Abstract
Baculovirus has traditionally been used for the production of recombinant protein and vaccine. However, more recently, baculovirus is emerging as a promising vector for gene therapy application. Here, baculovirus is produced by transient transfection of the baculovirus plasmid DNA (bacmid) in an adherent culture of Sf9 cells. Baculovirus is subsequently expanded in Sf9 cells in a serum-free suspension culture until the desired volume is obtained. It is then purified from the culture supernatant using heparin affinity chromatography. Virus supernatant is loaded onto the heparin column which binds baculovirus particles in the supernatant due to the affinity of heparin for baculovirus envelop glycoprotein. The column is washed with a buffer to remove contaminants and baculovirus is eluted from the column with a high-salt buffer. The eluate is diluted to an isotonic salt concentration and baculovirus particles are further concentrated using ultracentrifugation. Using this method, baculovirus can be concentrated up to 500-fold with a 25% recovery of infectious particles. Although the protocol described here demonstrates the production and purification of the baculovirus from cultures up to 1 L, the method can be scaled-up in a closed-system suspension culture to produce a clinical-grade vector for gene therapy application.
Keywords: Genetics, Issue 134, Baculovirus, Sf9 cells, bacmid, chromatography, purification, heparin column, ultracentrifuge.
Introduction
Baculovirus is primarily used for the production of recombinant proteins and vaccines in lepidopteran Spodoptera fugiperda (Sf)9 insect cells by using recombinant Autographa californica multicapsid nuclear polyhedrosis virus (AcMNPV)1,2,3,4. More recently, it is emerging as a promising vector for gene therapy application5. It is known to have a broad host and tissue tropism, infects both quiescent and proliferating cells, is non-pathogenic, and does not integrate into the host chromosome4,5,6. Moreover, baculovirus can be produced in serum-free suspension culture which is scalable and allows for closed system processing for future clinical production1.
The purity of baculovirus particles is important for achieving effective transduction while minimizing cytotoxicity7,8,9. Baculovirus can be concentrated by ultracentrifugation or tangential flow filtration (TFF) with limited impact on its infectivity. However, these procedures not only concentrate virus particles but also cellular debris and proteins from Sf9 culture, which can be toxic in vitro (personal observation) and may induce inflammation or an immune response when used in vivo. To avoid this, especially when using highly concentrated virus stocks, infectious baculovirus needs to be purified and separated from contaminating particles.
Several methods have been reported for the purification and concentration of baculovirus vectors10,11,12. Of the available approaches, heparin affinity chromatography allows for a single-step high level of purification with the low concentration of contaminating proteins12. The method is based on the identification of heparan sulfate as the receptor for baculovirus13,14. After loading Sf9 cell supernatant onto the column and binding of baculovirus, the column can be washed with physiologic (isotonic) buffer to remove unbound or loosely bound contaminating particles. Since the binding to heparin is reversible, baculovirus particles can be eluted with a high salt buffer, which is diluted immediately to physiologic (isotonic) salt concentration to prevent inactivation by osmotic shock12. Moreover, the production of baculovirus, as well as capture on and elution from the chromatography column, can be performed using a closed-system process which is compatible with current good manufacturing practices (cGMP).
Here, we provide a detailed protocol for the manufacture, purification, and concentration of infectious baculovirus using affinity chromatography and centrifugation. Briefly, we produce baculovirus by transfection of Sf9 cells with a baculovirus plasmid DNA in adherent culture and further expand the infectious baculovirus in serum-free suspension culture. We purify baculovirus using heparin affinity chromatography and use ultracentrifugation as the final step to highly concentrate the vector for gene therapy application.
Protocol
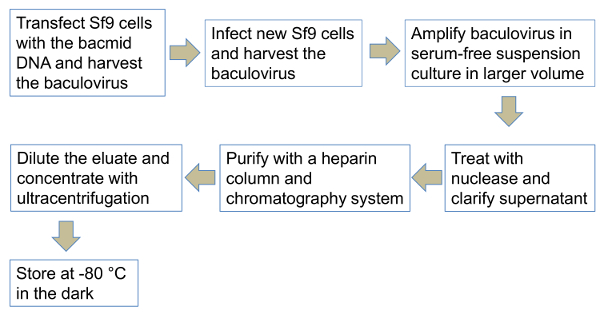
See Figure 1 for an illustration summarizing the protocol.
1. Purification of Baculovirus Plasmid DNA
Grow bacterial culture containing baculoviral plasmid DNA (bacmid)14 in 200 mL of LB broth with antibiotics; kanamycin (50 µg/mL), tetracycline (10 µg/mL), and gentamycin (7 µg/mL), for 16 h at 37 °C on an orbital shaker setting at 300 rpm.
Purify the bacmid DNA from the bacterial culture using standard alkaline lysis protocol15, and dissolve the bacmid in TE buffer.
2. Production of Baculovirus
Culture Sf9 cells in an orbital shaker incubator at 135 rpm and 28 °C in a polycarbonate Erlenmeyer flask containing serum-free insect culture medium1,2,3. Note: If Sf9 cells are thawed from frozen stock, allow at least two weeks for the cells to recover and enter the exponential phase of growth.
Count the Sf9 cells harvested at exponential phase of growth with a hemocytometer after staining with Trypan blue. Seed 1 x 106 viable Sf9 cells per well in a 6-well tissue culture-treated plate in 2 mL of medium. Allow the cell to attach for 1 h at 28 °C in an incubator.
Replace the growth medium with 1 mL of serum-free unsupplemented Grace's insect culture medium.
- Transfect the bacmid DNA into the Sf9 cells using insect cell transfection reagent.
- Mix 8 µL insect cell transfection reagent with 100 µL of Grace's Insect medium.
- Dilute 2 µg of bacmid DNA into 100 µL of unsupplemented insect medium, and mix gently by vortexing.
- Combine the diluted DNA with the diluted insect cell transfection reagent, and mix gently. Incubate for 15 to 30 min at room temperature.
- Add the DNA-lipid mixture dropwise onto the cells. Incubate at 28 °C in an incubator for approximately 5 h.
Remove the transfection mixture from the cells and wash the cells with 2 mL of PBS.
Add 2 mL of insect culture medium and continue to culture at 28 °C in an incubator without changing the media. Note: If the bacmid contains a fluorescent protein, its expression can be detected in cells 48 h post-transfection. Baculovirus is produced by transfected Sf9 cells and secreted into the culture medium which subsequently infects the untransfected cells. The entire cell population becomes infected with baculovirus in 5 days and shows signs of viral infection, also called cytopathic effect. These include increased cell diameter, vacuoles/granules in the cytoplasm, cessation of cell growth and dead or lysed cells are present in cell culture. However, if the transfection or infection efficiency is not optimal, the culture may not show an obvious cytopathic effect. The cytopathic effect can be visualized with an inverted phase microscope at 200 - 400X magnification. Baculovirus infected cell can be detected by staining with anti-gp64 antibody11.
Harvest baculovirus supernatant 5 days after transfection and label as the P1 virus stock.
Store the baculovirus supernatant, which is light sensitive, in the dark with 0.5% bovine serum albumin (BSA) in PBS. Store at 4 °C for the short-term (up to 90 days) or at -80 °C for the long-term.
Seed 6 × 106 Sf9 cells in a 10-cm tissue culture-treated plate in 10 mL of insect culture medium. Add 1 mL of initial baculovirus supernatant (P1) to the cells.
Harvest baculovirus supernatant (P2) at 5th day post-infection.
Seed 50 mL of Sf9 cells (3 x 106 cells/mL) in suspension culture in insect culture medium in a 250-mL polycarbonate Erlenmeyer flask and place the flask in an orbital shaker incubator. Add 2 mL of P2 stock to the cells and shake at 135 rpm while maintaining a constant temperature of 28 °C. Note: Three or four days after infection, the entire Sf9 cell population will show cytopathic effect, which is an indication of high-titer baculovirus production.
Sequentially amplify the baculovirus supernatants until the desired volume is obtained (P3, P4, ….) by increasing 10 to 50-fold volume of cell culture in each subsequent infection. Note: P3 is the 3rd round of infection in which 500 mL to 1 L of Sf9 cell culture can be infected with 10 to 20 mL of P2 baculovirus supernatant; P4 is the 4th round of infection in which 5 to 10 L of Sf9 cell culture can be infected with 100 to 200 mL of P3 baculovirus supernatant, and so on. Typically, Sf9 cells are seeded at 3 × 106 cells/mL in serum-free insect culture media.
Centrifuge the baculovirus supernatant at 1,000 x g for 30 min at 4 °C to remove cellular debris and treat the baculovirus supernatant with 50 units/mL nuclease (250 units/µL of stock) for 2 h at 37 °C to digest the genomic DNA and RNA. Filter the supernatants through a 0.45 μm filtration unit.
3. Preparation of the Chromatography System
Prepare the chromatography system by sequentially rinsing the sample and buffer lines each with sterile water, 1 N sodium hydroxide, water, and wash buffer (20 mM sodium phosphate buffer containing 150 mM sodium chloride, pH 7.0) at a linear flow rate of 50 mL/min.
Prepare a heparin 50 µm column (10 × 10 cm, 7.9 mL of column volume (CV)) by sequentially running column cleaning buffer (5 CV sterile 20 mM sodium phosphate buffer containing 2 M sodium chloride, pH 7.0) and 5 CV wash buffer at a linear flow rate of 7 mL/min.
4. Purification of Baculovirus Vector
- Set up the chromatography system as follows:
- Use the sample loading inlet port for loading the baculovirus supernatant.
- Use the buffer inlet port A1 for loading the wash buffer. Prepare a bottle with at least 500 mL of wash buffer and place the wash buffer line into the bottle.
- Use the buffer inlet port A2 for loading the elution buffer (20 mM sodium phosphate with 1.5 M sodium chloride, pH 8.0). Prepare a bottle with at least 500 mL of elution buffer and place the elution buffer line into the bottle.
- Insert several 50 mL conical tubes into the fraction collector to collect the column pass-through baculovirus supernatant, the wash buffer, and the eluted baculovirus.
Equilibrate the heparin column with five 7.9 mL column volumes (CVs) of wash buffer (40 mL) at a linear flow rate of 7.0 mL/min.
Load 250 mL of baculovirus supernatant onto the heparin column using the sample pump (inlet sample port) of the chromatography system at a linear flow rate of 2.0 mL/min.
Run 10 CVs (80 mL) of wash buffer through heparin column at a linear flow rate of 2.0 mL/min until the ultraviolet (UV) absorbance curve (280 nm) has returned to baseline and becomes stable.
Elute the baculovirus particles from the 7.9 mL heparin column with 5 CVs (40 mL) of elution buffer at a linear flow rate of 4.0 mL/min. Watch for a sharp elution peak of protein on the chromatogram when the baculovirus particles dissociate from the heparin column.
Post-elution, immediately dilute the eluted baculovirus supernatant 10-fold using 20 mM sodium phosphate buffer in water to prevent inactivation of baculovirus particles from osmotic shock during subsequent centrifugation.
Store 100 µL of each of the fractions, including the column flow-through collected during loading of the baculovirus supernatant, the wash buffer, and the eluate. Infect these baculovirus fractions to HT1080 to evaluate the purification process (See section 6).
Clean the column with column cleaning buffer and wash buffer. Finally, rinse the column with 5 CV sterile 20% ethanol in water at a linear flow rate of 7.0 mL/min and store at 4 °C.
Clean the chromatography system with water, 1 N sodium hydroxide, again with water, and 20% ethanol in water at a linear flow rate of 50.0 mL/min and store the system in 20% ethanol.
5. Concentration of Baculovirus
Pre-sterilize centrifuge tubes using an autoclave. Add an equal volume of baculovirus supernatant to each ultracentrifuge tubes in a tissue culture hood. Measure the tube weight by a balance and adjust the weight of the contents of the ultracentrifuge tubes with sterile PBS.
Place each of the ultracentrifuge tubes in opposing bucket of the SW 32 Ti rotor.
Run the ultracentrifuge at 80,000 × gfor 90 min at 4 °C.
Open the ultracentrifuge tubes in a tissue culture hood and aseptically aspirate the liquid without removing the baculovirus pellet.
Resuspend the pellet of each tube by vigorously pipetting up and down with 200 µL PBS containing 0.5% (w/vol) BSA.
Transfer the concentrated baculovirus to cryovials (50 to 100 µL per vial) and store at -80 °C.
6. Titration of Baculovirus
The day before infection, count HT1080 cells using a hemocytometer after staining cells with Trypan blue. Seed 1 x 105 HT1080 cells per well in a 6-well plate in 2 mL of Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal bovine serum (FBS). Seed several additional wells to be able to determine the exact number of cells present at the time of infection. Culture the cells in an incubator at 37 °C and 5% CO2. Note: HT1080 cells are used for titration as they express a higher level of heparan sulfate as compared to the other cell lines16. Since HT1080 cells are adherent, titration is simpler and less time consuming as compared to using the traditional Sf9-based titration.
On the day of infection, determine the number of cells per well by counting cells from wells. Infect the cells by adding the diluted original baculovirus which had been set aside prior to column purification, and with samples from the column flow-through, wash, and elution fractions. For each sample, determine the dilution and volume empirically based on infectious baculovirus particles, and infect each well in triplicate.
Two days after infection, analyze the cells for the expression of the gene of interest. Cells can be analyzed using a flow cytometer if they express a fluorescent protein (e.g. GFP)17.
Calculate the titers (infectious unit per mL) based on the number of cells present at the time of infection, the dilution factor, and percentages of fluorescent protein in cells using the formula: (Number of cells during infection × percentage of fluorescent protein positive cells × dilution factor)/volume of baculovirus in mL. Note: For example, if total number of cells per well at the time of infection is 1.2 × 105, the percentage of fluorescent protein is 5%, the dilution factor is 100, and the volume of diluted baculovirus added is 10 µL, the titer is 6 × 107 infectious units (IU)/mL.
Representative Results
The protocol presented is in a flow diagram (Figure 1). Steps include the transient transfection of Sf9 cells with bacmid DNA to produce baculovirus in adherent culture in a plate, the subsequent amplification in serum-free suspension culture, nuclease treatment and clarification by centrifugation and filtration, and the purification using the heparin affinity chromatography followed by concentration with ultracentrifugation.
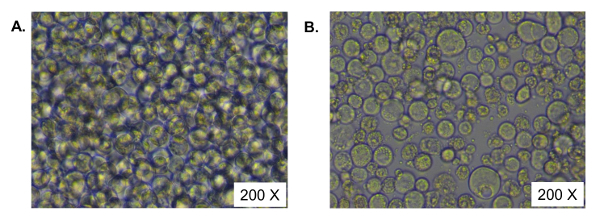
After thawing, Sf9 cells need at least two weeks to recover and entering into the exponential phase of growth. Actively growing Sf9 cells are transfected with bacmid DNA in adherent culture. Two days after transfection, expression of baculovirus envelope glycoproteins, gp64 is detected and subsequently, baculovirus production is initiated11. Since baculovirus is replication competent, the number of infected cells gradually increases due to a progressive infection of cells with the newly produced baculovirus that is secreted into the growth medium. Baculovirus containing cell supernatants are harvested 5 days after transfection when the entire cell population has become infected. This initial virus supernatant is designated as passage 1 (P1) stock. The P1 stock is used for the subsequent infection of a newly seeded adherent culture of Sf9 cells in a larger plate. The entire cell population shows sign of infection in 5 days which is called cytopathic effect (Figure 2). The supernatant from the second adherent cell culture is harvested and is designated as P2 stock. Baculovirus supernatant is further amplified in Sf9 suspension culture through ongoing infection of freshly added Sf9 cells in a shaker flask with serum-free insect cell culture medium. At the end of each infection cycle, most of the cells show cytopathic effect with an increased cellular diameter and dead or lysed cells, which are signs for completion of baculovirus infection. Baculovirus stock is sequentially amplified until the desired volume is obtained (P3, P4, ….). The supernatant is separated from the Sf9 cells by low-speed centrifugation, treated with a nuclease to reduce viscosity, filtered through a 0.45 μm membrane, before being used in the subsequent purification and concentration steps.
To purify baculovirus, supernatant from infected Sf9 cells is loaded onto a heparin column. The heparin column gradually becomes saturated with strongly bound baculovirus and loosely bound protein contaminants. The heparin column is rinsed with wash buffer to remove contaminants until the ultraviolet (UV) absorbance curve (280 nm) returns to baseline and becomes stable, indicating the removal of unbound and loosely bound materials from the column. Baculovirus particles on the other hand strongly bind to the heparin column. Therefore, no significant amount of virus is detected in the column wash buffer. Heparin-bound baculovirus particles are eluted with 1.5 M of sodium chloride at pH 8.0 and diluted 10-fold with buffer to attain isotonicity and prevent virus inactivation. A sharp peak of protein is observed during elution which corresponds to the baculovirus fraction (Figure 3). The optimized conditions for sample loading, washing, and elution used in this chromatography protocol yield more than 50% recovery of purified infectious baculovirus particles. Diluted supernatants are further concentrated up to 500-fold with ultracentrifugation and formulated in PBS containing 0.5 % BSA, which yields a net 25% recovery11.
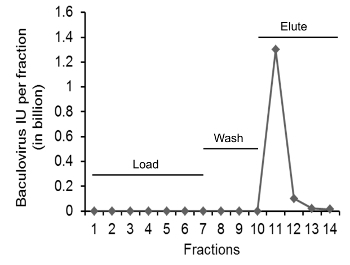
Figure 1. Schematic diagram for the production and purification of baculovirus. Sf9 cells are seeded in cell culture-treated plate transfected with bacmid DNA. Baculovirus supernatant is harvested and used to infect new Sf9 cells in adherent culture. Infected cells are subsequently propagated in serum-free suspension culture. Baculovirus supernatant is treated with a nuclease, centrifuged, and filtered. Baculovirus is purified by heparin affinity column chromatography, diluted in buffer, and concentrated aseptically by ultracentrifugation. Finally, baculovirus supernatant is stored at -80 °C. (The figure has been adapted from Nasimuzzaman et al., 2016, Mol Ther Methods Clin Dev. 2016; 3: 16071 with permission.) Please click here to view a larger version of this figure.
Figure 2. Cytopathic effects of baculovirus infected Sf9 cells. Baculovirus supernatant was used for infecting the Sf9 cells in a tissue-culture treated plate. Five days after infection, cells were visualized with an inverted phase microscope. A) Uninfected Sf9 cells as a control. B) Baculovirus infected Sf9 cells showing cytopathic effect. Cells are shown at 200X magnification. Please click here to view a larger version of this figure.
Figure 3. The number of infectious baculovirus in flow-through during heparin affinity chromatography. Baculovirus titers were estimated by infection of HT1080 cells. Samples tested were including the column run-through, wash, and eluate. Samples were diluted as needed. The line (dark diamonds) shows the total infectious units (IU) of baculovirus in each fraction. The fraction size of column flow-through sample and wash buffer were 40 mL in each tube, and eluate was 10 mL in each tube. (The figure has been adapted from Nasimuzzaman et al., 2016, Mol Ther Methods Clin Dev. 2016; 3: 16071 with permission.) Please click here to view a larger version of this figure.
Discussion
The protocol presented here describes the production of baculovirus in Sf9 cells in suspension culture and purification of baculovirus using a heparin affinity chromatography. The parameters used in this protocol maximize the yield and minimize the inactivation of infectious baculovirus. The protocol provided here shows a significantly improved recovery of baculovirus particles as compared to recoveries achieved by others9.
Due to broad host range and tissue tropism, several studies were conducted in the central nervous system, liver, eye, ovary, prostate, and testis with baculovirus-mediated gene delivery5,6,7,8. Baculovirus vectors containing therapeutic genes, such as suicide genes, tumor suppressor genes, and genes encoding tumor-specific antigens have successfully been conducted in preclinical animal models18,19. Although baculovirus can transduce human cells, it can only be propagated in insect cells and, as a result, does not pose a risk to human recipients.
Baculovirus is produced in insect cells by transient transfection of bacmid DNA. The quality of bacmid is critical for the production of high-titer baculovirus. Typically, freshly prepared bacmid performs better than those are stored in the refrigerator for long period of time. Actively growing Sf9 cells are transfected efficiently which produce high-titer baculovirus. During the amplification phase, it is important to optimize the cell concentration in the suspension culture and the volume of baculovirus supernatant used for infection. If the ratio of baculovirus particles to cells is not optimal, the yield of baculovirus may be lower than the expected (personal observation, MN).
While loading the baculovirus supernatant onto the heparin column, it is important to check the flow-through for virus particles that may be passing through the column. If that occurs, a lower column run speed may be necessary or smaller volume of supernatant should be loaded onto the column. Most of the contaminants present in the baculovirus supernatants are washed off during the washing step of the chromatography run. We evaluated the purity of baculovirus by silver staining of sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gel and electron microscopic study and found that our purified baculoviruses are good quality12.
We have carefully optimized the binding conditions and found that the POROS heparin medium is superior to other heparin media (e.g. HiTrap or Capto heparin) in its ability to capture baculovirus particles (personal observation). The ability of heparin to bind baculovirus at higher sample speed and capacity is important to minimize downstream processing time for the large-scale manufacturing process. Besides baculovirus, heparin medium also binds other contaminating proteins that have an affinity for heparin. Most of the low molecular weight contamination can be eliminated by including a tangential flow filtration (TFF) step downstream of the heparin chromatography run. TFF is also used as an alternative to centrifugation to concentrate the product20.
This protocol can be used for the purification of other viruses and proteins that have an affinity for heparin. However, modifications to the wash and elution buffer composition and the flow rate may be required.
Recombinant protein production works well using unpurified baculovirus supernatants directly. Therefore, crude baculovirus can be used for recombinant protein production. However, if baculoviruses are used as a vector for in vivo gene transfer, or as a vaccine, additional purification steps such as chromatography, gel filtration and/or ultracentrifugation are necessary to obtain pure and concentrated baculovirus particles and minimize producer cell- and culture media-derived impurities to avoid toxicity, inflammation, and an immune response.
In conclusion, we have described a simple protocol for the production and purification of baculovirus which can be scaled-up for the manufacturing recombinant proteins, vaccines, and gene therapy vectors.
Disclosures
The authors declare no conflict of interest.
Acknowledgments
This work is supported in part by the Start-Up funding from Cincinnati Children's Research Foundation (CCRF) to M.N. and Innovative Core Grant (ICG) supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1 TR001425. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Ikonomou L, Schneider YJ, Agathos SN. Insect cell culture for industrial production of recombinant proteins. Appl Microbiol Biotechnol. 2003;62(1):1–20. doi: 10.1007/s00253-003-1223-9. [DOI] [PubMed] [Google Scholar]
- Contreras-Gomez A, Sanchez-Miron A, Garcia-Camacho F, Molina-Grima E, Chisti Y. Protein production using the baculovirus-insect cell expression system. Biotechnol Prog. 2014;30(1):1–18. doi: 10.1002/btpr.1842. [DOI] [PubMed] [Google Scholar]
- Cox MM. Recombinant protein vaccines produced in insect cells. Vaccine. 2012;30(10):1759–1766. doi: 10.1016/j.vaccine.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce FM, Bucher NL. Baculovirus-mediated gene transfer into mammalian cells. Proc Natl Acad Sci U S A. 1996;93(6):2348–2352. doi: 10.1073/pnas.93.6.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airenne KJ, et al. Baculovirus: an insect-derived vector for diverse gene transfer applications. Mol Ther. 2013;21(4):739–749. doi: 10.1038/mt.2012.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung LY, et al. Efficient gene delivery into cell lines and stem cells using baculovirus. Nat Protoc. 2014;9(8):1882–1899. doi: 10.1038/nprot.2014.130. [DOI] [PubMed] [Google Scholar]
- Zeng J, Du J, Lin J, Bak XY, Wu C, Wang S. High-efficiency transient transduction of human embryonic stem cell-derived neurons with baculoviral vectors. Mol Ther. 2009;17(9):1585–1593. doi: 10.1038/mt.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Du J, Zhao Y, Palanisamy N, Wang S. Baculoviral vector-mediated transient and stable transgene expression in human embryonic stem cells. Stem Cells. 2007;25(4):1055–1061. doi: 10.1634/stemcells.2006-0616. [DOI] [PubMed] [Google Scholar]
- Wu C, Soh KY, Wang S. Ion-exchange membrane chromatography method for rapid and efficient purification of recombinant baculovirus and baculovirus gp64 protein. Hum Gene Ther. 2007;18(7):665–672. doi: 10.1089/hum.2007.020. [DOI] [PubMed] [Google Scholar]
- Grein TA, Michalsky R, Vega Lopez M, Czermak P. Purification of a recombinant baculovirus of Autographa californica M nucleopolyhedrovirus by ion exchange membrane chromatography. J Virol Methods. 2012;183(2):117–124. doi: 10.1016/j.jviromet.2012.03.031. [DOI] [PubMed] [Google Scholar]
- Nasimuzzaman M, Lynn D, van der Loo JC, Malik P. Purification of baculovirus vectors using heparin affinity chromatography. Mol Ther Methods Clin Dev. 2016;3:16071. doi: 10.1038/mtm.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkonen KE, Turkki P, Laakkonen JP, Yla-Herttuala S, Marjomaki V, Airenne KJ. 6-o- and N-sulfated syndecan-1 promotes baculovirus binding and entry into Mammalian cells. J Virol. 2013;87(20):11148–11159. doi: 10.1128/JVI.01919-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasimuzzaman M. Heparan sulfate in baculovirus binding and entry of mammalian cells. J Virol. 2014;88(8):4607–4608. doi: 10.1128/JVI.00024-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urabe M, et al. Scalable generation of high-titer recombinant adeno-associated virus type 5 in insect cells. J Virol. 2006;80(4):1874–1885. doi: 10.1128/JVI.80.4.1874-1885.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciello I, Chinali G. A modified alkaline lysis method for the preparation of highly purified plasmid DNA from Escherichia coli. Anal Biochem. 1993;212(2):394–401. doi: 10.1006/abio.1993.1346. [DOI] [PubMed] [Google Scholar]
- Nasimuzzaman M, Persons DA. Cell Membrane-associated heparan sulfate is a receptor for prototype foamy virus in human, monkey, and rodent cells. Mol Ther. 2012;20(6):1158–1166. doi: 10.1038/mt.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Americo JL, Moss B. Development and use of a vaccinia virus neutralization assay based on flow cytometric detection of green fluorescent protein. J Virol. 2003;77(19):10684–10688. doi: 10.1128/JVI.77.19.10684-10688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, et al. Suppression of tumor growth in xenograft model mice by programmed cell death 4 gene delivery using folate-PEG-baculovirus. Cancer Gene Ther. 2010;17(11):751–760. doi: 10.1038/cgt.2010.28. [DOI] [PubMed] [Google Scholar]
- Stanbridge LJ, Dussupt V, Maitland NJ. Baculoviruses as vectors for gene therapy against human prostate cancer. J Biomed Biotechnol. 2003;2003(2):79–91. doi: 10.1155/S1110724303209049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasimuzzaman M, et al. Production and purification of high-titer foamy virus vector for the treatment of leukocyte adhesion deficiency. Mol Ther Methods Clin Dev. 2015;3:16004. doi: 10.1038/mtm.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]


