Abstract
Cardiovascular diseases are a major cause of disability and they are currently responsible for a significant number of deaths in a large percentage of the world population. A large number of therapeutic options have been developed for the management of cardiovascular diseases. However, they are insufficient to stop or significantly reduce the progression of these diseases, and may produce unpleasant side effects. In this situation, the need arises to continue exploring new technologies and strategies in order to overcome the disadvantages and limitations of conventional therapeutic options. Thus, treatment of cardiovascular diseases has become one of the major focuses of scientific and technological development in recent times. More specifically, there have been important advances in the area of nanotechnology and the controlled release of drugs, destined to circumvent many limitations of conventional therapies for the treatment of diseases such as hyperlipidemia, hypertension, myocardial infarction, stroke and thrombosis.
Keywords: cardiovascular disease, controlled release of drugs, nanomedicine, nanotechnology
Introduction
Hypertension and hypercholesterolemia are the main factors of morbidity and mortality in today’s Western society, since they are two key risk factors for the emergence and development of cardiovascular diseases such as acute myocardial infarction, stroke and thrombosis, among others. Multiple drug therapies for the treatment of these conditions, in most cases based on the use of synthetic active ingredients, are known. However, the use of these drugs frequently results in the occurrence of adverse effects. This fact has led researchers to conduct numerous studies in order to find safer therapeutic alternatives.1
In this context, it is appealing to implement nanotechnology as an attractive and innovative tool for the treatment of cardiovascular diseases. The knowledge and application of nanoscience in medicine certainly aims to improve the treatment of these diseases.2 Thus, nanotechnology may provide a safe and effective platform for controlled drug delivery for a variety of active ingredients, directed to management of lipid disorders, inflammation and angiogenesis within atherosclerotic plaques, and prevention of thrombosis, among other diseases.3
An example is the development of iron oxide super-paramagnetic nanoparticles for use in remote magnetic drug targeting (MDT).4 The MDT approach is based on producing a bioactive molecule-magnetic nanoparticle injectable complex. This complex can be attracted to, and retained in, a desired target inside the body with the help of applied magnetic fields. These systems must provide appropriate magnetic gradients to increase the concentration of nanocomplex at the site of interest.5
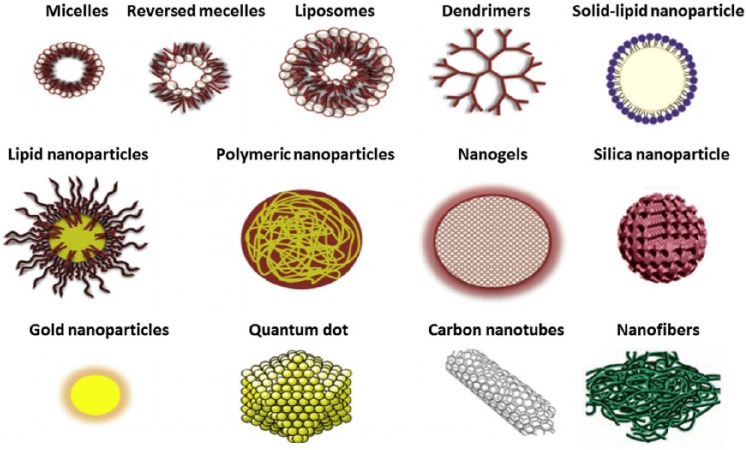
Moreover, gold and silica nanoparticles (Figure 1) have also been developed for improving the supply of nitric oxide (NO), for possible application in cardiovascular diseases such as hypertension, where low NO bioavailability is decisive for the occurrence and progression of this disease.6
Figure 1.
Size comparison and 3-D structures of different types of nanoparticles for drug delivery named in this review.
Additionally, antioxidant molecules are being investigated as tools to decrease the high levels of reactive oxygen species (ROS) often responsible for the progress of such diseases.7 This might improve the microvascular dysfunction associated with several cardiovascular diseases. From a therapeutic perspective, cerium dioxide nanoparticles (CeO2 NP) have a high antioxidant potential. After intravenous injection of CeO2 NP in animals, a significant decrease in the microvascular dysfunction and oxidative stress associated with hypertension was found. It should be noted that this antioxidant effect can only be observed when there are high levels of ROS before the exposure to these nanoparticles, while the results were contradictory in animals whose initial ROS concentration was low. Another factor to consider in the efficiency of the antioxidant activity of CeO2 NP is the nanoparticle primary size, which determines its permeability properties and toxicity. Hence, determining the size of the used nanoparticles is of great importance, since a small change in dimensions may alter both their physicochemical characteristics and their cellular and cardiovascular effects.8
Remarkably, new evidence suggests that both the innate and acquired immune system play an important role in hypertension, atherosclerosis and heart failure, among other diseases.9–11 Activation of dendritic cells has been shown to promote hypertension by stimulating of proliferation of T cells infiltrating the kidneys and the arterial wall. Similarly, macrophage infiltration in kidneys and arteries has been documented in models of experimental hypertension, and decreased macrophage infiltration is associated with an improvement of hypertensive disease in these models. Many studies have suggested that central nervous system (CNS) inflammation, at specific locations related to regulation of blood pressure, is involved in the pathogenesis of hypertension.7 Furthermore, the participation of numerous chemokines in the onset and progression of many cardiovascular diseases has been demonstrated.10
Taking into account the known influence of inflammatory mechanisms in the pathophysiology of cardiovascular diseases, this wealth of information can be exploited for the development of platforms nanostructured as carriers of anti-inflammatory and immunomodulatory drugs, giving rise to the design of highly effective therapeutic formulations.9–11
Applications of nanotechnology in hypertensive disease
Antihypertensive drugs currently used are classified in various categories such as angiotensin-converting enzyme (ACE) inhibitors, calcium antagonists, angiotensin antagonists, central sympathomimetic drugs, diuretics, alpha-adrenergic blockers, beta-adrenergic blockers and vasodilators.12 Within this broad classification, some of the most popular are ACE inhibitors such as enalapril, benazepril, ramipril and captopril. These drugs work by preventing the conversion of angiotensin I to angiotensin II and bradykinin to inactive metabolites.1
Most antihypertensive drugs have significant disadvantages, such as low bioavailability, relatively short half-life, low permeability and adverse side effects. For effective and safe administration of these drugs, delivery systems that can provide a low frequency of dosing, increased bioavailability, increased selectivity and reduced undesirable effects, are needed. Some oral drug administration systems based on nanotechnology provide alternative strategies to achieve those objectives.12
One example is the design of a formulation of curcumin in a nanoemulsion system with the aim of reducing its unfavorable solubility and bioavailability properties. Curcumin, a naturally occurring compound derived from turmeric, has become a promising therapeutic alternative, because its antioxidant and anti-inflammatory effects have been reported for various conditions, including inflammation, obesity and cardiovascular diseases such as hypertension. The activity of this new system was compared to captopril, a standard drug, as well as to pure curcumin in aqueous solution. Although results showed that curcumin in aqueous solution had no significant effect as ACE inhibitor compared to captopril (suggesting that curcumin produces its hypotensive effect by a different mechanism of action), the curcumin nanoemulsion showed increased ACE inhibition compared with pure curcumin. This finding demonstrates that the curcumin nanoemulsion system can improve its activity by enhancing its solubility, which results in increased bioavailability.1
Besides the development of nanoemulsions to improve therapeutic efficacy and safety of antihypertensive drugs, many additional formulations can be designed using nanotechnology. The use of solid lipidic nanoparticles (SLN) (Figure 1) containing carvedilol has also been proposed as a promising strategy for improving the bioavailability of the poorly soluble drug.13 In another study, Kumar and colleagues showed that intraduodenal administration of SLN loaded with nitrendipine (an antihypertensive drug with approximately 13% oral bioavailability) increased bioavailability three to four times compared with conventional formulations.14 Moreover, Gautam and Verma manufactured dendrimers (Figure 1) containing candesartan cilexetil, demonstrating a significant increase in water solubility of this formulation.15 Other studies showed similar results with the calcium antagonist nifedipine as the active drug. Additionally, nanosuspensions are systems with a short delay in the onset of action, improved surface area and higher adhesiveness to gastrointestinal epithelium, leading to improved oral absorption and bioavailability of lipophilic drugs. Thadkala and colleagues showed a higher dissolution rate and thus greater absorption of nebivolol hydrochloride by developing oral tablets from a nanosuspension.16 Other examples of nanosystems for controlled drug delivery consist of polymeric nanoparticles, carbon nanotubes, polymeric micelles, nanocrystals and liposomes (Figure 1).12 The design of nanoparticles charged with telmisartan has proven be effective in significantly increasing the oral bioavailability of this antihypertensive (up to ten times) as a result of enhanced solubility and dissolution speed.17
Furthermore, current limitations of the NO delivery systems stimulated a keen interest in the development of compounds that generate this vasoactive substance in a controlled and sustained manner for the treatment of cardiovascular diseases such as hypertension. A platform was designed using a sol-gel precursor in conjunction with a combination of chitosan and polyethylene glycol to form a fine powder of nanoparticles after drying by lyophilization. This nanomaterial retains NO or NO precursors (nitrites) in a stable form when dry. When exposed to moisture, these nanoparticles are slowly released, in a controlled and sustained manner (for several hours), furnishing therapeutic NO concentrations. After administration of this formulation the eluting NO nanoparticles decreased mean arterial pressure in a dose-dependent manner. Control nanoparticles (NO, NO-loaded) did not modify blood pressure. These data suggest that NO release from nanoparticles loaded with this substance was advantageous in relation to other compounds since their release is not dependent on chemical decomposition or enzymatic catalysis and it is only determined by the rate of hydration.18
Nanotechnological advances in atherosclerosis and hyperlipidemia
The experimental demonstration that some nanocarriers can be used as delivery drugs devices for reduction of major landmarks in the development of atherogenesis, including intimal hyperplasia, has led to advancing the use of nanotechnology as an appropriate tool for delivering bioactive agents to the vessel wall, whose pharmacologic profile predicts a potential benefit for the treatment of atherosclerosis.19
Besides its previously mentioned antihypertensive properties, it is known that curcumin encapsulated in a nanoemulsion also shows significant activity in reducing cholesterol, compared with pravastatin. Therefore, it has been suggested that curcumin could also have a potential use as alternative therapeutic compound for the treatment of hyperlipidemia.1
Some estrogens, like 17β-estradiol (17-βE), also interfere with the progression of coronary atherosclerosis. Major vasoprotective properties include reducing oxidative stress, improving vasodilatation and vascular tone, inhibiting inflammation, and changing the profile of circulating lipoproteins to increase high density lipoproteins (HDL) and to reduce low density lipoproteins (LDL), as well as an increased expression of endothelial nitric oxide synthase (eNOS) and NO production (a potent vasodilator and anti-thrombotic agent). These considerations allowed the design of a system based on a nanoemulsion loaded with 17-βE, which is rapidly internalized by vascular cells. Subsequently, the original system was modified to include in the nanoemulsion binding peptide cysteine-arginine-glutamic acid-lysine-alanine (CREKA) as guidance with binding selectivity for atherosclerotic plaques. The results of this study showed that treatment with the CREKA nanoemulsion, modified and charged with 17-βE, reduces luminal occlusion within the aortic valves and reduces the lipid content of the lesion, decreases the levels of circulating lipids (mainly cholesterol), regulates gene expression of various proinflammatory markers and lacks renal toxicity or hepatotoxicity at therapeutically effective doses.19
Faced with the need to reduce the size of the aortic atherosclerotic plaque, the use of potent anti-proliferative drugs, such as chemotherapeutic agents, has been suggested.3 Currently, the use of these drugs in high doses for the treatment of atherosclerotic cardiovascular diseases is unsafe due to their significant, even life-threatening, toxicity. However, this toxicity can be reduced by using optimized release systems. An example is paclitaxel. This drug normally causes hematologic toxicity characterized by a marked neutropenia, as well as neurotoxicity, arrhythmias, alopecia and hypersensitivity. This led to the development of a cholesterol-rich nanoemulsion (LDE) that mimics the lipid composition of the low density lipoproteins (LDL). When injected into the circulation, the nanoemulsion allows paclitaxel to concentrate in atherosclerotic lesions. In rabbits, after treatment with paclitaxel-LDE, the size of these lesions is reduced by 65%, as was their toxicity, which was low enough to allow its use in patients with cardiovascular disease.20
The enzyme 3-hydroxy-3-methylglutaryl-coenzyme A reductase is involved in cholesterol biosynthesis in the liver. Statins, which inhibit this enzyme, have become the main therapeutic tool to reduce cholesterol levels by decreasing the production of low density lipoproteins (LDL) in the liver.13 Lovastatin (LVT), a very lipophilic drug, belongs to this class of drugs and was the first clinically used statin. Pharmacokinetics and pharmacodynamics studies show that LVT in combination with nanostructured lipid carriers (LVT-CEN) resulted in a significant increase in drug bioavailability compared with free LVT. It was also shown that LVT-CEN maintains excellent stability without exhibiting any aggregation, precipitation or phase separation after 6 months of storage at 4°C.21 Additionally, Broz and colleagues have developed a polymeric nanovesicle system for targeted delivery of statins in high doses, thus reducing the toxicity caused by these drugs in other tissues. In this study, the nanovesicles were loaded with pravastatin and their surface was functionalized with an oligonucleotide having high affinity for inflammatory macrophages. The study demonstrated effective targeting of nanovesicles to macrophages, resulting in significant reduction of cytotoxicity (up to 15 times) in muscle cells.22
On the other hand, nanoparticles can also enhance the delivery of glucocorticoids, potent anti-inflammatory agents which reduce macrophage accumulation in atherosclerotic lesions. Glucocorticoids have unfavorable pharmacokinetic profiles, including short half-life times and a large volume of distribution, resulting in the need for frequent administration of high doses that produce significant adverse effects such as diabetes, hypertension and osteoporosis. The development of a liposomal formulation (Figure 1) loaded with glucocorticoids may overcome these limitations by prolonging the circulation time, thereby improving drug accumulation in the endothelium.23
Moreover, monocyte inflammatory activity plays a crucial role in the destabilization and rupture of atherosclerotic plaques. The administration of nanoparticles loaded with pitavastatin exerts its effect on circulating monocytes which are recruited to the site of inflammation, thereby inhibiting the destabilization and avoiding or delaying plaque rupture. Furthermore, the nanoparticles of a new nanoparticulate delivery drug system formulated from poly-(lactic-co-glycolic) acid (PLGA) were incorporated to a variety of relevant cells, such as monocytes, vascular smooth muscle cells, and endothelial cells. This new system enhances the therapeutic efficacy of pitavastatin at least 20 times compared with conventional daily oral administration of this drug.24
Of particular interest are studies on the administration of liposomes loaded with phosphatidylcholine (PC) for the treatment of atheromatous lesions. PC is a compound that increases the concentration of high density lipoproteins and decreases LDL oxidation in atherosclerotic plaque, thus limiting the inflammatory process occurring at this site. After 5 weeks of treatment with this liposomal formulation, there was a significant reduction in plaque volume and cholesterol levels in the aortic wall, suggesting that this formulation could become an attractive therapy to achieve regression of atherosclerotic plaques.3,25
Nanotechnology and pulmonary hypertension
Pulmonary arterial hypertension (PAH) is a devastating and progressive disease, usually characterized by remodeling of the pulmonary vasculature. This progressive vascular disease generally ends in right ventricular failure and the death of the patient. Endothelin is a potent vasoconstrictor that plays an important role in the pathogenesis of PAH. Also, endothelin is a mitogen for smooth muscle cells, and may both increase vascular tone and lead to hyperplasia.26 Bosentan is a selective and competitive antagonist of endothelin receptor used in the treatment of PAH.27 However, the water solubility of bosentan is very low, resulting in low bioavailability of this drug when it is orally administered. Due to an increase of its contact surface, nanoparticles can provide enhanced solubility and absorption, leading to improved therapeutic results. It should be remembered that nanosuspensions are pharmaceutical formulations that may be designed for oral, topical, inhalatory or parenteral administration. This characteristic may allow the development of various nanoparticulate dosage forms loaded with bosentan for administration by different routes.26
Also, some studies have shown that the concentration of the transcription factor NF-kappa B is increased in the presence of pulmonary arterial lesions in patients with PAH. Blocking receptors for NF-kappa B, by delivery of an antagonist of this compound through nanoparticles, not only prevents the development of pulmonary hypertension; it also improves the survival rate in patients with the disease. Therefore, this nanostructured platform can serve as a more effective and less invasive tool for the treatment of PAH.28
Nanotechnology applied to the treatment of acute myocardial infarction
Heart injuries are often permanent because of the low proliferation and limited capacity for self-repair of cardiomyocytes. Therefore, stem cell therapy has become an important alternative in the treatment of cardiovascular diseases.29 However, despite efforts to optimize this therapy, problems related to delivery and tracking or monitoring of cells injected into the myocardium still persist. Additionally, there are limitations for improving the survival of these transplanted cells, such as the lack of adequate techniques for the assessment of their viability. Moreover, less than 10% of the transplanted stem cells remain at the target site a few hours after injection. To overcome these drawbacks, new approaches are required for achieving a high concentration of cells in the injured tissue and monitoring their proliferation and viability. Magnetic resonance has become a reliable and safe technique for tracking these cells. Importantly, however, the sensitivity and the success of this technique largely depend on the contrast agent used. In this regard, due to their unique magnetic properties and excellent biocompatibility, iron oxide super-paramagnetic nanoparticles may be used for the orientation and monitoring of stem cells directed at the treatment of acute myocardial infarction (AMI). Iron oxide super-paramagnetic nanoparticles are recognized as one of the most promising contrast agents commercially available for stem cells labeling.30,31
The same limitations of stem cell therapy apply to the administration of placental growth factor (PlGF), a substance used to stimulate angiogenesis and improve heart function at the site of the injury caused by AMI. A good option to overcome these limitations is the use of chitosan-alginate nanoparticles, which are widely used for its unique biodegradability and biocompatibility properties, as well as its low toxicity potential. Additionally, these particles provide protection against local enzymatic degradation of PlGF, and are able to induce a slow and prolonged release of the containing material. Chitosan-alginate nanoparticles showed a high efficiency as a vehicle for PlGF delivery (by intramyocardial injection) when compared to the direct application of growth factor after AMI, resulting in an improvement of the beneficial effects of PIGF in the context of acute myocardial ischemia.32
Myocardial ischemia-reperfusion injury (IR) induces ROS generation, calcium overload and rapid modification of intracellular pH of myocardial cells; all these changes cause mitochondrial injury that subsequently leads to necrosis or apoptosis of cardiomyocytes in the early phase of IR injury. Recent reports suggest that, in the delayed phase of injury, monocyte recruitment occurs, which contributes to myocardial inflammation. Endogenous angiotensin II plays an important role in this process. Of special interest, a bioabsorbable nanoparticle formulation of PlGA loaded with irbesartan, an antagonist of angiotensin II type 1 receptors, has been developed. Intravenous infusion of these nanoparticles at the time of reperfusion is distributed on injured myocardium for ischemia inhibiting monocyte recruitment, reducing infarct size by anti-inflammatory mechanisms. Reperfusion therapy with nanoparticles loaded with irbesartan is a novel approach for the treatment of myocardial IR injury in patients with acute myocardial infarction.33
In addition, it has been found that adenosine exerts a potent cardioprotective effect when administered at the onset of cardiac reperfusion. However, the clinical use of adenosine for myocardial protection is limited because of its side effects, such as hypotension and bradycardia. In order to overcome this obstacle, a silica nanoparticle formulation aimed at facilitating drug delivery to the myocardium has been developed. Immobilization of adenosine on the surface of silica nanoparticles (Figure 1) resulted in further limitation of infarct size in an animal model. Additionally, the hypotensive effect of adenosine was attenuated after its adsorption on silica nanoparticles.34
Some injectable biomaterials designed to treat AMI during the early stages of myocardial remodeling are based on nanostructures that can reduce the debilitating effects that appear in the late stage of cardiovascular disease. Some injectable hydrogels are formed by nanofibers (Figure 1) that can facilitate the administration of therapeutic agents. By delivering the material through a catheter, invasive surgical procedures can be avoided, thus reducing patient recovery time and the incidence of infections. An example of injectable hydrogel as intramyocardial delivery system is the release of vascular endothelial growth factor (VEGF) in animals using RAD16-II (hydrogels based on self-assembling peptides) as the vehicle. Another example, this time for systemic delivery, is the design of liposomes loaded with VEGF. When liposomes are intravenously injected immediately after the onset of infarction, significant improvements were found in heart function and vascular density up to 4 weeks after administering the injection, compared to the groups receiving empty liposomes or free VEGF. Another example of immediate delivery was demonstrated with nanoparticles loaded with dodecafluoropentane for oxygen supply. Early intravenous injection of the said formulation produced a 60% decrease in infarct size, compared with patients treated with saline solution.35
Of special interest for this review, Harel-Adar and colleagues designed liposomes coated with phosphatidylserine (PS), a ligand exposed on the outer membrane of apoptotic cells and a trigger of anti-inflammatory responses in the presence of activated macrophages during inflammation. PS-liposomes were able to successfully minimize inflammatory responses triggered by macrophages, thus reducing collateral damage to adjacent healthy tissue after AMI.36
Nanomedicine applied to the treatment of stroke
Oxidative stress is the common underlying mechanism of damage in ischemic stroke. Furthermore, it has been shown that free radicals lead to injuries in various cell components, including proteins, lipids and DNA. In this sense, cerebral ischemia tests were conducted for 90 min by occluding the middle cerebral artery (MCAO), followed by 24 hours of reperfusion. Rats received fullerene nanoparticles before MCAO and just after the start of reperfusion. The administration of these nanoparticles significantly decreased infarct size. Ischemia also increased the content of malondialdehyde (MDA) and nitrates, events considerably reduced with the administration of fullerene nanoparticles. Furthermore, induction of MCAO significantly decreased glutathione content (GSH) and activity of superoxide dismutase (SOD), while the fullerene nanoparticles increased them. These findings indicate that these nanoparticles could be used as a powerful free radical scavenger (even more efficient than the cellular antioxidants) thereby protecting brain cells from injury by ischemia/reperfusion caused by cerebral infarction.37
Another novel nanotechnology application for the treatment of ischemic stroke consists of nanoparticles that can easily cross the blood brain barrier (BBB) without compromising its integrity. Studies show that cytidine 5’-diphosphate has neuroprotective capacity in ischemia-reperfusion. Incorporation of this drug in suitable nanoparticles could allow the drug to exert its effect in the CNS by passage of said nanoparticles through the BBB, increasing its therapeutic activity in the required site.38 Another class of drugs that may allow rescue neuronal cells after brain ischemia/reperfusion injury is, again, statins. A recent in vitro study evaluating the efficacy of statins as neuroprotective active ingredients concluded that both atorvastatin and rosuvastatin were effective in mitigating neuronal cell death. However, both drugs have very low permeability through the BBB. This obstacle can be overcome by formulating statins nanoparticulate carrier systems which greatly facilitate the penetration of these drugs in the CNS, a fundamental step that determines its usefulness in the treatment of ischemic stroke.39
Treatment of thrombosis using nanotechnology
Intravascular thrombosis leads to the formation of clots that can block blood vessels, with potentially lethal results. Therefore, rapid recanalization of occluded vessels is essential, but systemic fibrinolytic treatments available today, like intravenous infusion of tissue plasminogen activator (tPA), are associated with low efficacy and many side effects, including a high risk of bleeding complications. The design of a potent and long acting anticoagulant surface, facing the formation of a clot by using nanoparticles loaded with tPA directed to the thrombus site, offers a promising alternative for the treatment of acute thrombosis. The possibility of carrying out the magnetic orientation of tPA for local thrombolysis was investigated in an embolic rat model. Magnetite nanoparticles bound to tPA were administered intra-arterially. Their mobilization along the iliac artery was performed under the guidance of an external magnet. The tPA magnetic nanoparticles accumulated in the region of thrombus formation and were able to achieve thrombolysis. The dose of tPA nanoparticles required for clot dissolution was about 100 times less than that required to achieve a comparable effect with the free drug. Therefore, this strategy would lead to broad applicability of this approach to all occlusive vascular conditions.40,41
Additionally, Myerson and colleagues developed nanoparticles functionalized on its surface with PPACK, a synthetic peptide acting as an irreversible, very powerful and highly selective thrombin inhibitor. However, the use of PPACK has been largely limited due to its rapid elimination. By covalently binding PPACK to the surface of nanoparticles of long circulation time, significant improvements in antithrombotic activity in an animal model of arterial thrombosis were observed.42 Similarly, Peters and colleagues developed micellar nanoparticles (Figure 1) which encapsulate hirudin, a potent natural inhibitor of thrombin.43
Conclusion and prospects
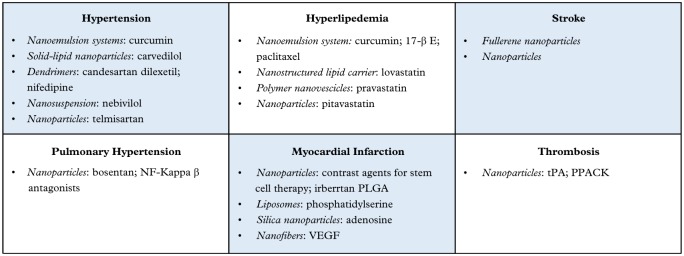
Deepening knowledge and increasing use of nanotechnology as a therapeutic tool for the treatment of many cardiovascular diseases will enable clinicians to achieve objectives that until recently seemed unattainable. This prospect turns nanomedicine, together with its advances and applications, into a real light at the end of the tunnel, giving great hope in the near future for the recovery in patients suffering from diseases such as hypertension and atherosclerosis, or who have suffered a cardiovascular event disabling sequelae such as AMI or stroke (Figure 2). At the same time, it is probable that even these important developments represent only the beginning of a tremendous growth and development in the field of public health worldwide.
Figure 2.
Summary of nanotechnological approaches by cardiovascular disease and drug.
PLGA, poly-(lactic-co-glycolic) acid; tPA, tissue plasminogen activator; VEGF, vascular endothelial growth factor
Acknowledgments
The authors acknowledge to Dr. Fernando Saraví for improving our manuscript.
Footnotes
Author contributions: All authors contributed to conception and design of the review, with substantial contribution to data, analysis and interpretation of the data, drafting of the article, and critical revision of the article for intellectual content.
Funding: This work was supported by grants from the Research and Technology Council of Cuyo University (SECyT), Mendoza, Argentina, and from the National Council of Scientific and Technical Research (CONICET) PIP 2010–2012, both of which were awarded to Walter Manucha. Grant no. PICT 2012-0234 Préstamo BID 2777 OC/AR.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Virna Margarita Martín Giménez, Instituto de Investigaciones en Ciencias Químicas, Facultad de Ciencias de la Alimentación, Bioquímicas y Farmacéuticas, Universidad Católica de Cuyo, Sede San Juan, Argentina.
Diego E. Kassuha, Instituto de Investigaciones en Ciencias Químicas, Facultad de Ciencias de la Alimentación, Bioquímicas y Farmacéuticas, Universidad Católica de Cuyo, Sede San Juan, Argentina
Walter Manucha, Instituto de Medicina y Biología Experimental de Cuyo, Consejo Nacional de Investigación Científica y Tecnológica (IMBECU-CONICET), Argentina; Laboratorio de Farmacología Experimental Básica y Traslacional, Área de Farmacología, Departamento de Patología, Facultad de Ciencias Médicas, Universidad Nacional de Cuyo, Centro Universitario, CP 5500, Mendoza, Argentina.
References
- 1. Rachmawati H, Soraya IS, Kurniati NF, et al. In vitro study on antihypertensive and antihypercholesterolemic effects of a curcumin nanoemulsion. Sci Pharm 2016; 84: 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Radomska A, Leszczyszyn J, Radomski MW. The nanopharmacology and nanotoxicology of nanomaterials: new opportunities and challenges. Adv Clin Exp Med 2016; 25: 151–162. [DOI] [PubMed] [Google Scholar]
- 3. Rhee JW, Wu JC. Advances in nanotechnology for the management of coronary artery disease. Trends Cardiovasc Med 2013; 23: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janko C, Dürr S, Munoz LE, et al. Magnetic drug targeting reduces the chemotherapeutic burden on circulating leukocytes. Int J Mol Sci 2013; 14: 7341–7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bietenbeck M, Florian A, Faber C, et al. Remote magnetic targeting of iron oxide nanoparticles for cardiovascular diagnosis and therapeutic drug delivery: where are we now? Int J Nanomedicine 2016; 11: 3191–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Das A, Mukherjee P, Singla SK, et al. Fabrication and characterization of an inorganic gold and silica nanoparticle mediated drug delivery system for nitric oxide. Nanotechnology 2010; 21: 305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith PM, Ferguson AV. Recent advances in central cardiovascular control: sex, ROS, gas and inflammation. F1000Res 2016; 5 pii: F1000 Faculty Rev-420. DOI: 10.12688/f1000research.7987.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Minarchick VC, Stapleton PA, Sabolsky EM, et al. Cerium dioxide nanoparticle exposure improves microvascular dysfunction and reduces oxidative stress in spontaneously hypertensive rats. Front Physiol 2015; 6: 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dieterlen MT, John K, Reichenspurner H, et al. Dendritic cells and their role in cardiovascular diseases: a view on human studies. J Immunol Res 2016; 2016: 5946807 DOI: 10.1155/2016/5946807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dusi V, Ghidoni A, Ravera A, et al. Chemokines and heart disease: a network connecting cardiovascular biology to immune and autonomic nervous systems. Mediators Inflamm 2016; 2016: 5902947 DOI: 10.1155/2016/5902947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barr TL, VanGilder RL, Seiberg R, et al. Systemic transcriptional alterations of innate and adaptive immune signaling pathways in atherosclerosis, ischemia stroke, and myocardial infarction. J Bioanal Biomed 2015; 7: 029–034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharma M, Sharma R, Jain DK. Nanotechnology based approaches for enhancing oral bioavailability of poorly water soluble antihypertensive drugs. Scientifica (Cairo) 2016; 2016: 8525679 DOI: 10.1155/2016/8525679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Venishetty VK, Chede R, Komuravelli R, et al. Design and evaluation of polymer coated carvedilol loaded solid lipid nanoparticles to improve the oral bioavailability: a novel strategy to avoid intraduodenal administration. Colloids Surf B Biointerfaces 2012; 95: 1–9. [DOI] [PubMed] [Google Scholar]
- 14. Kumar VV, Chandrasekar D, Ramakrishna S, et al. Development and evaluation of nitrendipine loaded solid lipid nanoparticles: influence of wax and glyceride lipids on plasma pharmacokinetics. Int J Pharm 2007; 335: 167–175. [DOI] [PubMed] [Google Scholar]
- 15. Gautam SP, Verma A. PAMAM dendrimers: novel polymeric nanoarchitectures for solubility enhancement of candesartan cilexetil. Pharm Sci 2012; 1: 1–4. [Google Scholar]
- 16. Thadkala K, Sailu C, Aukunuru J. Formulation, optimization and evaluation of oral nanosuspension tablets of nebivolol hydrochloride for enhancement of dissoluton rate. Der Pharmacia Lettre 2015; 7: 71–84. [Google Scholar]
- 17. Bajaj A, Rao MR, Pardeshi A, et al. Nanocrystallization by evaporative antisolvent technique for solubility and bioavailability enhancement of telmisartan. AAPS PharmSciTech 2012; 13: 1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cabrales P, Han G, Roche C, et al. Sustained release nitric oxide from long-lived circulating nanoparticles. Free Radic Biol Med 2010; 49: 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deshpande D, Kethireddy S, Janero DR, et al. Therapeutic efficacy of an ω-3-fatty acid-containing 17-β estradiol nano-delivery system against experimental atherosclerosis. PLoS One 2016; 11: e0147337 DOI: 10.1371/journal.pone.0147337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shiozaki AA, Senra T, Morikawa AT, et al. Treatment of patients with aortic atherosclerotic disease with paclitaxel-associated lipidnanoparticles. Clinics (Sao Paulo) 2016; 71: 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou J, Zhou D. Improvement of oral bioavailability of lovastatin by using nanostructured lipid carriers. Drug Des Devel Ther 2015; 9: 5269–5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Broz P, Ben-Haim N, Grzelakowski M, et al. Inhibition of macrophage phagocytotic activity by a receptor-targeted polymer vesicle-based drug delivery formulation of pravastatin. J Cardiovasc Pharmacol 2008; 51: 246–252. [DOI] [PubMed] [Google Scholar]
- 23. Lobatto ME, Fayad ZA, Silvera S, et al. Multimodal clinical imaging to longitudinally assess a nanomedical anti-inflammatory treatment in experimental atherosclerosis. Mol Pharm 2010; 7: 2020–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katsuki S, Matoba T, Nakashiro S, et al. Nanoparticle-mediated delivery of pitavastatin inhibits atherosclerotic plaque destabilization/rupture in mice by regulating the recruitment of inflammatory monocytes. Circulation 2014; 129: 896–906. [DOI] [PubMed] [Google Scholar]
- 25. Ruiz-Esparza GU, Flores-Arredondo JH, Segura-Ibarra V, et al. The physiology of cardiovascular disease and innovative liposomal platforms for therapy. Int J Nanomedicine 2013; 8: 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ghasemian E, Motaghian P, Vatanara A. D-optimal design for preparation and optimization of fast dissolving bosentan nanosuspension. Adv Pharm Bull 2016; 6: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Avellana P, Segovia J, Sufrate E, et al. Long-term (5 years) effects of bosentan in patients with pulmonary arterial hypertension. Rev Esp Cardiol 2011; 64: 667–673. [DOI] [PubMed] [Google Scholar]
- 28. Kimura S, Egashira K, Chen L, et al. Nanoparticle-mediated delivery of nuclear factor kappaB decoy into lungs ameliorates monocrotaline-induced pulmonary arterial hypertension. Hypertension 2009; 53: 877–883. [DOI] [PubMed] [Google Scholar]
- 29. La Francesca S. Nanotechnology and stem cell therapy for cardiovascular diseases: potential applications. Methodist Debakey Cardiovasc J 2012; 8: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santoso MR, Yang PC. Magnetic nanoparticles for targeting and imaging of stem cells in myocardial infarction. Stem Cells Int 2016; 2016: 4198790 DOI: 10.1155/2016/4198790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu K, Li J, Wang Y, et al. Nanoparticles-assisted stem cell therapy for ischemic heart disease. Stem Cells Int 2016; 2016: 1384658 DOI: 10.1155/2016/1384658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Binsalamah ZM, Paul A, Khan AA, et al. Intramyocardial sustained delivery of placental growth factor using nanoparticles as a vehicle for delivery in the rat infarct model. Int J Nanomedicine 2011; 6: 2667–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakano Y, Matoba T, Tokutome M, et al. Nanoparticle-mediated delivery of irbesartan induces cardioprotection from myocardial ischemia-reperfusion injury by antagonizing monocyte-mediated inflammation. Sci Rep 2016; 6: 29601 DOI: 10.1038/srep29601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Galagudza M, Korolev D, Postnov V, et al. Passive targeting of ischemic-reperfused myocardium with adenosine-loaded silica nanoparticles. Int J Nanomedicine 2012; 7: 1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nguyen MM, Gianneschi NC, Christman KL. Developing injectable nanomaterials to repair the heart. Curr Opin Biotechnol 2015; 34: 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harel-Adar T, Ben Mordechai T, Amsalem Y, et al. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci USA 2011; 108: 1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vani JR, Mohammadi MT, Foroshani MS, et al. Polyhydroxylated fullerene nanoparticles attenuate brain infarction and oxidative stress in rat model of ischemic stroke. EXCLI J 2016; 15: 378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Panagiotou S, Saha S. Therapeutic benefits of nanoparticles in stroke. Front Neurosci 2015; 9: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thompson BJ, Ronaldson PT. Drug delivery to the ischemic brain. Adv Pharmacol 2014; 71: 165–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cicha I. Thrombosis: novel nanomedical concepts of diagnosis and treatment. World J Cardiol 2015; 7: 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov 2014; 13: 813–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Myerson J, He L, Lanza G, et al. Thrombin-inhibiting perfluorocarbon nanoparticles provide a novel strategy for the treatment and magnetic resonance imaging of acute thrombosis. J Thromb Haemost 2011; 9: 1292–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peters D, Kastantin M, Kotamraju VR. Targeting atherosclerosis by using modular, multifunctional micelles. Proc Natl Acad Sci USA 2009; 106: 9815–9819. [DOI] [PMC free article] [PubMed] [Google Scholar]