Abstract
In response to the Ebola virus disease (EVD) outbreak in West Africa, the World Health Organization has advised all nations to prepare for the detection, investigation and management of confirmed and suspected EVD cases in order to prevent further spread through international travel. To gain insights into the state of preparedness of European hospitals, an electronic survey was circulated in August–September 2014 to 984 medical professionals representing 736 hospitals in 40 countries. The survey addressed the willingness and capacity to admit patients with suspected EVD as well as specific preparedness activities in response to the current Ebola crisis. Evaluable responses were received from representatives of 254 (32%) hospitals in 38 countries, mostly tertiary care centres, of which 46% indicated that they would admit patients with suspected EVD. Patient transfer agreements were in place for the majority of hospitals that would not admit patients. Compared with non-admitting hospitals, admitting hospitals were more frequently engaged in various preparedness activities and more often contained basic infrastructural characteristics such as admission rooms and laboratories considered important for infection control, but some gaps and concerns were also identified. The results of this survey help to provide direction towards further preparedness activities and prioritisation thereof.
Introduction
The unprecedented and devastating epidemic of Ebola virus disease (EVD) in West Africa, with over 15,000 reported cases and nearly 5,500 deaths as of 21 November 2014 [1], has ignited increasing global concerns about the potential introduction and further spread of the disease by international travel and repatriation [2–4]. For this reason, the World Health Organization (WHO) has advised all nations, including those not directly neighbouring currently-affected countries, to prepare for the detection, investigation and management of confirmed and suspected EVD cases [4]. In view of the non-specific nature of initial symptoms, suspected patients essentially include all travellers with unexplained febrile illness recently arrived from areas with ongoing EVD transmission, particularly when accompanied by gastrointestinal symptoms. The current assessment is that travel-associated cases will remain rare across Europe, but that the occurrence of EVD in returning healthcare workers is a realistic scenario [5,6]. The recent experiences with both types of EVD cases in the United States and Europe, with local transmission to healthcare workers, illustrate the importance of being prepared [7,8].
To gain insights into the preparedness of European hospitals and identify potential gaps in preparedness at hospital level, we conducted a survey of hospitals in 40 European and western Asian countries, focusing on the willingness and capacity to admit patients with suspected EVD and on specific preparedness activities of hospitals in response to the current Ebola crisis. It should be emphasised that the survey did not address preparedness for EVD at national levels but was solely intended to explore the preparedness at the hospital level.
This survey is an initiative of the PREPARE project. PREPARE (Platform for European Preparedness Against (Re-)emerging Epidemics) is an European Union (EU)-funded project that aims to establish preparedness for harmonised clinical research studies on epidemic infectious diseases, hence providing real-time evidence for clinical management of patients and to inform public health responses (www.prepare-europe.eu). PREPARE is a partnership of established and developing European clinical research networks, covering primary care (GRACE and TRACE) and hospital care (CAPNETZ, COMBACTE, ESICM and PENTA) in more than 40 European countries, including all EU Member States. The survey was performed in above-mentioned hospital care networks.
Methods
Survey
A questionnaire was developed in English, addressing: characteristics of the hospital such as the geographic location, type (primary, secondary or tertiary care) and size of the hospital; the availability and content of national and hospital guidelines or protocols for the management of patients with suspected or confirmed haemorrhagic fever; the performance of preparedness activities in response to the Ebola crisis (e.g. revision of protocols, exercises to test the protocols, formation of a hospital outbreak management team, training of healthcare workers, or immediate plans to do so); and arrangements for Ebola virus (EBOV) diagnostics.
In addition, the questionnaire asked whether hospitals would, in principle, admit patients with suspected EVD, and if not, whether local or national agreements were in place for transfer to another hospital. For hospitals that would admit patients with suspected EVD, additional questions were asked about the characteristics of admission rooms (e.g. presence of an anteroom, negative pressure, high-efficiency particulate air (HEPA) filtration). An open question was added to capture specific suggestions or needs in relation to EVD preparedness that could be addressed by the PREPARE project. Respondents could indicate whether or not permission was granted to use the anonymised results in reports or publications. The complete questionnaire is available upon request from the authors.
After pilot testing in three hospitals, an online link to the electronic questionnaire was circulated by email to 984 medical professionals representing 736 hospitals in 38 European and 2 western Asian countries (Turkey and Israel). All hospitals were affiliated with the PREPARE project through membership of one or more of the following clinical research networks: CAPNETZ (www.capnetz.de), COMBACTE (www.combacte.com), ESICM (www.esicm.org), and PENTA (www.penta-id.org).
The survey was started on 27 August 2014 and closed on 19 September 2014. Reminders to complete the survey were sent weekly during this three-week survey period.
Analysis
Descriptive statistics were used to analyse the survey data at the hospital level. In case of discrepant responses from multiple representatives of the same hospital, affirmative or negative answers took precedence over ‘do not know’ replies. Comparisons were made between hospitals that would admit patients with suspected EVD and those that would not or did not know. In addition, comparisons were made between hospitals in the four regions of Europe (eastern, northern, southern, western) and western Asia, as defined by the United Nations Statistics Division’s Geoscheme [9]. Differences between groups were analysed using chi-squared statistics. A p-value of less than 0.05 was considered significant. Analyses were performed using Microsoft Excel version 14.4.3 (Microsoft Corporation).
Results
Survey characteristics
Responses were received from 266 out of 984 (27%) medical professionals of whom 12 did not provide permission to use the data for reporting. The remaining 254 respondents represented 236 of 736 hospitals (32%) in 38 European and western Asian countries. The majority of respondents were intensivists (122, 48%), followed by internists/infectious disease specialists (49, 19%) and clinical microbiologists (42, 17%). Among the remaining respondents were infection control specialist (19, 8%) and paediatricians (9, 4%). Hospitals represented in the survey were mostly tertiary care centres (78%) and were widely distributed across Europe and western Asia (Table 1, Figure 1).
Table 1. Admission, guidelines and preparedness for patients with suspected Ebola virus disease in European hospitals, results from survey of representatives from 236 hospitals in 38 European and western Asian countries, August–September 2014.
| Total (%) | Would admit patient with suspected EVD (%) | Would not admit patient with suspected EVD (%) | Do not know (%) | p-value | |
|---|---|---|---|---|---|
| Hospital type | 236 | 111 (47.0) | 99 (42.0) | 26 (11.0) | - |
| Primary | 5 (2.1) | 2 (1.8) | 2 (2.0) | 1 (3.8) | - |
| Secondary | 46 (19.5) | 13 (11.7) | 23 (23.2) | 10 (38.5) | - |
| Tertiary | 185 (78.4) | 96 (86.5) | 74 (74.7) | 15 (57.7) | - |
| National guidelines | |||||
| Yes | 181 (76.7) | 90 (81.1) | 75 (75.8) | 16 (61.5) | 0.047 |
| No | 30 (12.7) | 14 (12.6) | 13 (13.1) | 3 (11.5) | - |
| Do not know | 25 (10.6) | 7 (6.3) | 11 (11.1) | 7 (26.9) | - |
| Topics covered | |||||
| Triage criteria | 165 (91.2) | 83 (92.2) | 70 (93.3) | 12 (75.0) | 0.78 |
| EBOV diagnostics | 160 (88.4) | 84 (93.3) | 64 (85.3) | 12 (75.0) | 0.09 |
| Other diagnostics | 143 (79.0) | 79 (87.7) | 54 (72.0) | 10 (62.5) | 0.01 |
| Infection control | 174 (96.1) | 89 (98.9) | 72 (96.0) | 13 (81.2) | 0.23 |
| Clinical management | 137 (75.7) | 76 (84.4) | 52 (69.3) | 9 (56.2) | 0.02 |
| Hospital guidelines | |||||
| Yes | 153 (64.8) | 93 (83.8) | 52 (52.5) | 8 (30.8) | < 0.01 |
| No | 60 (25.4) | 13 (11.7) | 36 (36.4) | 11 (42.3) | - |
| Do not know | 23 (9.7) | 5 (4.5) | 11 (11.1) | 7 (26.9) | - |
| Topics covered | |||||
| Triage criteria | 146 (95.4) | 90 (96.8) | 49 (94.2) | 7 (87.5) | 0.46 |
| EBOV diagnostics | 123 (80.4) | 82 (88.2) | 38 (73.1) | 3 (37.5) | 0.02 |
| Other diagnostics | 133 (86.9) | 90 (96.8) | 38 (73.1) | 5 (62.5) | < 0.01 |
| Infection control | 151 (98.7) | 93 (100) | 51 (98.1) | 7 (87.5) | 0.18 |
| Clinical management | 118 (77.1) | 79 (84.9) | 34 (65.4) | 5 (62.5) | < 0.01 |
| Preparedness efforts | |||||
| Revision protocols | 168 (71.2) | 95 (85.6) | 64 (64.6) | 9 (34.6) | < 0.01 |
| Training HCW | 131 (55.5) | 81 (73.0) | 46 (46.5) | 4 (15.4) | < 0.01 |
| Hospital OMT | 121 (51.3) | 79 (71.2) | 41 (41.4) | 1 (3.8) | < 0.01 |
| National OMT | 89 (37.7) | 57 (51.4) | 31 (31.3) | 1 (3.8) | < 0.01 |
| Exercise | 67 (28.4) | 51 (45.9) | 16 (16.2) | 0 (0) | < 0.01 |
EVD: Ebola virus disease; EBOV: Ebola virus; HCW: healthcare worker; OMT: outbreak management team.
Primary care: general practice and basic district hospital services; secondary care: district hospitals with basic specialty functions; tertiary care: specialised care, usually on referral from primary or secondary care, with facilities for special investigations and treatment.
Figure 1. Geographic distribution and numbers of responding hospitals, survey on willingness and capacity to admit patients with suspected Ebola virus disease, August–September 2014 (n=236).
Admission of patients with suspected EVD and characteristics of admission rooms
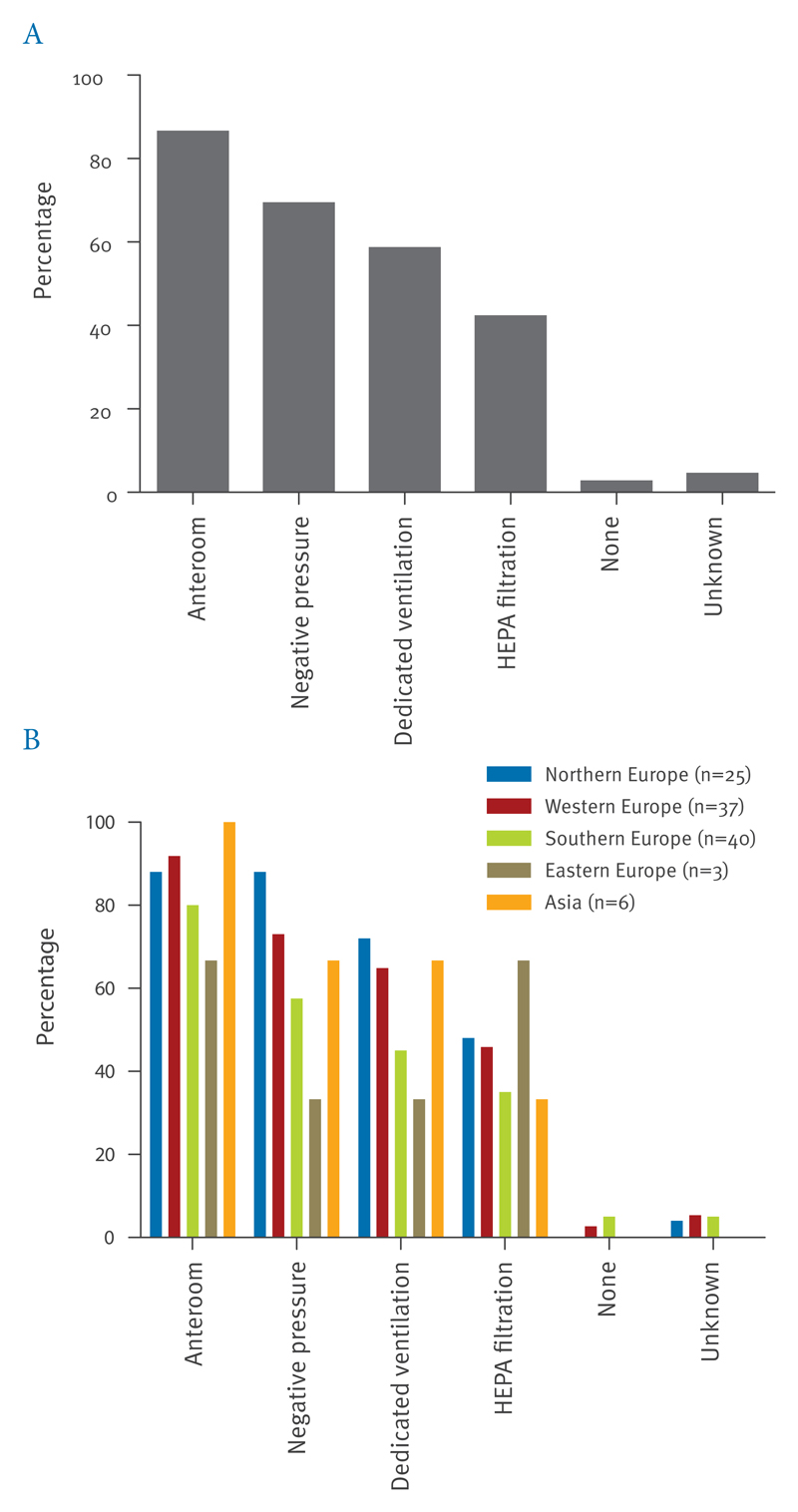
Of 236 hospitals, 111 (47%) stated that they would admit suspected EVD patients, 99 (42%) indicated that they would not admit such patients, and 26 (11%) did not know whether such patients would be admitted (Table 1). In the 99 hospitals indicating they would not admit patients, local or national agreements for transfer of patients were in place in the majority (local 25 (25%), national 67 (68%)). Admission rooms of most of the 111 admitting hospitals, the majority of which were tertiary care centres (87%), had an anteroom (87%), availability of negative pressure (69%), and/or the presence of dedicated ventilation systems (59%) (Figure 2A). Less than half used HEPA filtration of exhausted air (42%). In five hospitals (5%), none of these assets were available.
Figure 2. Characteristics of admission facilities in hospitals admitting Ebola virus disease-suspected patients, survey on willingness and capacity to admit patients with suspected Ebola virus disease, August–September 2014 (n=111).
HEPA: high-efficiency particulate air.
Percentages are represented overall (A) and per region (B).
National and hospital guidelines for management of EVD patients
Respondents from 181 hospitals (77%) were aware of the existence of national guidelines for management of patients with haemorrhagic fever (including EVD) while 30 hospitals (13%) indicated these were not available. The remaining respondents did not know (Table 1). Available guidelines were based on those from WHO (63%), the European Centre for Disease Prevention and Control (ECDC) (43%) and/or the US Centers for Disease Control and Prevention (CDC) (34%), and covered triage criteria and infection control practices in more than 90% of guidelines, while diagnostics and clinical management were covered less frequently (Table 1).
Local hospital guidelines were available in 153 of 236 hospitals (65%), not available in 60 hospitals (25%) and the remaining respondents did not know (Table 1). Guidelines were based on national guidelines in 81% and on international guidelines in the remaining cases (19%). Similar to national guidelines, triage criteria and infection control practices were covered in more than 95% of local guidelines with less frequent coverage of other topics.
The availability of national, and even more so of hospital guidelines was highest in hospitals that would admit patients with suspected EVD compared with those that would not admit patients or did not know (Table 1).
Laboratory infrastructure and Ebola virus diagnostics Microbiology laboratories were present in nearly all hospitals (98%) (Table 2). In these laboratories, biosafety level (BSL) 2 and 3 facilities were available in 57% and 24%, respectively and not available in 11% and 40%, respectively. In the remaining cases respondents were not aware of the biosafety levels of the laboratory (32% and 36%, respectively). Availability of BSL 2 and 3 facilities was higher in hospitals that would admit patients (70% and 36%, respectively) compared with those that did not (51% and 14%, respectively).
Table 2. Laboratory infrastructure and diagnostics for patients with suspected Ebola virus disease in European hospitals, results from survey of representatives from 236 hospitals in 38 European and western Asian hospitals, August–September 2014.
| Total (n=236) (%) | Would admit patient with suspected EVD (n=111) (%) | Would not admit patient with suspected EVD (n = 99) (%) | Do not know (n = 26) (%) | |
|---|---|---|---|---|
| Microbiology laboratory present | 231 (97.9) | 109 (98.2) | 97 (98.0) | 25 (96.2) |
| BSL2 | ||||
| Yes | 132 (57.1) | 76 (69.7) | 49 (50.5) | 7 (28.0) |
| No | 26 (11.3) | 9 (8.3) | 16 (16.5) | 1 (4.0) |
| Do not know | 73 (31.6) | 24 (22.0) | 32 (33.0) | 17 (68.0) |
| BSL3 | ||||
| Yes | 56 (24.2) | 39 (35.8) | 14 (14.4) | 3 (12.0) |
| No | 93 (40.3) | 43 (39.4) | 46 (47.4) | 4 (16.0) |
| Do not know | 82 (35.5) | 27 (24.8) | 37 (38.1) | 18 (72.0) |
| Ebola virus diagnostics | ||||
| On site | 17 (7.2) | 14 (12.6) | 1 (1.0) | 2 (7.7) |
| National reference laboratory | 140 (59.3) | 64 (57.7) | 65 (65.7) | 11 (42.3) |
| International reference laboratory | 30 (12.7) | 22 (19.8) | 8 (8.1) | 0 (0) |
| Not performed | 4 (1.7) | 3 (2.7) | 1 (1.0) | 0 (0) |
| Do not know | 45 (19.1) | 8 (7.2) | 24 (24.2) | 13 (50.0) |
EVD: Ebola virus disease; BSL: biosafety level.
EBOV diagnostics were performed on site in 17 hospitals, which included 14 hospitals that would admit patients, 1 that would not admit patients and 2 that did not know. For the majority of remaining hospitals, agreements and procedures were in place for performance of Ebola diagnostics in national (59%) or international (13%) reference laboratories.
Preparedness activities
Preparedness activities in response to the EVD outbreak included revision of hospital protocols or guidelines in 168 hospitals (71%), education and training of healthcare workers (HCWs) in 131 (56%), formation of an outbreak management team (OMT) in 121 (51%) and participation in regional or national preparedness committees in 89 (38%) (Table 1). In 67 hospitals (28%), exercises to test procedures and protocols were completed or planned in the immediate future. All preparedness activities were performed more frequently in hospitals that would admit patients (Table 1, Figure 3).
Figure 3. Preparedness activities for patients with suspected Ebola virus disease in European hospitals, results from survey of representatives from 236 hospitals in 38 European and western Asian countries, August–September 2014.
HCW: healthcare workers; OMT: outbreak management team.
Percentages are shown separately for admitting and non-admitting hospitals and for those not aware whether Ebola virus disease-suspected patients would be admitted.
Regional differences in Europe
Northern and western Europe had the highest proportions of hospitals that would admit patients with suspected EVD (57% and 56% respectively) and this proportion was lowest in eastern European states (12%) (Table 3). Differences were noted between regions with respect to availability of national and local guidelines, laboratory infrastructure and preparedness activities, with highest frequencies mostly observed in western European countries, followed by southern, northern and eastern European states (Table 3, Figure 2B).
Table 3. Geographical comparisons of hospitals and willingness and capacity to admit patients with suspected Ebola virus disease, results from survey of representatives from 236 hospitals in 38 European and western Asian countries, August–September 2014.
| Geographical regiona | |||||
|---|---|---|---|---|---|
| Northern Europe | Southern Europe | Eastern Europe | Western Europe | Western Asia | |
| Number of hospitals (%) | |||||
| Received questionnaire | 138 | 257 | 106 | 219 | 16 |
| Responded | 44 (31.8 | 93 (36.2) | 26 (24.5) | 66 (30.1) | 7 (43.8) |
| Would admit suspected EVD patient | 25 (56.8) | 40 (43.0) | 3 (11.5) | 37 (56.1) | 6 (85.7) |
| Would not admit suspected EVD patient | 14 (31.8) | 41 (44.1) | 18 (69.2) | 25 (37.9) | 1 (14.30 |
| Do not know | 5 (11.4) | 12 (12.9) | 5 (19.2) | 4 (6.1) | 0 (0) |
| National guidelines | |||||
| Yes | 34 (77.3) | 71 (76.3) | 12 (46.2) | 57 (86.4) | 7 (100) |
| No | 3 (6.8) | 15 (16.1) | 6 (23.1) | 6 (9.1) | 0 (0) |
| Do not know | 7 (15.9) | 7 (7.5) | 8 (30.8) | 3 (4.5) | 0 (0) |
| Hospital guidelines | |||||
| Yes | 26 (59.1) | 57 (61.3) | 12 (46.2) | 53 (80.3) | 5 (71.4) |
| No | 11 (25.0) | 28 (30.1) | 10 (38.5) | 10 (15.2) | 1 (14.3) |
| Do not know | 7 (15.9) | 8 (8.6) | 4 (15.4) | 3 (4.5) | 1 (14.3) |
| Preparedness efforts | |||||
| Revision of protocols | 26 (59.1) | 65 (69.9) | 14 (53.8) | 56 (84.8) | 6 (85.7) |
| Training HCWs | 17 (38.6) | 50 (53.8) | 15 (57.7) | 44 (66.7) | 5 (71.4) |
| Hospital OMT | 18 (40.1) | 46 (49.5) | 9 (34.6) | 43 (65.2) | 5 (71.4) |
| National OMT | 10 (22.7) | 33 (35.5) | 6 (23.1) | 37 (56.1) | 3 (42.9) |
| Exercise | 8 (18.2) | 24 (25.8) | 3 (11.5) | 31 (47.0) | 1 (14.3) |
| Admission rooms | |||||
| Anteroom | 22 (88.0) | 32 (80.0) | 2 (66.7) | 34 (91.9) | 6 (100) |
| Negative pressure | 22 (88.0) | 23 (57.5) | 1 (33.3) | 27 (73.0) | 4 (66.7) |
| Dedicated ventilation | 18 (72.0) | 18 (45.0) | 1 (33.3) | 24 (64.9) | 4 (66.7) |
| HEPA filtration | 12 (48.0) | 14 (35.0) | 2 (66.7) | 17 (45.9) | 2 (33.3) |
| None | 0 (0) | 2 (5.0) | 0 (0) | 1 (2.7) | 0 (0.0) |
| Unknown | 1 (4.0) | 2 (5.0) | 0 (0) | 2 (5.4) | 0 (0.0) |
| Laboratories | |||||
| Microbiology laboratory | 44 (100) | 92 (98.9) | 24 (92.3) | 65 (98.5) | 7 (100) |
| BSL2 | |||||
| Yes | 18 (40.9) | 51 (55.4) | 11 (45.8) | 46 (70.8) | 6 (85.7) |
| No | 4 (9.1) | 16 (17.4) | 2 (8.3) | 3 (4.6) | 1 (14.3) |
| Unknown | 22 (50.0) | 25 (27.2) | 11 (45.8) | 16 (24.6) | 0 (0) |
| BSL3 | |||||
| Yes | 10 (22.7) | 19 (20.7) | 2 (8.3) | 24 (36.9) | 1 (14.3) |
| No | 11 (25.0) | 44 (47.8) | 11 (45.8) | 21 (32.3) | 6 (85.7) |
| Unknown | 23 (52.3) | 29 (31.5) | 11 (45.8) | 20 (30.8) | 0 (0) |
BSL: biosafety level; EVD: Ebola virus disease; HCW: healthcare worker; HEPA: high-efficiency particulate air; OMT: outbreak management team.
European regions according to United Nations Geoscheme (United Nations Statistics Division, http://unstats.un.org/unsd/methods/m49/m49regin.htm) [7]. Included Asian countries are Israel and Turkey.
Inventory of needs and suggestions
Suggestions were received from 60 of 266 respondents, of whom 42 (70%) emphasised the need for education, information and harmonised guidelines for infection control, diagnostic procedures and clinical management. Most remaining suggestions pertained to the need for support and clinical research in affected West African countries.
Discussion
Our exploratory survey was initiated less than three weeks after WHO’s Public Health Emergency of International Concern (PHEIC) declaration on 8 August 2014 [4], to provide initial insights into the state of EVD preparedness in European hospitals at that time. It should be emphasised that this survey explored the preparedness to admit patients with suspected EVD at the level of hospitals and no inferences can be made from the results of this survey with regards to preparedness at national levels.
At the time of the survey (August–September 2014), the vast majority of admitting hospitals were engaged in various preparedness activities such as revision of protocols, training of HCWs and implementation of a local OMT. Recent healthcare-associated cases in the US and Spain have demonstrated the importance of training of HCWs in personal protective equipment regimens [7,8], and the finding that 27% of hospitals indicated they had not performed or planned training of HCWs shows room for improvement. At the time of the survey, 46% of admitting hospitals had planned or carried out exercises to test protocols. Given the complexity of issues surrounding admission of patients with suspected EVD, such exercises are essential. Preparedness activities were significantly less frequent in hospitals that would not admit patients or were not sure whether they would. Although unlikely, suspected EVD patients may present at any healthcare setting, and so awareness of initial management of suspected cases is important for all settings, including non-admitting centres. Almost all respondents indicated the availability of initial triage protocols, suggesting that undetected hospitalisations are unlikely. However, some training of HCWs for this scenario also in non-admitting hospitals seems prudent.
Technical characteristics of admission rooms varied across admitting hospitals, with differences observed between European regions. Admission rooms in a substantial proportion of hospitals lacked one or more characteristics considered to be important for control of highly infectious pathogens and 5% of hospitals appeared to have none of these characteristics. The required conditions for treatment of EVD patients is an issue of some debate: EBOV is not considered to be transmitted by aerosol, which is the underlying assumption in the design of high-containment patient rooms, but the intensive-care setting may include exceptional circumstances where infectious droplets or aerosols may be generated, e.g. during intubation and ventilation [10,11]. Therefore, while standard contact precautions would generally suffice for management of EVD patients, this may differ for such high-care settings. Our analysis did not provide this level of detail. Of note, the proportions of hospital admission rooms with characteristics such as the presence of an anteroom and availability of negative pressure were higher than observed in a previous survey of emergency departments in 14 European countries (87% and 69% vs 46% and 42%, respectively) but, not unexpectedly, lower than those observed in a survey of 48 isolation facilities for highly infectious diseases in 16 European countries (100% and 90% respectively) [12,13].
With regards to laboratory infrastructure, our survey data lacked the resolution to assess in detail whether and to what extent laboratories are compliant with recommendations from WHO, ECDC and/or CDC. However, it should be noted that 8% of admitting hospitals did not appear to have the absolute minimal level laboratory containment (BSL2) needed for handling specimens from EVD patients, which indicates less than optimal capacity for biocontainment during processing blood specimens for EBOV diagnostics and/or routine supportive diagnostics. During the course of illness, clinical specimens can contain very high viral loads for extended periods of time [14,15], and a careful assessment of the risks for processing such specimens in the local laboratories is crucial. Laboratories without BSL2 containment should therefore be encouraged to upgrade their facilities and refer samples to laboratories with BSL2 or preferably BSL3 facilities in the meantime.
Availability of national and local hospital guidelines for management of patients with (suspected) haemorrhagic fever was indicated by a majority of respondents with highest availabilities observed in admitting hospitals and in western European countries. Of note, discordant responses from the same country in relation to availability of national guidelines were observed on several occasions (data not shown), indicating that differences in awareness of guidelines exist within countries. This might illustrate the importance and challenges of dissemination of guidelines, also at national levels. At the same time, the need and desire for guidance was illustrated by responses to our open request for suggestions, the vast majority of which emphasised a need for education, information and harmonised guidelines, especially for diagnostic issues and clinical management of patients.
Our survey has several limitations. First of all, although the geographical distribution of participating hospitals across Europe was excellent, the survey results may not be fully representative of European medical professionals and hospitals for several reasons. The survey was circulated only to hospitals actively participating in established clinical networks and these may not be representative of European hospitals overall. Furthermore, the response rate was fairly low: responses were received from 27% of colleagues representing 32% of hospitals, which means that the survey results may also not be fully representative of hospitals to which the survey was circulated. The majority of responses (78%) were from tertiary care hospitals, which might suggest overrepresentation of tertiary care settings. However, the extent of this possible overrepresentation could not be determined since no information was available about the settings (i.e. primary, secondary or tertiary care) of hospitals that did not participate in the survey. Nevertheless, as tertiary care centres generally have a central and leading role in preparedness efforts for emerging health crises, our survey results do serve as important indicators of the state of preparedness in Europe. Secondly, several of the questions in our survey remained unanswered (‘do not know’) a substantial proportion of respondents, likely due in large part to differences in medical background of respondents (ranging from intensive care specialists to clinical microbiologists) and the variety of topics addressed. However, close collaboration between these specialists is clearly needed to provide optimal and safe care for EVD patients. Thirdly, as the number of participating hospitals differed substantially between regions, with relatively low numbers from eastern Europe and western Asia, geographical differences in the results of this survey should be interpreted with caution. Finally, this survey represents a snapshot of the state of affairs six months after the EVD outbreak in West Africa became apparent to the world and three weeks after it had been declared a PHEIC. Since then, preparedness activities of hospitals, including training and exercises, will undoubtedly have intensified globally given the continuing and expanding crisis in West Africa and emergence of travel-associated cases elsewhere. It will be interesting to assess whether this is indeed the case in a future follow-up survey.
In summary, this survey has provided important initial insights into the preparedness and capacity to admit patients suspected for EVD in European hospitals. These results, including identified gaps or concerns, help to provide direction towards further preparedness activities and prioritisation thereof.
Acknowledgements
We are greatly indebted to all colleagues who have participated in this survey, and to Julia Bielicki and Mike Sharland for helpful comments on the questionnaire.
This work received funding from the European Union Seventh Framework Programme (FP7) under the project PREPARE (grant agreement No 602525).
Footnotes
Platform for European Preparedness against (re-) emerging epidemics (PREPARE), and affiliated clinical networks Community-Acquired Competence Network (CAPNETZ www.capnetz.de), European Society of Intensive Care Medicine (ESICM www.esicm.org), COMbatting BACTerial resistance in Europe (COMBACTE www.combacte.com) and Pediatric European Network for the Treatment of AIDS (PENTA http://www.penta-id.org).
Conflict of interest
None declared.
Authors’ contribution
Designed the study: MDdJ, PH, MK, HG. Executed the survey: MB, J-DC, CQ, TW, FL, JS. Prepared and analysed data: MDdJ, CR, FL, JS. Interpreted the results: MDdJ, CR, PH, MK, HG. Wrote the first draft: MDdJ. All authors reviewed, provided comments and approved the final manuscript.
References
- 1.WHO. Situation report 21 November 2014. Geneva: World Health Organization; 2014. [accessed 26 November 2014]. http://www.who.int/csr/disease/ebola/en/ [Google Scholar]
- 2.Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, Magassouba N, et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med. 2014;371(15):1418–25. doi: 10.1056/NEJMoa1404505. [DOI] [PubMed] [Google Scholar]
- 3.WHO Ebola Response Team. Ebola virus disease in West Africa--the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371(16):1481–95. doi: 10.1056/NEJMoa1411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briand S, Bertherat E, Cox P, Formenty P, Kieny MP, Myhre JK, et al. The international Ebola emergency. N Engl J Med. 2014;371(13):1180–3. doi: 10.1056/NEJMp1409858. [DOI] [PubMed] [Google Scholar]
- 5.Sprenger M, Coulombier D. Preparedness is crucial for safe care of Ebola patients and to prevent onward transmission in Europe - outbreak control measures are needed at its roots in West Africa. Euro Surveill. 2014;19(40):20925. doi: 10.2807/1560-7917.ES2014.19.40.20925. [DOI] [PubMed] [Google Scholar]
- 6.ECDC. Outbreak of Ebola virus disease in West Africa. Stockholm: European Centre for Disease Prevention and Control; 2014. [accessed 26 November 2014]. Seventb update, 17 October 2014. http://www.ecdc.europa.eu/en/publications/Publications/ebola-Sierra-Leone-Liberia-Guinea-Spain-United-States-risk-assessment.pdf/ [Google Scholar]
- 7.WHO. Ebola virus disease – Spain. Geneva: World Health Organization; 2014. [accessed 4 November 2014]. http://www.who.int/csr/don/09-october-2014-ebola/en/ [Google Scholar]
- 8.McCarthy M. Texas healthcare worker is diagnosed with Ebola. BMJ. 2014 Oct 13;349(6):g6200. doi: 10.1136/bmj.g6200. [DOI] [PubMed] [Google Scholar]
- 9.UN. Composition of macro geographical (continental) regions. New York City: UN Statistics Division; 2013. [accessed 4 November 2014]. http://unstats.un.org/unsd/methods/m49/m49regin.htm. [Google Scholar]
- 10.Fowler RA, Fletcher T, Fischer WA, 2nd, Lamontagne F, Jacob S, Brett-Major D, et al. Caring for critically ill patients with ebola virus disease. Perspectives from West Africa. Am J Respir Crit Care Med. 2014;190(7):733–7. doi: 10.1164/rccm.201408-1514CP. [DOI] [PubMed] [Google Scholar]
- 11.Martin-Moreno JM, Llinás G, Hernández JM. Is respiratory protection appropriate in the Ebola response? Lancet. 2014;384(9946):856. doi: 10.1016/S0140-6736(14)61343-X. [DOI] [PubMed] [Google Scholar]
- 12.Fusco FM, Schilling S, De Iaco G, Brodt HR, Brouqui P, Maltezou HC, et al. EuroNHID Working Group Infection control management of patients with suspected highly infectious diseases in emergency departments: data from a survey in 41 facilities in 14 European countries. BMC Infect Dis. 2012;12(1):27. doi: 10.1186/1471-2334-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schilling S, Fusco FM, De Iaco G, Bannister B, Maltezou HC, Carson G, et al. European Network for Highly Infectious Diseases project members Isolation facilities for highly infectious diseases in europe - a cross-sectional analysis in 16 countries. PLoS ONE. 2014;9(10):e100401. doi: 10.1371/journal.pone.0100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreuels B, Wichmann D, Emmerich P, Schmidt-Chanasit J, de Heer G, Kluge S, et al. A Case of Severe Ebola Virus Infection Complicated by Gram-Negative Septicemia. N Engl J Med. 2014 doi: 10.1056/NEJMoa1411677. [DOI] [PubMed] [Google Scholar]
- 15.Schieffelin JS, Shaffer JG, Goba A, Gbakie M, Gire SK, Colubri A, et al. KGH Lassa Fever Program; Viral Hemorrhagic Fever Consortium; WHO Clinical Response Team Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014;371(22):2092–100. doi: 10.1056/NEJMoa1411680. [DOI] [PMC free article] [PubMed] [Google Scholar]