Abstract
Chronic pain is difficult to treat and new approaches to resolve persistent pain are urgently needed. Anti-inflammatory cytokines are promising candidates for treating debilitating pain conditions due to their capacity to regulate aberrant neuro-immune interactions. However, physiologically they work in a network of various cytokines, and therefore their therapeutic effect may not be optimal when used as stand-alone drugs. To overcome this limitation, we developed a fusion protein of the anti-inflammatory cytokines IL4 and IL10. Here, we describe the methods for production and quality control of IL4-10 recombinant fusion protein and we test the effectiveness of the IL4-10 fusion protein to resolve pain in a mouse model of persistent inflammatory pain.
Keywords: Medicine, Issue 134, Chronic pain, drug development, neuroimmunology, fusion protein, anti-inflammatory cytokines, protein
Introduction
Chronic pain remains one of the most debilitating and under-treated medical problems of the 21st century, affecting >20% of the adult population1,2. However, treatments to provide relief from chronic pain are often ineffective or must be discontinued due to severe side effects3. Importantly, currently available drugs only provide symptomatic relief, but do not significantly modify or cure chronic pain. Although chronic pain appears to be a neurological disorder, evidence suggests involvement of the immune system in chronic pain development4,5. Moreover, immune-based approaches to treat pain are emerging. For example, anti-inflammatory cytokines inhibit pain in several models of chronic pain6,7,8. However, anti-inflammatory cytokines have a short half-life, reducing their potential pain-inhibiting effects. Moreover, anti-inflammatory cytokines work most optimally in concert with each other. To overcome these limitations, we recently fused the anti-inflammatory cytokines interleukin-4 (IL4) and interleukin-10 (IL10) into one molecule. The IL4-10 fusion protein shows superior efficacy in inhibiting chronic inflammatory and neuropathic pain compared to the individual cytokines9. Here we describe how such fusion protein is produced, purified, and how its quality is controlled.
IL4-10 fusion protein is produced in human cells by transient transfection of HEK293-F cells with a pUPE expression vector carrying the cDNA sequence coding the IL4-10 fusion protein. HEK293-F cells are chosen to allow for post-translational modification of the protein, something that does not occur in bacterial expression systems. To optimize glycan capping with sialic acid, cDNA coding beta-galactoside-2, 3-sialyl-transferase is incorporated in the vector as a second transgene. The fusion protein is purified using affinity protein purification of the culture supernatant because it is more powerful than purification by other methods e.g. size-exclusion or ion exchange chromatography10,11. To purify the IL4-10 fusion protein, we used in-house made monoclonal antibodies against IL4. Evaluation of the purity and bioactivity of purified IL4-10 fusion protein is performed as a part of quality control. The purity of produced batches is evaluated by Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and High Pressure Size Exclusion Chromatography (HP-SEC). The bioactivity of the IL4-10 fusion protein is evaluated by measuring its capacity to inhibit lipopolysaccharide (LPS)-induced tumor necrosis factor-alpha (TNFα) production in whole blood cultures, and comparing it to the combination of the individual cytokines.
Finally, in order to test the capacity of IL4-10 fusion proteins to inhibit chronic pain, we describe how the fusion protein can be tested as analgesic in widely used mouse models of persistent inflammatory pain12,13,14. Here we describe the methods of an inflammatory pain model. However, it is important to note that other pain models can be used (e.g., neuropathic pain models), depending on the research questions that need to be answered. To assess pain in these models, it is important to use a variety of behavioral measures that include evoked and non-evoked pain measures. Here, we described the methods of assessment for changes in mechanically and thermally evoked behavioral responses. Mechanical sensitivity to innocuous stimuli is assessed using the Von Frey test, whilst thermal sensitivity is assessed using the Hargreaves test. Importantly, non-evoked hyperalgesia/allodynia is measured using the Dynamic Weight Bearing test. These measures are widely accepted as pain measures and yield important information on pain thresholds and potential pain experienced by the animals15,16,17. Other measures to assess non-evoked pain (e.g., stimulus independent), such as the conditioned place preference test, may be valuable18. To assess the potential of the drug to inhibit pain, we performed intrathecal administration of the fusion protein, as with this route of administration less protein dose is needed to reach pain-related areas and avoid systemic (side-) effects19,20.
Protocol
All animal experiments were performed in accordance with international guidelines and prior approval from the local experimental ethical committee. Whole blood was obtained from the Mini Donor Service (Mini Donor Dienst, MDD) at the University Medical Center Utrecht (UMCU) in the Netherlands. The MDD has received positive approval from the Medical Ethics Committee of the UMCU (Medisch Ethische Toetscommissie) for the protocol number 07-125/C.
1. Protein Production and Characterization
- Cell culture Note: See the Table of Materials for culture medium used.
- Thaw the HEK293-F cells at 37 °C until there is a small clump of ice. Transfer the thawed cells immediately to 10 mL of ice-cold medium (4 °C) and centrifuge the cell suspension at room temperature (RT) for 4 minutes (min) at 500 x g.
- Remove the medium and resuspend the cell pellet in 10 mL of fresh medium at RT, followed by centrifugation at 500 x g for 4 min.
- Remove the supernatant and resuspend the cell pellet in 5 mL of medium at RT. Add the cell suspension directly into a 125 mL flask containing 25 mL of pre-warmed medium (37 °C). Culture the cells at 37 °C, 8% CO2, and 95% humidity on an orbital shaker rotating at a speed of 125 rpm.
- Subculture the cells twice a week. Use an automated cell counter (see Table of Materials) to assess cell number and cell viability. Seed viable cells at a concentration of 3 x 105 cells/mL into Erlenmeyer flasks. Keep the total volume of the culture medium at 1/5 of the total volume of culture Erlenmeyer flasks at all times to keep aeration optimal.
- Transfection Note: Subculture the cells three to four times prior to transfection and transfect them when the cell viability is >90%. Cell viability is assessed by an automated (electric field multi-channel) cellcounting system based on the integrity of plasma membrane. See the Table of Materials for the transfection reagent.
- Centrifuge the cells at RT for 4 min at 500 x g; resuspend the cell pellet in 10 mL of pre-warmed culture medium (37 °C) and dilute the cell suspension to ~1.0 x 106 cells/mL in a total volume of 1 L. Aliquot the cell suspension in 10 aliquots of 100 mL in 500 mL Erlenmeyer flasks.
- Dilute 1 mg of plasmid DNA in 33 mL reduced-serum minimal essential medium (MEM, see Table of Materials) by gentle mixing. In a separate tube, dilute 1,330 µL of transfection reagent in MEM by gentle mixing. Incubate both solutions at RT for 5 min. Add and mix the DNA diluted in MEM to the transfection reagent in MEM and incubate the mix for 30 min at RT.
- Add the DNA- transfection reagent mix to the cells (cultured in section 1); add 6.6 mL of the mix to each 500 mL Erlenmeyer flask containing 100 mL of cells in culture medium. Maintain the cells in the previously mentioned culture conditions. Harvest the culture supernatant containing secreted IL4-10 fusion protein three days after transfection.
- Determine the concentration of the IL4-10 fusion protein in the culture supernatant by performing an IL10 ELISA according to manufacturer's protocol (see Table of Materials). NOTE: In optimal culture conditions, the concentration of the fusion protein can be expected to be between 2.5 and 5 µg/mL.
- Protein purification by affinity chromatography NOTE: Affinity chromatography is performed at room temperature. However, all fractions (culture supernatant, load, flow through, and elution fractions) are kept on ice during the procedure.
- Couple 10 mg of an in-house-made monoclonal antibody against IL4 to 1 g of CNBr- activated sepharose 4B, according to the manufacturer's protocol (see Table of Materials). Pour 2.5 mL of coupled sepharose beads slowly into the glass chromatography column (30 cm length and 1.5 cm inner diameter, see Table of Materials).
- Block the remaining binding sites of the beads by passing 50 mL of phosphate buffered saline (PBS) containing 1% Bovine Serum Albumin through the column. Wash the column with 25 mL of PBS (pH = 7.4) followed by 12.5 mL of 0.1 M Glycine buffer (pH = 2.5) and 25 mL of PBS (pH = 7.4).
- Pass 100 mL of 10-fold concentrated HEK293 cell culture supernatant through the column at a flow rate of 1 mL/min. Wash the column with 50 mL of PBS.
- Elute the bound IL4-10 fusion protein with 12.5 mL of 0.1 M Glycine buffer (pH = 2.5) at a flow rate of 1 mL/min and collect fractions of 2.5 mL. Add 350 µL of 1 M Tris buffer (pH = 9) to each fraction to bring the pH to 7. Dialyze neutralized fractions overnight against 2 L of PBS (pH = 7.4).
- Use dialysis tubing with 16 mm dry diameter and a 3.5 K molecular weight cut-off. Sterile filter the dialyzed fractions using disposable filters with 0.45 µM pore diameter. Store sterile IL4-10 fusion protein solution in aliquots at -80 °C.
- Evaluate the purity of eluted IL4-10 fusion protein by Coomassie-stained 12% SDS-PAGE gel and HP-SEC (3 µm SEC-2000 column). For HP-SEC analysis, load 40 µL of protein at the concentration of ~1 mg/mL on the column. Use 100 mM Sodium Phosphate Buffer with 150 mM Sodium Chloride (pH = 6.8) as a mobile phase. Set the flow rate at 1 mL/min.
- Determine the concentration of each batch of purified IL4-10 fusion protein by IL10 ELISA and Bicinchoninic Acid Protein Assay (BCA Protein Assay Kit, see Table of Materials) according to the manufacturer's protocols.
- Use recombinant human IL10 to create a standard curve in the ELISA assay. To correct for the size difference between recombinant IL10 (18 kDA) and IL4-10 fusion protein (34 kDa), multiply the obtained concentration by a factor of 1.8.
- Bioactivity of IL4-10 fusion protein NOTE: The whole blood assay is performed for each batch of IL4-10 fusion protein produced and the activity of different batches is compared as a part of quality control. The whole blood assay is performed in fresh human blood collected the day of the assay in heparin tubes. Blood was obtained from healthy volunteers in our in-house donor service. Samples should be handled as potentially biological hazards.
- Prepare 3-fold serial pre-dilutions of the purified IL4-10 fusion protein in RPMI medium (see Table of Materials) over a concentration range 5 - 1,215 ng/mL.
- Prepare pre-dilutions of LPS (100 ng/mL) and human blood that is diluted 4 times in RPMI medium.
- Pipette the pre-dilutions of the IL4-10 fusion protein, LPS, and human blood into a 48-well plate. To each well, add 50 µL IL4-10 fusion protein (final concentration 1 - 243 ng/mL), 75 µL RPMI medium, 25 µL LPS (final concentration 10 ng/mL), and 100 µL human blood (final diluted). NOTE: Total volume per well is 250 µL.
- Incubate the plate at 37 °C, 8% CO2, and 95% humidity for 18 h.
- Evaluate the concentration of TNFα in culture supernatants using a TNFα ELISA, according to the manufacturer's protocol.
- Calculate the inhibition of the inflammatory response by IL4-10 fusion protein according to the formula: % inhibition = (1-(A-B)/(C-B)) x 100 NOTE: Here A = TNFα levels in LPS stimulated cultures treated with IL4-10 fusion protein, B = TNFα levels in unstimulated culture, and C = TNFα levels in LPS stimulated culture.
2. Mouse Model for Persistent Inflammatory Pain
NOTE: It is important to use both male and female mice (C57Bl/6) because this will enable the identification of sex-dependent effects. In general, the mice used are between 8 - 16 weeks old. To determine the number of animals required, power calculations should be performed. Web-based tools to perform such calculations are readily found on the internet (e.g., http://www.powerandsamplesize.com; http://www.sample-size.net).
- Induction of chronic inflammatory pain NOTE: Induce persistent inflammatory pain in adult mice with intraplantar injections of carrageenan or Complete Freud's Adjuvant (CFA). The different tests do not interfere with each other.
- Prepare carrageenan (2% w/v) by dissolving λ-carrageenan (see Table of Materials) in 0.9% NaCl by passing the solution through a 23-gauge needle to ensure that the carrageenan is properly dissolved. Prepare a fresh solution directly before the injection is performed.
- Alternatively, if CFA is used, mix the CFA solution properly before injecting it intraplantarly, because the CFA is a water-in-oil emulsion containing 1 mg of Mycobacterium tuberculosis (H37Ra) heat killed and dried, 0.85 mL paraffin oil, and 0.15 mL mannide monooleate (see Table of Materials). NOTE: Glass Hamilton syringes with metal plungers are recommended for injecting this compound because rubber plungers may react with the oil in the adjuvant.
- Intraplantar injection
- Maintain the compound (steps 2.1.1 or 2.1.2) in the syringe at RT for at least 15 min before injection to allow adjustment to RT. Inject 20 µL of the compound (or dissolvent as a control) subcutaneously into the hind paw of the non-anesthetized mouse using a 30-gauge needle.
- Insert the needle at the base of the thumb directing to the heel and inject the compound when the needle is inserted approximately 0.5 cm. After injection, slowly retract the needle whilst slightly rotating the syringe to avoid leakage of solution out of the injection site.
3. Pain Measurements
Note: Ensure that researchers performing the behavioral experiments are blind to the mouse treatment. It is important to acclimatize the animals to the testing environment before performing any measurement. Preferably, the week before starting the experiments, animals are placed 1 - 2 times for 15 - 30 min in each of the different devices used for performing the tests. When handling males and females in the same experiment, evaluate males independently of females. Clean cages and surfaces extensively between the different groups to avoid potential unwanted effects on behavior of the different sexes. Measure baseline withdrawal latencies or mechanical thresholds two to three times to accurately determine baseline thresholds and identify whether these are stable before intraplantar injection of any compound.
- Von Frey test: mechanical sensitivity to innocuous stimuli
- Place the animals individually into acrylic cages on a wire mesh stand and allow the mice to acclimatize for at least 15 min prior to measurements.
- Assess mechanical sensitivity by measuring the paw withdrawal threshold in response to a calibrated series of von Frey hairs ranging from 0.02 to 4 g. Consider any movement of the paw away from applied hair as a positive withdrawal response. Apply the filaments perpendicular to the paw surface with sufficient force to cause slight bending against the skin and hold for ~3 s. Make sure the hair does not twist during application to the paw.
- Calculate the 50% paw-withdrawal threshold using the up-and-down method15,21. NOTE: The first filament to apply is 0.4 g (other starting filaments may be used depending on the thresholds these mice display at baseline). A lack of response to a filament indicates that the next thicker filament is used in the following stimulation, while a positive response indicates the use of the next thinner filament. The number of hair presentations is determined by the first differential response; after that, 5 more applications are performed. Calculate the 50% threshold according to the methods described previously15,21.
- Thermal sensitivity: Hargreaves test
- Place the animals into cages on a pre-warmed (30 °C) glass plate and allow the mice to acclimatize for at least 15 min prior to measurements, waiting until the animals sit still.
- Determine heat withdrawal latency times by heating the paw of the animals by a focused visible light beam using a Hargreaves apparatus (see Table of Materials). NOTE: The intensity of the beam is set to 12%, because with this experimentally determined intensity setting, the light beam evokes a withdrawal response of an average time of 8 s at baseline on C57Bl/6 mice. The intensity setting should be determined per individual machine and mouse strain. Maximal cut-off times of 20 s or more are used to avoid injury to the animals.
- Measure the withdrawal latency times ~4 times per animal. Average all responses measured. Measure each paw independently, and with at least 1 min intervals between subsequent measurements in the same paw.
- Postural deficits: dynamic weight bearing test
- Measure the body weight of each animal before the test, since weight bearing analysis requires the body weight of each individual animal as an input parameter.
- Place the animals individually in a floor-instrumented dynamic weight-bearing system assembled onto a cover supporting a camera that will record mouse movements. Let the animal acclimatize for 0.5 to 1 min before recording for 5 min. NOTE: In this test, only one freely moving animal can be evaluated at a time.
- Adjust settings to perform weight bearing and analyze the data. NOTE: In these experiments, we use the following parameters: (i) Low weight threshold of 0.6 g: this is the minimum weight necessary to activate one of the cells of the sensor. (ii) High weight threshold of 1 g: this number indicates the weight needed to fully activate one cell. (iii) Surface threshold of 2 cells: this number indicates the number of cells adjacent to each other that need to be activated to be taken into account. (iv) Minimum number of images is 5: it takes 0.1 s to capture every image. The number indicates the minimal number of frames in which the mouse does not move. As such, a value of 5 means that the mouse posture needs to be stable for >0.5 s to obtain a measurement that is taken into account for analysis.
- Perform the analysis of each video by replaying the video, and ensure that each limb is recognized correctly by the software at the time frame indicated by the software. NOTE: A minimum of 1 min of the recorded time needs to be validated. The dynamic weight bearing software calculates and provides the following parameters: (i) The weight borne by each individual paw in grams, and as a percentage of the whole body's weight. (ii) The weight borne by the combined front paw or the combined rear paws in grams and as a percentage of the whole body's weight. (iii) The ratio of left/right paws weight bearing or front/rear weight bearing. (iv) The surface area of each paw or combined front/rear paws that activated the pressure sensitive pad. (v) Mean and standard deviation for each parameter. (vi) Duration of different postures (rearing, 3 paws, or 4 paws) during the whole experiment. (vii) Time (s) each paw carries weight during the whole experiment.
4. Intrathecal Injection of IL4-10 fusion protein for analgesia
NOTE: Confirm that the ventilation to the room is open to ensure proper air flow. To test the efficacy of the IL4-10 fusion protein (1 µg/mouse; 5 µL total injection volume) it should be tested against vehicle injections and injections of the combination of the individual cytokines (IL4 + IL10, 0.5 µg + 0.5 µg/mouse, 5 µL total injection volume).
Anesthetize the mouse with 3 - 4% isoflurane with an oxygen flow rate of 2 L/min in the induction chamber. Check that the mouse is properly anesthetized by pinching the paw to ensure that the animal is not responsive.
Place the mouse on the table with its head placed in the nose cone of the apparatus and maintain the mouse sedation with 1.5 - 2% isoflurane with an oxygen flow rate of 2 L/min whilst performing the intrathecal injection.
Back-fill the injectable compounds into a clean 25 µL glass Hamilton syringe connected to a 27-gauge needle.
- Hold the mouse firmly by the spine posterior to the ribs and slightly lift the mouse. Place the needle vertically between the lumbar vertebrae L5 and L6 and carefully insert it through the skin right at the middle of the lumbar vertebrae. Place the needle into the intervertebral space. NOTE: Finding the intervertebral space may require some 'searching' by move the needle along the rostral ventral axis, lightly pressing the needle until a loss of resistance of bone tissue is felt.
- Confirm correct targeting by verifying that a tail flick occurred. Inject 5 µL of the compound slowly, wait a couple of seconds, and then slowly retract the needle. If only a single paw flick is observed, the needle is likely not placed correctly.
After injection, check the mouse closely for the first 30 min after recovery from anesthesia to ensure the absence of any motor impairment/paralysis. NOTE: Pain behaviors can be measured (as described in section 3) 15 minutes after recovery.
Representative Results
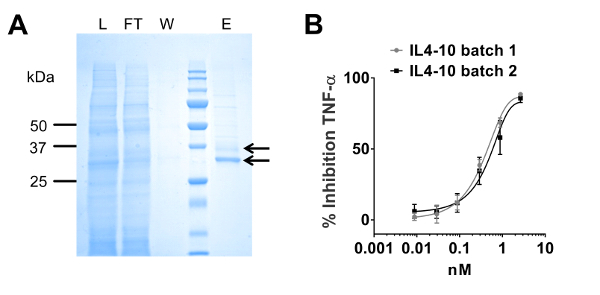
A representative picture of an SDS-PAGE gel containing different fractions obtained during affinity chromatography purification is shown in Figure 1A. In the load (L) and flow through (FT) fractions, all proteins present in the HEK293 supernatant are observed. No protein is observed in the wash (W) fraction. In the elution (E) fraction, two bands of 35 and 37 kDa are observed corresponding to two different glycoforms of IL4-10 fusion protein (arrows). In Figure 1B, a representative potency assay in a whole blood assay of two different IL4-10 fusion protein batches is shown. A dose-dependent inhibition of LPS-induced TNFα release is observed, showing comparable bioactivity for the two IL4-10 fusion protein batches.
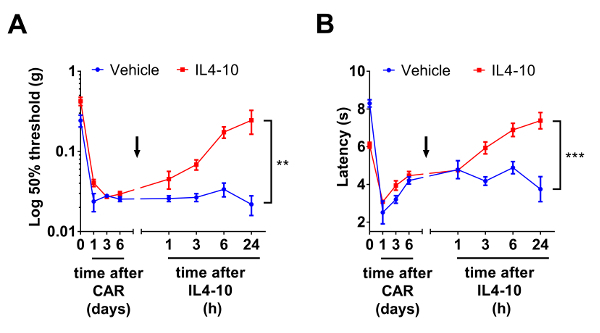
Next, an example of test results of representative experiments in which the IL4-10 fusion protein is tested to inhibit persistent inflammatory pain is shown (Figure 2). Persistent inflammatory pain was induced by intraplantar injection of 2% carrageenan. Mechanical (Figure 2A) as well as thermal (Figure 2B) hyperalgesia was followed over time. At day 6 after induction of inflammatory pain, mice were injected intrathecally with the IL4-10 fusion protein or vehicle as a control. IL4-10 significantly resolved both mechanical (Figure 2A) and thermal (Figure 2B) hyperalgesia, and reached its maximal effect after 24 h after injection. Ideally, the efficacy of the fusion protein in pain inhibition should be also tested against equimolar concentrations of the individual cytokines to test whether its capacity to inhibit pain is better than the sum of the individual cytokines. These data are not displayed here, but we refer to a recent publication that displays this data9.
The effectiveness of the IL4-10 fusion protein was recently tested in the CFA-induced inflammatory pain model9. In this experiment, multiple injections of the IL4-10 fusion protein were administered intrathecally. The multiple injections completely resolved CFA-induced persistent inflammatory hyperalgesia, measured using von Frey and Hargreaves tests, demonstrating the potential of the IL4-10 fusion protein as an analgesic treatment9. Here we show that intraplantar CFA injection also induced a reduction in weight bearing of the affected paw (Figure 3A-B). Intrathecal injection of IL4-10 fusion protein at day 7 after intraplantar CFA injection attenuated the reduction in weight bearing of the affected paw compared to vehicle-injected mice 2 days after IL4-10 fusion protein administration (Figure 3B).
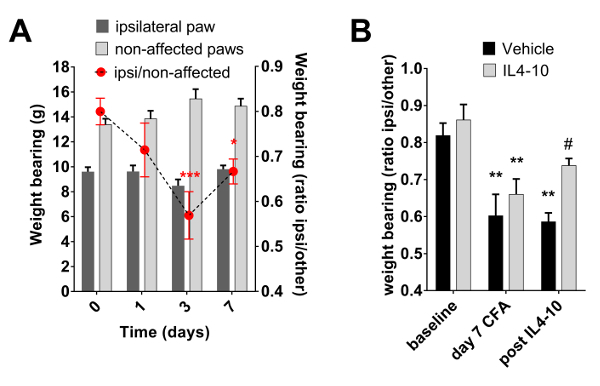
Figure 1: Purification and characterization of IL4-10 fusion protein. (A) Coomassie-stained SDS-PAGE analysis of the affinity chromatography fractions: L: load, FT: flow through, W: wash, E: elution. (B) Functional assay: IL4-10 fusion protein dose dependently inhibits TNFα production in LPS stimulated whole blood culture (expressed as percent inhibition compared to cultures stimulated with LPS alone). Data is represented as mean ± SD. Please click here to view a larger version of this figure.
Figure 2: Inhibition of inflammatory hyperalgesia by the IL4-10 fusion protein. Inflammatory pain was induced by an intraplantar injection of 20 µL of 2% carrageenan (CAR). Six days after intraplantar injection mice received an intrathecal injection (arrow) of 1 µg IL4-10 fusion protein or vehicle (PBS). (A) Mechanical hypersensitivity (Log 50% threshold (g)) was measured over time using Von Frey test and (B) thermal sensitivity (latency (s)) was measured using Hargreaves test. Data is represented as mean ± SEM. Asterisks represent significant differences analyzed by two-way ANOVA, between vehicle and IL4-10 injected animals. **, *** = p <0.01 and p <0.001, respectively. Please click here to view a larger version of this figure.
Figure 3: Inflammation-induced changes in weight bearing. Inflammatory pain was induced by an intraplantar injection of 20 µL of Complete Freud's Adjuvant (CFA). Weight bearing on each paw was measured using the dynamic weight bearing device. Weight bearing in grams of the affected paw (ipsilateral hind paw) and the total weight bearing of the non-affected paws (front paws and contralateral paw) is displayed, as well as the ratio of weight bearing of the ipsilateral paw compared to the other 3 paws. (A) Course of weight bearing during CFA-induced inflammation (n = 10). (B) At day 7 after intraplantar CFA, mice received an intrathecal injection of 1 µg IL4-10 fusion protein or vehicle (PBS). Weight bearing was measured at 0 and 7 days after intraplantar CFA, and 2 days after administration of IL4-10 fusion protein. Weight bearing of the ipsilateral paw compared to the other 3 paws is represented (n = 3 - 4). Data is represented as mean ± SEM. *** = p <0.001; ** = p <0.01; * = p <0.05 compared to baseline (day 0). # = p <0.05 compared to vehicle treated animals. Please click here to view a larger version of this figure.
Discussion
This manuscript describes methods for the production and characterization of a recombinant IL4-10 fusion protein, and methods to test its efficacy in inhibiting inflammatory hyperalgesia in mouse models of persistent inflammatory pain. The production and purification of the IL4-10 fusion protein is performed on a small scale. HEK293 cells are selected as an expression system for protein production because they enable post-translational modifications that cannot be achieved in prokaryotic expression systems. Post-translational modifications are relevant for protein function, and the absence of such modifications potentially could affect the therapeutic efficacy of the IL4-10 fusion protein. The protein is purified from culture supernatant by affinity chromatography. We chose to use in-house made monoclonal antibody against IL4 for affinity purification and not to produce an IL4-10 fusion protein containing a tag to allow for purification with an affinity tag system. The reason for this was to avoid possibility of tag-related changes in protein folding and function. Nevertheless, if a highly selective monoclonal antibody is lacking, a protein with a tag needs to be developed, e.g. a his tag. In that case, it will be important to determine whether the C- or N-terminus of the protein is best suited for the tag to avoid affecting protein function.
From 1 L of HEK293 supernatant, approximately 3 mg of purified IL4-10 fusion protein is obtained, but this may vary from batch to batch. The column for affinity purification can be used for several purification cycles before discarding. The most sensitive step in the purification procedure is the elution of IL4-10 fusion protein from the column by low pH, because the low pH may induce irreversible protein denaturation and protein precipitation. Thus, the maintenance of native protein structure and the absence of protein aggregates is essential for good quality batches. To that end, evaluation of the bioactivity of the fusion protein needs to be tested in vitro before and after purification with different elution buffers. After purification of the IL4-10 fusion protein, the bioactivity was not reduced compared to non-purified protein. Moreover, aggregate formation was absent when the elution step was performed with 0.1 M glycine buffer (pH = 2.5), and the pH of the elution fractions was immediately neutralized with 1 M Tris buffer (pH = 9).
The concentration of purified IL4-10 fusion protein is estimated on the basis of human IL10 ELISA results. BCA protein assay is used to confirm the protein concentrations. Recovery of the protein after purification is evaluated based on concentrations measured in load, flow through, wash and elution fractions by IL10 ELISA. The BCA protein concentration determination of IL4-10 fusion protein can be evaluated only in dialyzed elution fractions. Non-dialyzed elution fractions contain imidazole, a compound that affects the BCA measurement. Non-purified fractions contain other proteins from the HEK293 culture medium.
The determination of the bioactivity of the IL4-10 fusion protein is performed in a whole blood assay. The bioactivity of IL4-10 fusion protein should be evaluated in the whole blood of several donors to determine whether functional activity is retained. Several donors are required because donor to donor variance exists in the capacity of IL10 and IL4 to inhibit LPS-induced TNFα release. Moreover, expression of IL4R and IL10R may differ between donors, affecting the capacity of IL4-10 fusion protein to inhibit LPS-induced TNFα production.
In the described methods, we detailed 2 mouse models of persistent inflammatory pain to evaluate the efficacy of IL4-10 fusion protein to resolve pain in vivo. Nevertheless, for full evaluation of the analgesic potential of recombinant fusion proteins, the molecules should be tested in other models of chronic pain as well. These models include, but are not limited to, models of nerve-injury and chemotherapy-induced neuropathic pain9,22. Although the von Frey test and Hargreaves test are commonly used for testing analgesic drugs, it has become clear that an additional test (e.g. dynamic weight bearing test) should be included to assess the effects on non-evoked pain behaviorsand also with the operant pain assay (e.g. conditioned place preference (CPP) that detect non-evoked pain behaviors17,18). To perform CPP, the onset of the pain-inhibiting effects of the fusion protein should be taken into account, because conditioning of the animals by pain relief requires quick acting analgesics (which exert effects in less than 15 min). Nevertheless, if the test compound has a slow analgesic onset, but is likely to induce long-lasting pain inhibition (more than 4 days), CPP can still be used to test whether the compound inhibited pain. In this case, compound-induced loss of conditioning with fast acting analgesics needs to be assessed.
Intrathecal injections are selected as a route of administration, as the compound will reach pain relevant areas such as the dorsal root ganglia and spinal cord. Notably, intrathecal delivery of analgesic drugs has become common practice as a treatment of patients with chronic pain, as this approach reduces the dose of analgesic drug needed and decreases toxicity and systemic side effects. Nevertheless, this administration route has some limitations; it requires well-trained personnel and, due to the small subarachnoid space, the volume of injection should be limited to 5 µL in mice, requiring highly concentrated solutions of the fusion protein. The technique of intrathecal injections requires training. Such training can be performed on non-recovering anesthetized animals with injection of a dye to verify correct intrathecal injection.
Disclosures
The authors have nothing to disclose.
Acknowledgments
Part of this work has been funded by a Utrecht University Life Sciences grant
References
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Gerdle B, et al. Prevalence of widespread pain and associations with work status: a population study. BMC Musculoskelet Disord. 2008;9:102. doi: 10.1186/1471-2474-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Aasted CM, Burstein R, Becerra L. Migraine Mistakes: Error Awareness. Neuroscientist. 2014;20(3):291–304. doi: 10.1177/1073858413503711. [DOI] [PubMed] [Google Scholar]
- Raoof R, Willemen HL, Eijkelkamp N. Divergent roles of immune cells and their mediators in pain. Rheumatology. 2017. [DOI] [PMC free article] [PubMed]
- Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16(11):1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Penzkover KR, Soderquist RG, Mahoney MJ. Spinal interleukin-10 therapy to treat peripheral neuropathic pain. Neuromodulation. 2012;15(6):520–526. doi: 10.1111/j.1525-1403.2012.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, et al. Behavioral assessment of neuropathic pain, fatigue, and anxiety in experimental autoimmune encephalomyelitis (EAE) and attenuation by interleukin-10 gene therapy. Brain Behav Immun. 2017;59:49–54. doi: 10.1016/j.bbi.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi N, et al. Peripheral administration of interleukin-13 reverses inflammatory macrophage and tactile allodynia in mice with partial sciatic nerve ligation. J Pharmacol Sci. 2017;133(1):53–56. doi: 10.1016/j.jphs.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Eijkelkamp N, et al. IL4-10 Fusion Protein Is a Novel Drug to Treat Persistent Inflammatory Pain. J Neurosci. 2016;36(28):7353–7363. doi: 10.1523/JNEUROSCI.0092-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott H. Affinity Chromatography: Template Chromatography of Nucleic Acids and Proteins. New York: Dekker; 1984. [Google Scholar]
- Scopes RK. Strategies for protein purification. Curr Protoc Protein Sci. 2001. Chapter 1 Unit 1 2. [DOI] [PubMed]
- Gregory NS, et al. An overview of animal models of pain: disease models and outcome measures. J Pain. 2013;14(11):1255–1269. doi: 10.1016/j.jpain.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, et al. Balancing GRK2 and EPAC1 levels prevents and relieves chronic pain. J Clin Invest. 2013;123(12):5023–5034. doi: 10.1172/JCI66241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemen HL, et al. Microglial/macrophage GRK2 determines duration of peripheral IL-1beta-induced hyperalgesia: contribution of spinal cord CX3CR1, p38 and IL-1 signaling. Pain. 2010;150(3):550–560. doi: 10.1016/j.pain.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Robinson I, Sargent B, Hatcher JP. Use of dynamic weight bearing as a novel end-point for the assessment of Freund's Complete Adjuvant induced hypersensitivity in mice. Neurosci Lett. 2012;524(2):107–110. doi: 10.1016/j.neulet.2012.07.017. [DOI] [PubMed] [Google Scholar]
- He Y, Tian X, Hu X, Porreca F, Wang ZJ. Negative reinforcement reveals non-evoked ongoing pain in mice with tissue or nerve injury. J Pain. 2012;13(6):598–607. doi: 10.1016/j.jpain.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottros MM, Christo PJ. Current perspectives on intrathecal drug delivery. J Pain Res. 2014;7:615–626. doi: 10.2147/JPR.S37591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp N, et al. A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat Commun. 2013;4:1682. doi: 10.1038/ncomms2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman MJ, Ferrini F, Salio C, Merighi A. Practical mechanical threshold estimation in rodents using von Frey hairs/Semmes-Weinstein monofilaments: Towards a rational method. J Neurosci Methods. 2015;255:92–103. doi: 10.1016/j.jneumeth.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Krukowski K, et al. CD8+ T Cells and Endogenous IL-10 Are Required for Resolution of Chemotherapy-Induced Neuropathic Pain. J Neurosci. 2016;36(43):11074–11083. doi: 10.1523/JNEUROSCI.3708-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


