Abstract
In addition to tumouricidal activity, radiotherapy is now recognized to display potent immunostimulatory properties that can contribute to the generation of anti-cancer immune responses. Treatment with radiation can induce a variety of pro-immunogenic and phenotypic changes in malignant cells, and recalibrate the immune contexture of the tumour microenvironment, leading to enhanced activation of the innate immune system, and priming of tumour-specific T-cell immunity. The immune-dependent effects of radiotherapy provide a sound rationale for the development of combination strategies, whereby the immunomodulatory properties of radiation can be exploited to augment the activity of immunotherapeutic agents. Encouraged by the recent success of breakthrough therapies such as immune checkpoint blockade, and a wealth of experimental data demonstrating the efficacy of radiotherapy and immunotherapy combinations, the clinical potential of this approach is now being explored in numerous trials. Successful translation will require careful consideration of the most suitable dose and fractionation of radiation, choice of immunotherapy and optimal sequencing and scheduling regimen. Immunological control of cancer is now becoming a clinical reality. There is considerable optimism that the development of effective radiotherapy and immunotherapy combinations with the capacity to induce durable, systemic immunity will further enhance patient outcome and transform the future management of cancer.
Keywords: combination therapy, immune checkpoint, immunotherapy, radiotherapy
Introduction
Utilizing immunotherapy to exploit endogenous immune surveillance mechanisms and drive anti-cancer immune responses is an attractive proposition for the eradication of malignant disease. The most successful approach to emerge in recent years, targeting of immune checkpoints, has revitalized the field in light of unheralded clinical success in a range of malignancies, including melanoma and non-small cell lung cancer, which have proven effective even in previously treated, relapsed or refractory disease.1 However, while impressive, durable responses suggestive of long-term immune-mediated tumour control are observed in a subset of patients, current trial data indicates that over half of patients fail to demonstrate a significant improvement in response to checkpoint blockade.2 Combining immunotherapy with other treatments capable of modifying phenotypic, genomic and microenvironmental factors to potentiate immune responses is therefore an attractive strategy. In this respect, radiotherapy (RT) has emerged as a leading candidate. RT is a principle treatment option for many malignancies, with over 50% of cancer patients receiving RT as part of their clinical management programme, either as monotherapy or as part of a multimodal approach. Historically, RT has been delivered with the primary objective of debulking or eradicating disease via instigation of DNA damage and tumour cell death. While the radiobiological effects of RT are well characterized, there has been considerably less regard for any immune adjuvant properties, presumably, at least in part, due to the known immunosuppressive side effects of RT, many of which can contribute to immune escape.3 However, this oversight is now being challenged by the growing realization that RT can stimulate multiple aspects of both the priming and effector phase of the immune response involved in the induction of anti-tumour immunity, leading to the generation of local and systemic responses. Consequently, this has provided a firm rationale for integrating RT regimen with immunotherapy and the development of combination treatment strategies. A wealth of pre-clinical data demonstrates that RT can potentiate the activity of a diverse range of immunotherapeutics, including dendritic cell (DC) vaccination4; Toll-like receptor (TLR) agonists5–7; adoptive T-cell transfer8; cytokines9–11; co-stimulatory antibodies12–14; and checkpoint blockade.15–18 In view of this experimental evidence, a growing number of clinical trials are now rigorously testing RT and immunotherapy combinations in a range of malignancies.19 Here, we will describe the evidence suggesting that localized RT may instigate immunogenic, phenotypic and environmental changes to create ‘the perfect storm’, conducive for the synergistic induction of anti-tumour immunity in combination with immunotherapy, and define the current challenges faced by the field.
Radiotherapy as a driver of anti-cancer immune responses
In addition to potent tumouricidal effects, it is increasingly recognized that RT can directly contribute to immune-mediated rejection of tumour (summarized in Figure 1). This concept is supported by numerous observations demonstrating that RT has a profound influence on multiple aspects of the anti-cancer response, with the summative effect of enhancing the processing and cross-presentation of tumour antigen; sensitizing tumour cells to immune recognition and attack; and altering the immune contexture of the tumour microenvironment (TME) in favour of T-cell recruitment, activation and functionality. Many of these effects are orchestrated at the level of the innate immune system, with RT augmenting the cross-priming capability of antigen-presenting DC due to the concomitant release of tumour-associated antigen (TAA), and inflammatory mediators which can stimulate DC maturation and activation. Evidence from experimental models, predominantly using single, ablative doses (10–25 Gy) indicates that RT can induce the activation of intra-tumoural DC,20 and promote the migration and accumulation of tumour-antigen specific DC in the draining lymph nodes,21 which are endowed with enhanced antigen presentation capabilities22 leading to the effective cross-priming of TAA-specific lymphocytes. The molecular pathways which underlie these phenomena are gradually being elucidated.
Figure 1.
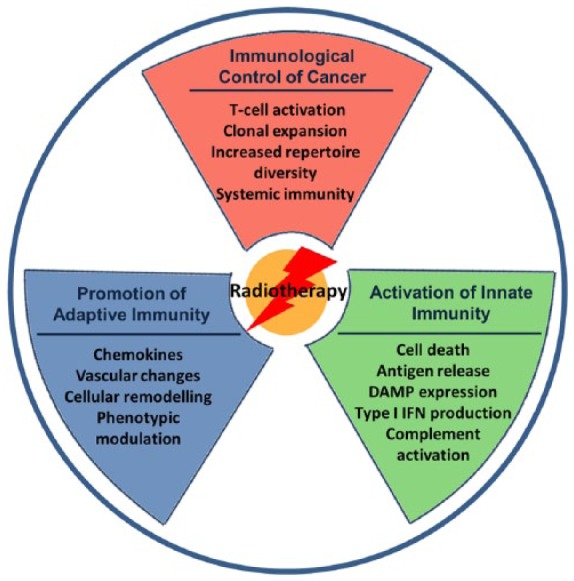
Immune modifying effects of radiotherapy. Treatment of cancer with radiotherapy results in multiple pro-immunogenic, immune-modulatory and microenvironmental changes which contribute to the generation of anti-tumour immunity. Exposure of tumour cells to radiation induces antigen release, hallmarks of immunogenic cell death including damage-associated molecular pattern and type I interferon release, and complement activation, which contribute to recruitment, maturation and enhanced antigen processing by dendritic cells. Tumour cells may undergo phenotypic modulation, increasing expression of surface MHC I, ICAM, death receptors and NKG2D ligands, making them more susceptible to immune attack. In addition, radiation can recalibrate the immune contexture of the tumour microenvironment, promoting T-cell recruitment through release of pro-inflammatory cytokines and cellular remodelling. These molecular changes bridge innate and adaptive immunity, reshaping the T-cell repertoire and inducing clonal expansion and infiltration of tumour-specific lymphocytes, which can mediate eradication of primary and distal disease, and generate durable immunity.
Activation of the innate immune system
Tumour cells dying after RT display hallmarks of immunogenic cell death (ICD), a form of cellular demise elicited through a combination of caspase-activation, oxidative and ER stress, and macroautophagy.23 Initially defined as a property of certain chemotherapeutic agents,24 ICD is characterized by the temporal expression of damage-associated molecular patterns (DAMP), including ectopic-surface calreticulin exposure,25,26 and extracellular release of ATP27 and high mobility group box 1 (HMGB1).28 DAMP function primarily as immune ‘danger signals’, working in concert to promote the recruitment, differentiation and effective acquisition, processing and presentation of tumour antigen by specialized CD11b+ CD11c+ DC within the TME.29 Immunogenic modulation by RT has subsequently been demonstrated to occur in human cell lines30 and can be augmented when delivered concurrently with pro-immunogenic chemotherapy.31
Induction of adaptive immunity by RT is further choreographed by the production of type I interferon (IFN) within the TME, an additional facet of ICD,32 which activates the innate immune system and facilitates effective antigen cross-presentation and priming of tumour-specific CD8+ T-cells.33,34 Induction of IFNβ by RT is dependent upon the sensing of cytosolic DNA via a signalling cascade involving cyclic GMP-AMP synthase (cGAS) and stimulator of interferon genes (STING).35 Moreover, this pathway has recently been shown to be attenuated by the DNA exonuclease, Trex1, in a radiation dose-dependent manner.36 While single doses above 12–18 Gy induce Trex1, fractionated doses below this threshold amplify IFNβ production, leading to optimal activation of Batf3 transcription factor positive DC and priming of tumour-specific immunity. Type I IFN mediate a wide range of effects which can contribute to priming of tumour-specific T-cells,37 and enhanced MHC I expression following induction of IFNβ signalling pathways by localized RT can restore therapeutic responses to anti-PD-1 resistant tumours.38
Maturation of DC following RT has also been shown to be dependent upon the transient, acute activation of components of the complement cascade. Localized production of the pro-inflammatory C3a and C5a anaphylatoxins within the TME occurs as a consequence of RT-induced tumour cell death and is essential for optimal therapeutic efficacy39 – although, in contrast to ICD which is a subtype of apoptosis, the primary cell death modality required for the generation of complement proteins appears to be necrosis.39
Phenotypic and environmental changes
In addition to environmental cues that function to recruit and activate DC within the TME, tumour cells exposed to sublethal doses of RT may undergo immuno-phenotypic changes rendering them more susceptible to immune attack. RT has been shown to induce a dose-dependent enhancement in the level of surface MHC class I expression,40,41 coupled with a biphasic increase in the intracellular peptide pool – initially due to rapid protein degradation as a consequence of oxidation, and subsequently due to activation of the mammalian target of rapamycin (mTOR) pathway which enhances protein synthesis.41 Enhanced expression of TAA or novel, RT-induced antigenic peptides results in increased killing by cognate HLA-restricted CD8+ T-cells. Effective cytolysis by effector T-cells post-RT is also potentiated by increased expression of the adhesion molecule ICAM140,42 and multiple death receptors including FAS40,42–44 and TRAILR1/TRAILR2 receptors (DR4 and DR5),44 which sensitizes tumour cells to apoptosis. Similarly, induction of the DNA damage response has been linked to the upregulation of stress ligands for activatory NKG2D receptors, which can enhance lysis by both natural killer (NK) cells and CD8+ T-cells.45
These immunogenic and phenotypic changes are coupled with microenvironmental remodelling, which contributes to the trafficking of T-cells into the TME. For example, RT can trigger the production of pro-inflammatory chemokines including CXCL9, CXCL1046 and CXCL16,47 resulting in the chemotactic recruitment of effector CD8+ T-cells into the TME. Release of chemokines such as Mig and IP10 post-RT contribute to restoration of the vascular network and T-cell extravasation.48 Likewise, low-dose RT (2 Gy) can reprogram tumour-resident macrophages from an immunosuppressive (‘M2’) phenotype to a TH1 polarizing iNOS+ (M1) phenotype.49 iNOS+ macrophages mediate vascular normalization, promoting T-cell recruitment and subsequent rejection of tumours. The direct contribution of infiltrating versus resident T-cells to overall tumour control remains to be clarified. The number and function of tumour-infiltrating lymphocytes has been shown to increase following single, ablative doses of RT.20–22 Likewise, both high single-dose and fractionated RT have been shown to impact on T-cell repertoire diversity and clonality, predominantly leading to the enrichment of T-cell clones already resident within the TME.18,50
The cumulative pro-immunogenic effects of RT mean that tumour cells dying after irradiation act as an in situ vaccine, which by its very nature is personalized to the individual patient and tumour, capable of eliciting a cancer-specific CD8+ T-cell response and generating immunological memory. Moreover, in murine cancer models, responses to RT have been observed to extend beyond the site of irradiation, leading to abscopal responses: systemic immune-mediated clearance of distal, non-irradiated tumours.51 Induction of abscopal responses is a major goal for clinical cancer therapy, enabling the control of metastatic and occult disease. While such responses are rarely observed in the clinic following RT alone, the immune response-modifying effects of RT favour the induction of systemic immunity when delivered in combination with immunotherapy, such as checkpoint blockade.50–52 Moreover, combination of RT with inhibition of CTLA-4 and PD-1/PD-L1 can enhance peripheral expansion of T-cell clones and T-cell receptor diversity in non-irradiated tumours, suggesting that dual treatment can induce systemic changes in the repertoire of responding CD8+ effector T-cells.18,50 An additional benefit of localized, focal delivery of RT is that it lacks the systemic toxicity of chemotherapy, potentially sparing responding lymphocytes, and making it a more attractive and effective partner for immunotherapy, despite the overlapping immunomodulatory effects of certain immunogenic chemotherapy regimen.
Optimizing RT and immunotherapy combinations for clinical application
While the potential for RT to modify anti-cancer immune responses and augment immunotherapy is clear, how best to integrate these two modalities to maximize clinical responsiveness remains uncertain. The optimal generation of an in situ vaccine will likely be dependent on the dose and fractionation of RT employed, and influenced by factors such as size of field, volume of tumour irradiated, involvement of regional lymph nodes and metastatic burden. Inherent properties of the tumour itself including anatomical location, stage, radiosensitivity, immunogenicity and immune status may also potentially impact on the ability of RT to incite a favourable immune response. Chemotherapy has been shown to influence intratumoural mutational heterogeneity, driving the evolution of tumours with enhanced subclonal neoantigen expression that correlates with poor response53; the influence of RT on clonal diversity and the potential impact this may have on combination therapy with checkpoint blockade remains to be determined. Careful selection of appropriate patient populations, RT parameters and rationalized integration with immunotherapy are therefore key to success.
RT dose fractionation
Conventional radiotherapy given with curative intent is delivered as small daily fractions of 2 Gy over several weeks. Whether this is conducive to optimal stimulation of the immune system is a matter of debate, accompanied by the theoretical concern that repeated exposure of the tumour to RT may eliminate resident and infiltrating lymphocytes, negating any beneficial therapeutic effects.6,17 Modern advancements in image-guided delivery techniques, enabling highly accurate precision targeting, have permitted the delivery of much higher doses per fraction over a shorter time period. For example, stereotactic body radiotherapy (SBRT) approaches are becoming more commonly used clinically in several disease sites.54 Moreover, high-dose RT appears to be particularly good at eliciting favourable immunogenic modulation of tumours55 and due to the shorter period of delivery, may avoid continued eradication of responding lymphocytes.
Experimental data indicate that RT regimens using high dose per fraction are highly effective at augmenting immunotherapy. A hypofractionated RT protocol giving 8 Gy in three daily doses was able to induce anti-tumour immunity and abscopal responses when used to treat murine breast cancer in combination with CTLA-4 blockade.56 Comparable responses were observed when using this regime to treat a range of solid malignancies in combination with anti-PD-1 or CD137 antibody.57 Treatment of mammary or colorectal carcinoma with a single 12 Gy dose of RT led to a reduction in tumour-infiltrating myeloid-derived suppressor cells (MDSC), an effect that was potentiated by the addition of anti-PD-1 therapy.16 Environmental depletion of MDSC was dependent on production of TNF by CD8 T-cells, activated by the combination therapy. Localized 12 Gy RT delivered as one or two fractions together with anti-CTLA-4 antibody has also been shown to induce T-cell-dependent clearance of metastatic breast cancer.15 Similar immunomodulatory effects have been observed following treatment of lymphoma with 10 Gy single-dose RT in combination with a TLR-7 agonist6 and with 20 Gy single-dose RT in combination with anti-CTLA-4 and PD-L1/PD-1 blockade.18 Clinical trials to assess the efficacy of high-dose RT delivered by SBRT in combination with immunotherapy are ongoing and it will be of interest to see whether this approach translates to more effective clinical outcomes. Likewise, emerging RT treatments such as proton therapy are also able to induce immunomodulatory changes in pre-clinical tumour models,58 although the extent to which proton beam therapy can effectively enhance tumour-specific immune responses in collaboration with immunotherapy remains to be determined.
Sequencing and scheduling
The optimal sequencing and scheduling of RT with respect to immunotherapy also needs defining. Despite remaining a major question for trial design, relatively few studies have sought to address this issue. What evidence exists substantiates the notion that sequencing will need to be tailored according to the specific mechanism of action of the immunotherapeutic agent employed. Tumour cells are known to upregulate the PD-L1 immune checkpoint in response to T-cell derived IFNγ as a mechanism of adaptive resistance.59 This phenomenon can be driven by RT, providing a rationale for combination with antibodies targeting the PD-L1/PD-1 axis.16,17 Dual radiation and checkpoint blockade can overcome resistance and elicit potent anti-tumour immunity and immunological memory, but only when given concurrently or immediately following RT; delayed administration of anti-PD-L1 therapy by 7 days post-completion of RT abrogated any therapeutic benefit.17 This response likely relates to the temporal kinetics of PD-1 expression post-RT, with checkpoint blockade unable to overcome T-cell exhaustion and re-invigorate the response if delayed. In contrast, when targeting the CTLA-4 checkpoint in combination with RT, optimal responses were observed when immunotherapy was administered 7 days prior to single-dose RT, in part due to depletion of regulatory T-cells.60 For therapeutic interventions targeting the OX40 co-stimulatory pathway together with RT, synergistic responses were only observed when OX40 agonism was achieved during a narrow therapeutic window immediately post-RT to coincide with radiation-induced antigen release.60 The situation is further complicated by the observation that optimal therapeutic responses may require multiple checkpoint blockade.61 Accordingly, high-dose RT given with dual checkpoint blockade is incrementally more effective than targeting of a single pathway,18,62 but adds an additional level of complexity with regards to sequencing and scheduling.
Consideration of adverse immune reactions
The safety of combination strategies is a possible concern, given the potential for toxicity associated with the creation of a pro-inflammatory milieu and perturbation of effector T-cell responses, which may break tolerance to self-antigen and give rise to autoimmune-type reactions. Addition of RT to immune checkpoint blockade is the focus for current trials, given the success of these immunotherapeutics as single-agent monotherapy. Evocation of toxicity by immune checkpoint inhibitors is well characterized, with the majority of patients displaying signs of low-grade immune-related adverse effects (irAEs) that can be readily managed; however, occasionally more severe toxicity (grade 3 and above) is observed.1 While the severity of these adverse events is likely dependent upon the nature of the specific immunotherapeutic treatment employed,63 it is conceivable that toxicity may be further enhanced by the addition of radiation. The propensity towards the development of irAEs following combination therapy may well be influenced by the site and nature of the tumour being treated, and more evident where there are overlapping tissue-related toxicities. For example, anecdotal evidence from lung cancer patients treated with the anti-PD-1 antibody, nivolumab, indicate that the induction of durable immunity may be associated with the risk of pneumonitis in previously irradiated tissue.64 However, the extent to which irAEs are an issue for combination therapy remains to be determined.
Conclusions
The confluence of RT with immunotherapy offers tremendous potential for the generation of effective immune-mediated control of cancer. The development of next-generation immunotherapeutics and advancements in radiation delivery enable greater precision and provide grounds for considerable optimism that pre-clinical observations will translate to clinical success. However, currently, we remain some way from understanding how best to utilize these approaches for maximal patient benefit. The relationship between RT dose, fractionation and scheduling warrants further investigation in innovative clinical trials. The extent to which the immune-modulatory effects of RT are recapitulated in patient tumours needs clarification, and may guide selection of the most appropriate immunotherapy and help define how best to integrate the two therapeutic components. The ability of emerging RT modalities such as proton therapy to trigger phenotypic and immunogenic changes in cancer cells also requires exploration in the clinical setting. Development of appropriate predictive and prognostic biomarkers together with immune profiling techniques will be essential to ensure favourable patient selection and stratification, identify immunological correlates of therapeutic outcome and aid the development of personalized combination approaches to enhance efficacy. The ability of RT to augment durable, systemic, tumour-specific immunity in combination with immunotherapy is an exciting proposition, currently being investigated in a multitude of clinical trials, the results of which will inform the future development of this potentially highly effective treatment for cancer.
Footnotes
Funding: Funding was provided by a Cancer Research-UK programme grant to TI/JH (C431/A17737) and the National Institute of Health Research Biomedical Research Centre to TI.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Jamie Honeychurch  https://orcid.org/0000-0001-6938-0839
https://orcid.org/0000-0001-6938-0839
Contributor Information
Jamie Honeychurch, Targeted Therapy Group, Division of Cancer Sciences, Manchester Cancer Research Centre, Christie Hospital, Manchester Academic Health Sciences Centre, National Institute of Health Research Biomedical Research Centre, Manchester, M20 4BX, UK.
Timothy M. Illidge, Targeted Therapy Group, Division of Cancer Sciences, Manchester Cancer Research Centre, Christie Hospital, Manchester Academic Health Sciences Centre, National Institute of Health Research Biomedical Research Centre, Manchester, UK
References
- 1. Honeychurch J, Cheadle EJ, Dovedi SJ, et al. Immuno-regulatory antibodies for the treatment of cancer. Expert Opin Biol Ther 2015; 15: 787–801. [DOI] [PubMed] [Google Scholar]
- 2. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015; 33: 1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wennerberg E, Lhuillier C, Vanpouille-Box C, et al. Barriers to radiation-induced in situ tumor vaccination. Front Immunol 2017; 8: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Teitz-Tennenbaum S, Li Q, Rynkiewicz S, et al. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res 2003; 63: 8466–8475. [PubMed] [Google Scholar]
- 5. Dewan MZ, Vanpouille-Box C, Kawashima N, et al. Synergy of topical toll-like receptor 7 agonist with radiation and low-dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clin Cancer Res 2012; 18: 6668–6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dovedi SJ, Melis MH, Wilkinson RW, et al. Systemic delivery of a TLR7 agonist in combination with radiation primes durable antitumor immune responses in mouse models of lymphoma. Blood 2013; 121: 251–259. [DOI] [PubMed] [Google Scholar]
- 7. Milas L, Mason KA, Ariga H, et al. CpG oligodeoxynucleotide enhances tumor response to radiation. Cancer Res 2004; 64: 5074–5077. [DOI] [PubMed] [Google Scholar]
- 8. Ward-Kavanagh LK, Zhu J, Cooper TK, et al. Whole-body irradiation increases the magnitude and persistence of adoptively transferred T cells associated with tumor regression in a mouse model of prostate cancer. Cancer Immunol Res 2014; 2: 777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang AY, Keng PC. Potentiation of radiation cytotoxicity by recombinant interferons, a phenomenon associated with increased blockage at the G2-M phase of the cell cycle. Cancer Res 1987; 47: 4338–4341. [PubMed] [Google Scholar]
- 10. van den Heuvel MM, Verheij M, Boshuizen R, et al. NHS-IL2 combined with radiotherapy: preclinical rationale and phase Ib trial results in metastatic non-small cell lung cancer following first-line chemotherapy. J Transl Med 2015; 13: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zegers CM, Rekers NH, Quaden DH, et al. Radiotherapy combined with the immunocytokine L19-IL2 provides long-lasting antitumor effects. Clin Cancer Res 2015; 21: 1151–1160. [DOI] [PubMed] [Google Scholar]
- 12. Honeychurch J, Glennie MJ, Johnson PW, et al. Anti-CD40 monoclonal antibody therapy in combination with irradiation results in a CD8 T-cell-dependent immunity to B-cell lymphoma. Blood 2003; 102: 1449–1457. [DOI] [PubMed] [Google Scholar]
- 13. Shi W, Siemann DW. Augmented antitumor effects of radiation therapy by 4-1BB antibody (BMS-469492) treatment. Anticancer Res 2006; 26: 3445–3453. [PubMed] [Google Scholar]
- 14. Yokouchi H, Yamazaki K, Chamoto K, et al. Anti-OX40 monoclonal antibody therapy in combination with radiotherapy results in therapeutic antitumor immunity to murine lung cancer. Cancer Sci 2008; 99: 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res 2005; 11: 728–734. [PubMed] [Google Scholar]
- 16. Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014; 124: 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014; 74: 5458–5468. [DOI] [PubMed] [Google Scholar]
- 18. Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015; 520: 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer 2016; 4: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gupta A, Probst HC, Vuong V, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol 2012; 189: 558–566. [DOI] [PubMed] [Google Scholar]
- 21. Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009; 114: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lugade AA, Moran JP, Gerber SA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 2005; 174: 7516–7523. [DOI] [PubMed] [Google Scholar]
- 23. Kepp O, Senovilla L, Vitale I, et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 2014; 3: e955691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 2005; 202: 1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13: 54–61. [DOI] [PubMed] [Google Scholar]
- 26. Obeid M, Panaretakis T, Joza N, et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ 2007; 14: 1848–1850. [DOI] [PubMed] [Google Scholar]
- 27. Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 2009; 15: 1170–1178. [DOI] [PubMed] [Google Scholar]
- 28. Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007; 13: 1050–1059. [DOI] [PubMed] [Google Scholar]
- 29. Ma Y, Adjemian S, Mattarollo SR, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 2013; 38: 729–741. [DOI] [PubMed] [Google Scholar]
- 30. Gameiro SR, Jammeh ML, Wattenberg MM, et al. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 2014; 5: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Golden EB, Frances D, Pellicciotta I, et al. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 2014; 3: e28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sistigu A, Yamazaki T, Vacchelli E, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med 2014; 20: 1301–1309. [DOI] [PubMed] [Google Scholar]
- 33. Burnette BC, Liang H, Lee Y, et al. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res 2011; 71: 2488–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gerber SA, Sedlacek AL, Cron KR, et al. IFN-gamma mediates the antitumor effects of radiation therapy in a murine colon tumor. Am J Pathol 2013; 182: 2345–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deng L, Liang H, Xu M, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 2014; 41: 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 2017; 8: 15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fuertes MB, Woo SR, Burnett B, et al. Type I interferon response and innate immune sensing of cancer. Trends Immunol 2013; 34: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang X, Schoenhals JE, Li A, et al. Suppression of type I IFN signaling in tumors mediates resistance to anti-PD-1 treatment that can be overcome by radiotherapy. Cancer Res 2017; 77: 839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Surace L, Lysenko V, Fontana AO, et al. Complement is a central mediator of radiotherapy-induced tumor-specific immunity and clinical response. Immunity 2015; 42: 767–777. [DOI] [PubMed] [Google Scholar]
- 40. Garnett CT, Palena C, Chakraborty M, et al. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res 2004; 64: 7985–7994. [DOI] [PubMed] [Google Scholar]
- 41. Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006; 203: 1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol 2003; 170: 6338–6347. [DOI] [PubMed] [Google Scholar]
- 43. Chakraborty M, Abrams SI, Coleman CN, et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res 2004; 64: 4328–4337. [DOI] [PubMed] [Google Scholar]
- 44. Ifeadi V, Garnett-Benson C. Sub-lethal irradiation of human colorectal tumor cells imparts enhanced and sustained susceptibility to multiple death receptor signaling pathways. PLoS One 2012; 7: e31762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gasser S, Orsulic S, Brown EJ, et al. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005; 436: 1186–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Meng Y, Mauceri HJ, Khodarev NN, et al. Ad.Egr-TNF and local ionizing radiation suppress metastases by interferon-beta-dependent activation of antigen-specific CD8+ T cells. Mol Ther 2010; 18: 912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Matsumura S, Wang B, Kawashima N, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 2008; 181: 3099–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ganss R, Ryschich E, Klar E, et al. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res 2002; 62: 1462–1470. [PubMed] [Google Scholar]
- 49. Klug F, Prakash H, Huber PE, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013; 24: 589–602. [DOI] [PubMed] [Google Scholar]
- 50. Dovedi SJ, Cheadle EJ, Popple AL, et al. Fractionated radiation therapy stimulates antitumor immunity mediated by both resident and infiltrating polyclonal T-cell populations when combined with PD-1 blockade. Clin Cancer Res 2017; 23: 5514–5526. [DOI] [PubMed] [Google Scholar]
- 51. Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004; 58: 862–870. [DOI] [PubMed] [Google Scholar]
- 52. Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366: 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016; 351: 1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Walshaw RC, Honeychurch J, Illidge TM. Stereotactic ablative radiotherapy and immunotherapy combinations: turning the future into systemic therapy? Br J Radiol 2016; 89: 20160472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Filatenkov A, Baker J, Mueller AM, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res 2015; 21: 3727–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009; 15: 5379–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rodriguez-Ruiz ME, Rodriguez I, Garasa S, et al. Abscopal effects of radiotherapy are enhanced by combined immunostimulatory mAbs and are dependent on CD8 T cells and crosspriming. Cancer Res 2016; 76: 5994–6005. [DOI] [PubMed] [Google Scholar]
- 58. Gameiro SR, Malamas AS, Bernstein MB, et al. Tumor cells surviving exposure to proton or photon radiation share a common immunogenic modulation signature, rendering them more sensitive to T cell-mediated killing. Int J Radiat Oncol Biol Phys 2016; 95: 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4: 127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Young KH, Baird JR, Savage T, et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One 2016; 11: e0157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016; 7: 10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim JE, Patel MA, Mangraviti A, et al. Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin Cancer Res 2017; 23: 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kong YC, Wei WZ, Tomer Y. Opportunistic autoimmune disorders: from immunotherapy to immune dysregulation. Ann N Y Acad Sci 2010; 1183: 222–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shibaki R, Akamatsu H, Fujimoto M, et al. Nivolumab induced radiation recall pneumonitis after two years of radiotherapy. Ann Oncol 2017; 28: 1404–1405. [DOI] [PubMed] [Google Scholar]


