). In the analysis of the Western blot data, was a covariate to control for total protein in the sample. Intracellular and were modeled as -transformed intensity values, and secreted hCG variants were modeled as log-transformed concentrations. To estimate subunit-specific effects, we calculated ratios of the , , and hyperglycosylated hCG to intact hCG. This calculation was performed to normalize for overall hCG production, which serves as an indicator of quantity and viability of the cells. We used Spearman rank correlations to quantitate the relationship between mRNAs and intracellular and secreted proteins that were normalized for internal controls. In all mixed effects models, we estimated empirical standard errors that are more robust when the assumption of equal covariance across experiments cannot be confirmed (Verbeke and Molenberghs 2000). Analyses were performed using SAS (version 9.3; SAS Institute Inc.).
Results
The intracellular and secreted hCG protein levels from the TBPCs were primarily below the level of detection and were not analyzed (data not shown). All mRNA and protein end points were well above the detection limit in the vCTBs. Differences in cellular morphology and cell density were not observed in treated versus control cells. We present the sex-specific means (Ct values, protein intensities, and secreted protein concentrations) of cells treated with single metabolites and with the combined metabolites.
mRNA
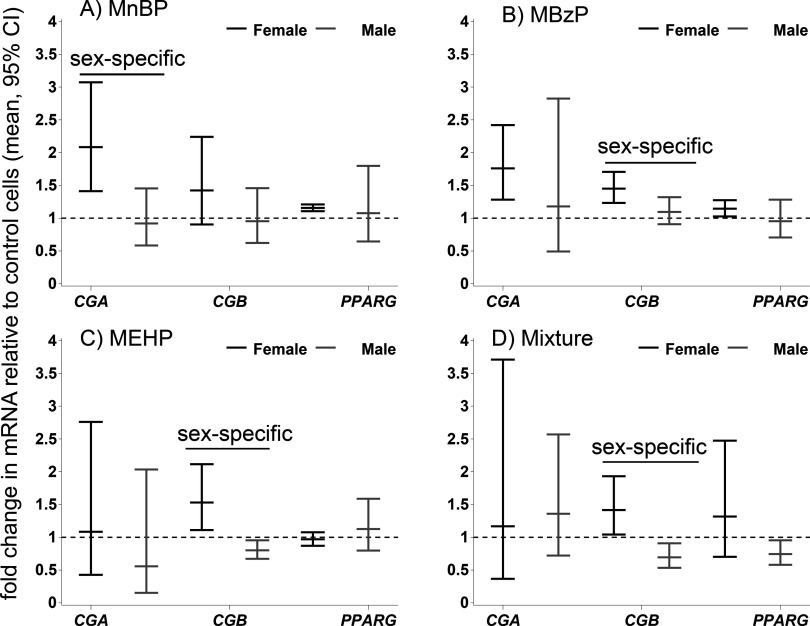
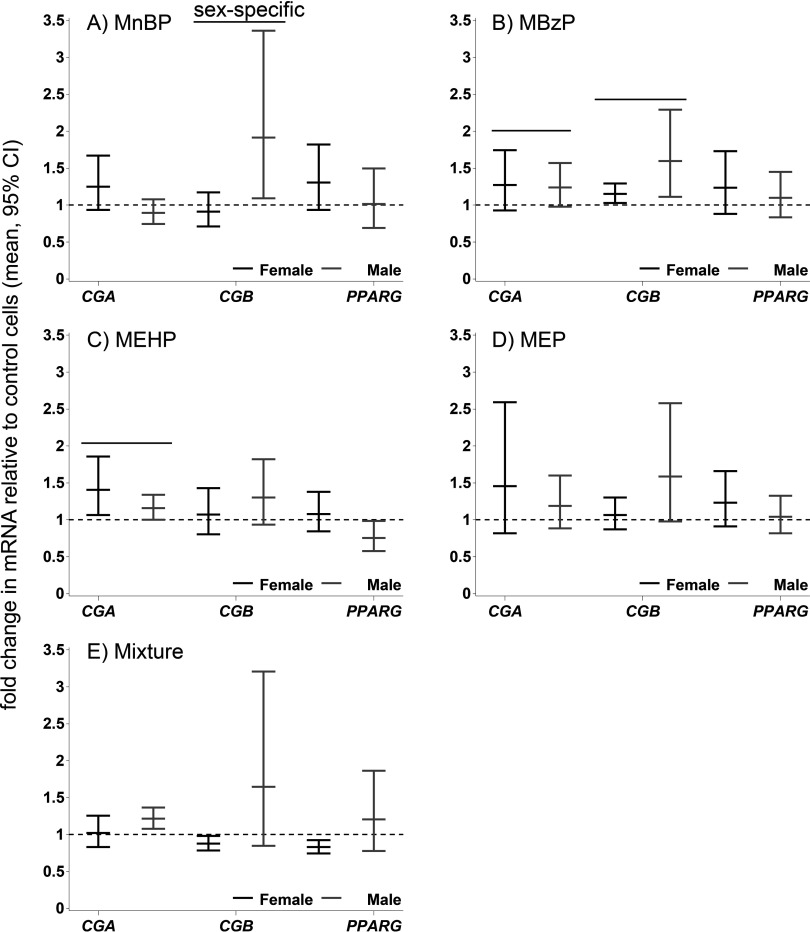
MnBP stimulated the CGA mRNA expression in female TBPCs (2.1-fold; 95% CI: 1.4, 3.1) and vCTBs (1.3-fold; 95% CI: , 1.7) (Figures 1A, 2A; see also Tables S1 and S2), whereas an opposite and weaker effect was observed in male cells (TBPCs ; 95% CI: , 1.5; vCTBs ; 95% CI: , 1.1). This was the only sex-specific effect on mRNA that was common to both cell types (Figures 1, 2). In the TBPCs, the phthalate mixture increased the CGB levels in female cells 1.4-fold (95% CI: 1.0, 1.9) and decreased the levels in male cells 0.7-fold (95% CI: , ). In the female cells, the mixture’s effect on CGB resembled the average effect of the single metabolites (1.4-fold vs. 1.5-fold in the mixture-dosed cells). In the male cells, the effect of the mixture was similar to that of MEHP, which significantly reduced CGB ( by MEHP alone vs. in the mixture-dosed cells). Conversely, in the female vCTBs, the mixture lowered CGB mRNA (95% CI: , ) and increased CGB in the male vCTBs 1.7-fold (95% CI: 0.9, 3.2) relative to the controls. There was inadequate biologic replication for the MEP-dosed TBPCs (see Table S5, results not shown).
Figure 1.
Phthalate effects on mRNAs. The effects are expressed as mean relative fold change () and 95% confidence intervals (CIs) compared with DMSO-treated control cells in undifferentiated trophoblasts (TBPCs). (A) MnBP; (B) MBzP; (C) MEHP; (D) mixture of all four metabolites (MnBP, MBzP, MEHP, MEP). The black lines indicate female-specific effects, and the gray lines indicate male-specific effects. Overall effects that were significant () are indicated by a line and marked “sex-specific” if the phthalate effect differed in male and female cells. CG, chorionic gonadotropin; DMSO, dimethyl sulfoxide; MBzP, monobenzyl phthalate; MEHP, mono-2-ethylhexyl phthalate; MEP, monoethyl phthalate; MnBP, mono-n-butyl phthalate; PPARG, peroxisome proliferator activated receptor gamma.
Figure 2.
Phthalate effects on mRNAs. The effects are expressed as mean relative fold change () and 95% confidence intervals (CIs) compared with DMSO-treated control cells in differentiated cytotrophoblasts (vCTBs). (A) MnBP; (B) MBzP; (C) MEHP; (D) MEP; (E) mixture of all four metabolites. The black lines indicate female-specific effects, and the gray lines indicate male-specific effects. Overall effects that were significant () are indicated by a line and marked “sex-specific” if the phthalate effect differed in male and female cells. CG, chorionic gonadotropin; DMSO, dimethyl sulfoxide; MBzP, monobenzyl phthalate; MEHP, mono-2-ethylhexyl phthalate; MEP, monoethyl phthalate; MnBP, mono-n-butyl phthalate; PPARG, peroxisome proliferator activated receptor gamma.
In the vCTBs, we analyzed three genes that are targets of phthalates in other cell types (Howdeshell et al. 2007; Lovekamp and Davis 2001; Schlezinger et al. 2004) and that are important to placental function: CYP19A1 and CYP11A1 are involved in steroidogenesis and in xenobiotic metabolism, respectively (Hakkola et al. 1998; Miller 1998), and PTGS2 (COX-2) is involved in prostaglandin production by the placenta (Challis et al. 2005). CYP19A1 and CYP11A1 were up-regulated by phthalates in both sexes. MBzP increased CYP11A1 levels in the female cells 1.4-fold (95% CI: 1.2, 1.6) compared with the control cells (see Figure S1). PTGS2 levels were increased in female cells by MBzP 1.2-fold (95% CI: , 1.6) and decreased in male cells (95% CI: , 1.0). In male and female cells combined, MEHP lowered PTGS2 (95% CI , , ).
Intracellular Proteins
MnBP and MBzP increased (2.5-fold and 2.2-fold, respectively) and (1.5-fold and 1.8-fold, respectively) (Table 1, Figure 3). The mixture dose had a stronger sex-specific effect on than the single metabolites. was increased in female cells [1.7-fold (95% CI: 1.1, 2.4)] and was decreased in male vCTBs [ (95% CI: , 1.1)].
Table 1.
Mean log intensities of protein (95% CI) measurements of intracellular and protein expression in vCTBs treated with phthalate metabolites compared with DMSO-treated control cells.
| Protein | Metabolite | Females | Males | Overall | p-Value | Sex-specific p-value |
|---|---|---|---|---|---|---|
| 0.1% DMSO | 3.43 (1.40, 5.47) | 4.51 (3.08, 5.94) | 3.91 (2.83, 5.43) | Reference | Reference | |
| MnBP | 4.63 (4.12, 5.13) | 5.00 (3.90, 6.11) | 4.81 (4.11, 5.52) | 0.08 | 0.38 | |
| MBzP | 3.94 (2.64, 5.23) | 5.43 (4.16, 6.71) | 4.68 (3.57, 5.79) | 0.04* | 0.46 | |
| MEHP | 4.50 (3.84, 5.16) | 4.39 (3.14, 5.64) | 4.44 (3.57, 5.32) | 0.35 | 0.19 | |
| MEP | 4.18 (2.88, 5.48) | 4.72 (3.45, 5.99) | 4.44 (3.23, 5.65) | 0.35 | 0.59 | |
| 0.4% DMSO | 4.00 (2.41, 5.60) | 4.93 (3.28, 6.57) | 4.47 (3.45, 5.48) | Reference | Reference | |
| Mixture | 4.41 (3.81, 5.00) | 5.21 (3.90, 6.52) | 4.81 (4.11, 5.50) | 0.34 | 0.85 | |
| 0.1% DMSO | 5.80 (4.50, 7.10) | 5.39 (3.11, 7.68) | 5.60 (4.32, 6.87) | Reference | Reference | |
| MnBP | 6.05 (4.76, 7.34) | 5.99 (4.31, 7.67) | 6.02 (4.95, 7.08) | 0.01* | 0.29 | |
| MBzP | 6.34 (5.57, 7.10) | 6.03 (3.95, 8.10) | 6.18 (5.12, 7.24) | 0.01* | 0.81 | |
| MEHP | 6.16 (5.01, 7.31) | 5.53 (3.71, 7.34) | 5.84 (4.81, 6.87) | 0.37 | 0.65 | |
| MEP | 5.79 (5.14, 6.45) | 5.27 (4.15, 6.39) | 5.59 (4.83, 6.34) | 0.98 | 0.87 | |
| 0.4% DMSO | 6.36 (5.36, 7.37) | 5.94 (4.82, 7.07) | 6.11 (5.39, 6.83) | Reference | Reference | |
| Mixture | 6.87 (5.95, 7.79) | 5.51 (4.67, 6.34) | 6.23 (5.45, 7.01) | 0.38 | 0.01* |
Note: p-Values are reported for the overall and sex-specific effects of the phthalate dose on secreted hCG. *. Means were estimated by using a mixed effects model with a random intercept for experiment, allowing for control for between- versus within-placenta variability in protein expression. The final sample included three female and three male biologic replicates. Dose groups: MnBP, ; MBzP, ; MEHP, ; MEP, . The mixture includes all 4 concentrations. CI, confidence interval; DMSO, dimethyl sulfoxide; hCG, human chorionic gonadotropin; MBzP, monobenzyl phthalate; MEHP, mono-2-ethylhexyl phthalate; MEP, monoethyl phthalate; MnBP, mono-n-butyl phthalate; , peroxisome proliferator activated receptor gamma; vCTB, villous cytotrophoblast cells.
Secreted hCG Isoforms and Subunits
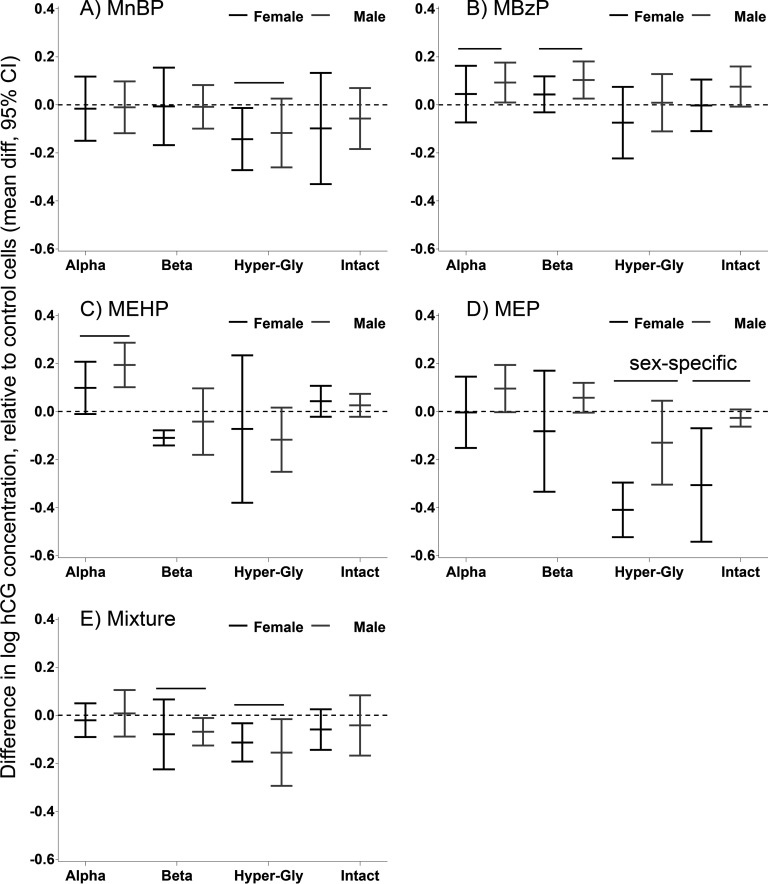
Phthalate effects on secreted hCG levels in the conditioned media differed by metabolite (Table 2, Figure 4). MEP had the strongest effect, with a decrease (95% CI: , ) in hCG-h secreted from female cells. In male cells, MEHP increased secretion 1.21-fold (95% CI: 1.11, 1.33). The mixture significantly suppressed (; 95% CI: , ) and hCG-h ( 95% CI: , ) levels in cells of both sexes.
Table 2.
Mean log concentrations (95% CI) of hCG in the conditioned media of vCTBs treated with four phthalate metabolites, a mixture of the metabolites, and with DMSO.
| Protein | Metabolite | Females | Males | Overall | p-Value | Sex-specific p-value |
|---|---|---|---|---|---|---|
| 0.1% DMSO | 7.06 (6.77, 7.35) | 6.74 (6.54, 6.94) | 6.90 (6.72, 7.07) | Reference | Reference | |
| MnBP | 7.04 (6.81, 7.28) | 6.73 (6.55, 6.91) | 6.88 (6.73, 7.04) | 0.71 | 0.95 | |
| MBzP | 7.10 (6.75, 7.45) | 6.83 (6.65, 7.02) | 6.97 (6.77, 7.16) | 0.05* | 0.49 | |
| MEHP | 7.16 (6.83, 7.48) | 6.94 (6.72, 7.15) | 7.04 (6.84, 7.24) | 0.001* | 0.18 | |
| MEP | 7.05 (6.78, 7.33) | 6.84 (6.63, 7.04) | 6.94 (6.77, 7.12) | 0.29 | 0.26 | |
| 0.4% DMSO | 7.05 (6.73, 7.37) | 6.87 (6.59, 7.15) | 6.96 (6.76, 7.15) | Reference | Reference | |
| Mixture | 7.03 (6.64, 7.42) | 6.88 (6.57, 7.19) | 6.95 (6.73, 7.18) | 0.94 | 0.58 | |
| 0.1% DMSO | 5.14 (4.00, 6.29) | 5.62 (4.88, 6.36) | 5.38 (4.73, 6.03) | Reference | Reference | |
| MnBP | 5.14 (4.10, 6.17) | 5.61 (4.82, 6.40) | 5.37 (4.73, 6.00) | 0.79 | 0.98 | |
| MBzP | 5.19 (4.10, 6.27) | 5.72 (4.96, 6.49) | 5.45 (4.82, 6.09) | 0.01* | 0.26 | |
| MEHP | 5.03 (3.86, 6.21) | 5.58 (4.82, 6.34) | 5.30 (4.63, 5.97) | 0.08 | 0.33 | |
| MEP | 5.06 (3.90, 6.22) | 5.68 (4.88, 6.47) | 5.37 (4.69, 6.04) | 0.88 | 0.27 | |
| 0.4% DMSO | 5.19 (3.50, 6.88) | 5.94 (4.84, 7.04) | 5.56 (4.64, 6.48) | Reference | Reference | |
| Mixture | 5.11 (3.44, 6.78) | 5.87 (4.77, 6.97) | 5.49 (4.57, 6.41) | 0.03 | 0.87 | |
| hCG-h | 0.1% DMSO | 4.47 (3.68, 5.27) | 4.65 (4.06, 5.23) | 4.55 (4.08, 5.02) | Reference | Reference |
| hCG-h | MnBP | 4.33 (3.44, 5.22) | 4.53 (3.91, 5.15) | 4.42 (3.91, 4.94) | 0.02* | 0.79 |
| hCG-h | MBzP | 4.40 (3.46, 5.33) | 4.66 (4.04, 5.28) | 4.53 (3.99, 5.07) | 0.69 | 0.37 |
| hCG-h | MEHP | 4.40 (3.36, 5.44) | 4.53 (3.92, 5.15) | 4.47 (3.87, 5.06) | 0.34 | 0.78 |
| hCG-h | MEP | 4.06 (3.19, 4.93) | 4.52 (3.92, 5.11) | 4.28 (3.78, 4.79) | 0.001* | 0.01* |
| hCG-h | 0.4% DMSO | 4.47 (2.97, 5.98) | 4.84 (4.04, 5.64) | 4.66 (3.88, 5.44) | Reference | Reference |
| hCG-h | Mixture | 4.36 (2.93, 5.79) | 4.69 (3.88, 5.49) | 4.52 (3.76, 5.28) | 0.01* | 0.55 |
| Intact hCG | 0.1% DMSO | 5.15 (4.48, 5.83) | 5.22 (4.76, 5.68) | 5.18 (4.78, 5.57) | Reference | Reference |
| Intact hCG | MnBP | 5.05 (4.48, 5.62) | 5.16 (4.72, 5.61) | 5.10 (4.75, 5.46) | 0.21 | 0.74 |
| Intact hCG | MBzP | 5.15 (4.44, 5.86) | 5.30 (4.87, 5.72) | 5.23 (4.83, 5.62) | 0.13 | 0.25 |
| Intact hCG | MEHP | 5.20 (4.52, 5.87) | 5.25 (4.80, 5.70) | 5.22 (4.81, 5.63) | 0.11 | 0.66 |
| Intact hCG | MEP | 4.85 (4.31, 5.38) | 5.19 (4.72, 5.67) | 5.01 (4.67, 5.36) | 0.04* | 0.02* |
| Intact hCG | 0.4% DMSO | 5.20 (4.27, 6.12) | 5.38 (4.78, 5.97) | 5.29 (4.77, 5.80) | Reference | Reference |
| Intact hCG | 0.1% DMSO | 5.14 (4.15, 6.13) | 5.33 (4.76, 5.91) | 5.24 (4.71, 5.76) | 0.21 | 0.79 |
Note: p-Values are reported for the overall and sex-specific effects of the phthalate dose on secreted hCG. *. Means were estimated by using a mixed effects model with a random intercept for experiment, allowing for control for between- versus within-placenta variability in hCG secretion. The final sample included three female and four male biologic replicates. Dose groups: MnBP, ; MBzP, ; MEHP, ; MEP, . The mixture includes all 4 concentrations. CI, confidence interval; DMSO, dimethyl sulfoxide; hCG, human chorionic gonadotropin; MBzP, monobenzyl phthalate; MEHP, mono-2-ethylhexyl phthalate; MEP, monoethyl phthalate; MnBP, mono-n-butyl phthalate; , peroxisome proliferator activated receptor gamma; vCTB, villous cytotrophoblast cells.
Figure 4.
Phthalate effects on secreted hCG forms in the conditioned media of differentiated trophoblasts (villous cytotrphoblasts, vCTBs). The effects are expressed as the difference in mean natural log concentration and 95% confidence intervals (CIs) compared with DMSO-treated control cells. (A) MnBP; (B) MBzP; (C) MEHP; (D) MEP; (E) mixture of all four metabolites. The black lines indicate female-specific effects, and the gray lines indicate male-specific effects. Overall effects that were significant () are indicated by a line and marked “sex-specific” if the phthalate effect differed in male and female cells. DMSO, dimethyl sulfoxide; hCG human chorionic gonadotropin; MBzP, monobenzyl phthalate; MEHP, mono-2-ethylhexyl phthalate; MEP, monoethyl phthalate; MnBP, mono-n-butyl phthalate.
To evaluate specific effects of phthalates on the levels of hCG subunits, we used ratios of the subunits to intact hCG as our end points (see Figure S2). Here, intact hCG serves as an indicator of overall hCG production. We did not detect sex-specific effects. MnBP significantly up-regulated (1.07-fold; 95% CI: 1.00, 1.15), and MEHP significantly down-regulated (; 95% CI: , ). MEP up-regulated (1.22-fold; 95% CI: 1.06, 1.42) and (1.17-fold; 95% CI: 1.07, 1.28). The mixture did not significantly alter the hCG subunits.
Correlations between mRNA and Intracellular and Secreted Protein
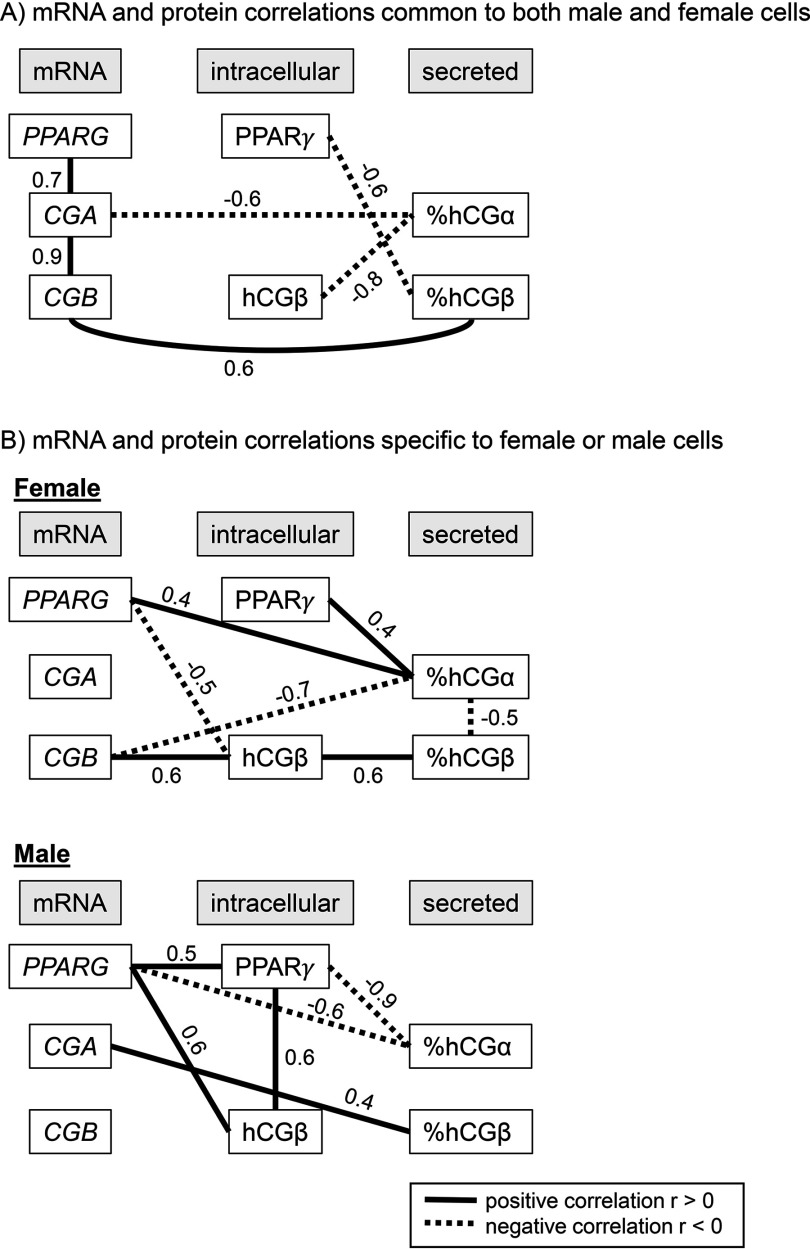
Even though it is only a snapshot, we can evaluate these correlations as indicators of potential sex differences in the underlying in vitro mechanisms of hCG synthesis and secretion. A direct and positive correlation between CGB mRNA and intracellular was only observed in females (23 kDa , ; Figure 5; see also Table S3). This finding is similar to measurements obtained by others who used this antibody, but without consideration of the sex of the placental cells (Uhlén et al. 2015). In males, there was no correlation between levels of mRNAs for hCG and the intracellular protein levels. Correlations that were common to males and females were the negative correlation between CGA and secreted and the positive correlation between CGB and secreted . PPARG mRNA was positively correlated with its encoded protein only in the male (, ) but not the female vCTBs (, ). These are univariate correlations and do not take into account clustering within placentas or sources of variability in the correlations other than the sex of the cells.
Figure 5.
Spearman rank correlations between levels of mRNAs, intracellular proteins, and secreted proteins in female and male differentiated trophoblasts (villous cytotrphoblasts, vCTBs). (A) Correlations that are common to male and female vCTBs; (B) Correlations detected only in female and in male cells (). Positive correlations are drawn as solid black lines, and negative correlations are drawn as dotted lines. hCG, human chorionic gonadotropin; PPAR, peroxisome proliferator activated receptor.
Discussion
Using experimental methods, we generated data that reproduced, in cell models, observed relationships between prenatal exposure to MnBP, MBzP, and MEHP and genes essential to placental gonadotropin synthesis (CGA), placental progesterone synthesis (CYP11A1), and placental prostaglandin production (PTGS2). To further evaluate the biological relevance of these changes in mRNA, we measured two corresponding proteins: hCG and . MnBP and MBzP changed , and MEHP changed , in ways that were consistent with mRNA effects. To maximize the two-way translational value of our findings to human pregnancy, in the present study, we used primary human placental cells and dosed them with phthalate metabolites at concentrations found in the urine of pregnant women exposed to environmental levels of phthalates. Compared with working with homogenous or immortalized cell lines or high doses of phthalates, this approach presents unique experimental and statistical challenges, yet it produces results with greater translational significance to pregnancy.
In two birth cohort studies, we previously reported that maternal urinary phthalates were associated with higher levels of mRNA and protein in female placentas/fetuses and with lower levels in male placentas/fetuses (Adibi et al. 2015a, 2017). In the present study, we explored this further by studying mRNA and protein effects in tandem and by including a transcription factor that regulates hCG in the placenta that is also activated by phthalates— (Fournier et al. 2011; Handschuh et al. 2007; Hurst and Waxman 2003). It has been hypothesized that may be the mechanism by which phthalates can exert endocrine-disrupting effects (Desvergne et al. 2009; Lovekamp-Swan and Davis 2003).
Unexpectedly, we observed sex-specific relationships between and hCG. PPARG mRNA and protein were positively correlated with in male but not female cells. hCG synthesis also differed by sex. In female cells, CGB mRNA was positively correlated with intracellular and secreted , as expected. In male cells, CGB mRNA was not correlated with intracellular . and are subunits of intact hCG but may also have unique functions independent of classical LH/hCG-receptor (Blithe and Nisula 1987; Blithe et al. 1991; Hussa 1980, 1982; Lee et al. 2013). and were highly positively correlated at the mRNA level in both sexes, but not at the protein level. In female cells, the subunits were inversely correlated, and they were not correlated in male cells. hCG subunit variation may be relevant to sex-specific hCG regulation or to other types of posttranscriptional regulation of hCG.
may be a key intermediary between phthalate exposure and placental hCG levels, explaining why hormonal effects are opposite in direction for males and females. The effects of the mixture dose on were opposite in males and females. The sex difference in the correlation of PPARG with and could explain the opposite effects of phthalates on hCG. These are important and novel insights that give rise to testable hypotheses that can be further studied using biomarkers in human pregnancy and in vitro by using experimental techniques. Additional levels of complexity in this relationship should be considered, such as the epigenetic regulation of PPARG (Lendvai et al. 2016), mitochondrial expression of PPARG in the placenta (Calabuig-Navarro et al. 2016), and sex-specific mitochondrial dysfunction in response to maternal exposures (Muralimanoharan et al. 2015).
We based our experimental doses on phthalate concentrations in maternal urine that were correlated with placental tissue CGA mRNA to compare the two sets of results and to evaluate reproducibility (Adibi et al. 2017). In three cases, the sex-specific associations between phthalates and placental mRNA expression were supported by the in vitro replication. For CYP11A1, the association with MBzP was stronger in magnitude than the in vitro effect. PTGS2 was down-regulated by MEHP in male placentas in both studies. Discrepancy in results between the two study designs may indicate that isolated trophoblast cells, cultured in the absence of fetal tissue and signals from the fetal pituitary/adrenal/gonadal cells, exhibit weaker or even a reversal of hCG sex differences measured in vivo. We observed heterogeneity in the direction and magnitude of the hCG effects by phthalate metabolite, by sex, by hCG subunit, and by differentiated versus undifferentiated Tbs. We offer these as testable hypotheses to be pursued in future studies in vitro and in human populations.hCG is an essential hormone for pregnancy maintenance and is correlated with many obstetric outcomes (Filicori et al. 2005; Yaron et al. 2002b), yet it has not been considered in studies of fetal endocrine disruption to the same degree as androgens, estrogens, and progesterone. In both sexes, there is evidence that hCG can act as a potent gonadotropin at different points in development. In females, hCG is used to stimulate ovulation for the purpose of in vitro fertilization (Yen et al. 2014). In males, hCG has been used to induce virilization and penile growth in prepubertal males with hypogonadotrophic hypogonadism (Bistritzer et al. 1989) and to induce spermatogenesis in adult life. In normal pregnancy, hCG binds to the luteinizing hormone/chorionic gonadotropin (LH/CGR) receptor in the male fetus during the first trimester, stimulating testicular steroidogenesis and thereby indirectly guiding genital differentiation (Huhtaniemi et al. 1977). If the LH/CGR receptor is inactive because of mutation of its gene, males are born with defective genital masculinization (XY, disorder of sexual differentiation) (Kremer et al. 1995). Women with 20% lower circulating hCG had an increased chance of giving birth to cryptorchid boys (Chedane et al. 2014). Taken together, these findings support the idea that disruption of hCG production and function by phthalates or by other endocrine-disrupting chemicals during pregnancy may have effects on fetal sex differentiation.
There are not likely to be real-life situations where a person would only be exposed to one phthalate metabolite at a time; therefore, we evaluated and compared the effects of phthalate mixtures and of single phthalates. We used a nonbalanced approach (i.e., nonequivalent doses) in the design of our doses to accurately reflect real-life exposures during pregnancy (Evans et al. 2012). We detected three cases of a significant effect of the mixture. In the case of CGB mRNA in undifferentiated trophoblasts, the mixture effect was analogous to the single-metabolite effects. We interpret this to mean that there was a common mechanism that was not overwhelmed by the phthalate concentrations used. In the case of intracellular in the differentiated trophoblasts, the mixture effect was stronger than the single-metabolite effects, sex-specific, and opposite in direction. The mechanism of activation may differ in the case of multiple versus single phthalates. In the case of the female cells, the different metabolites may have synergized to increase the strength of the positive effect on (i.e., agonism). In the case of the male cells, the metabolites may have competed for or antagonized (or both) a common mechanism to cause the down-regulation of . This latter scenario may also apply to secreted , where the negative effect of the mixture was a reversal of the positive effect of MBzP. Comparisons between the effects of single metabolites versus mixtures are critical in establishing which metabolites are more biologically potent and should be prioritized in efforts to reduce risks to the placenta and fetus.
Sex and gestational variation in hCG have been previously established at the population level in analyses of hCG biomarker data (Adibi et al. 2015b; Bremme and Eneroth 1983; Buckberry et al. 2014; Clements et al. 1976; Cowans et al. 2009; Nagy et al. 1994a, 1994b; Steier et al. 1999; Yaron et al. 2002a). This type of variation is generally not considered when analyzing in vitro experimental data. We observed in our primary tissue cultures that the sex and gestational-age variation in mRNA and secreted protein levels that were present at baseline persisted and rendered our biologic replicates less comparable. For this reason, we controlled for these variables in the data analysis using basic multivariate statistical techniques.
There are noteworthy caveats in making comparisons between observational and experimental findings. In the in vitro study, our controls received no phthalates, whereas in the observational studies, we compared pregnancies with lower but not zero exposure because all subjects were environmentally exposed. Similarly, all of the pregnant women were exposed to a phthalate mixture even though we estimated associations with single metabolites to which we compared the results of the present study (Adibi et al. 2017). Phthalate concentrations in placental tissue are most likely lower than the urine concentrations modeled here [phthalates are not measured in blood owing to a short half-life and to a high risk of phthalate contamination in the sampling process (Calafat et al. 2015)]. In a small pilot study, we estimated that MnBP, MBzP, and MEP were higher, but within an order of magnitude, in urine than in placental tissue by 14-, 28-, and 8-fold respectively. MEHP was 4-fold higher in placental tissue; therefore, we may have slightly underestimated the true exposure to the placenta in this study (J. Adibi and N. Snyder, unpublished data, 2017); this may increase the translational value over previously published studies that dosed with concentrations of MEHP that are 1–3 orders of magnitude higher than urinary levels (Meruvu et al. 2016; Tetz et al. 2013; Wang et al. 2016).
These relationships and the relatively small effect sizes are supported by studies conducted by other investigators. In a study of immature rat Leydig cells, CYP11A1 mRNA was 30–40% higher than in controls at MnBP (Li et al. 2016), similar to the effect we found in male placental cells (33%) in the present study. In an immortalized first-trimester cell line (sex not specified), PTGS, the gene that encodes the COX-2 protein, increased approximately 2- to 3-fold at a MEHP dose (129-fold as high as the dose used in our study) (Tetz et al. 2013). In another study, the effects of MEHP on the COX-2 protein were not detected within the dose range that we used, but only at higher doses (Wang et al. 2016). We measured a significant reduction in PTGS2 by MEHP. Results cannot be easily compared owing to the large differences in dose and to a high likelihood of different mechanisms at low versus high doses. In a review of 35 published studies that reported associations of prenatal phthalate exposures with obstetric outcomes, the authors indicated that knowledge of a mechanism and of ways to measure specific biologic intermediaries in human pregnancy are lacking (Marie et al. 2015). Our findings address this gap by offering biologic insight into correlations of prenatal phthalate exposure with placental end points.
Conclusion
In conclusion, we moved one step beyond an observational association by showing sex-specific relationships between MnBP and placental CGA, and between MBzP and placental CYP11A1 using experimental methods with primary cells. The finding that MnBP can alter chorionic gonadotropin (CGA) was extended to other phthalate metabolites (MBzP, MEHP, and MEP) and to intracellular and secreted hCG and its subunits. The sex-specific effects of phthalates may be challenging to reproduce in vitro in the absence of the fetus. However, our results support the hypothesis that hCG is altered by low concentrations of single and combined phthalates, which is relevant for environmental exposure to phthalates. We are the first to report sex differences in hCG transcription and translation, which we believe to be partially regulated by . This finding provides a testable hypothesis to better understand why the hormonal effects of phthalates are opposite in direction between males and females. In future studies, it will be important to quantify the functional significance (for cells, for organs, and for the future child) of small perturbations in hCG by ubiquitous phthalate exposures.
Supplemental Material
Acknowledgments
We acknowledge S. Fisher and O. Genbacev for their mentoring in placental biology and to Y. Zhou, M. Gormley, N. Hunkapiller, B. Hromatka, and members of the Fisher Lab who all provided valuable mentoring, technical training resources, and feedback on this project. We thank the D. Lewis and S. Gollin laboratories in the Department of Human Genetics and the University of Pittsburgh Genomics Core for support in completing experiments at our new university; we also thank T. Grönholm for technical assistance with hCG immunoassays. Funding was received from the National Institute of Envirnomental Health Sciences/National Institutes of Health (NIEHS/NIH) (grant nos. 1K99 ES017780-01, 5R00ES017780 – 06) and from the Science Innovation Fund of the Passport Foundation (J.J.A.). Funds were provided by the Department of Epidemiology at the University of Pittsburgh to complete this project. Contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- Adibi JJ, Hauser R, Williams PL, Whyatt RM, Calafat AM, Nelson H, et al. 2009. Maternal urinary metabolites of di-(2-ethylhexyl) phthalate in relation to the timing of labor in a US multicenter pregnancy cohort study. Am J Epidemiol 169(8):1015–1024, PMID: 19251754, 10.1093/aje/kwp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi JJ, Whyatt RM, Hauser R, Bhat HK, Davis BJ, Calafat AM, et al. 2010. Transcriptional biomarkers of steroidogenesis and trophoblast differentiation in the placenta in relation to prenatal phthalate exposure. Environ Health Perspect 118(2):291–296, PMID: 20123604, 10.1289/ehp.0900788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi JJ, Lee MK, Naimi AI, Barrett E, Nguyen RH, Sathyanarayana S, et al. 2015a. Human chorionic gonadotropin partially mediates phthalate association with male and female anogenital distance. J Clin Endocrinol Metab 100(9):E1216–E1224, PMID: 26200238, 10.1210/jc.2015-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi JJ, Lee MK, Saha S, Boscardin WJ, Apfel A, Currier RJ. 2015b. Fetal sex differences in human chorionic gonadotropin fluctuate by maternal race, age, weight and by gestational age. J Dev Orig Health Disease 6(6):493–500, PMID: 26242396, 10.1017/S2040174415001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi JJ, Buckley JP, Lee MK, Williams PL, Just AC, Zhao Y, et al. 2017. Maternal urinary phthalates and sex-specific placental mRNA levels in an urban birth cohort. Environ Health 16(1):35, PMID: 28381288, 10.1186/s12940-017-0241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfthan H, Haglund C, Dabek J, Stenman UH. 1992. Concentrations of human choriogonadotropin, its beta-subunit, and the core fragment of the beta-subunit in serum and urine of men and nonpregnant women. Clin Chem 38(10):1981–1987, PMID: 1382894. [PubMed] [Google Scholar]
- Bistritzer T, Lunenfeld B, Passwell JH, Theodor R. 1989. Hormonal therapy and pubertal development in boys with selective hypogonadotropic hypogonadism. Fertil Steril 52(2):302–306, PMID: 2753178, 10.1016/S0015-0282(16)60859-2. [DOI] [PubMed] [Google Scholar]
- Blithe DL, Nisula BC. 1987. Similarity of the clearance rates of free alpha-subunit and alpha-subunit dissociated from intact human chorionic gonadotropin, despite differences in sialic acid contents. Endocrinology 121(4):1215–1220, PMID: 3653025, 10.1210/endo-121-4-1215. [DOI] [PubMed] [Google Scholar]
- Blithe DL, Richards RG, Skarulis MC. 1991. Free alpha molecules from pregnancy stimulate secretion of prolactin from human decidual cells: a novel function for free alpha in pregnancy. Endocrinology 129(4):2257–2259, PMID: 1717245, 10.1210/endo-129-4-2257. [DOI] [PubMed] [Google Scholar]
- Bremme K, Eneroth P. 1983. Fetal sex dependent hormone levels in early pregnant women with elevated maternal serum alpha-fetoprotein. Int J Gynaecol Obstet 21(6):451–457, PMID: 6198221, 10.1016/0020-7292(83)90034-6. [DOI] [PubMed] [Google Scholar]
- Buckberry S, Bianco-Miotto T, Bent SJ, Dekker GA, Roberts CT. 2014. Integrative transcriptome meta-analysis reveals widespread sex-biased gene expression at the human fetal-maternal interface. Mol Hum Reprod 20(8):810–819, PMID: 24867328, 10.1093/molehr/gau035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabuig-Navarro V, Puchowicz M, Glazebrook P, Haghiac M, Minium J, Catalano P, et al. 2016. Effect of ω-3 supplementation on placental lipid metabolism in overweight and obese women. Am J Clin Nutr 103(4):1064–1072, PMID: 26961929, 10.3945/ajcn.115.124651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Longnecker MP, Koch HM, Swan SH, Hauser R, Goldman LR, et al. 2015. Optimal exposure biomarkers for nonpersistent chemicals in environmental epidemiology. Environ Health Perspect 123(7):A166–A168, PMID: 26132373, 10.1289/ehp.1510041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE, Meeker JD, Ferguson KK, Mukherjee B, Hauser R, McElrath TF. 2016. Urinary concentrations of bisphenol A and phthalate metabolites measured during pregnancy and risk of preeclampsia. Environ Health Perspect 124(10):1651–1655, PMID: 27177253, 10.1289/EHP188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis JR, Bloomfield FH, Bocking AD, Casciani V, Chisaka H, Connor K, et al. 2005. Fetal signals and parturition. J Obstet Gynaecol Res 31(6):492–499, PMID: 16343248, 10.1111/j.1447-0756.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- Chedane C, Puissant H, Weil D, Rouleau S, Coutant R. 2014. Association between altered placental human chorionic gonadotrophin (hCG) production and the occurrence of cryptorchidism: a retrospective study. BMC Pediatr 14:191, PMID: 25064170, 10.1186/1471-2431-14-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JA, Reyes FI, Winter JS, Faiman C. 1976. Studies on human sexual development. Iii. Fetal pituitary and serum, and amniotic fluid concentrations of LH, CG, and FSH. J Clin Endocrinol Metab 42(1):9–19, PMID: 1249196, 10.1210/jcem-42-1-9. [DOI] [PubMed] [Google Scholar]
- Cowans NJ, Stamatopoulou A, Maiz N, Spencer K, Nicolaides KH. 2009. The impact of fetal gender on first trimester nuchal translucency and maternal serum free beta-hCG and PAPP-A MoM in normal and trisomy 21 pregnancies. Prenat Diagn 29(6):578–581, PMID: 19288535, 10.1002/pd.2246. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Feige JN, Casals-Casas C. 2009. PPAR-mediated activity of phthalates: A link to the obesity epidemic?. Mol Cell Endocrinol 304(1–2):43–48, PMID: 19433246, 10.1016/j.mce.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Ejaredar M, Nyanza EC, Ten Eycke K, Dewey D. 2015. Phthalate exposure and childrens neurodevelopment: a systematic review. Environ Res 142:51–60, PMID: 26101203, 10.1016/j.envres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Evans RM, Scholze M, Kortenkamp A. 2012. Additive mixture effects of estrogenic chemicals in human cell-based assays can be influenced by inclusion of chemicals with differing effect profiles. PloS One 7(8):e43606, PMID: 22912892, 10.1371/journal.pone.0043606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Meeker JD. 2014. Environmental phthalate exposure and preterm birth. JAMA Pediatr 168(1):61–67, PMID: 24247736, 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. 2015. Phthalate metabolites and bisphenol-A in association with circulating angiogenic biomarkers across pregnancy. Placenta 36(6):699–703, PMID: 25913709, 10.1016/j.placenta.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filicori M, Fazleabas AT, Huhtaniemi I, Licht P, Rao ChV, Tesarik J, et al. 2005. Novel concepts of human chorionic gonadotropin: reproductive system interactions and potential in the management of infertility. Fertil Steril 84(2):275–284, PMID: 16084861, 10.1016/j.fertnstert.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Fournier T, Guibourdenche J, Handschuh K, Tsatsaris V, Rauwel B, Davrinche C, et al. 2011. PPARγ and human trophoblast differentiation. J Reprod Immunol 90(1):41–49, 10.1016/j.jri.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Donne M, Kapidzic M, Gormley M, Lamb J, Gilmore J, et al. 2011. Establishment of human trophoblast progenitor cell lines from the chorion. Stem Cells 29(9):1427–1436, PMID: 21755573, 10.1002/stem.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Larocque N, Ona K, Prakobphol A, Garrido-Gomez T, Kapidzic M, et al. 2016. Integrin α4-positive human trophoblast progenitors: functional characterization and transcriptional regulation. Hum Reprod 31(6):1300–1314, PMID: 27083540, 10.1093/humrep/dew077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkola J, Pelkonen O, Pasanen M, Raunio H. 1998. Xenobiotic-metabolizing cytochrome P450 enzymes in the human feto-placental unit: role in intrauterine toxicity. Crit Rev Toxicol 28(1):35–72, PMID: 9493761, 10.1080/10408449891344173. [DOI] [PubMed] [Google Scholar]
- Handschuh K, Guibourdenche J, Tsatsaris V, Guesnon M, Laurendeau I, Evain-Brion D, et al. 2007. Human chorionic gonadotropin produced by the invasive trophoblast but not the villous trophoblast promotes cell invasion and is down-regulated by peroxisome proliferator-activated receptor-γ. Endocrinology 148(10):5011–5019, PMID: 17628005, 10.1210/en.2007-0286. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Furr J, Lambright CR, Rider CV, Wilson VS, Gray LE. Jr. 2007. Cumulative effects of dibutyl phthalate and diethylhexyl phthalate on male rat reproductive tract development: altered fetal steroid hormones and genes. Toxicol Sci 99(1):190–202, PMID: 17400582, 10.1093/toxsci/kfm069. [DOI] [PubMed] [Google Scholar]
- Huber A, Hudelist G, Knöfler M, Saleh L, Huber JC, Singer CF. 2007. Effect of highly purified human chorionic gonadotropin preparations on the gene expression signature of stromal cells derived from endometriotic lesions: potential mechanisms for the therapeutic effect of human chorionic gonadotropin in vivo. Fertil Steril 88(4 Suppl):1232–1239, PMID: 17561010, 10.1016/j.fertnstert.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi IT, Korenbrot CC, Jaffe RB. 1977. HCG binding and stimulation of testosterone biosynthesis in the human fetal testis. J Clin Endocrinol Metab 44(5):963–967, PMID: 192755, 10.1210/jcem-44-5-963. [DOI] [PubMed] [Google Scholar]
- Hunkapiller NM, Fisher SJ. 2008. Chapter 12. Placental remodeling of the uterine vasculature. Methods Enzymol 445:281–302, PMID: 19022064, 10.1016/S0076-6879(08)03012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst CH, Waxman DJ. 2003. Activation of PPARα and PPARγ by environmental phthalate monoesters. Toxicol Sci 74(2):297–308, PMID: 12805656, 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- Hussa RO. 1980. Biosynthesis of human chorionic gonadotropin. Endocr Rev 1(3):268–294, PMID: 6262070, 10.1210/edrv-1-3-268. [DOI] [PubMed] [Google Scholar]
- Hussa RO. 1982. Clinical utility of human chorionic gonadotropin and alpha-subunit measurements. Obstet Gynecol 60(1):1–12, PMID: 6283446. [PubMed] [Google Scholar]
- Kremer H, Kraaij R, Toledo SP, Post M, Fridman JB, Hayashida CY, et al. 1995. Male pseudohermaphroditism due to a homozygous missense mutation of the luteinizing hormone receptor gene. Nat Genet 9(2):160–164, PMID: 7719343, 10.1038/ng0295-160. [DOI] [PubMed] [Google Scholar]
- Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, et al. 2003. Exposure to di(2-ethylhexyl)phthalate in humans during pregnancy. A preliminary report. Biol Neonate 83(1):22–24, PMID: 12566679. [DOI] [PubMed] [Google Scholar]
- Lee CL, Chiu PC, Hautala L, Salo T, Yeung WS, Stenman UH, et al. 2013. Human chorionic gonadotropin and its free β-subunit stimulate trophoblast invasion independent of LH/hCG receptor. Mol Cell Endocrinol 375(1–2):43–52, PMID: 23684886, 10.1016/j.mce.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Lendvai A, Deutsch MJ, Plösch T, Ensenauer R. 2016. The peroxisome proliferator-activated receptors under epigenetic control in placental metabolism and fetal development. Am J Physiol Endocrinol Metab 310(10):E797–E810, PMID: 26860983, 10.1152/ajpendo.00372.2015. [DOI] [PubMed] [Google Scholar]
- Li L, Chen X, Hu G, Wang S, Xu R, Zhu Q, et al. 2016. Comparison of the effects of dibutyl and monobutyl phthalates on the steroidogenesis of rat immature Leydig cells. Biomed Res Int 2016:1376526, PMID: 27148549, 10.1155/2016/1376526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovekamp TN, Davis BJ. 2001. Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicol Appl Pharmacol 172(3):217–224, PMID: 11312650, 10.1006/taap.2001.9156. [DOI] [PubMed] [Google Scholar]
- Lovekamp-Swan T, Davis BJ. 2003. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect 111(2):139–145, PMID: 12573895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltepe E, Bakardjiev AI, Fisher SJ. 2010. The placenta: transcriptional, epigenetic, and physiological integration during development. J Clin Invest 120(4):1016–1025, PMID: 20364099, 10.1172/JCI41211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie C, Vendittelli F, Sauvant-Rochat MP. 2015. Obstetrical outcomes and biomarkers to assess exposure to phthalates: a review. Environ Int 83:116–136, PMID: 26118330, 10.1016/j.envint.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Meruvu S, Zhang J, Bedi YS, Choudhury M. 2016. Mono-(2-ethylhexyl) phthalate induces apoptosis through miR-16 in human first trimester placental cell line HTR-8/SVneo. Toxicol In Vitro 31:35–42, PMID: 26597031, 10.1016/j.tiv.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Miller WL. 1998. Steroid hormone biosynthesis and actions in the materno-feto-placental unit. Clin Perinatol 25(4):799–817, PMID: 9891616. [PubMed] [Google Scholar]
- Muralimanoharan S, Guo C, Myatt L, Maloyan A. 2015. Sexual dimorphism in miR-210 expression and mitochondrial dysfunction in the placenta with maternal obesity. Int J Obes (Lond) 39(8):1274–1281, PMID: 25833255, 10.1038/ijo.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy AM, Glinoer D, Picelli G, Delogne-Desnoeck J, Fleury B, Courte C, et al. 1994a. Total amounts of circulating human chorionic gonadotrophin α and β subunits can be assessed throughout human pregnancy using immunoradiometric assays calibrated with the unaltered and thermally dissociated heterodimer. J Endocrinol 140(3):513–520, PMID: 7514205. [DOI] [PubMed] [Google Scholar]
- Nagy AM, Jauniaux E, Jurkovic D, Meuris S. 1994b. Placental production of human chorionic gonadotrophin α and β subunits in early pregnancy as evidenced in fluid from the exocoelomic cavity. J Endocrinol 142(3):511–516, PMID: 7525825. [DOI] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. 2004. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303(5658):682–684, PMID: 14726596, 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- Racca AC, Camolotto SA, Ridano ME, Bocco JL, Genti-Raimondi S, Panzetta-Dutari GM. 2011. Krüppel-like factor 6 expression changes during trophoblast syncytialization and transactivates βhCG and PSG placental genes. PloS One 6(7):e22438, PMID: 21799854, 10.1371/journal.pone.0022438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawn SM, Cross JC. 2008. The evolution, regulation, and function of placenta-specific genes. Annu Rev Cell Dev Biol 24:159–181, PMID: 18616428, 10.1146/annurev.cellbio.24.110707.175418. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, Howard GJ, Hurst CH, Emberley JK, Waxman DJ, Webster T, et al. 2004. Environmental and endogenous peroxisome proliferator-activated receptor γ agonists induce bone marrow B cell growth arrest and apoptosis: interactions between mono(2-ethylhexyl)phthalate, 9-cis-retinoic acid, and 15-deoxy-Δ12,14-prostaglandin J2. J Immunol 173(5):3165–3177, PMID: 15322177, 10.4049/jimmunol.173.5.3165. [DOI] [PubMed] [Google Scholar]
- Senol-Cosar O, Flach RJ, DiStefano M, Chawla A, Nicoloro S, Straubhaar J, et al. 2016. Tenomodulin promotes human adipocyte differentiation and beneficial visceral adipose tissue expansion. Nat Commun 7:10686, PMID: 26880110, 10.1038/ncomms10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steier JA, Myking OL, Bergsjø PB. 1999. Correlation between fetal sex and human chorionic gonadotropin in peripheral maternal blood and amniotic fluid in second and third trimester normal pregnancies. Acta Obstet Gynecol Scand 78(5):367–371, PMID: 10326878. [PubMed] [Google Scholar]
- Swan SH, Sathyanarayana S, Barrett ES, Janssen S, Liu F, Nguyen RH, et al. 2015. First trimester phthalate exposure and anogenital distance in newborns. Hum Reprod 30(4):963–972, PMID: 25697839, 10.1093/humrep/deu363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetz LM, Cheng AA, Korte CS, Giese RW, Wang P, Harris C, et al. 2013. Mono-2-ethylhexyl phthalate induces oxidative stress responses in human placental cells in vitro. Toxicol Appl Pharmacol 268(1):47–54, PMID: 23360888, 10.1016/j.taap.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetz LM, Aronoff DM, Loch-Caruso R. 2015. Mono-ethylhexyl phthalate stimulates prostaglandin secretion in human placental macrophages and THP-1 cells. Reprod Biol Endocrinol 13:56, PMID: 26036283, 10.1186/s12958-015-0046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirkill TL, Douglas GC. 1997. Differentiation of human trophoblast cells in vitro is inhibited by dimethylsulfoxide. J Cell Biochem 65(4):460–468, PMID: 9178096, . [DOI] [PubMed] [Google Scholar]
- Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. 2015. Tissue-based map of the human proteome. Science 347(6220):1260419, PMID: 25613900, 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G. 2000. Linear Mixed Models for Longitudinal Data. New York: Springer. [Google Scholar]
- Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM. 2003. First and second trimester antenatal screening for Down's syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). J Med Screen 10(2):56–104, PMID: 14746340, 10.1258/096914103321824133. [DOI] [PubMed] [Google Scholar]
- Wang XK, Agarwal M, Parobchak N, Rosen A, Vetrano AM, Srinivasan A, et al. 2016. Mono-(2-ethylhexyl) phthalate promotes pro-labor gene expression in the human placenta. PloS One 11(1):e0147013, PMID: 26751383, 10.1371/journal.pone.0147013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YV, Wade M, Wong E, Li YC, Rodewald LW, Wahl GM. 2007. Quantitative analyses reveal the importance of regulated Hdmx degradation for p53 activation. Proc Natl Acad Sci USA 104(30):12365–12370, PMID: 17640893, 10.1073/pnas.0701497104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn VD, Haimov-Kochman R, Paquet AC, Yang YJ, Madhusudhan MS, Gormley M, et al. 2007. Gene expression profiling of the human maternal–fetal interface reveals dramatic changes between midgestation and term. Endocrinology 148(3):1059–1079, PMID: 17170095, 10.1210/en.2006-0683. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM. 2011. Environmental chemicals in pregnant women in the united states: NHANES 2003–2004. Environ Health Perspect 119(6):878–885, PMID: 21233055, 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaron Y, Lehavi O, Orr-Urtreger A, Gull I, Lessing JB, Amit A, et al. 2002a. Maternal serum HCG is higher in the presence of a female fetus as early as week 3 post-fertilization. Hum Reprod 17(2):485–489, PMID: 11821300, 10.1093/humrep/17.2.485. [DOI] [PubMed] [Google Scholar]
- Yaron Y, Ochshorn Y, Heifetz S, Lehavi O, Sapir Y, Orr-Urtreger A. 2002b. First trimester maternal serum free human chorionic gonadotropin as a predictor of adverse pregnancy outcome. Fetal Diagn Ther 17(6):352–356, PMID: 12393965, 10.1159/000065384. [DOI] [PubMed] [Google Scholar]
- Yen SSC, Strauss JF, Barbieri RL. 2014. Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 7th ed Philadelphia, PA:Elsevier/Saunders, 243–271. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.