Abstract
Chronic airway infection with Pseudomonas aeruginosa is a major cause of increased morbidity and mortality in patients with cystic fibrosis (CF). The development and widespread use of nebulized antibacterial therapies, including tobramycin inhalation solution (TIS), has led to improvements in lung function and quality of life. However, the use of nebulizers is associated with various challenges, including extended administration times and the need for frequent device cleaning and disinfection. Multiple therapies are required for patients with CF, which poses a considerable burden to patients, and adherence to the recommended treatments remains a challenge. Tobramycin inhalation powder (TIP), delivered via the T-326 Inhaler, has been shown to have similar clinical efficacy and safety as compared to TIS, with improved patient convenience, satisfaction, and treatment adherence. Long-term safety studies have shown that TIP was well tolerated with no unexpected adverse events in patients with CF. This review of the TIP pivotal and postmarketing studies reinforces the well-established efficacy and safety profile of TIP and its ease of use.
Keywords: cystic fibrosis, equipment contamination, nebulizers and vaporizers, patient compliance, patient preference, Pseudomonas aeruginosa, tobramycin
Background
Cystic fibrosis (CF), an autosomal recessive genetic disorder, is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene located on chromosome 7 that encodes for a complex chloride channel found in exocrine tissues.1,2 Mutations in the CFTR gene lead to decreased chloride and bicarbonate secretion, and enhanced sodium absorption across the epithelial surfaces, resulting in dysfunction in salt–water balance and consequently thick and viscous secretions.3 Deranged ion transport in the lungs and other organs (pancreas, liver, intestine, and reproductive tract) that express CFTR causes various clinical complications.4
An estimated 70,000 patients worldwide have CF,5 including 28,983 patients in the US.6 The majority of these patients develop respiratory failure due to progressive lung disease caused by chronic bacterial infection and concomitant airway inflammation.7 Staphylococcus aureus (including methicillin-resistant S. aureus), Pseudomonas aeruginosa, Haemophilus influenzae, and Stenotrophomonas maltophilia are the most frequently isolated bacteria in patients with CF.6 P. aeruginosa has been considered as the predominant pathogen contributing to chronic lung disease,8 and airway infection caused by P. aeruginosa is a major predictor of morbidity and mortality in patients with CF.7,9 The inherent resistance of P. aeruginosa to antibacterial therapies10 and its persistence through production of a mucoid alginate matrix makes it difficult to eradicate from chronically infected airways.11,12 However, during the early colonization phase, P. aeruginosa strains are generally nonmucoid and are present at lower bacterial density, which makes them more susceptible to antibiotics.13,14 In clinical practice, treatment of chronic P. aeruginosa infection and P. aeruginosa eradication have been associated with increased survival of patients with CF.11,15
Several antibiotics administered via different routes (parenteral, inhaled, and oral) are available for the treatment of CF patients and have been found to be efficacious;16 however, the optimal regimen and duration of therapy remain unclear. Moreover, inhalation of antibiotics has been recognized as a suitable option for delivering relatively high doses directly to the site of infection while minimizing systemic exposure, and achieving adequate local antibiotic concentrations to kill microbes.15,17 In recent years, new drug formulations and inhalation devices have been developed for effective management of P. aeruginosa infection in patients with CF.18–20 Inhaled antibiotics such as tobramycin, colistimethate sodium (COLI, approved only in the European Union), aztreonam, and levofloxacin are approved for the treatment of chronic P. aeruginosa lung infection in CF patients (Table 1).21 The dry powder inhalation formulation of tobramycin [tobramycin inhalation powder (TIP)] has also been approved in Europe and the US under the brand name TOBI® Podhaler™ (Novartis Pharma AG, Basel, Switzerland) for the management of CF patients with P. aeruginosa infection (Table 1).22
Table 1.
Inhaled antibiotics approved in Europe and the US.
| Antibiotic | Agency | Approved indications | References | |
|---|---|---|---|---|
| 1 | Aztreonam inhalation solution (Cayston®) | EMA/FDA | To suppress chronic pulmonary infections due to
Psedomonas aeruginosa in patients with CF,
⩾6 years of age and FEV1 25–75% predicted
(EU) To improve respiratory symptoms in CF patients with P. aeruginosa, ⩾7 years and with FEV1 25–75% predicted (US) Treatment schedule is 28-days-on drug alternating with 28-days-off drug |
CAYSTON® summary of product characteristics23
CAYSTON® prescribing information24 |
| 2 | Colistimethate sodium inhalation solution (Colistin) | EMA | Colistin: For management of chronic infections due to P. aeruginosa in patients with CF, adults and children | Promixin® summary of product characteristics25 |
| Colistimethate sodium inhalation powder (Colobreathe®) | Colobreathe®: For management of chronic infections
due to P. aeruginosa in patients with CF aged
⩾6 years Treatment schedule is a continuous regimen |
Colobreathe® summary of product characteristics26 | ||
| 3 | Levofloxacin nebulizer
solution (QUINSAIR®) |
EMA/FDA | For management of chronic pulmonary infections due to P.
aeruginosa in adult patients with
CF Treatment schedule is 28-days-on drug alternating with 28-days-off drug |
Quinsair® summary of product characteristics27 |
| 4 | Tobramycin inhalation solution
(TOBI®) Tobramycin inhalation powder (TOBI® Podhaler™) |
EMA/FDA | TOBI®: For management of CF patients with P.
aeruginosa, ⩾6 years and with FEV1
25–75% predicted TOBI® Podhaler™: For management of CF patients with P. aeruginosa, ⩾6 years and with FEV1 25–80% predicted (US) or 25–75% predicted (EU) Treatment schedule is 28-days-on drug alternating with 28-days-off drug |
TOBI® prescribing information28
TOBI® Podhaler™ prescribing information22 |
CF, cystic fibrosis; EMA, European Medicines Agency; EU, European Union; FDA, US Food and Drug Administration; FEV1, forced expiratory volume in 1 second; US, United States.
The efficacy and safety of tobramycin inhalation solution (TIS) are well established in patients with CF aged ⩾6 years.29,30 Therefore, the US treatment guidelines strongly recommend chronic use of inhaled tobramycin in patients with CF who have moderate-to-severe lung disease with persistent P. aeruginosa-positive airway cultures.31,32 As per the European consensus guideline recommendations, the therapeutic options for chronic P. aeruginosa infection in CF patients include either an intermittent (1-month on and 1-month off) regimen of inhaled aminoglycoside or continuous administration of inhaled colistin.33 The European Cystic Fibrosis Society Standards of Care best practice guidelines reinforced the US treatment guideline recommendations for the use of TIS on alternate months in CF patients aged ⩾6 years and further acknowledged that TIP has been shown to have similar efficacy to TIS.34
Nebulized antibiotics and associated challenges in patients with cystic fibrosis
Nebulized antibiotics have been established as effective treatment options for chronic P. aeruginosa infection and are recommended for chronic use to improve lung function and quality of life in patients with CF.32 Despite guideline recommendations, there are several challenges associated with the real-world use of nebulized antibiotics.35 Treatment burden is a major challenge for patients with CF, as they require daily administration of multiple inhaled therapies including bronchodilators, mucolytics, hypertonic saline, and antibiotics. Adult subjects have reported spending an average of 2 hours [108 minutes (SD ± 58 min)] on daily CF treatments.36,37 Time hindrance results in poor treatment adherence, which is an important cause of increased pulmonary exacerbations and hospitalization in patients with CF.38 In general, aerosolized antibiotics require a compressor and a nebulizer, and take approximately 20 minutes per dose (excluding cleaning and sterilization). Furthermore, nebulizers require regular cleaning after each use to prevent device contamination and to further ensure that the device performance is not compromised. As most patients do not clean their nebulizer as directed, this can lead to device contamination and potentially, transport of pathogens to the lower airways.39,40
Tobramycin inhalation powder
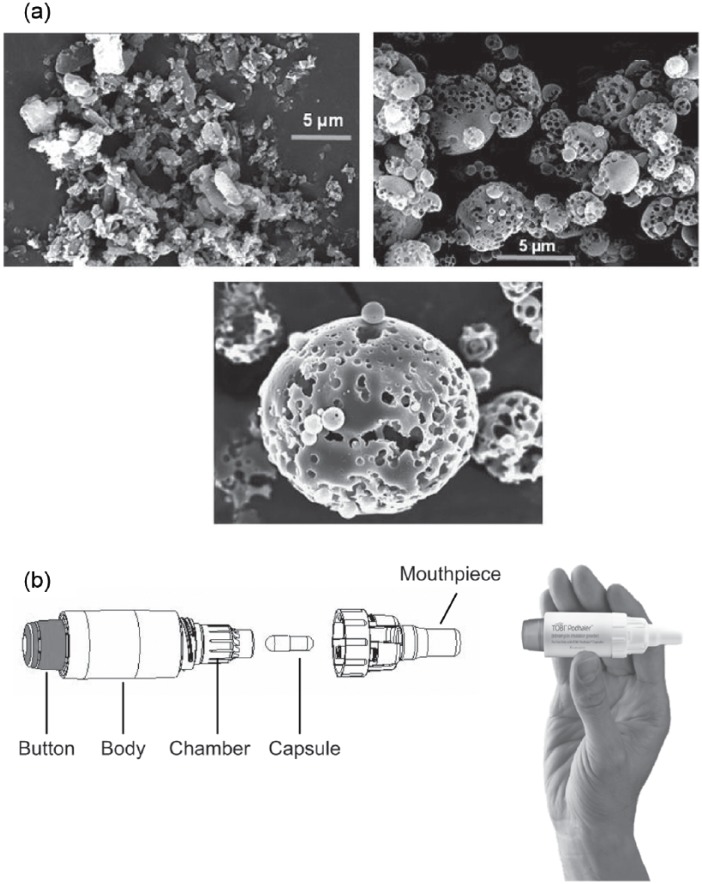
TIP, an innovative drug–device combination, was developed with an aim to overcome the challenges associated with nebulized antibiotics, to minimize treatment burden, and to improve treatment adherence in patients with CF. TIP was developed using PulmoSphere™ (Novartis Pharma AG, Basel, Switzerland) technology (Figure 1).35,41
Figure 1.
(a) Scanning electron microscopic image of typical micronized drug particles, TIP particles, and TIP particle (close up) and (b) T-326 Inhaler for use with TIP (TOBI® Podhaler™) (images included with permission from Geller et al.).35
TIP, tobramycin inhalation powder.
TIP is manufactured via an emulsion-based spray-drying process that yields uniform-sized, spherical hollow porous particles (pulmospheres). It is delivered via the breath-actuated T-326 Inhaler, a portable, mechanical, capsule-based dry powder inhaler (DPI).35 The drug delivery is largely independent of the patient’s peak inspiratory flow rate (PIFR), thus reducing dosing variability.35
Studies have shown that TIP improves intrapulmonary deposition efficiency and shorter administration time, when compared with nebulized tobramycin (TIS).42,43 In addition, inhalation via DPIs can be more convenient compared with nebulizers, as the DPIs are portable and do not require special storage conditions or any electrical sources.35
A phase I pharmacokinetic study of TIP in patients with CF reported a mean administration time of 4.9 minutes with the T-326 Inhaler as compared to 15.8 minutes with nebulizers, excluding the nebulizer cleaning and disinfection time.41 Similarly, in a multicenter, randomized, open-label, phase III trial (EAGER), the mean administration time was significantly lower for TIP as compared with TIS (5.6 versus 19.7 min; p < 0.0001), resulting in a time saving of 28 minutes per day, or 13 hours per cycle, excluding the nebulizer maintenance time.43 A recent real-world study in patients with CF suggested that treatment adherence may be associated with improved clinical outcomes.37 Collectively, these benefits can significantly decrease the treatment burden in patients with CF.
This comprehensive review, based on data from both pivotal clinical trials and real-world studies describes the efficacy, safety, and additional benefits (convenience, adherence, quality of life, and minimal device contamination) associated with the use of TIP in CF patients with chronic P. aeruginosa infection. The clinical studies identified include globally conducted phase III trials (EVOLVE, EDIT, and EAGER), phase IV studies across various countries (2403, FREE, FR01, GB01, and BR01), and a long-term safety study (ETOILES) to discuss the characteristics of TIP use in patients with CF (Figure 2).
Figure 2.
Overview of phase III and IV studies and evaluated endpoint.
ITA-CFq, Italian Treatment Adherence Cystic Fibrosis Questionnaire; CFQ-R, cystic fibrosis questionnaire: revised scale; TIP, tobramycin inhalation powder.
Pharmacokinetics
The pharmacokinetic profile of TIP was evaluated in different clinical studies. In a multicenter, open-label, active-controlled, single-dose escalation, phase I study in CF patients aged ⩾6 years with FEV1 ⩾40% predicted, administration of TIP 112 mg (4 × 28 mg) capsules resulted in similar systemic exposure to that of TIS at the standard dose of 300 mg/5 ml.41 Furthermore, serum tobramycin concentrations were assessed in the EVOLVE study44 and both serum and sputum tobramycin concentrations were assessed in the EDIT and EAGER studies.43,45 Blood and sputum samples were collected between 0–6 and 0–2 hours postdose, respectively; such samples were also collected predose. Tobramycin was analyzed at a central laboratory. In the EVOLVE study, there was no evidence of serum tobramycin accumulation with successive cycles of TIP 112 mg (tobramycin peak levels: cycle 1, 1.99 ± 0.59 μg/ml and cycle 2, 1.64 ± 0.96 μg/ml; tobramycin trough levels: cycle 1, 0.29 ± 0.27 μg/ml and cycle 2, 0.38 ± 0.44 μg/ml).44 In the EDIT study, the mean peak and trough serum concentrations of tobramycin after 28 days of treatment were 1.48 and 0.41 μg/ml, respectively, and the mean maximum sputum concentrations of tobramycin were 1140 and 1739 μg/g at days 1 and 29, respectively.45 Importantly, systemic levels were low, relative to those associated with toxicity with intravenous tobramycin (10–12 μg/ml).35,46 Moreover, the EAGER study showed that serum tobramycin concentrations were similar for TIP and TIS, and that sputum tobramycin concentrations were generally greater for TIP 30 minutes postdose on day 28 of the third cycle of treatment (mean ± SD: TIP, 1979 ± 2770 μg/g; TIS, 1074 ± 1182 μg/g). Of note, serum-to-sputum tobramycin concentrations were comparable in both the EDIT and EAGER studies (data on file). In the EAGER study, the majority (>91%) of TIP patients had P. aeruginosa isolates with a minimum inhibitory concentration ⩽64 µg/ml at baseline, that is, at least 20 times lower than the mean sputum concentration observed within 30 minutes of the first dose of TIP.43
Clinical and microbiologic efficacy in phase III studies
The phase III clinical trials reviewed in this article primarily focused on the efficacy and safety of a drug–device combination of TIP (tobramycin 112 mg delivered via the T-326 Inhaler). TIP was evaluated in two placebo-controlled trials [EVOLVE and EDIT (latter included two extensions)] in relatively treatment-naïve patients (Table 2).44,45,47 Both EVOLVE and EDIT were similar in study design. Cycle 1 comprised a double-blind, placebo-controlled period with 28-days-on and 28-days-off drug, whereas cycles 2 and 3 were open-label, crossover periods for EVOLVE and open-label extensions for EDIT. TIP was also evaluated in a comparative noninferiority study (EAGER) that compared TIP and TIS over a 24-week treatment period (three cycles each consisting of 28-days-on and 28-days-off drug).43
Table 2.
Summary of key phase III studies.
| Parameters | EVOLVE | EDIT, including extensions | EAGER |
|---|---|---|---|
| Study design | Randomized, double-blind, placebo-controlled trial* | Core study: Randomized, double-blind, placebo-controlled trial | Randomized, open-label, active-controlled, noninferiority trial |
| Treatment arms (28-days-on/28-days-off drug) | TIP: 112 mg bid (n = 46); placebo (n = 49) | TIP: 112 mg bid (n = 30); placebo (n = 32) |
TIP: 112 mg bid (n = 308); TIS: 300 mg/5 ml bid (n = 209) |
| Primary objective | To demonstrate the efficacy of a 28-day bid dosing regimen of TIP versus placebo, as measured by the relative change in FEV1% predicted from baseline to the end of cycle 1 dosing | To evaluate the efficacy of TIP manufactured by an improved process versus placebo, assessed by relative change in FEV1% predicted from baseline to day 29 | To evaluate the safety of bid dosing of TIP delivered with the T-326 inhaler, versus TOBI delivered with the PARI-LC® PLUS Jet nebulizer and DeVilbiss PulmoAide® compressor |
| Duration | 24 weeks (1 cycle TIP or placebo followed by 2 cycles open-label TIP) | Core study: 8 weeks (1 cycle TIP or placebo) Extensions: Each consisted of 3 additional cycles of TIP |
24 weeks (3 cycles TIP or TIS) |
| Patients | 95 patients aged 6–21 years | 62 patients aged 6–21 years | 517 patients aged ⩾6 years |
| Key efficacy endpoint | |||
| Primary | |||
| Change in FEV1% predicted (mean treatment difference) | Baseline to day 28–TIP versus placebo: 13.3% (95% CI: 5.3 to 21.3; p = 0.0016) | Baseline to day 29–TIP versus placebo: 5.9% (95% CI: –2.2 to 14.0; p = 0.148) | Baseline to day 28 of cycle 3–TIP versus TIS†: 1.1% relative change (least squares mean difference) |
| Secondary | |||
| Antibiotic (antipseudomonal) use | Antibiotic use–TIP versus placebo: 13.0% versus 18.4% | Antibiotic use–TIP versus placebo: 6.7% versus 12.5% | Antibiotic use–TIP versus TIS: 64.9% versus 54.5% |
| Pseudomonas aeruginosa sputum density log10 CFU/g | Nonmucoid–TIP versus placebo: −1.91 (SD: 2.54)
versus –0.15 (0.68); mucoid–TIP versus placebo: –2.61 (2.53) versus −0.43 (1.05) |
Sum of all biotypes–TIP versus placebo): −1.2 versus 0 (p = 0.002) | Nonmucoid–TIP versus TIS: −1.77 versus −0.73; mucoid–TIP versus TIS: −1.6 versus −0.92 |
| Safety | |||
| Overall AEs | TIP versus placebo: 23 (50.0%) versus 37 (75.5%) | TIP versus placebo: 8 (26.7%) versus 11 (34.4%) | TIP versus TIS†: 278 (90.3%) versus 176 (84.2%) |
| Cough | TIP versus placebo: 6 (13.0%) versus 13 (26.5%) | TIP versus placebo: 3 (10.0%) versus 0 | TIP versus TIS: 149 (48.4%) versus 65 (31.1%) |
| Pulmonary exacerbation | TIP versus placebo: Cycle 1: 5 (10.9%) versus 6 (12.2%) | TIP versus placebo: 1 (3.3%) versus 0 | TIP versus TIS: 104 (33.8%) versus 63 (30.1%) |
| Discontinuation | TIP versus placebo: 7 (15.2%) versus 9 (18.4%) | TIP versus placebo: 1 (3.3%) versus 1 (3.1%) | TIP versus TIS: 83 (26.9%) versus 38 (18.2%) |
| References | Konstan et al.44 | Galeva et al.45; data on file | Konstan et al.43 |
Cycle 1: double-blind, placebo-controlled; cycles 2 and 3: open-label. †The primary endpoint for the EAGER trial related to safety (efficacy was a secondary endpoint).
AE, adverse event; bid, twice daily; CFU/g, colony forming units per gram; FEV1, forced expiratory volume in 1 second; TIP, tobramycin inhalation powder; TIS, tobramycin inhalation solution; SD, standard deviation.
In the EVOLVE study, TIP showed an improvement in forced expiratory volume in 1-second percent predicted (FEV1 % predicted) versus placebo at day 28 of cycle 1 (least squares mean difference, 13.3; p = 0.0016). Similar changes in FEV1% predicted were observed in patients switching from placebo to TIP in cycle 2, and these improvements were maintained through the end of the study. In addition, TIP also reduced P. aeruginosa sputum density, respiratory-related hospitalizations, and use of additional antipseudomonal antibiotics compared with placebo.44 The results of the EDIT study showed that TIP improved FEV1% predicted compared with placebo after 28 days of treatment in cycle 1 (least squares mean difference, 5.9; p = 0.148). Patients who switched from placebo in the core trial to TIP in the study extensions had a mean relative increase in FEV1% predicted of 13% after 28 days of treatment, and this was maintained for up to 7 cycles of treatment.45,47 In addition, TIP reduced P. aeruginosa sputum density and the need for other antipseudomonal antibiotics, as well as the incidence of respiratory-related hospitalizations compared with placebo. Furthermore, in addition to sustained improvement in lung function, the 1-year extension of the EDIT study showed sustained suppression of P. aeruginosa sputum density.47
The EAGER study enrolled patients with prior exposure to inhaled antipseudomonal antibiotics. The increases in FEV1% predicted from baseline to day 28 of cycle 3 were similar between the two arms. The results demonstrated similar efficacy and safety profiles for TIP and TIS over a 6-month study period with a significantly reduced administration time for TIP versus TIS (mean: 5.6 versus 19.7 minutes).43 A significant improvement was reported in patient convenience and adherence with TIP when compared with TIS.37,43 Furthermore, subgroup analyses of the EAGER study based on gender and age group were also performed. The analysis by gender showed a trend toward greater improvements in FEV1% predicted with TIP in male patients than in female patients, and in patients with FEV1% predicted <50% compared with those with FEV1% predicted >50% (10.1% versus −0.5%).48 In the analysis by age, patients were categorized into three groups [⩾6 to <13 years (children), ⩾13 to <20 years (adolescents), and ⩾20 years (adults)]. Comparable efficacy was observed in all age groups for both TIP and TIS. Improvements in FEV1% predicted from baseline to end of cycle 3 were largest in children [4.7%; confidence interval (CI): −1.2 to 10.6], and patient-reported convenience was higher in patients receiving TIP versus TIS across all age groups.49
Microbiologic endpoints were included to evaluate the efficacy of TIP and TIS in all three trials, with sampling of oropharyngeal swabs and sputum following consistent methodology and cultures done at the same central laboratory. In the EVOLVE and EDIT studies, P. aeruginosa sputum density was significantly reduced in the TIP arm compared with placebo.44,45 In the EAGER study, a greater decline in mucoid and nonmucoid sputum P. aeruginosa densities from baseline to day 28 in the third cycle was observed in the TIP arm.43 The details of phase III studies are presented in Table 2.
Phase IV studies
Several studies (ETOILES, 2403, FREE, FR01, BR01, and GB01) have been conducted to collect real-world data to investigate whether the features of TIP translate into real benefits when used in routine clinical practice as demonstrated in previous studies.37,50 The results of these real-world studies (ETOILES, 2403, FREE, FR01, and GB01) showed that TIP treatment was associated with lung function benefits and suppression of P. aeruginosa sputum density, which is consistent with the published reports from the phase III trials (Table 3).43–45 The 2403 study also compared the ease of use, device contamination, and safety parameters associated with TIP treatment versus TIS and COLI treatment. Similar to the phase III study results, study 2403 showed that the T-326 Inhaler used to deliver TIP was easy to use and required shorter total administration time as compared with nebulizers used to deliver TIS or COLI (Table 3).43,51 The ETOILES study evaluated the safety profile of TIP and the BR01 study evaluated device contamination with nebulizers. In addition, three phase IV studies (2403, FREE, and GB01) evaluated patient-reported outcomes assessing patient satisfaction and adherence with TIP treatment.
Table 3.
Summary of key phase IV studies.
| Parameters | ETOILES | 2403 | FREE | FR01 | GB01 | BR01 |
|---|---|---|---|---|---|---|
| Study design | Single-arm, open-label, multicenter, phase IV study* | Open-label, crossover, interventional study | Observational, longitudinal, multicenter cohort study | Postmarketing noninterventional, prospective, multicenter study in France | Combined prospective and retrospective, observational study in the United Kingdom | A descriptive study with a cross-sectional approach to assess the contamination profile of home nebulizers in Brazil |
| Objective | To assess the safety of TIP over 6 cycles of treatment in terms of the incidence of treatment-emergent AEs | To compare the ease-of-use of the T-326 Inhaler device used to deliver TIP with nebulizers used to deliver TIS, COLI, or other inhaled medications | To describe the adherence to treatment with TIP in patients with CF using ITA-CFq questionnaire | To evaluate the adherence to treatment with TIP, as measured using the Morisky score | To describe the change in treatment burden domain score of the revised CFQ–R at 1 month and 5 months after initiation of TIP | To evaluate the microbiologic contamination profile of the nebulizer device |
| Treatment arms (28-days-on /28-days-off drug) |
TIP: 112 mg bid | TIP: 112 mg bid; TIS: 300 mg bid; COLI: 1 million or 2 million units bid/tid (cyclical or noncyclical regimen during cycle 1) |
TIP: 112 mg bid | TIP: 112 mg bid; inhaled TIS, COLI and aztreonam | TIP: 112 mg bid | Not applicable |
| Duration | 48 weeks (6 cycles) | 20 weeks | 6 months | 12 months | 6 months | 12 months |
| Patients | 157 patients aged ⩾6 years | 60 patients aged ⩾6 years TIS/TIP (n = 14); COLI/TIP (n = 28); TIP/TIP (n = 18) | 72 patients aged ⩾12 and ⩽35 years | 126 patients aged ⩾6 years | 87 patients aged ⩾14 years | 77 patients aged ⩾6 years (using same nebulizer for at least 3 consecutive months) |
| Primary clinical outcome† | No deaths and no new emerging safety signals were reported over the 1-year study period | Mean total administration time‡ of TIP compared to nebulized antibiotics; TIS/TIP: 37.0
versus 5.0 min; mean difference: −32.7
(23.9) (95% CI: −54.8 to −10.6; p = 0.0112);
COLI/TIP: 16.4 versus 3.8 min; mean difference:
−13.3 (10.4) (95% CI: 20.3–6.4; p =
0.0016); TIP/TIP: 4.2 versus 3.4 min |
Adherence to TIP at the end of cycle 3 as measured by ITA-CFq scores was high (Table 4) | At 12-month follow-up: Adherence was better in children and teenagers compared to adults: 80%, 75% and 48.3%, respectively (Table 4) |
All four domains of TSQM questionnaire were scored high indicating greater patient satisfaction (Table 4) | Cleaning nebulizers under tap water was a risk factor, increasing the chance of contamination by 4.29 (95% CI: 1.13–16.28; p = 0.03) |
| Secondary/exploratory outcome | ||||||
| FEV1 % predicted | FEV1 % predicted decreased from baseline to cycle 6: −1.9% (14.6);p = 0.199 | FEV1 % predicted change: Cycle 1: TIS/TIP and
COLI/TIP: Increase from baseline (2.2% and 3.9%,
respectively); TIP/TIP: Decrease from baseline (−2.8%) Cycle 2: Stable across the treatment arms |
FEV1 % predicted: 73.8 ± 23.9% at enrollment, 78.1 ± 20.7% at first follow-up visit, and 77.2 ± 17.9% at the second follow-up visit | FEV1 % predicted improved in 47.4% of patients, stabilized in 21.6%, and worsened in 30.9% | FEV1 % predicted change: Not statistically significant | Not evaluated |
| Pseudomonas aeruginosa sputum density | After 6 cycles of TIP treatment, significant reductions in CFUs:
Mucoid: 1.4-log10 reduction, p <
0.001 Sum of all biotypes: 1.2-log10 reduction, p < 0.001 |
Sum of all biotypes (cycle 1): TIS/TIP: mean log reduction of
1.4 log10 CFU; COLI/TIP: mean log reduction of 0.6
log10 CFU; TIP/TIP: mean log reduction of 1.7
log10 CFU Cycle 2: slightly lower for the TIS/TIP arm and was similar for the other two arms |
Not evaluated | Not evaluated | Not evaluated | Not evaluated |
| Tobramycin MIC values | MIC50: Stable at 2 μg/ml (no change from baseline); MIC90: Increased 4-fold (from 128 μg/ml at baseline to 512 μg/ml at the end of the study driven by a small subset of patients) | TIS/TIP and COLI/TIP: MIC50 and MIC90:
1-fold dilution increase; TIP/TIP: MIC50
and MIC90 tobramycin values were stable up to cycle 2 |
Increased from enrollment to the second follow-up visit | Not evaluated | Not evaluated | Not evaluated |
| Safety | ||||||
| AEs, n (%) | 134 (85.4)* | Cycle 1: 36 (60.0%); Cycle 2:30 (57.7%) | 26 (31.7) | 42 (35.3) | 51 AEs reported in 43 patients | No AEs were reported during study |
| Cough | 37 (23.6) | Cycle 1: 6 (10 %); Cycle 2: 3 (5.8%) | 3 (3.7) | 18 (15.1) | 3 episodes | |
| Pulmonary exacerbation | 87 (55.4) | Cycle 1: 17 (28.3); Cycle 2: 11 (21.1) |
7 (8.5) | 3 (2.5) | 1 episode | |
| References | Sommerwerck et al.52 | Greenwood et al.51; Greenwood et al.53 | Data on file | Data on file | Data on file | Data on file |
Safety was the primary objective of this study. †Patient reported outcomes are described in Table 4. ‡Time including the time required to set up the device, administer the drug, and clean the delivery device.
AE, adverse event; bid, twice daily; CFQ-R, cystic fibrosis questionnaire: revised; CI, confidence interval; CFU, colony forming unit; COLI, colistimethate sodium; FEV1, forced expiratory volume in 1 second; ITA-CFq, Italian Treatment Adherence Cystic Fibrosis Questionnaire; MIC, minimum inhibitory concentration; tid, thrice daily; TIP, tobramycin inhalation powder; TIS, tobramycin inhalation solution.
Patient-reported outcomes
Patient satisfaction, convenience and adherence were evaluated in phase III (EAGER) and phase IV (2403, FREE, FR01, and GB01) studies using different questionnaires such as the modified-treatment satisfaction questionnaire for medication (TSQM),54,55 ACCEPTance,56 the revised cystic fibrosis questionnaire (CFQ-R),57 the Italian CFQ (ITA-CFq), and the Morisky scale.58 EAGER, 2403, FREE, and GB01 studies utilized the TSQM questionnaire for evaluating patients reported outcomes (effectiveness, satisfaction, convenience, and side effects). ACCEPTance and patient preference questionnaires were used in the 2403 study. Furthermore, CFQ-R and ITA-CFq were used in the GB01 and FREE studies, respectively (Table 4). The TSQM scores for the treatment satisfaction domain were significantly higher for TIP with regard to effectiveness, convenience, and global satisfaction in EAGER (Table 4). Similarly, the 2403 study showed high scores for TSQM in cycle 1, which were either sustained or further improved in cycle 2 for the majority of domains, indicating greater treatment satisfaction in patients receiving TIP over TIS and COLI.51 In the GB01 study, increases were seen in the scores for all four domains of TSQM (data on file).
Table 4.
| Patient-reported outcome | Key finding | |
|---|---|---|
| TSQM (median scores for domains): TIP versus TIS | ||
| EAGER (phase III) | Effectiveness: 74.8 versus 65.4 (difference
9.36, SE = 1.46; p = 0.0001) Global satisfaction: 76.2 versus 71.0 (difference 5.20, SE = 1.66; p = 0.002) Convenience: 82.7 versus 58.4 (difference 24.35, SE = 1.55; p < 0.0001) Side effects: 92.1 versus 92.6 (difference 0.50, SE = 1.22; p = 0.6833) |
Overall, patients showed greater treatment satisfaction with TIP |
| 2403 (phase IV) | TIS/TIP arm: Cycle 2 (after crossover to TIP): Median scores were improved from cycle 1 only for convenience (13.9 units) COLI/TIP arm: Cycle 2 (after crossover to TIP): Median scores were improved from cycle 1 [effectiveness (8.3 units), convenience (22.2 units), and global satisfaction (10.7 units)] TIP/TIP arm: Effectiveness and convenience scores: cycle 2 scores were unchanged from those of cycle 1 Global satisfaction: median score decreased slightly (7.2 units) in cycle 2 from cycle 1 Side-effects: maximum score (100 units on a scale of 0–100 ) for all the treatment arms in both cycles, meaning patients did not report experiencing side effects from their medication |
|
| FREE (phase IV) | Convenience domain: domain with highest improvement (enrollment:
74.2 ± 17.1; end of study: 77.8 ± 15.9 at the end of study);
mean increase: 2.8 ± 17.9 (95% CI: −2.4 to 8.0) Efficacy domain: slight increase following the introduction of TIP (enrollment: 63.6 ± 19.8; end of study: 67.5 ± 15.1 at the end of study); mean increase: 1.8 ± 22.4 (95% CI: –4.6 to 8.2) Global satisfaction and side effects domains revealed no significant changes during the study when compared with baseline |
|
| GB01 (phase IV) | All four domains were scored high, indicating greater patient
satisfaction Mean increase of scores in four domains: Effectiveness: 5.0 points (SD: ± 24.1) at 1 month and 10.2 points (SD: ± 19.9) at 5 months Side effects: 5.2 points (SD: ± 18.7) at 1 month and 5.6 points (SD: ± 15.9) at 5 months Convenience: 25.6 points (SD: ± 23.5) at 1 month and 29.7 (SD: ± 24.6) points at 5 months global satisfaction: 12.9 points (SD: ± 23.6) at 1 month and 19.9 points (SD: ± 22.9) at 5 months |
|
| ACCEPtance (median scores for domains) | ||
| 2403 (phase IV) | TIS/TIP arm: Cycle 2: Median scores were improved from cycle 1 (visit 3) for the domains of medication inconvenience (10 units), long-term treatment (8.3 units), and regime constraints (10 units) Median score for the domain of side effects reached the maximum satisfaction level (on a scale of 100 units) in both cycle 1 and cycle 2Median scores decreased in cycle 2 for the domains of effectiveness and general COLI/TIP arm: Cycle 2: Median scores were improved for the domains of medication inconvenience (20 units), long-term treatment (12.5 units), and regime constraints (10 units) Median scores decreased in cycle 2 for the domain of side effects TIP/TIP arm: Cycle 2: Median scores were improved from cycle 1 for the domains of medication inconvenience (5 units), regime constraints (2.5 units), effectiveness (16.7 units), and general (8.3 units) The median score for the domain of side effects was the same in both cycles (90 units on a 0–100 scale) Median score for the domain of long-term treatment remained the same in both cycles (66.7 units on a 0–100 scale) |
After crossover to TIP (cycle 2), TIS/TIP and COLI/TIP arms showed greater acceptance for TIP |
| Patient preference | ||
| 2403 (phase IV) | The majority of patients demonstrated either ‘strong’ or
‘somewhat’ treatment preference for TIP in the TIS/TIP (9 out of
12 patients, 75.0%) and COLI/TIP (18 out of 23 patients, 78.3%)
arms The majority (⩾78.3%) of patients demonstrated preference for TIP for the efficiency of saving time, portability, simplicity, and other advantages |
Patient preference was high for TIP |
| FR01 | 12-month follow up (TIP versus previous
treatments): 61 (88.4%) versus 4
(5.8%) Patient preference for TIP was higher in adult than in younger patients: 48 (87.3%) versus 12 (66.7%) |
|
| ITA-CFq | ||
| FREE (phase IV) | Adherence to TIP at the end of cycle 3 as measured by ITA-CFq
scores was high: Enrollment: mean compliance score to nebulized antibiotics: 7.8 ± 3.2 (95% CI: 6.9–8.7) Follow-up visits 1 and 2: mean compliance scores to TIP: 9.4 ± 1.2 (95% CI: 9.0–9.7) and 9.5 ± 1.2 (95% CI: 9.1–9.8), respectively After initiation of TIP, treatment adherence increased by 20.5% at the first follow up at 3 months (9.4 ± 1.2), then remained stable up to the end of study (9.5 ±1.2) |
Patients were more compliant with TIP |
| CFQ-R | ||
| FREE (phase IV) | No substantial impact of TIP was observed in terms of QoL, as
assessed by the CFQ-R All domains remained roughly unchanged from baseline to study end |
|
| GB01 (phase IV) | Mean increase from baseline in CFQ-R treatment burden domain scores‡ at 1 and 5 months after initiation of TIP: 7.9 points (SD: ± 19.2) at 1 month (p < 0.01) and 6.5 points (SD: ± 20.3) at 5 months | |
| Morisky score | ||
| FR01 (phase IV) | Adherence to TIP as measured by the Morisky
score: Baseline visit: 50.0% of patients were compliant with previous inhaled treatment; mean Morisky score was 2.5 (±1.2) 12-month follow up: 59.6% of patients were compliant with TIP treatment (95% CI: 45.3–72.4%); mean Morisky score was 2.7 (SD: ± 1.0) At 12-month follow up, adherence was better in children and teenagers compared with adults: 80%, 75%, and 48.3% of adherent patients with mean Morisky scores of 3.1 (SD: ± 0.7), 3.0 (SD: ± 0.8), and 2.5 (SD: ± 1.1), respectively |
|
| Patient’s diary | ||
| FREE (phase IV) | Average number of missed antibiotic doses per patient during the past week was 0.5 ± 0.7 at first cycle, 0.4 ± 0.5 at second cycle, and 0.4 ± 0.8 at third cycle (the average number of antibiotic capsules used for each dose was always 4) | |
Konstan et al. 2011a. † Data on file. ‡Domains are scored out of 100 with a higher score indicating greater patient satisfaction.
AEs, adverse events; CFQ-R, cystic fibrosis questionnaire: revised; CI, confidence interval; COLI, colistimethate sodium; ITA-CFq, Italian Treatment Adherence Cystic Fibrosis Questionnaire; QoL, quality of life; SE, standard error; SD, standard deviation; TIP, tobramycin inhalation powder; TIS, tobramycin inhalation solution; TSQM, treatment satisfaction questionnaire for medication.
A real-world study by Harrison and colleagues showed that the proportion of participants reporting ‘excellent adherence’ was increased by twofold after switching from TIS to TIP (43–83%).37 Another real-world study showed that the majority of patients expressed satisfaction with TIP administration time (100%), time to clean (97.1%), portability (97.1%), and ease of use (94.3%). Overall, the patient preference for TIP was based on shorter administration time, convenience, and ease of use.59 In summary, results from various real-world studies are in line with the phase III clinical trial data that suggest improved patient adherence with TIP as compared with TIS.43 The detailed patient reported outcomes from various studies are presented in Table 4.
Inhalation-device contamination
Various studies have investigated the role of home nebulizers as a source of contamination in patients with CF.39,40,60,61 An open-label, crossover, interventional phase IV study (2403) analyzed the contamination profile of both nebulizers and the T-326 inhaler, while a descriptive study, BR01, analyzed the contamination profile of nebulizers with regard to methods of cleaning to minimize contamination. In the 2403 study, microbial contamination of the nebulizers was assessed at the start and end of the first treatment period, second treatment period, and at the discontinuation visit if applicable. For patients on TIP, the T-326 Inhaler used in the last week of TIP treatment was cultured. Device samples were obtained from four locations on the nebulizer (mouthpiece, reservoir cup, filter, and tubing) and from one location on the T-326 Inhaler (mouthpiece). A central laboratory performed all device cultures, as well as sputum cultures from patients. The results of this study showed that the T-326 Inhaler used to deliver TIP was much less frequently contaminated than the nebulizers, thus potentially reducing the sources of pathogenic bacteria in patients with CF.53 In the BR01 study, microbial contamination of the nebulizers was assessed using samples taken from the mouthpiece and the reservoir cup, and cultures were performed at a central laboratory. The latter study concluded that cleaning nebulizers with tap water increased the chance of contamination by 4.29 fold (Table 5). Assessment of contamination of various parts of the nebulizer showed that the frequency of contamination was 60.8% in the mouthpiece and 62.2% in the cup, which was consistent with the reported pattern of contamination profile of nebulizers.39,60
Table 5.
Summary of key phase IV studies device contamination results.
| Study number | 2403 | BR01 |
|---|---|---|
| Treatment arms | TIS/TIP (n = 14); COLI/TIP (n = 28); TIP/TIP (n = 18) | Nebulizers (n = 77) |
| Results | Contamination frequency: n = 12 (20%): COLI/TIP: 9 (32.1%); TIS/TIP: 2 (14.3%); TIP/TIP: 1 (5.6%) | Contamination frequency: n = 53 (71.6%) |
| TIS/TIP: Cycle 1: No pathogen isolated from the devices Cycle 2: Pseudomonas aeruginosa was isolated from both the nebulizer (medication not specified) and the sputum Staphylococcus aureus was the only pathogen isolated (light growth) from one T-326 Inhaler and it was not present in the patient’s sputum COLI/TIP: Cycle 1: The majority of pathogens were isolated (only once) from the devices that delivered COLI. Except for one patient with S. aureus in the COLI/TIP arm, no patient had the same pathogen isolated from the delivery device and sputum at visits 2 and 3 Cycle 2: No contamination was observed in the T-326 inhaler during cycle 2 TIP/TIP: Cycle 2: No contamination was observed in the T-326 Inhaler during cycles 1 or 2 P. aeruginosa was isolated from nebulizers (used for other nonspecified medication) |
Frequently isolated pathogen Pseudomonas putida: 6 (8.1%), Stenotrophomonas maltophilia: 5 (6.8%); Chryseobacterium indologenes: 5 (6.8%); nonalbicans Candida species: 16 (21.6%) Environmental fungal contaminants: 9 (12.2%) 97.4% of patients reported cleaning their nebulizers Cleaning methods: Lathering and rinsing with tap water (66.2%) Tap water only (60.8%) Immersion in boiling water (52.7%) Cleaning nebulizers under tap water was a risk factor for device contamination (OR = 4.29; 95% CI: 1.13–16.28, p = 0.03) |
CI, confidence interval; COLI, colistimethate sodium; OR, odds ratio; TIP, tobramycin inhalation powder; TIS, tobramycin inhalation solution.
Safety and tolerability
Inhaled tobramycin preparations have the advantage of minimal systemic exposure, and hence have a different adverse event (AE) profile compared with parenteral formulations.62 The phase III (EVOLVE, EDIT, and EAGER) and phase IV (ETOILES, 2403, FREE, FR01, and GB01) studies suggested that there were no unexpected safety signals with TIP treatment. In the EVOLVE study, the incidence of AEs reported among TIP-treated patients was lower (50.0%) when compared with placebo-treated patients (75.5%) in cycle 1.44 The incidence of lung disorders (preferred term mainly for pulmonary exacerbations) was comparable in cycle 1; however, the frequency appeared to be higher for any given cycle in the TIP arm compared with the placebo arm (Table 2). The incidence rates of overall AEs were generally higher in the placebo arm versus the TIP arm in the first cycle of treatment.44 A similar trend was observed for AEs and serious AEs (SAEs) in the EDIT study.45 In the EAGER study, AEs were observed more frequently in the TIP arm than in the TIS arm throughout the three treatment cycles; however, the incidence decreased successively with each cycle.43
In the ETOILES study (n = 157), safety was evaluated over a period of 48 weeks, along with supportive efficacy endpoints during 6 cycles of therapy in patients with CF. No new emerging safety signals were reported.52 The study findings were consistent with results from the 1-year extension of the EDIT study conducted to evaluate the safety and tolerability profile of TIP; no increased incidence of the different types of AEs with longer exposure to TIP was reported.47
The data from phase IV studies showed that TIP was well tolerated, and safety findings from real-world studies are consistent with results of the phase III studies.
In general, the most common AEs in patients receiving TIP were cough and pulmonary exacerbations.43,45 Cough was the most common AE in the TIP groups in all three studies, EVOLVE, EDIT, and EAGER. However, there were no treatment discontinuations due to this AE (Table 2).44,45
Postinhalation cough is reported as a common side effect associated with both wet- and dry-powder inhalation in patients with CF in various clinical studies.43,44,52 The reason for the relatively high incidences of cough, dysphonia, and dysgeusia could be the delivery of a relatively high powder load or deposition of tobramycin to the posterior pharynx causing irritation, which decreases with time.43 Therefore, ETOILES used a specific case-report form to record and characterize postinhalation events (including cough), capturing time of onset and duration. In this study, 78 patients reported postinhalation cough, which was highest in cycle 1 (31.4%) and subsequently decreased during cycles 4–6 (21–22%). In most cases, the postinhalation cough was generally of short duration (<4 min) and decreased over time, with no action required, possibly due to patients becoming more experienced with the administration of TIP.52 Furthermore, there is growing evidence that proper inhalation techniques may result in minimization of postinhalation cough for ‘high-dose’ dry powder products like TIP. Reduction in postinhalation cough was observed for inhaled drugs when a higher resistance DPI with a lower PIFR was used. The flow rate independence in total lung dosein vitro observed for TIP should allow patients to inhale comfortably without cough, while maintaining consistent drug delivery to the lungs.63–65 The ETOILES study reconfirmed that TIP continues to be well tolerated in patients with CF, with no increase in the frequency of AEs in the second year of treatment, and no new emerging safety signals.66 Moreover, the postinhalation cough events were not associated with bronchospasm events.43,52
Common measures to minimize postinhalation cough utilized during clinical trials were drinking water, less forceful inhalation, and correction of inhalation technique.52 Additional cough-mitigation strategies include: (1) avoid pressing button more than once; (2) tilt head back slightly during inhalation; and (3) inhalation with a single, slow, and deep breath to minimize cough.
Conclusion
Nebulized antibiotics have significantly contributed to increasing the life expectancy in CF patients with chronic airway infection; however, the high treatment burden and nebulizer contamination are major concerns. TIP administered via the T-326 Inhaler is efficacious for the management of chronic pulmonary P. aeruginosa infection in patients with CF and may help alleviate this treatment burden. Controlled clinical and real-world studies have demonstrated comparable efficacy and safety of TIP with TIS treatment. TIP is considered easy to use by some patients as the total administration time in patients with CF was considerably less compared with that with TIS. Additionally, unlike nebulizers, the T-326 Inhaler does not require disinfection. Moreover, greater patient satisfaction demonstrated in various clinical trials suggests that the convenience and lower treatment burden associated with TIP use may result in improved adherence to therapy. Long-term safety and real-world studies suggest that TIP was well tolerated and its safety profile was generally consistent with the established safety profile from the phase III studies. Although postinhalation cough was the most commonly reported AE with TIP in clinical trials, it seemed to decrease over time and with proper administration measures that are important components of patient education. In addition to comparable efficacy and safety, TIP administered via the T-326 Inhaler may offer a therapeutic advantage over traditional nebulized formulations by demonstrating improved convenience and treatment adherence.
Acknowledgments
The authors acknowledge Anupama Tamta (Novartis Healthcare Pvt. Ltd., Hyderabad, India) for providing medical writing assistance for this manuscript.
Footnotes
Funding: The study was sponsored by Novartis Pharma AG.
Conflict of interest statement: KH and LD are full-time employees of Novartis Pharmaceuticals Corporation.
Contributor Information
Kamal Hamed, Novartis Pharmaceuticals Corporation, One Health Plaza, East Hanover, NJ 07936-1080, USA.
Laurie Debonnett, Novartis Pharmaceuticals Corporation, One Health Plaza, East Hanover, NJ 07936-1080, USA.
References
- 1. Vallières E, Elborn JS. Cystic fibrosis gene mutations: evaluation and assessment of disease severity. Adv Genomics Genet 2014; 4: 161–172. [Google Scholar]
- 2. Proesmans M. Best practices in the treatment of early cystic fibrosis lung disease. Ther Adv Respir Dis 2016; pii: 1753465816680573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goss CH, Ratjen F. Update in cystic fibrosis 2012. Am J Respir Crit Care Med 2013; 187: 915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ashlock MA, Beall RJ, Hamblett NM, et al. A pipeline of therapies for cystic fibrosis. Semin Respir Crit Care Med 2009; 30: 611–626. [DOI] [PubMed] [Google Scholar]
- 5. Cystic Fibrosis Foundation (CFF). Patient registry 2012 annual data report. Bethesda: Cystic Fibrosis Foundation, 2013. [Google Scholar]
- 6. Cystic Fibrosis Foundation (CFF). Patient registry 2015 annual data report. Bethesda: Cystic Fibrosis Foundation, 2016. [Google Scholar]
- 7. Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev 2002; 15: 194–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cystic Fibrosis Foundation (CFF). Patient registry 2008 annual data report. Bethesda: Cystic Fibrosis Foundation, 2009. [Google Scholar]
- 9. Emerson J, Rosenfeld M, McNamara S, et al. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 2002; 34: 91–100. [DOI] [PubMed] [Google Scholar]
- 10. Tumbarello M, Repetto E, Trecarichi EM, et al. Multidrug-resistant Pseudomonas aeruginosa bloodstream infections: risk factors and mortality. Epidemiol Infect 2011; 139: 1740–1749. [DOI] [PubMed] [Google Scholar]
- 11. Doring G, Hoiby N. Early intervention and prevention of lung disease in cystic fibrosis: a European consensus. J Cyst Fibros 2004; 3: 67–91. [DOI] [PubMed] [Google Scholar]
- 12. Doring G, Conway SP, Heijerman HG, et al. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. Eur Respir J 2000; 16: 749–767. [DOI] [PubMed] [Google Scholar]
- 13. Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 2003; 168: 918–951. [DOI] [PubMed] [Google Scholar]
- 14. Rosenfeld M, Ramsey BW, Gibson RL. Pseudomonas acquisition in young patients with cystic fibrosis: pathophysiology, diagnosis, and management. Curr Opin Pulm Med 2003; 9: 492–497. [DOI] [PubMed] [Google Scholar]
- 15. UK Cystic Fibrosis Trust Antibiotic Working Group. Antibiotic treatment for cystic fibrosis. 3rd ed. London: UK Cystic Fibrosis Trust, 2009. [Google Scholar]
- 16. Das RR, Kabra SK, Singh M. Treatment of pseudomonas and Staphylococcus bronchopulmonary infection in patients with cystic fibrosis. ScientificWorldJournal 2013: 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Geller DE. Aerosol antibiotics in cystic fibrosis. Respir Care 2009; 54: 658–670. [DOI] [PubMed] [Google Scholar]
- 18. Anderson P. Emerging therapies in cystic fibrosis. Ther Adv Respir Dis 2010; 4: 177–185. [DOI] [PubMed] [Google Scholar]
- 19. Ballmann M, Smyth A, Geller DE. Therapeutic approaches to chronic cystic fibrosis respiratory infections with available, emerging aerosolized antibiotics. Respir Med 2011; 105(Suppl. 2): S2–S8. [DOI] [PubMed] [Google Scholar]
- 20. Heijerman H, Westerman E, Conway S, et al. Inhaled medication and inhalation devices for lung disease in patients with cystic fibrosis: a European consensus. J Cyst Fibros 2009; 8: 295–315. [DOI] [PubMed] [Google Scholar]
- 21. Elborn JS, Vataire AL, Fukushima A, et al. Comparison of inhaled antibiotics for the treatment of chronic Pseudomonas aeruginosa lung infection in patients with cystic fibrosis: systematic literature review and network meta-analysis. Clin Ther 2016; 38: 2204–2226. [DOI] [PubMed] [Google Scholar]
- 22. TOBI® Podhaler: prescribing information (tobramycin inhalation powder). East Hanover: Novartis Pharmaceuticals Corp, 2015. [Google Scholar]
- 23. CAYSTON®: summary of product characteristics (aztreonam for inhalation solution). Ireland: Gilead Sciences, 2016. [Google Scholar]
- 24. CAYSTON®: prescribing information (aztreonam for inhalation solution). Foster City: Gilead Sciences, Inc, 2014. [Google Scholar]
- 25. Promixin®: summary of product characteristics. Chichester: Profile Pharma Limited, 2015. [Google Scholar]
- 26. Colobreathe®: summary of product characteristics (colistimethate sodium inhalation powder). Dartford: Forest Laboratories UK Ltd, 2016. [Google Scholar]
- 27. Quinsair®: information summary of product characteristics. European Medicines Agency. Novato: Raptor Pharmaceuticals Inc, 2015. [Google Scholar]
- 28. TOBI®: prescribing information (tobramycin inhalation solution). East Hanover: Novartis Pharmaceuticals Corp, 2015. [Google Scholar]
- 29. Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med 1999; 340: 23–30. [DOI] [PubMed] [Google Scholar]
- 30. Moss RB. Administration of aerosolized antibiotics in cystic fibrosis patients. Chest 2001; 120(Suppl. 3): 107S–113S. [DOI] [PubMed] [Google Scholar]
- 31. Flume PA, O’Sullivan BP, Robinson KA, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med 2007; 176: 957–969. [DOI] [PubMed] [Google Scholar]
- 32. Mogayzel PJ, Jr, Naureckas ET, Robinson KA, et al. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am J Respir Crit Care Med 2013; 187: 680–689. [DOI] [PubMed] [Google Scholar]
- 33. Doring G, Flume P, Heijerman H, et al. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros 2012; 11: 461–479. [DOI] [PubMed] [Google Scholar]
- 34. Smyth AR, Bell SC, Bojcin S, et al. European cystic fibrosis society standards of care: best practice guidelines. J Cyst Fibros 2014; 13(Suppl. 1): S23–S42. [DOI] [PubMed] [Google Scholar]
- 35. Geller DE, Weers J, Heuerding S. Development of an inhaled dry-powder formulation of tobramycin using PulmoSphere technology. J Aerosol Med Pulm Drug Deliv 2011; 24: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. J Cyst Fibros 2009; 8: 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harrison MJ, McCarthy M, Fleming C, et al. Inhaled versus nebulised tobramycin: a real world comparison in adult cystic fibrosis (CF). J Cyst Fibros 2014; 13: 692–698. [DOI] [PubMed] [Google Scholar]
- 38. Eakin MN, Bilderback A, Boyle MP, et al. Longitudinal association between medication adherence and lung health in people with cystic fibrosis. J Cyst Fibros 2011; 10: 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blau H, Mussaffi H, Mei Zahav M, et al. Microbial contamination of nebulizers in the home treatment of cystic fibrosis. Child Care Health Dev 2007; 33: 491–495. [DOI] [PubMed] [Google Scholar]
- 40. Lester MK, Flume PA, Gray SL, et al. Nebulizer use and maintenance by cystic fibrosis patients: a survey study. Respir Care 2004; 49: 1504–1508. [PubMed] [Google Scholar]
- 41. Geller DE, Konstan MW, Smith J, et al. Novel tobramycin inhalation powder in cystic fibrosis subjects: pharmacokinetics and safety. Pediatr Pulmonol 2007; 42: 307–313. [DOI] [PubMed] [Google Scholar]
- 42. Newhouse MT, Hirst PH, Duddu SP, et al. Inhalation of a dry powder tobramycin PulmoSphere formulation in healthy volunteers. Chest 2003; 124: 360–366. [DOI] [PubMed] [Google Scholar]
- 43. Konstan MW, Flume PA, Kappler M, et al. Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: the EAGER trial. J Cyst Fibros 2011; 10: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Konstan MW, Geller DE, Minic P, et al. Tobramycin inhalation powder for P. aeruginosa infection in cystic fibrosis: the EVOLVE trial. Pediatr Pulmonol 2011; 46: 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Galeva I, Konstan MW, Higgins M, et al. Tobramycin inhalation powder manufactured by improved process in cystic fibrosis: the randomized EDIT trial. Curr Med Res Opin 2013; 29: 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sweetman SC. Martindale: the complete drug reference. London: Pharmaceutical Press, 2016, http://www.medicinescomplete.com. [Google Scholar]
- 47. Konstan MW, Flume PA, Galeva I, et al. One-year safety and efficacy of tobramycin powder for inhalation in patients with cystic fibrosis. Pediatr Pulmonol 2016; 51: 372–378. [DOI] [PubMed] [Google Scholar]
- 48. Konstan M, Parkins M, Angyalosi G, et al. Tobramycin inhaltion powder is as effective as Tobramycin inhalation solution in patients with cystic fibrosis: a subgroup analysis of the EAGER trial. Poster (39401) presented at American Thoracic Society, Philadelphia, PA, 2013. [Google Scholar]
- 49. Geller DE, Nasr SZ, Piggott S, et al. Tobramycin inhalation powder in cystic fibrosis patients: response by age group. Respir Care 2014; 59: 388–398. [DOI] [PubMed] [Google Scholar]
- 50. Bilton D, Nash EF, Peckham D, et al. 74 An evaluation of treatment burden following initiation of TOBI Podhaler; in patients with CF. J Cyst Fibros 2014; 13: S65. [Google Scholar]
- 51. Greenwood JSC, Sommerwerck U, Nash EF, et al. Ease of use of tobramycin inhalation powder compared with nebulized tobramycin and colistimethate sodium: a phase IV crossover study in cystic fibrosis patients with Pseudomonas aeruginosa infection. Pediatr Pulmonol 2016; 51: S361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sommerwerck U, Virella-Lowell I, Angyalosi G, et al. Long-term safety of tobramycin inhalation powder in patients with cystic fibrosis: phase IV (ETOILES) study. Curr Med Res Opin 2016; 32: 1789–1795 [DOI] [PubMed] [Google Scholar]
- 53. Greenwood JSC, Sommerwerck U, Nash EF, et al. Microbial contamination profile of TOBI Podhaler versus nebulizers used in cystic fibrosis patients with chronic Pseudomonas aeruginosa infection: a real-world study. Pediatr Pulmonol 2016; 51: S315. [Google Scholar]
- 54. Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2004; 2: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Regnault A, Balp MM, Kulich K, et al. Validation of the Treatment Satisfaction Questionnaire for Medication in patients with cystic fibrosis. J Cyst Fibros 2012; 11: 494–501. [DOI] [PubMed] [Google Scholar]
- 56. Arnould B, Gauchoux R, Meunier J, et al. Validation of ACCEPT, a new generic measure to assess how patients with chronic diseases balance between the advantages and disadvantages of following the recommended treatment regimen in real-life. ISPOR 16th Annual European Congress, Dublin, Ireland, 2013. [Google Scholar]
- 57. Quittner AL, Buu A, Messer MA, et al. Development and validation of The Cystic Fibrosis Questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest 2005; 128: 2347–2354. [DOI] [PubMed] [Google Scholar]
- 58. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986; 24: 67–74. [DOI] [PubMed] [Google Scholar]
- 59. Greenberg J, Palmer JB, Chan WW, et al. Treatment satisfaction in cystic fibrosis: early patient experience with tobramycin inhalation powder. Patient Prefer Adherence 2016; 10: 2163–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Della Zuana A, Oliveira Garcia D, Juliani RC, et al. Effect that an educational program for cystic fibrosis patients and caregivers has on the contamination of home nebulizers. J Bras Pneumol 2014; 40: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vassal S, Taamma R, Marty N, et al. Microbiologic contamination study of nebulizers after aerosol therapy in patients with cystic fibrosis. Am J Infect Control 2000; 28: 347–351. [DOI] [PubMed] [Google Scholar]
- 62. Hagerman JK, Hancock KE, Klepser ME. Aerosolised antibiotics: a critical appraisal of their use. Expert Opin Drug Deliv 2006; 3: 71–86. [DOI] [PubMed] [Google Scholar]
- 63. Tiddens HA, Geller DE, Challoner P, et al. Effect of dry powder inhaler resistance on the inspiratory flow rates and volumes of cystic fibrosis patients of six years and older. J Aerosol Med 2006; 19: 456–465. [DOI] [PubMed] [Google Scholar]
- 64. Jaques A, Daviskas E, Turton J, et al. Inhaled mannitol improves lung function in cystic fibrosis. Chest 2008; 133: 1388–1396. [DOI] [PubMed] [Google Scholar]
- 65. Haynes A, Geller D, Weers J, et al. Inhalation of tobramycin using simulated cystic fibrosis patient profiles. Pediatr Pulmonol 2016; 51: 1159–1167. [DOI] [PubMed] [Google Scholar]
- 66. Virella-Lowell I, Sommerwerck U, Debonnett L, et al. Long-term safety and efficacy of tobramycin inhalation powder hard capsules (TIPTM) in patients with cystic fibrosis: an extension to the ETOILES study. Pediatric Pulmonol 2015; 50: S364–S365. [Google Scholar]