Abstract
This cohort study presents serological data with regard to 2 novel glial targets (myelin oligodendrocyte glycoprotein and glial fibrillary acidic protein) in recurrent longitudinally extensive transverse myelitis.
A 2014 study1 reported that autoimmune aquaporin 4 (AQP4) channelopathy was the most common cause of immune-mediated recurrent longitudinally extensive transverse myelitis (rLETM), with seropositivity in 42 of 47 cases (89%). Autoimmune myelin oligodendrocyte glycoproteinopathy (MOG-opathy) with serum antibodies targeting MOG (MOG-IgG) has recently been reported in AQP4-IgG seronegative longitudinally extensive transverse myelitis (LETM).2 Another autoimmune astrocytopathy with glial fibrillary acidic protein (GFAP-IgG) as a biomarker may include myelitis sometimes longitudinally extensive as a component of a meningoencephalomyelitis.3 We present additional serological data with regard to 2 novel glial targets (MOG and GFAP) in patients with rLETM.
Methods
We conducted a cohort study investigating MOG-IgG and GFAP-IgG serostatus in patients with rLETM. Adult (age, ≥16 years) patients fulfilling the following criteria were included: (1) 2 or more radiologically confirmed consecutive episodes of LETM (≥3 contiguous vertebral segments in length); (2) infectious, rheumatologic, vascular, and neoplastic causes excluded after a variety of serological, microbiological, and radiological tests were performed as deemed clinically appropriate; and (3) serum available. The study protocol was reviewed and approved by the Mayo Clinic institutional review board. Patients provided written consent for passive use of their medical records for research purposes.
Patients from the 2014 study,1 2005 to 2011, for whom serum samples were available (n = 41) were included; 29 patients identified from 2011 to 2017 were added. These patients were identified through the Mayo Clinic medical records database using the search terms LETM and recurrent LETM. For comparison, consecutive patients with onset of single-episode LETM (sLETM) (2008-2017) were included. Serostatus for AQP4-IgG and MOG-IgG1 was determined with a Clinical Laboratory Improvement Amendments–validated flow cytometry assay using live M1-AQP4–transfected and full-length MOG–transfected HEK293 cells.4,5
Indirect immunofluorescence assay was used to screen for GFAP-IgG.6 Frequency of glial autoantibody detection was determined and compared in the different LETM phenotypes; 50 healthy individuals and 50 patients with multiple sclerosis (MS) served as control patients.
Results
We identified 70 consecutive patients with rLETM and 81 with sLETM, grouped by phenotype (Table): (1) 59 with rLETM with inflammation restricted to spinal cord (rLETM alone); (2) 11 with rLETM with subsequent relapses outside spinal cord (rLETM+ [eg, optic neuritis and brainstem syndromes]); (3) 57 with sLETM with inflammation restricted to spinal cord (sLETM alone); and (4) 24 with sLETM with subsequent relapses outside spinal cord (sLETM+ [eg, optic neuritis and brainstem syndromes]) (Figure, A and B). Patients who were positive for MOG–IgG1 were younger at LETM onset, lacked female predominance, and had higher cerebrospinal fluid white blood cell count compared with the seropositive for AQP4-IgG patients. Identification of the patients with rLETM occurred between January 2005 and March 2017. Identification of the patients with sLETM occurred between July 2008 and July 2017.
Table. Demographic and Clinical Features in AQP4-IgG–Positive, MOG-IgG1–Positive, and Double-Negative Adult Patients With LETM.
| Characteristic | rLETM | sLETM at Onset | ||||||
|---|---|---|---|---|---|---|---|---|
| AQP4-IgG Positive (n = 65) | MOG-IgG1 Positive (n = 1) | Double-Negative (n = 4) | P Valuea | AQP4-IgG Positive (n = 40) | MOG-IgG1 Positive (n = 7) | Double-Negative (n = 34) | P Valuea | |
| Age at onset, median (IQR), y | 51 (43-57) | 37 | 35 (31-46) | .11 | 54 (42-60) | 28 (23-30) | 43 (33-53) | .001 |
| Sex ratio, female:male | 7:1 | 1:0 | 1:1 | .25 | 2:1 | 2:5 | 3:2 | .14 |
| White race/ethnicity, No. (%) | 47 (75) | 1 (100) | 1 (25) | .19 | 27 (71) | 5 (83) | 28 (88) | .33 |
| Follow-up after the first LETM, median (IQR), mob | 85 (35-165) | 7 | 77 (21-120) | .26 | 30 (10-79) | 38 (14-79) | 13 (5-38) | .21 |
| Relapse characteristics | ||||||||
| No. of attacks, median (range) | 3 (2-22) | 3 | 2 (2-7) | .45 | 1 (1-4) | 1 (1-9) | 1 (1-3) | .37 |
| Spinal cord lesion, median (IQR), vertebral segment | 6 (4-8) | 7 | 7 (5-8) | .89 | 7 (4-11) | 6 (5-17) | 7 (4-10) | .78 |
| Subsequent demyelinating attacks, No. | ||||||||
| Optic neuritis | 7c | 1 | 0 | .26 | 12 | 2 | 5 | .16 |
| Area postrema syndrome | 3c | 0 | 0 | 0 | 0 | 0 | ||
| Encephalitis | 0 | 0 | 0 | 0 | 1 | 1 | ||
| CSF findings, No. of patientsd | ||||||||
| Sample available | 42 | 1 | 2 | 29 | 6 | 29 | ||
| WBC count >5/mL | 22 | 1 | 2 | .18 | 16 | 5 | 18 | .45 |
| WBC count, median (range), cells/mL | 6 (0-3200) | 284 | 20 (12-27) | .50 | 33 (1-1727) | 71 (4-373) | 13 (1-943) | .70 |
| Neutrophils >40% | 3 | 1 | NA | >.99 | 2 | 0 | 4 | .77 |
| Protein, median (range), mg/dL | 48 (18-515) | 35 | 80 (44-85) | .47 | 76 (22-261) | 87 (63-125) | 56 (35-229) | .29 |
| ≥4 Oligoclonal bands positive | 5 | 0 | 0 | >.99 | 5 | 0 | 3 | .69 |
| Elevated IgG index (>0.85) | 3 | 0 | 0 | >.99 | 4 | 0 | 1 | .31 |
| Brain MRI findings, No. of patients | ||||||||
| Available | 61 | 1 | 4 | >.99 | 37 | 7 | 32 | .94 |
| Normal results | 25 | 1 | 1 | .67 | 17 | 4 | 16 | .39 |
| Nonspecific and small vessel disease | 32 | 0 | 3 | 18 | 3 | 16 | ||
| Multiple sclerosis–like | 4 | 0 | 0 | 2 | 0 | 1 | ||
| Preventive medications, No. of patientse (%) | ||||||||
| Immunosuppressive drugsf | 52 (80) | 0 | 2 (50) | .75 | 18 (45) | 2 (29) | 8 (24) | .19 |
| Immunomodulatory drugsg | 9 (14)h | 0 | 0 | >.99 | 0 | 0 | 1 (3) | .51 |
| Rituximab/monoclonal antibodiesi | 24 (37) | 1 (100) | 0 | .11 | 19 (48) | 1 (14) | 4 (12) | .002 |
| Disability outcome at last follow-up, No. (%) of patients | ||||||||
| EDSS score ≥6j | 29 (45) | 0 | 1 (25) | .79 | 7 (18) | 0 | 6 (17) | .74 |
| EDSS score ≥8j | 14 (22) | 0 | 0 | .66 | 3 (8) | 0 | 6 (17) | .35 |
Abbreviations: AQP4, aquaporin 4; CSF, cerebrospinal fluid; EDSS, Expanded Disability Status Scale; IQR, interquartile range; LETM, longitudinally extensive transverse myelitis; MOG, myelin oligodendrocyte glycoprotein; MRI, magnetic resonance imaging; MS, multiple sclerosis; NA, not available; rLETM, recurrent LETM; sLETM, single-episode LETM; WBC, white blood cell count.
Analysis of continuous data by Wilcoxon rank sum test and categorical data by Fisher exact test.
There was a statistically significant difference between patients with rLETM and those with sLETM, P < .001.
Subsequent demyelinating attack after the second episode of LETM.
Where not otherwise indicated, values for CSF finding indicate numbers of patients.
Certain patients had more than 1 treatment during the follow-up period.
Long-term immunosuppressive drugs included azathioprine, mycophenolate mofetil, and cyclophosphamide.
Long-term immunomodulatory drugs included interferon beta groups, fingolimod, glatiramer acetate, teriflunomide, and dimethyl fumarate.
Misdiagnosed as MS.
Long-term monoclonal antibodies included ocrelizumab and alemtuzumab.
An EDSS score of 6 indicates that the patient requires intermittent or unilateral assistance (cane, crutches, or braces) to walk 100 m with or without resting. An EDSS score of 8 indicates that the patient is restricted to bed or chair or perambulated in a wheelchair but may be out of bed much of the day, retains many self-care functions, and generally has effective use of his or her arms.
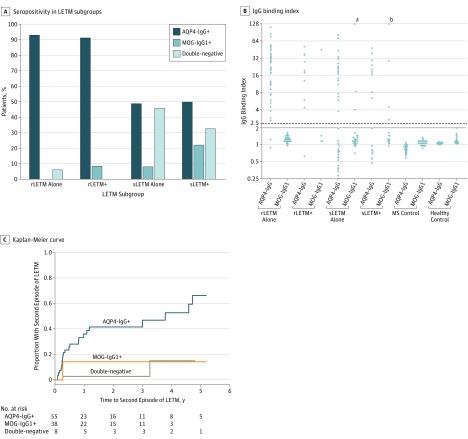
Figure. Seroprevalence and Outcome of Longitudinally Extensive Transverse Myelitis (LETM) Stratified According to Aquaporin 4 (AQP4) and Myelin Oligodendrocyte Glycoprotein IgG (MOG-IgG) Serostatus.
A, Frequency of AQP4-IgG and MOG-IgG1 seropositivity in LETM subgroups. B, IgG binding index of AQP4-IgG and MOG-IgG1 stratified according to disease and control subgroups: recurrent longitudinally extensive transverse myelitis (rLETM alone), rLETM with subsequent other demyelinating attacks (rLETM+), single-episode longitudinally extensive transverse myelitis (sLETM alone), sLETM with subsequent other demyelinating attacks (sLETM+), multiple sclerosis (MS), and healthy controls. The IgG binding index cut point of positivity of M1-AQP4-IgG was 2 (solid line) and of MOG-IgG1 was 2.5 (dashed line).4 C, Kaplan-Meier curve estimates of years from the first episode of LETM to second episode. All patients with sLETM as onset attack between 2008 and 2017 seen at Mayo Clinic were included and analyzed. Five years after the first episode of LETM, a second episode of LETM was estimated to occur in a significantly higher proportion of AQP4-IgG–positive patients (66%) compared with double-seronegative (15%) and MOG-IgG1–positive patients (14%) (P < .001).
aThe IgG binding index value for this individual is 280.
bThe IgG binding index value for this individual is 190.
For the total rLETM cohort, AQP4-IgG was detected in 65 of 70 patients (93%) and MOG-IgG1 was detected in only 1 of 57 patients (2%) who subsequently developed optic neuritis. The frequency of AQP4-IgG was significantly higher than MOG-IgG (P < .001, Figure, A and C).
For rLETM alone, no patient was MOG-IgG1 positive. Of 11 rLETM+ patients, 10 (91%) were AQP4-IgG positive and 1 patient (9%) was MOG-IgG1 positive (Figure, A and B).
For sLETM alone, the frequency of AQP4-IgG was 28 of 57 patients (49%) and of MOG-IgG1 was 3 of 39 patients (8%). For sLETM+, the frequency of AQP4-IgG was 12 of 24 patients (50%) and of MOG-IgG1 was 4 of 18 patients (22%).
None of the patients (including 62 with cerebrospinal fluid samples) tested positive for GFAP-IgG. None of the 50 healthy controls and 50 patients with MS tested positive for MOG, AQP4, or GFAP-IgG.
Discussion
This study, the first to our knowledge to investigate MOG-IgG1 serostatus in patients with rLETM, reports that autoimmune MOG-opathy can present with LETM, but recurrent sequential LETM is uncommon among patients with MOG-IgG (Figure, C). The single patient with rLETM and MOG-IgG1 subsequently developed optic neuritis.
The 15% (7 of 47) frequency of MOG-IgG1 in sLETM patients in this study is similar to that reported by Cobo-Calvo and colleagues2 (MOG-IgG1 in 13 of 56 patients [23%]). The lack of patients with sLETM or rLETM who were seropositive for GFAP-IgG suggests that isolated LETM does not fall within the autoimmune GFAP astrocytopathy spectrum. Accepting potential confounding by referral bias, autoimmune AQP4 channelopathy is the cause of half of sLETM cases and 93% of rLETM cases.
References
- 1.Jiao Y, Fryer JP, Lennon VA, et al. Aquaporin 4 IgG serostatus and outcome in recurrent longitudinally extensive transverse myelitis. JAMA Neurol. 2014;71(1):48-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cobo-Calvo Á, Sepúlveda M, Bernard-Valnet R, et al. Antibodies to myelin oligodendrocyte glycoprotein in aquaporin 4 antibody seronegative longitudinally extensive transverse myelitis: clinical and prognostic implications. Mult Scler. 2016;22(3):312-319. [DOI] [PubMed] [Google Scholar]
- 3.Flanagan EP, Hinson SR, Lennon VA, et al. Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: analysis of 102 patients. Ann Neurol. 2017;81(2):298-309. [DOI] [PubMed] [Google Scholar]
- 4.Fryer JP, Lennon VA, Pittock SJ, et al. AQP4 autoantibody assay performance in clinical laboratory service. Neurol Neuroimmunol Neuroinflamm. 2014;1(1):e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen JJ, Tobin WO, Majed M, et al. Myelin oligodendrocyte glycoprotein and aquaporin-4-IgG serostatus of patients in the Optic Neuritis Treatment Trial [published online February 22, 2018]. JAMA Ophthalmol. doi: 10.1001/jamaophthalmol.2017.6757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang B, McKeon A, Hinson SR, et al. Autoimmune glial fibrillary acidic protein astrocytopathy: a novel meningoencephalomyelitis. JAMA Neurol. 2016;73(11):1297-1307. [DOI] [PubMed] [Google Scholar]