Abstract
Increasing evidence supports the idea that bone morphogenetic proteins (BMPs) regulate cartilage maintenance in the adult skeleton. The aim of this study is to obtain insight into the regulation of BMP activities in the adult skeletal system. We analyzed expression of Noggin and Gremlin1, BMP antagonists that are known to regulate embryonic skeletal development, in the adult skeletal system by Noggin-LacZ and Gremlin1-LacZ knockin reporter mouse lines. Both reporters are expressed in the adult skeleton in a largely overlapping manner with some distinct patterns. Both are detected in the articular cartilage, pubic symphysis, facet joint in the vertebrae, and intervertebral disk, suggesting that they regulate BMP activities in these tissues. In a surgically-induced knee osteoarthritis model in mice, expression of Noggin mRNA was lost from the articular cartilage, which correlated with loss of BMP2/4 and pSMAD1/5/8, an indicator of active BMP signaling. Both reporters are also expressed in the sterna and rib cartilage, suggesting an extensive role of BMP antagonism in adult cartilage tissue. Moreover, Noggin-LacZ was detected in sutures in the skull and broadly in the nasal cartilage, while Gremlin1-LacZ exhibits a weaker and more restricted expression domain in the nasal cartilage. These results suggest broad regulation of BMP activities by Noggin and Gremlin1 in cartilage tissues in the adult skeleton, and that BMP signaling and its antagonism by NOGGIN play a role in osteoarthritis development.
Keywords: Bone morphogenetic protein antagonist, cartilage tissue, Noggin, Gremlin1, osteoarthritis
INTRODUCTION
BMP signaling in skeletal development and maintenance
Bone morphogenetic proteins (BMPs) are secreted extracellular signaling molecules, which constitute the largest subfamily of the transforming growth factor ß superfamily 1; 2. Among their diverse roles, regulation of skeletogenesis is one of the well-appreciated functions of BMPs. For instance, genetic experiments in mice have demonstrated that Bmp2, Bmp4 and Bmp7 play a major role in the patterning and development of the limb and craniofacial skeletons3–5. In vitro differentiation assays using embryonic mesenchymal progenitor cells also demonstrated that BMP2, BMP4, BMP6, BMP7 and GDF5 stimulate chondrogenesis and osteogenesis 6; 7. In addition, BMPs are important regulators in the maintenance and repair of the adult bone and cartilage8–10.
BMP signaling is initiated by ligand binding to transmembrane Ser/Thr kinase receptors, which triggers intracellular canonical and non-canonical pathways2. BMP signaling is regulated at various levels along the signaling pathway. These regulations are found at the transmembrane receptor level, at the level of controlling SMAD signal transducers, and at the level of transcriptional regulation 1; 11; 12. In addition, regulation by extracellular antagonists plays important roles in controlling BMP signaling13. For instance, extracellular antagonists bind BMPs, which limits BMPs interaction with their receptors, thereby, inhibiting BMP signaling1.
Function of Nog and Grem1 in embryonic skeletal development
Among various extracellular antagonists, gene-targeting experiments have demonstrated that Noggin (Nog) and Gremlin1 (Grem1) play a role in the regulation of BMP signaling during embryonic skeletal development. For instance, Nog null embryos exhibited excess chondrogenesis14. Moreover, overexpressing Nog in developing cartilage resulted in the lack of most of cartilaginous components. Nog overexpression in bone caused decreased osteoclast number as well as a reduced rate of bone formation 15; 16. During these processes, NOGGIN inhibition of BMP2 and BMP4 regulates activities of chondrocytes, osteoblasts, and osteoclasts 15; 16. These reports collectively point to a critical role of Nog in the regulation of skeletogenesis in developing embryos.
Grem1 null embryos exhibited appendicular skeletal defects, which is a consequence of defects of early limb bud patterning17 rather than a direct effect on chondrocytes or osteoblasts. However, later in development, Grem1 is expressed in osteoblasts18, suggesting its involvement in embryonic skeletogenesis.
Functions of Nog and Grem1 in adult tissues
In addition to embryonic skeletal development, both Nog and Grem1 function in postnatal skeletal development and maintenance. Loss and gain of function of Nog in adult mice resulted in osteopenia or osteoporosis19–21. These phenotypes were thought to be caused by a failure to regulate BMP activities, suggesting a role of NOG in the regulation of BMP signaling in the adult skeletal system. Grem1 knockout mice die due to kidney defects, however, a small fraction of Grem1−/− mice survive and exhibit osteopenia22. Moreover, gain of function of Grem1 in osteoblasts caused reduced bone formation23. These reports suggest a role of GREM1 in skeletal homeostasis. Furthermore, osteoblasts in postnatal mice express Nog 24; 25 and Grem123 in response to stimulation by BMPs. These reports collectively provide evidence that BMP activities in the skeletal system are fine-tuned by these antagonists. However, it is not well understood whether such regulation occurs in a localized manner in select skeletal elements or broadly throughout the skeletal system.
To gain a better understanding of BMP antagonism by Nog and Grem1 in the adult skeleton, we examined their expression in adult mice by using LacZ reporter knockin mouse lines 14; 17. The LacZ cassette is knocked into the Nog or Grem1 locus, and faithfully mirrors their endogenous expression. Our analysis indicates that these BMP antagonists are broadly expressed in the body and exhibit largely redundant expression in the articular joint, rib and sternum, while they exhibit an overlapping and distinct expression pattern in the vertebrae and the craniofacial region. Moreover, expression of Nog and BMP2/4 was lost in the articular cartilage in surgically-induced osteoarthritis. Broad expression of Nog and Grem1 in the adult skeleton suggests their role in regulating BMP activities for proper and broad maintenance of the adult skeletal system.
METHODS
Animal breeding and LacZ staining
LacZ knockin mouse lines for Nog14 and Grem117 were outcrossed to the CD1 background. Mice are maintained and euthanized in accordance with the University of Minnesota Institutional Animal Care and Use Committee.
The whole mount skeletal LacZ staining protocol was kindly provided by Dr. Brian Harfe at the University of Florida. Briefly, after euthanizing adult mice, the skin, internal organs and muscles were resected. The skeleton was fixed in 0.2% paraformaldehyde at 4°C overnight on a shaker. After washing, LacZ staining was performed at room temperature overnight. The stained skeletons were fixed in 4% paraformaldehyde, washed and decalcified in TBD-2 solution (Thermo Scientific) for three days, and cryo-sectioned at 10-14 μm thickness. Sections were counterstained with nuclear fast red.
Immunohistochemistry and immunofluorescence
For anti-LacZ immunostaining, hindlimbs were fixed in 4% paraformaldehyde at 4°C overnight after removing the skin and muscle. The samples were washed and decalcified in 20% EDTA for 2 weeks with the solution changed every 4-5 days and then processed for paraffin sectioning at 6 μm thickness. Sections were baked at 60°C for 20 min, de-paraffinized and blocked with 10% goat serum in PBS + 0.1% Triton X100. The sections were further blocked by the streptavidin-biotin blocking kit (Vector Laboratories), reacted with rabbit anti-LacZ (MP Biosciences, #8559762, 1:2000), followed by biotinylated anti-rabbit IgG (Vector laboratories, #BA-1000, 1:300) and HRP-streptavidin (Vector laboratories, SA-5004, 1:200). Signals were detected by using ImmPACT DAB substrate kit (Vector laboratories, #SK-4105). For detection of pSMAD1/5/8 (Millipore, AB3848-1 or 06-702, 1:50) or BMP2/4 (R&D, AF-355, 1:100) by immunohistochemistry, sections were treated similarly without baking, and signals were detected by using ImmPACT DAB substrate kit. For immunofluorescent detection, Alexa488-anti-rabbit IgG (Invitrogen, A-11008, 1:1000) was used as the secondary antibody, and signals were detected by Zeiss LSM710 laser confocal microscope.
Surgically-induced osteoarthritis model in mice
Experiments on mice subjected to knee surgery were performed according to protocols approved by the Institutional Animal Care and Use Committee at The Scripps Research Institute. The surgical osteoarthritis model was induced in 4-6 month-old C57Bl/6J mice by transection of the medial meniscotibial ligament and the medial collateral ligament26, and the animals were euthanized 4 or 8 weeks later. Knee joints from osteoarthritis models and age-matched control mice were resected from both hindlimbs, fixed in 10% zinc-buffered formalin (Z-Fix; Anatech, Battle Creek, MI) for two days, and decalcified in TBD-2 solution, followed by paraffin embedding. Serial sections (4 μm) from 10 mice per group were stained with Safranin O, and examined for histopathological changes in the tibial plateau and femoral condyle using the OARSI scoring system27. The result is shown using summed score of each animal, and statistical examination was performed by One-way ANOVA and Tukey test.
RESULTS
Broad expression of Nog-LacZ and Grem1-LacZ in the articular joints of the appendicular skeleton
In order to examine expression of Nog and Grem1 in the adult skeleton, we performed whole mount skeletal LacZ staining, and detected LacZ signals in joints of the limb, rib, sternum, vertebral column and skull. Therefore, we further examined the Nog-LacZ and Grem1-LacZ reporter signals by sectioning the stained skeleton.
In the hindlimb of young adults (2-3 months of age), the Nog-LacZ and Grem1-LacZ signals were detected in the knee joint. The signals were located at the surface of the femoral and tibial articular cartilage (Figure 1A, J). The specificity of staining was confirmed by the lack of LacZ signals in the skeleton in wild type mice at ages of 1, 3 and 7 month old, which were further validated by the lack of signals in both femoral and tibial articular cartilage after sectioning (Figure 1S, T). Both signals were also detected on the surface of the patellar cartilage (Figure 1B, K). These results indicate that both Nog-LacZ and Grem1-LacZ are in the cartilage tissue in the limb. Although we introduced cracks in the femur and tibia before the LacZ staining procedure, LacZ signals in the growth plate, another cartilage tissue in the adult limb, were not consistently detected. Therefore, we performed anti-LacZ immunohistochemistry after sectioning hindlimbs of Nog-LacZ and Grem1-LacZ mice, and detected anti-LacZ staining in the articular cartilage and growth plate in both Nog-LacZ and Grem1-LacZ hindlimbs (Figure 1C, D, L, M). The immunoreactivities at the articular cartilage were detected in deeper zone compared to the LacZ reporter signals, as well as the surface of the meniscus (Figure C, E, L, N). Such a difference of signals in the deeper zone is likely caused by limited accessibility of reagents in whole mount samples (LacZ signals), compared to sectioned samples (immunoreactivities).
Figure 1. Expression of the Nog-LacZ and Grem1-LacZ reporters in the joints of the adult hindlimb.

A, B: Sagittal sections of LacZ-stained 2 month-old Nog+/LacZ mouse knee. The LacZ signal is detected in the articular cartilage of the tibia (ti), femur (fe), and patella (pa). The Nog-LacZ is also detected in the growth plate (gp).
C-E: LacZ immunoreactivity of a sagittal section of Nog+/LacZ mice at 2 month-old.
F-I: Sagittal section of LacZ stained 2 month-old Nog+/LacZ mouse foot (E). G, H, and I show closeup of indicated areas. LacZ staining is observed in various joints.
J, K: Sagittal sections of LacZ-stained 2 month-old Grem1+/LacZ mouse knee. The LacZ signal is detected in the articular cartilage of the tibia, femur, and patella. No staining is observed in the growth plate (gp).
L-N: LacZ immunoreactivity of a sagittal section of Grem1+/LacZ mice at 2 month-old.
O-R: Sagittal section of LacZ-stained 2 month-old Grem1+/LacZ mouse foot (M). P, Q, and R show closeup of indicated areas. LacZ staining is observed in various joints.
Arrowheads point to LacZ signals in the articular cartilage. Black arrows point to signals in the growth plate in A, D and M. Red arrows (B, K) and blue arrows (E, N) point to signals in the patella and meniscus, respectively.
S, T: Lack of LacZ signals in the tibial (S) and femoral (T) cartilage of wild-type mice at 3 month-old.
Abbreviations: ca; calcaneum, cu; cuneiform bone, cub; cuboid, fe; femur, gp; growth plate, mt; metatarsal bone, n; navicular bone, pa; patella, ta; talus, ti; tibia
Scale bars, A, C, D, E, G- I, J, L-N, P-R: 100 μm, B, K: 250μm, F, O: 500 μm.
In order to examine whether the reporters are expressed in other joints in the limb, we sectioned the LacZ-stained foot plate. Both Nog-LacZ and Grem1-LacZ signals were detected on the surface of the joints of small bone elements (Figure 1F, O). For instance, in tibial-tarsal joints (Figure 1G, P), the joint of the navicular bone and cuneiform bone (Figure 1H, Q), and joints of the cuneiform bone and metatarsal bone (Figure 1I, R) both Nog-LacZ and Grem1-LacZ are expressed in the cartilage.
Forelimbs and hindlimbs share basic skeletal patterns. Therefore, we also examined LacZ signals in forelimbs. Similar to the knee, both Nog-LacZ and Grem1-LacZ reporter signals were detected on the surface of the humerus, radius, and ulna of the elbow joint (Figure 2A, B, G, H). In the articulating joints of the hand plate, both Nog-LacZ and Grem1-LacZ signals were detected, similar to the foot plate (Figure 2C, I). These include joints between the navicular bone and the capitate bone (Figure 2D, J), the joints of metacarpal and proximal phalange (Figure 2E, K), and the joints between proximal and distal phalanges (Figure 2F, L).
Figure 2. Expression of Nog-LacZ and Grem1-LacZ reporters in the joints of the adult forelimb.

A, B: Sagittal section of LacZ-stained elbow of 2 month-old Nog+/LacZ mice. B shows a closeup of the indicated area in A. LacZ staining is detected in the articular cartilage where the humerus (hu) meets the radius(r) and the ulnar (u).
C-F: Sagittal section of LacZ-stained 2 month-old Nog+/LacZ hand plate. D, E, F show closeup of the indicated areas in C. LacZ staining is observed in various joint areas.
G, H: Sagittal section of LacZ-stained elbow of 2 month-old Grem1+/LacZ mice. H shows close up of the indicated area. LacZ staining is detected in the articular cartilage where the humerus (hu) meets the radius(r) and the olecranon (o).
I-L: Sagittal section of LacZ-stained 2 month-old Grem1+/LacZ hand plate (I). J, K, L show closeup of the indicated areas. LacZ staining is observed in various joint areas.
Arrowheads point to LacZ signals in the articular cartilage.
Abbreviations, cap; capitate bone, dp; distal phalangeal bone, hu; humerus, mc; metacarpal bone, n; navicular bone, o; olecranon, pp; proximal phalangeal bone, r; radius, se; sesamoid bone, u; ulnar
Scale bars, A, G: 250 μm, C, I: 500 μm, B, D-F, H, J-L: 100 μm.
Similar staining was detected in 9 month-old Nog+/LacZ and Grem1+/LacZ mice, suggesting that both Nog and Grem1 are expressed in the matured adult skeleton as well. These results illustrate that both Nog-LacZ and Grem1-LacZ reporter signals are broadly detected in the cartilage tissue in various articular joints and the growth plate in the appendicular skeletal system.
Nog and Grem1 regulates local BMP signaling
Given that NOG and GREM1 are BMP antagonists, we next examined expression of BMP ligands and phosphorylated SMAD1/5/8, as markers of active BMP signaling, in the knee of 3-4 month old mice (Figure 3). We detected BMP2/4 and pSMAD1/5/8 immunoreactivity in the articular cartilage of wild type mice (Figure 3D, G). In Nog+/LacZ and Grem1+/LacZ knees, both BMP2/4 and pSMAD1/5/8 signals were detected in the LacZ positive area with slightly stronger intensities than wild type (Figure 3B-I). Both BMP2/4 and pSMAD1/5/8 signals were also detected in the LacZ positive area in Nog+/LacZ and Grem+/LacZ tibia growth plate (Figure 3 K,L,N,O,Q,R). Similar to articular cartilage, BMP2/4 and pSMAD1/5/8 signals were stronger in growth plate cartilage in Nog+/LacZ and Grem+/LacZ mice than in wild type mice (Figure 3M-R, weak BMP2/4 signals were pointed by arrows in M). In both articular cartilage and growth plate more cells with BMP2/4 or pSMAD1/5/8 signals were detected in Nog+/LacZ and Grem+/LacZ than wild type. These results support the idea that NOG and GREM1 regulate BMP signaling in these cartilage tissues.
Figure 3. Colocalization of Nog-LacZ and Grem-LacZ signals with BMP2/4 and pSMAD1/5/8 in the articular cartilage and growth plate.

Immunofluorescence of LacZ (A-C), BMP2/4 (D-F) and pSMAD1/5/8 (pSMAD1, G-I) in the tibia articular cartilage in wild type (A, D, G), Nog+/LacZ (B, E, H) and Grem1+/LacZ (C, F, I) mice.
Immunofluorescence of LacZ (J-L), BMP2/4 (M-O) and pSMAD1/5/8 (P-R) in the tibia growth plate in wild type (J, M, P), Nog+/LacZ (K, N, Q) and Grem1+/LacZ (L, O, R) mice.
Green and blue signals indicate immunoreactivities and DAPI-stained nuclear DNA, respectively. For simplicity, only nuclear pSMAD1/5/8 signals (G-I, P-R) and weak BMP2/4 signals in M were pointed by arrowheads and arrows, respectively. Obvious LacZ (B, C, K, L) and BMP2/4 (D-F, N, O) signals were not pointed. Panels A-I are in the same magnifications, and panels J-R are in the same magnifications. Scale bar: 50 μm.
Loss of Nog expression in joints with surgically-induced OA
Previous studies showed that BMP antagonism might have a role in osteoarthritis pathogenesis and/or progression in humans and animal models28. The broad expression of the Nog-LacZ and Grem1-LacZ reporters in the articular cartilage suggests their involvement in osteoarthritis in mice. In order to investigate this possibility, we examined their mRNA expression, as well as expression of BMP2/4 and pSMAD1/5/8, in a surgically-induced knee osteoarthritis model26. Compared to evident signals of BMP2/4, pSMAD1/5/8 and Nog mRNA in the articular joint in control mice (Figure 4B, C, F, I, J), these signals were significantly reduced 4 weeks after surgery with a few cells with weak BMP2/4 or pSMAD1/5/8 signals (Figure 4D, G, K). OARSI scoring indicates development of osteoarthritis by this time point (Figure 4A). Eight weeks after surgery the articular cartilage exhibited further progression of osteoarthritis (Figure 4A). At this time, signals of BMP2/4, pSMAD1/5/8 and Nog were undetectable except for a few cells with weak signals (Figure 4E, H, L). In contrast, Grem1 mRNA signals were faint in the knee articular cartilage in both control and osteoarthritis models (Figure 4M, N, O). Correlation between osteoarthritis severity and downregulation of expression of BMP2/4, pSMAD1/5/8 and Nog suggests that BMP antagonism by Nog might have a role in osteoarthritis pathogenesis.
Figure 4. Expression of Nog and Grem1 in normal and Osteoarthritis articular cartilage.

A: OARSI scoring of control, 4 weeks post surgery and 8 weeks post surgery knees. n=10, ***: p<0.001.
B-H: Immunohistochemical analysis of BMP2/4 (C-E) and pSMAD1/5/8 (F-H) of wild type (B, C, F), 4 weeks post surgery (D, G) and 8 weeks post surgery (E, H) knees. Panel B shows a control with non-immunized IgG.
I-O: in situ hybridization of Nog (J-L) and Grem1 (M-O) of wild type (I, J, M), 4 weeks post surgery (K, N) and 8 weeks post surgery (L, O) knees. Panel I shows a control without probe.
Arrowheads in C, F and J point to expression of BMP2/4, pSMAD1/5/8 and Nog mRNA, respectively. Arrowheads in D, E and G, H point to weak signals of BMP2/4 and pSMAD1/5/8, respectively. fe: femur, fi: fibula, ti: tibia. Scale bar: 100 μm
Expression of the Nog-LacZ and Grem1-LacZ reporters in the rib and sterna
In the whole mount stained skeleton, strong signals were detected in the xiphoid cartilage and the rib in both Nog+/LacZ and Grem1+/LacZ mice at 2-3 months of age (Figure 5A, B, I, J). Cross section images show that both Nog-LacZ and Grem1-LacZ signals are present in the rib (Figure 5C, D, K, L). Sagittal section analysis shows that strong signals of Nog-LacZ and Grem1-LacZ are also present in the xiphoid process of the sternum (Figure 5E, F, M, N). Given the strong LacZ reporter signals, we analyzed BMP signaling in this tissue. In the rostral part of the xiphoid process, strong LacZ reporter signals and pSMAD1/5/8 signals were detected, while the signals were reduced in the caudal part both in Nog+/LacZ and Grem1+/LacZ mice (Figure 5 G-H’, O-P’). These results demonstrate that Nog-LacZ and Grem1-LacZ reporters are also expressed in the cartilage in the rib and sternum, and indicate that expression of Nog and Grem1 in the adult skeletal system is not restricted to the articular joint cartilage.
Figure 5. Expression of Nog-LacZ and Grem1-LacZ reporters in the sterna and the rib.

A, B: Frontal view (A) and side view (B) of whole mount LacZ-stained Nog+/LacZ mouse skeleton.
C, D: Cross-section of 2 month-old LacZ stained Nog+/LacZ mouse rib (C). D shows closeup of the indicated area.
E, F: Sagittal section of 2 month old LacZ stained Nog+/LacZ mouse sternum (E). Staining is detected along the entire sternum (E). F shows closeup of the indicated area.
G-H’: Sagittal-section immunofluorescence of 2 month-old sterna for LacZ (G, H) and pSMAD1/5/8 (G’, H’). Corresponding areas are indicated as dotted boxes in E.
I, J: Frontal view (I) and side view (J) of whole mount LacZ-stained Grem1+/LacZ mouse skeleton.
K, L: Cross-section of 3 month-old LacZ-stained Grem1+/LacZ mouse rib (K). L shows closeup of the indicated area.
M, N: Sagittal section of 2 month-old LacZ-stained Grem1+/LacZ sternum. Staining is detected along the entire sternum (M). N shows closeup of the indicated area.
O-P’: Sagittal-section immunofluorescence of 2 month-old sterna for LacZ (O, P) and pSMAD1/5/8 (O’, P’). Corresponding areas are indicated as dotted boxes in M.
Labeled regions identify as: xc; xiphoid cartilage and st; sterna.
Scale bars, A, B, I, J: 5 mm, C, E, K, M: 500 μm, D, L: 100 μm, F, N: 50 μm. G-H’ and O-P’ are in the same magnification with a 50 μm sale bar in G.
Expression of Nog-LacZ and Grem1-LacZ in the pubic symphysis
Given that both LacZ reporters are detected in the hyaline cartilage of the articular joints of the diathrosis, we examined whether the LacZ reporters are expressed in another type of cartilage in the joint. We examined the pubic symphysis, an immobile joint located between the pubis. Both Nog-lacZ and Grem1-LacZ signals were detected in the pubic symphysis of mature 5-7 month-old mice (Figure 6). This result indicates that the Nog-LacZ and Grem1-LacZ signals are present in joints that are rich with fibrocartilage, in addition to hyaline cartilage-rich articular joints and the growth plate.
Figure 6. Expression of Nog-LacZ and Grem1-LacZ reporters in the pubic symphysis.

A, B: Coronal section of pubic symphysis of LacZ-stained 7 month-old Nog+/LacZ mice (A). The LacZ signal is detected in the pubic symphysis (ps). B shows closeup of the indicated area.
C, D: Coronal section of pubic symphysis of LacZ-stained 7 month-old Grem1+/LacZ mice. The LacZ signal is detected in the pubic symphysis (ps). D shows closeup of indicated area.
Abbreviations: ps; pubic symphysis
Scale bars, A,C: 500 μm, B,D: 100 μm
Expression of Nog-LacZ and Grem1-LacZ in the vertebrae
The vertebral column is made up of individual vertebrae and articulation between them allows for movement. The facet joints, aligned at the back of the spinal column, link each vertebra. We detected both Nog-LacZ and Grem1-LacZ signals in the facet joint (Figure 7A, F). Coronal section analysis of the lumber vertebrae revealed that both signals are present in the cartilaginous endplate of the vertebrae (Figure 7B, C, G, H). Moreover, strong Grem1-LacZ signals were detected in the nucleus pulposus, while Nog-LacZ signals were localized to the periphery in the annulus fibrosus (Figure 7D, E, I, J). These results indicate that Nog-LacZ and Grem1-LacZ are also present in the joints of vertebrae and in the intervertebral disc.
Figure 7. Expression of Nog-LacZ and Grem1-LacZ reporters in the vertebrae.

A: Ventral view of whole mount LacZ-stained vertebrae of 2 month-old Nog+/LacZ mice.
B, C: Coronal section of LacZ-stained transverse process (tp) of Nog+/LacZ mice (B). C shows closeup of the indicated area in B. LacZ staining is observed in the transverse process.
D, E: Coronal section of LacZ-stained intervertebral space of Nog+/LacZ mice (D). E shows closeup of the indicated areas in D. LacZ staining is observed in the cartilaginous endplate of the vertebrae.
F: Ventral view of whole mount LacZ-stained vertebrae of 2 month-old Grem1+/LacZ mice.
G, H: Coronal section of LacZ stained transverse process of Grem1+/LacZ mice (G). H shows closeup of the indicated area in G. LacZ staining is observed in the transverse process.
I, J: Coronal section of LacZ-stained intervertebral space of Grem1+/LacZ mice (I). J shows closeup of the indicated area in I. Strong LacZ staining is detected in the nucleus pulposus.
Abbreviations, bv; body of vertebra, np: nucleus pulposus, tp; transverse process.
Scale bars, A, F: 1 mm, B, D, E, G, I, J: 100 μm, C, H: 50 μm.
Expression of Nog-LacZ and Grem1-LacZ in the craniofacial skeleton
In addition to the appendicular skeleton, rib, vertebrae and pubic symphysis, the craniofacial region also exhibited strong LacZ signals. The Nog-LacZ and Grem1-LacZ signals in the craniofacial region exhibited overlapping and non-overlapping patterns. Specifically, strong Nog-LacZ signals were detected in the sagittal suture, coronal suture, and anterior lambdoid suture (Figure 8A). Contrary to this observation, the Grem1-LacZ signal was not detected in these suture structures (Figure 8F).
Figure 8. Expression of Nog-LacZ and Grem1-LacZ reporter in the skull.
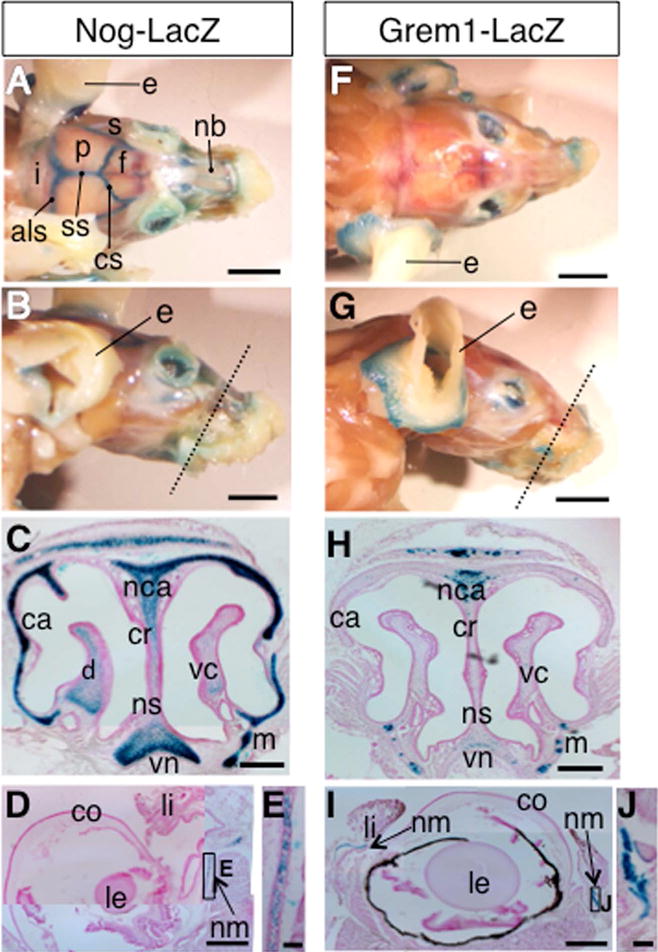
A, B: Top view (A) and side view (B) of whole mount LacZ-stained skull of the Nog+/LacZ mice. Dotted line in B indicates the approximate section plane in C. The Nog-LacZ signal was detected in the anterior lambdoid suture (als) coronal suture (cs) and sagittal suture (ss).
C: Coronal section of 2 month-old Nog+/LacZ mouse skull around the nose area. The LacZ signal is detected in the nasal cartilage (nca), nasal capsule (ca), vomeronasal cartilage (vn), and maxilla (m). Light staining can also be detected in the nasal crest (cr), dorsal nasal concha (d), nasal septum (ns) and ventral nasal concha (vc).
D, E: Coronal section of 2 month old Nog+/LacZ mouse eye (D). E shows closeup of the indicated area, showing LacZ staining in the cartilage of nictitating membrane (nm).
F, G: Top view (F) and side view (G) of whole mount of LacZ-stained skull of Grem1+/LacZ mice. Dotted line in G indicates the approximate section plane in H.
H: Coronal section of 2 month-old Grem1+/LacZ skull around the nose area. The LacZ signal is detected in the nasal cartilage and maxilla (m). Light staining can be seen in the vomeronasal cartilage (vn)
I, J: Coronal section of 2 month-old eye (I). J shows closeup image of the indicated area, showing LacZ-staining in the cartilage of nictitating membrane (nm).
Abbreviations: als: anterior lambdoid suture, ca; nasal capsule, co; cornea, cr; nasal crest, cs: coronal suture, d; dorsal nasal concha, f; frontal bone, i; interparietal bone, le; lens, li; eyelid, m; maxilla, nb; nasal bone, nca; nasal cartilage, nm: cartilage of nictitating membrane, ns; nasal septum, p; parietal bone, s; squamosal bone, ss: sagittal suture, vc; ventral nasal concha, vn; vomeronasal cartilage.
Scale bars, A, B, F, G: 5 mm, C, H: 250 μm, D, I: 500 μm, E, J: 50 μm
Coronal sections of the nose region show overlapping, but substantially different intensities of LacZ signals in Nog+/LacZ and Grem1+/LacZ mice at 2 to 3 months of age. The Nog-LacZ signal was observed in the nasal cartilage (nca), nasal capsule (ca), vomeronasal cartilage (vn), and maxilla (m) (Figure 8B, C). Lower intensity Nog-LacZ signals were also detected in the nasal crest (cr), dorsal nasal concha (d), nasal septum (ns) and ventral nasal concha (vc). The nasal cartilage is hyaline, and the results here demonstrate that the Nog-LacZ reporter is broadly expressed in the nasal cartilage. In contrast, the Grem1-LacZ signals were detected in a more localized manner (Figure 8G, H). The signals were observed in the nasal cartilage (nca) and maxilla (m). Weak signals were also observed in the vomeronasal cartilage (vn). The Grem1-LacZ signals partially overlap with those of Nog-LacZ with lower intensities.
In addition to the nasal cartilage, we observed both Nog-LacZ and Grem1-LacZ in the nictitating membrane in the eye (Figure 8D, E, I, J). The nictitating membrane possesses cartilage, which is covered by loose connective tissue and epithelium29. Contrary to this, both Nog-LacZ and Grem1-LacZ were not detected in the external ear (e) (Figure 8A, B, F, G), which is rich in elastic cartilage.
DISCUSSION
BMP antagonism in the articular joints
In this study, we investigated expression of Nog and Grem1 by means of LacZ reporter signals in the whole adult skeletal system. Using the sensitive LacZ staining protocol, we detected expression of Nog and Grem1 broadly in articular cartilage.
Previous studies reported expression of Nog and Grem1 in articular cartilage with contradictions. The NOG mRNA by RT-PCR and NOG immunoreactivities have been reported in articular chondrocytes in humans and mice, respectively30. Similarly, a recent study also reported the presence of Nog-LacZ reporter signals in the knee articular cartilage in adult mice31. By contrast, another study reported that NOG was undetectable in normal human chondrocytes from articular cartilage by qRT-PCR analysis32. In the case of GREM1, its expression in normal human chondrocytes from articular cartilage was detected by qRT-PCR32, however, its expression in normal cartilage in the dog is low33. The cause of such discrepancies is unclear, and it might be due to differences in species or detection methodology. Nonetheless, by using sensitive LacZ reporter signals, our study provides a comprehensive view of the presence of BMP antagonism by NOG and GREM1 in the joint cartilage. First, our data shows that Nog is expressed not only in the long bone joints of the hindlimbs but also broadly in the articular joints of skeletal elements both in forelimbs and hindlimbs, including those in the hand plate and foot plate (Figure 1, 2). Second, we found that Grem1 is also expressed broadly in articular joints of the forelimbs and hindlimbs, similar to Nog (Figure 1, 2, 3). Although mRNA in situ hybridization showed a faint signal of Grem1 in the normal knee articular cartilage of mice (Figure 4), a sensitive LacZ reporter system allowed for the additional detection of Grem1 expression in the joint. Third, both Nog-LacZ and Grem1-LacZ are expressed in the pubic symphysis (Figure 6), where the pubis forms a joint. Lastly, Nog-LacZ and Grem1-LacZ were detected in the facet joint of the vertebral column (Figure 7). These expression patterns suggest a role of BMP antagonism by NOG and GREM1 in integrity and/or maintenance of various joint tissues in the body.
Analysis of expression of Nog and Grem1 in the knee cartilage pointed to a different pattern of signals between the LacZ reporter and immunohistochemistry/in situ hybridization detections. Although the LacZ reporter signals allowed for sensitive detection in the whole mount skeleton of the entire body, the signals were restricted to the surface of joint cartilage, compared to staining on sectioned samples. This difference is likely caused by limited penetration of reagents during whole mount LacZ staining of the skeleton. Therefore, analysis on sectioned samples would allow for visualization of a more detailed spatial expression pattern in deeper zone of skeletal elements. In such analyses, mRNA expression and protein expression may be affected by their different stabilities. In particular, LacZ is a stable protein, while endogenous proteins and mRNA have different stabilities. Nonetheless, whole mount LacZ staining could provide sensitive detection broadly in the entire skeletal system, which is useful to study gene expression in the entire skeletal system. Analyses of sectioned samples (immunostaining and in situ hybridization) allows for more detailed view of spatial distributions. These different methods can provide different results, which may potentially confound data interpretation. Therefore, careful interpretation of results using these different approaches would be useful to study gene/protein expression in the skeletal system.
Regulation of BMP activities in joints
A genetic study has demonstrated that BMP signaling is required for postnatal maintenance of articular cartilage in the limb8. Studies of human tissues showed an increase of BMP2 levels in osteoarthritic cartilage and suggested that BMP2 acts as a stimulus of anabolic activities in normal and osteoarthritic chondrocytes 34; 35. These studies, as well as mouse genetic studies, suggest that BMP activity is involved in chondro-protective and/or cartilage repair in response to cartilage damage 28; 30; 36. Contrary to this idea, BMPs are shown to be disease promoting candidate molecules for joint ankylosis. In a mouse model, Bmp2, Bmp6 and Bmp7 are expressed during the progression of joint ankylosis. Furthermore, reducing BMP activities by delivering a Nog-expression plasmid into the joint inhibited the onset and progression of this disease37. Collectively, these reports indicate that BMPs can act as both chondro-protective factors for articular cartilage and disease-promoting factors for joints28. Based on this idea, the broad expression of both Nog and Grem1 in the articular cartilage, the faucet joint in the vertebrae, and the pubic symphysis (Figure 1, 6, 7) suggests that they function to fine-tune extracellular BMP activities. Moreover, our observation of more cells with higher levels of pSMAD1/5/8 in the articular joint and growth plate in Nog+/LacZ or Grem1+/LacZ than wild type mice also supports the idea that NOG and GREM1 regulates BMP signaling in these cartilage tissues in adults (Figure 3). Taken together, the overlapping expression pattern of Nog and Grem1 in joints would contribute to maintaining proper levels of BMP activity for normal cartilage maintenance.
BMP antagonism and osteoarthritis
Given the broad expression of Nog and Grem1, they likely participate in regulation of BMP activities in the joint and might have a role in joint diseases, such as osteoarthritis. However, it is controversial whether BMP antagonists have positive or negative roles in osteoarthritis. For instance, expression of GREM1 and FOLLISTATIN was significantly upregulated in human osteoarthritis samples and surgically-induced dog models of osteoarthritis 32; 33. Contrary to these reports, another report showed decreased GREM1 mRNA levels in human osteoarthritic cartilage38. In the case of the mouse experiments shown here, although Grem1-LacZ was detected in the articular cartilage, Grem1 mRNA signals were faint in normal mouse knee joints, and the surgically-induced osteoarthritis model did not result in an evident increase of Grem1 mRNA (Figure 4). Such discrepancies might be derived from different experimental systems as discussed with respect to endogenous expression. Humans, dogs and mice have different mechanical loading on the knee joint, and Grem1 expression might be regulated in response to different mechanical loading in these models. Alternatively, ageing related osteoarthritis and surgically-induced osteoarthritis might involve different responses for gene regulation.
Compared to these reports on Grem1, studies on Nog and osteoarthritis are limited. A functional study suggested that reduced Nog gene dosage in Nog+/LacZ mice contributed to cartilage protection in an inflammation-driven joint destruction30. This model differs from surgically-induced osteoarthritis models with respect to mechanisms to induce joint degeneration, and it was unknown whether Nog expression changes in osteoarthritis cartilage. Our result here showed significant downregulation of Nog mRNA in a surgically-induced mouse knee osteoarthritis model and suggests that regulation of BMP activities by NOG is involved in osteoarthritis. Correlations between reduced expression of Nog and BMP2/4 suggest that Nog expression and BMP signaling may form a feedback regulation in osteoarthritis cartilage. Therefore, the relationship between osteoarthritis development and regulation of BMP signaling might be mutually interactive, rather than a simple cause and effect.
BMP antagonism in the rib, sternum, eyelid and vertebral columns
Our analysis showed strong expression of both Nog-LacZ and Grem1-LacZ in the rib, sternum and the xiphoid process (Figure 5). In addition, both reporter signals were detected in the cartilage of the nictitating membrane in the eye (Figure 8D, E, I, J). Similarly, BMPs and their receptors are detected in the intervertebral disk in mice 39; 40. Extensive studies have been carried out to investigate the relationship between BMPs and degenerative disk diseases (reviewed in41), which suggested roles of BMP antagonism in the vertebrae for regulation of proper levels of BMP activities. The presence of BMP antagonism by Nog and Grem1 in these tissues suggests that fine-tuning of BMP signaling might be important in these tissues, similar to those in the articular cartilage.
BMP antagonism in the craniofacial structures
Genetic studies have demonstrated that BMP signaling plays a crucial role broadly in craniofacial morphogenesis during embryonic development 42; 43. We observed Nog-LacZ but not Grem1-LacZ signals in the adult cranial sutures (Figure 8A). Nog expression has been reported in patent sutures, such as the sagittal suture and the coronal suture44. Moreover, misexpression of Nog in the posterior frontal cranial suture, which fuses in 45 days after birth in mice, prevented fusion of the posterior frontal suture44. These studies demonstrated an important role of Nog in cranial suture fusion. The lack of Grem1-LacZ signals in the cranial suture (Figure 7F) suggests that, unlike joint cartilage, rib and sterna, BMP antagonism depends on Nog without redundancy by Grem1. Although both NOG and GREM1 bind BMP2, BMP4 and BMP7, NOG but not GREM1 binds additional ligands, such as GDF5 and GDF613. Interestingly, Gdf6 is expressed in the coronal suture in perinatal mouse fetus45, although its expression in postnatal stages is not known. Thus, NOG might specifically regulate GDF6 in the coronal suture during postnatal skeletal development.
It has been recently demonstrated that an aberrant increase in BMP signaling in neural crest cells during embryonic development caused a shorter nasal septum and discontinuous nasal cartilage with an increase in cell death46. In the adult nasal cartilage, both Nog and Grem1 are expressed but with significantly higher levels of Nog-LacZ reporter signals than Grem1-LacZ signals. Such an observation suggests that, in addition to embryonic development, BMP antagonism by NOG and GREM1 plays a role in adult nasal cartilage maintenance.
Implantation of the autologous chondrocyte, isolated from the articular cartilage biopsy47, has been appreciated as a successful procedure for the repair of focal chondral lesions in the large joints. It is suggested that auricular cartilage might serve as an alternative source of cells for joint repair, however, it has been indicated that chondrocytes from different cartilaginous sites maintain their characteristics of tissue origins48. The lack of Nog-LacZ and Grem1-LacZ signals in the ear illustrates a difference of the elastic cartilage in the ear, compared to hyaline cartilage in articular joints. Such a difference suggests that ear cartilage and chondrocytes may have different behavior from other cartilages49, and that its responses may not be generalizable as cartilage.
Conclusions
Our study identified broad expression of Nog and Grem1 in the adult mouse skeletal system, especially in the cartilage tissue. Numerous studies have identified that BMPs function for both cartilage repair 8; 36 and accelerating chondrogenic differentiation50. These seemingly contradictory functions might be derived from the ability of BMPs to regulate multiple steps of chondrogenic differentiation, which include pre-chondrogenic condensation, differentiation into chondrocytes, chondrocyte proliferation and matrix synthesis, and chondrocyte maturation and terminal differentiation7. As the terminal differentiation causes matrix degradation through upregulation of matrix metalloproteinases and chondrocyte death, chondrocyte differentiation must be tightly regulated in the adult skeleton to maintain the cartilage. Although it remains unknown how exactly these multiple functions of BMPs are balanced, it is conceivable that BMP activities should be tightly regulated for proper maintenance of articular cartilage, especially with respect to chondro-protective and differentiation-promoting functions. Overlapping expression of Nog and Grem1 would contribute to not only the fine regulation of BMP activities but also securing BMP antagonism in joint cartilage in the adult body.
Acknowledgments
We thank Drs. Laura Gammill, Yasushi Nakagawa, Michael O’Connor and Jonathan Slack for the use of their equipment. We are grateful to Drs. Michael O’Connor and Aidan Peterson for help in pSMAD1/5/8 staining, to Midori Usuki-Filiz, Yumi Motokura, Holly Johnson, Kristina Weimer, Jenna Matson, Sho Kawakami, Malina Peterson and Elizabeth West for excellent technical assistance, to the Medical Student Internship Program at the Okayama University School of Medicine, Japan. We also thank Dr. Richard Harland for Nog-LacZ and Grem1-LacZ knockin mouse lines, and Dr. Brian Harfe for the whole mount LacZ staining protocol. Research reported in this publication was supported by the National Institute of Arthritis, Musculoskeletal and Skin Diseases to YK (AR064195) and National Institute on Aging to ML (AG007996). Xiaodan Yu was partially supported by the Undergraduate Research Opportunity Program at the University of Minnesota. The authors have no conflicts to disclose.
Footnotes
Author Contributions Statement: All authors have made substantial contributions to research design, or the acquisition, analysis or interpretation of data. XY, ML and YK drafted the paper, and all authors approved the final version of the manuscript.
References
- 1.Bragdon B, Moseychuk O, Saldanha S, et al. Bone morphogenetic proteins: a critical review. Cell Signal. 2011;23:609–620. doi: 10.1016/j.cellsig.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Wharton K, Derynck R. TGFbeta family signaling: novel insights in development and disease. Development. 2009;136:3691–3697. doi: 10.1242/dev.040584. [DOI] [PubMed] [Google Scholar]
- 3.Bandyopadhyay A, Tsuji K, Cox K, et al. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006;2:e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonilla-Claudio M, Wang J, Bai Y, et al. Bmp signaling regulates a dose-dependent transcriptional program to control facial skeletal development. Development. 2012;139:709–719. doi: 10.1242/dev.073197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shu B, Zhang M, Xie R, et al. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J Cell Sci. 2011;124:3428–3440. doi: 10.1242/jcs.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isaacs MJ, Kawakami Y, Allendorph GP, et al. Bone morphogenetic protein-2 and -6 heterodimer illustrates the nature of ligand-receptor assembly. Mol Endocrinol. 2010;24:1469–1477. doi: 10.1210/me.2009-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon BS, Lyons KM. Multiple functions of BMPs in chondrogenesis. J Cell Biochem. 2004;93:93–103. doi: 10.1002/jcb.20211. [DOI] [PubMed] [Google Scholar]
- 8.Rountree RB, Schoor M, Chen H, et al. BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biol. 2004;2:e355. doi: 10.1371/journal.pbio.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuji K, Bandyopadhyay A, Harfe BD, et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 10.Mi M, Jin H, Wang B, et al. Chondrocyte BMP2 signaling plays an essential role in bone fracture healing. Gene. 2013;512:211–218. doi: 10.1016/j.gene.2012.09.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller TD, Nickel J. Promiscuity and specificity in BMP receptor activation. FEBS Lett. 2012;586:1846–1859. doi: 10.1016/j.febslet.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 12.Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–3714. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- 13.Gazzerro E, Canalis E. Bone morphogenetic proteins and their antagonists. Rev Endocr Metab Disord. 2006;7:51–65. doi: 10.1007/s11154-006-9000-6. [DOI] [PubMed] [Google Scholar]
- 14.Brunet LJ, McMahon JA, McMahon AP, et al. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto M, Murai J, Yoshikawa H, et al. Bone morphogenetic proteins in bone stimulate osteoclasts and osteoblasts during bone development. J Bone Miner Res. 2006;21:1022–1033. doi: 10.1359/jbmr.060411. [DOI] [PubMed] [Google Scholar]
- 16.Tsumaki N, Nakase T, Miyaji T, et al. Bone morphogenetic protein signals are required for cartilage formation and differently regulate joint development during skeletogenesis. J Bone Miner Res. 2002;17:898–906. doi: 10.1359/jbmr.2002.17.5.898. [DOI] [PubMed] [Google Scholar]
- 17.Khokha MK, Hsu D, Brunet LJ, et al. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat Genet. 2003;34:303–307. doi: 10.1038/ng1178. [DOI] [PubMed] [Google Scholar]
- 18.Pereira RC, Economides AN, Canalis E. Bone morphogenetic proteins induce gremlin, a protein that limits their activity in osteoblasts. Endocrinology. 2000;141:4558–4563. doi: 10.1210/endo.141.12.7851. [DOI] [PubMed] [Google Scholar]
- 19.Canalis E, Brunet LJ, Parker K, et al. Conditional inactivation of noggin in the postnatal skeleton causes osteopenia. Endocrinology. 2012;153:1616–1626. doi: 10.1210/en.2011-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devlin RD, Du Z, Pereira RC, et al. Skeletal overexpression of noggin results in osteopenia and reduced bone formation. Endocrinology. 2003;144:1972–1978. doi: 10.1210/en.2002-220918. [DOI] [PubMed] [Google Scholar]
- 21.Wu XB, Li Y, Schneider A, et al. Impaired osteoblastic differentiation, reduced bone formation, and severe osteoporosis in noggin-overexpressing mice. J Clin Invest. 2003;112:924–934. doi: 10.1172/JCI15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canalis E, Parker K, Zanotti S. Gremlin1 is required for skeletal development and postnatal skeletal homeostasis. J Cell Physiol. 2012;227:269–277. doi: 10.1002/jcp.22730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gazzerro E, Pereira RC, Jorgetti V, et al. Skeletal overexpression of gremlin impairs bone formation and causes osteopenia. Endocrinology. 2005;146:655–665. doi: 10.1210/en.2004-0766. [DOI] [PubMed] [Google Scholar]
- 24.Gazzerro E, Gangji V, Canalis E. Bone morphogenetic proteins induce the expression of noggin, which limits their activity in cultured rat osteoblasts. J Clin Invest. 1998;102:2106–2114. doi: 10.1172/JCI3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abe E, Yamamoto M, Taguchi Y, et al. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J Bone Miner Res. 2000;15:663–673. doi: 10.1359/jbmr.2000.15.4.663. [DOI] [PubMed] [Google Scholar]
- 26.Carames B, Hasegawa A, Taniguchi N, et al. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis. 2012;71:575–581. doi: 10.1136/annrheumdis-2011-200557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Lories RJ, Luyten FP. Bone morphogenetic protein signaling and arthritis. Cytokine Growth Factor Rev. 2009;20:467–473. doi: 10.1016/j.cytogfr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Schlegel T, Brehm H, Amselgruber WM. The cartilage of the third eyelid: a comparative macroscopical and histological study in domestic animals. Ann Anat. 2001;183:165–169. doi: 10.1016/S0940-9602(01)80041-8. [DOI] [PubMed] [Google Scholar]
- 30.Lories RJ, Daans M, Derese I, et al. Noggin haploinsufficiency differentially affects tissue responses in destructive and remodeling arthritis. Arthritis Rheum. 2006;54:1736–1746. doi: 10.1002/art.21897. [DOI] [PubMed] [Google Scholar]
- 31.Pregizer SK, Mortlock DP. Dynamics and cellular localization of Bmp2, Bmp4, and Noggin transcription in the postnatal mouse skeleton. J Bone Miner Res. 2015;30:64–70. doi: 10.1002/jbmr.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tardif G, Hum D, Pelletier JP, et al. Differential gene expression and regulation of the bone morphogenetic protein antagonists follistatin and gremlin in normal and osteoarthritic human chondrocytes and synovial fibroblasts. Arthritis Rheum. 2004;50:2521–2530. doi: 10.1002/art.20441. [DOI] [PubMed] [Google Scholar]
- 33.Tardif G, Pelletier JP, Boileau C, et al. The BMP antagonists follistatin and gremlin in normal and early osteoarthritic cartilage: an immunohistochemical study. Osteoarthritis Cartilage. 2009;17:263–270. doi: 10.1016/j.joca.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 34.Nakase T, Miyaji T, Tomita T, et al. Localization of bone morphogenetic protein-2 in human osteoarthritic cartilage and osteophyte. Osteoarthritis Cartilage. 2003;11:278–284. doi: 10.1016/s1063-4584(03)00004-9. [DOI] [PubMed] [Google Scholar]
- 35.Fukui N, Zhu Y, Maloney WJ, et al. Stimulation of BMP-2 expression by pro-inflammatory cytokines IL-1 and TNF-alpha in normal and osteoarthritic chondrocytes. J Bone Joint Surg Am. 2003;85-A(Suppl 3):59–66. doi: 10.2106/00004623-200300003-00011. [DOI] [PubMed] [Google Scholar]
- 36.Blaney Davidson EN, Vitters EL, van Lent PL, et al. Elevated extracellular matrix production and degradation upon bone morphogenetic protein-2 (BMP-2) stimulation point toward a role for BMP-2 in cartilage repair and remodeling. Arthritis Res Ther. 2007;9:R102. doi: 10.1186/ar2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lories RJ, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 2005;115:1571–1579. doi: 10.1172/JCI23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leijten JC, Bos SD, Landman EB, et al. GREM1, FRZB and DKK1 mRNA levels correlate with osteoarthritis and are regulated by osteoarthritis-associated factors. Arthritis Res Ther. 2013;15:R126. doi: 10.1186/ar4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura Y, Nakaya H, Saito N, et al. Coordinate expression of BMP-2, BMP receptors and Noggin in normal mouse spine. J Clin Neurosci. 2006;13:250–256. doi: 10.1016/j.jocn.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Takae R, Matsunaga S, Origuchi N, et al. Immunolocalization of bone morphogenetic protein and its receptors in degeneration of intervertebral disc. Spine (Phila Pa 1976) 1999;24:1397–1401. doi: 10.1097/00007632-199907150-00002. [DOI] [PubMed] [Google Scholar]
- 41.Than KD, Rahman SU, Vanaman MJ, et al. Bone morphogenetic proteins and degenerative disk disease. Neurosurgery. 2012;70:996–1002. doi: 10.1227/NEU.0b013e318235d65f. discussion 1002. [DOI] [PubMed] [Google Scholar]
- 42.Komatsu Y, Yu PB, Kamiya N, et al. Augmentation of Smad-dependent BMP signaling in neural crest cells causes craniosynostosis in mice. J Bone Miner Res. 2013;28:1422–1433. doi: 10.1002/jbmr.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu W, Sun X, Braut A, et al. Distinct functions for Bmp signaling in lip and palate fusion in mice. Development. 2005;132:1453–1461. doi: 10.1242/dev.01676. [DOI] [PubMed] [Google Scholar]
- 44.Warren SM, Brunet LJ, Harland RM, et al. The BMP antagonist noggin regulates cranial suture fusion. Nature. 2003;422:625–629. doi: 10.1038/nature01545. [DOI] [PubMed] [Google Scholar]
- 45.Settle SH, Jr, Rountree RB, Sinha A, et al. Multiple joint and skeletal patterning defects caused by single and double mutations in the mouse Gdf6 and Gdf5 genes. Dev Biol. 2003;254:116–130. doi: 10.1016/s0012-1606(02)00022-2. [DOI] [PubMed] [Google Scholar]
- 46.Hayano S, Komatsu Y, Pan H, et al. Augmented BMP signaling in the neural crest inhibits nasal cartilage morphogenesis by inducing p53-mediated apoptosis. Development. 2015;142:1357–1367. doi: 10.1242/dev.118802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Demoor M, Ollitrault D, Gomez-Leduc T, et al. Cartilage tissue engineering: molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim Biophys Acta. 2014;1840:2414–2440. doi: 10.1016/j.bbagen.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 48.Isogai N, Kusuhara H, Ikada Y, et al. Comparison of different chondrocytes for use in tissue engineering of cartilage model structures. Tissue Eng. 2006;12:691–703. doi: 10.1089/ten.2006.12.691. [DOI] [PubMed] [Google Scholar]
- 49.Chung C, Erickson IE, Mauck RL, et al. Differential behavior of auricular and articular chondrocytes in hyaluronic acid hydrogels. Tissue Eng Part A. 2008;14:1121–1131. doi: 10.1089/ten.tea.2007.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Kraan PM, Blaney Davidson EN, van den Berg WB. Bone morphogenetic proteins and articular cartilage: To serve and protect or a wolf in sheep clothing's? Osteoarthritis Cartilage. 2010;18:735–741. doi: 10.1016/j.joca.2010.03.001. [DOI] [PubMed] [Google Scholar]


