Abstract
Lung cancer remains a common and deadly disease. Many modalities are available to the bronchoscopist to evaluate and stage lung cancer. We review the role of bronchoscopy in the staging of the mediastinum with convex endobronchial ultrasound (EBUS) and discuss emerging role of esophageal ultrasonography as a complementary modality. In addition, we discuss advances in scope technology and elastography.
We review the bronchoscopic methods available for the diagnosis of peripheral lung nodules including radial EBUS and navigational bronchoscopy (NB) with a consideration of the basic methodologies and diagnostic accuracies. We conclude with a discussion of the comparison of the various methodologies.
Keywords: biopsy, bronchoscopy, cancer staging, lung cancer
Introduction
Lung cancer is the most lethal cancer in the United States. It is projected to account for more than one in four cancer deaths and an estimated 224,390 new cases in 2016 in the United States.1 Initial diagnostic and staging recommendations focus on obtaining tissue in a systematic manner in order to minimize risk and maximize utility.2,3 These recommendations were issued in recognition of the increasing number of lung cancer evaluation modalities and the importance of use of the correct staging paradigm.
The purpose of this review is to present the current and emerging bronchoscopic techniques and technologies available for the diagnosis and staging of lung cancer. We first focus on the role of bronchoscopy in the staging of the mediastinum with convex endobronchial ultrasound (EBUS) with transbronchial needle aspiration (EBUS-TBNA). We then shift our focus to the role of bronchoscopy in the diagnosis of peripheral nodules with a discussion of the various available methodologies including radial endobronchial ultrasound (rEBUS) and navigational bronchoscopy (NB), specifically, electromagnetic navigational bronchoscopy (ENB) and virtual navigational bronchoscopy (VNB). We provide a brief description of the performance of each technique followed by a review of major publications assessing its diagnostic accuracy. We conclude by discussing the next wave of potential advances in bronchoscopic technology and/or techniques.
Mediastinal and hilar lymph node staging
Convex EBUS-TBNA was introduced in its modern form in 2003.4 EBUS-TBNA is used to examine and biopsy mediastinal and hilar lymph nodes (LNs) as well as central parenchymal lung lesions. This modality allows for accurate minimally invasive nodal staging in patients with suspected lung cancer. The recommended staging paradigm is one that begins with biopsy of the highest stage LN (N3) then descends in stage until a diagnosis is achieved or all LN stations amenable to staging are sampled.5,6
Conventional EBUS bronchoscopes have an ultrasonographic tip with a camera and white light source offset at an angle of 35°. A similarly offset working channel allows for biopsy under real time ultrasound visualization of the needle within the target lesion. Visualization of the target LN is assisted by inflation of a saline-filled balloon. Biopsy is achieved with specially designed needles which attach to the bronchoscope.7 (Figure 1).
Figure 1.
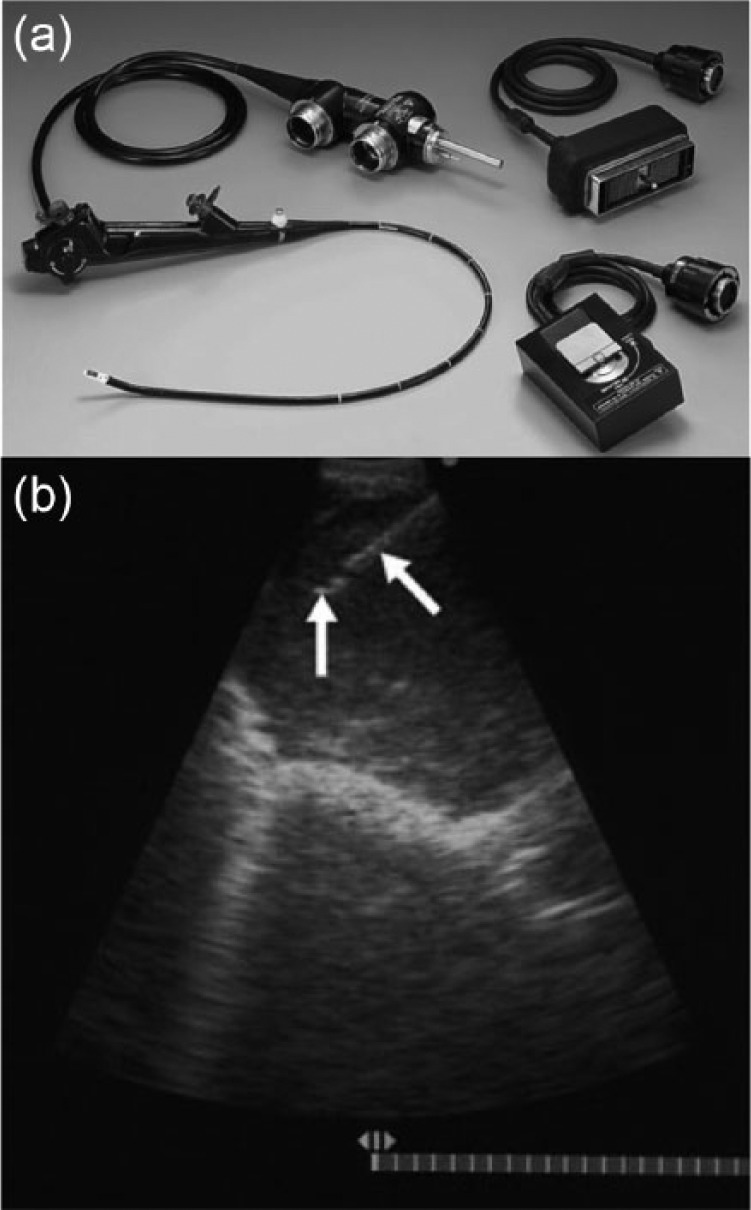
Convex endobronchial ultrasound (EBUS). (a) EBUS scope with associated white light and ultrasound cables. (b) Intraprocedural view of a biopsy. The white arrows point to the needle. Images © Georg Thieme Verlag KG.
EBUS-TBNA diagnosis of the mediastinum has a long and well established track record. A meta-analysis published in 2012 including 14 studies and 1658 patients reported a pooled sensitivity and specificity of 92% and 100%, respectively. In addition, the positive likelihood ratio was 5.1 and negative likelihood ratio was 0.13. There was no significant effect on the performance of the test with the use of rapid on site evaluation (ROSE). Only one serious complication occurred in a patient who developed intra-procedural hypoxemia and stridor. Three patients had minor hemorrhage.8
The results of the American College of Chest Physicians (ACCP) Quality Improvement Registry, Evaluation, and Education (AQuIRE) registry for EBUS-TBNA were released in 2011; the AQuiRE registry included data on 891 patients undergoing TBNA, the vast majority of which were EBUS-TBNA, from six different hospital centers. Using a rigorous definition of obtaining a specific diagnosis, they determined that the gross diagnostic yield was 50%. Hospital volume, patient smoking status, increased LN size, biopsy of more than two sites, and positron emission tomography/computed tomography positivity were all associated with higher yields.9 A safety analysis of the AQuiRE registry was published in 2013 and included the results of 1317 patients enrolled at six different hospital centers. Complications occurred in 19 patients, one of which was fatal. Pneumothorax was the most common complication and occurred in seven patients.10
In comparison, EBUS-TBNA has been shown to be equal to or better than mediastinoscopy in regards to the diagnostic yield of mediastinal LN sampling. That said, the two techniques should be considered synergistic as several studies have shown that each method is able to detect disease missed by the other.11–15
The accuracy of EBUS-TBNA in the restaging of treated lung cancer is less robust. Herth and colleagues studied 124 consecutive patients with stage IIIa disease with N2 nodal disease who underwent restaging EBUS-TBNA and then went on to have curative-intent surgery. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy of EBUS-TBNA in this setting were 76%, 100%, 100%, 20%, and 77%, respectively.16
Future directions of mediastinal staging
Single scope staging examinations
One of the major limitations of EBUS-TBNA is its inability to reach stations LN stations 8 and 9, potentially missing critical staging information. By combining EBUS-TBNA with endoscopic ultrasound guided biopsy (EUS-FNA), the reach of the ultrasonographic staging procedure is greatly increased.17 A meta-analysis of eight separate trials including 822 patients published in 2013 found that combined EBUS-TBNA and EUS-FNA staging outperformed either technique alone. In addition, the only complications detected were one pneumothorax and one procedure-induced LN infection.18
Hybrid EBUS scope
The hybrid EBUS (H-EBUS) scope was developed to address several limitations of conventional convex EBUS (C-EBUS) scopes, including the exaggerated oblique forward view and limited flexibility. A H-EBUS scope has been introduced which has a 10° viewing angle versus the traditional 35° angle, an anterior flexion angle of 130° and a slightly slimmer insertion diameter19 (Figure 2).
Figure 2.
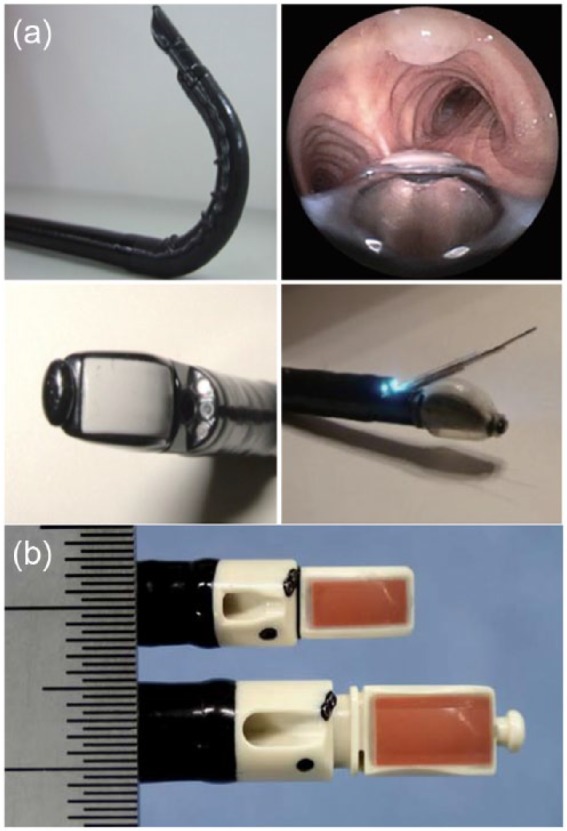
Endobronchial ultrasound (EBUS) scope advances. (a) Hybrid EBUS (H-EBUS) scope demonstrating augmented flexibility and the more acute viewing angle. Images adapted with permission from AME publishing company. (b) Size comparison of the standard EBUS scope (bottom) with the thin convex probe EBUS scope (top). Images © Georg Thieme Verlag KG.
The performance of the H-EBUS scope was evaluated in a single-center randomized trial in which the scope’s ability to perform a complete airway examination was compared with a conventional C-EBUS scope. The H-EBUS scope was significantly better able to visualize more airways than C-EBUS. This was especially true with visualization of the lower lobes. There was no difference in specimen adequacy or diagnostic yield.20
Thin convex probe EBUS bronchoscope
Thin convex probe EBUS (TCP-EBUS) scopes seek to access more lobar and interlobar LNs as well as more distal intraparenchymal lesions than are accessible with C-EBUS scopes. A TCP-EBUS scope has recently been introduced which has a 20° oblique viewing angle, a distal diameter of 5.9 mm versus 6.9 mm of the C-EBUS scope, and a 170° angle of flexion. These characteristics require a trade off in which the working channel is reduced from 2.2 to 1.7 mm and the maximum needle size is reduced from 21 to 25 gauge (G).21 No data currently exist comparing the diagnostic yield or specimen adequacy of a 21 G or 22 G to a 25 G EBUS needle in lung parenchyma. Several studies in the gastroenterology literature have addressed the adequacy of 25 G needle biopsies compared with larger needles and have found similar yields.22,23
The TCP-EBUS scope has been tested in a porcine model in which the TCP-EBUS scope was found to have a 14.7 mm greater endoscopic viewing range and 16.0 mm greater reach; this allowed the scope to traverse one to three bronchial generations deeper than a C-EBUS scope.21 These observations were repeated in a small study of explanted human lungs.24
Both the H-EBUS and TCP-EBUS scopes represent advancements in scope design which, coupled with the increasing cumulative experience with EBUS-TBNA, will likely continue to improve the ability of EBUS to acquire targets that were previously not within reach.
Elastography
Elastography has recently been applied to the evaluation of mediastinal LNs in lung cancer. Elastography measures the stiffness of tissue in response to local forces applied by physiologic activity such as cardiac motion. Elastography can potentially differentiate lung cancer-infiltrated LNs from normal LNs because infiltrated LNs are stiffer than noninfiltrated LNs.25
Izumo and colleagues conducted a study in which they evaluated the performance characteristics of a novel three-level elastography scale; the ability of the scale to predict malignancy was then evaluated. A total of 75 LNs were evaluated using this method and clinicopathologic correlation was obtained. The sensitivity, specificity, PPV, NPV, and diagnostic accuracy of elastography were 100%, 92%, 95%, 100%, and 97%, respectively. Based on these data, the authors have suggested a possible role for elastography as a tool to help select LNs for biopsy during EBUS-TBNA.26 These results are exciting but clearly require more study to evaluate their clinical utility.
Peripheral nodule biopsy
The solitary pulmonary nodule is a challenge frequently encountered by chest physicians, with approximately 150,000–1,500,000 detected each year in the United States.27,28 There are multiple possible avenues of evaluation for these nodules, accounting for part of the challenge in evaluating and managing them. The gold standard remains surgical resection in patients who are good surgical candidates with a high pre-test probability of malignant disease. In the setting of low to intermediate risk nodules or patients that either cannot have or do not want surgery, less invasive evaluation may be appropriate.29 These less invasive techniques include CT-guided biopsy (CTGBx) and guided bronchoscopic techniques.
CTGBx is the primary nonbronchoscopic method available for biopsy of lung nodules. The generally reported yield is over 90% for all nodules.30 The yield for lesions less than 2 cm is decreased and has been reported to be 70–77%, depending on the precise size of the nodules examined.31–33 The pneumothorax of CT-guided biopsy has been reported to be as high as 44.6%.34 More recent reports put the rate at approximately 25%.30,32,35
rEBUS and NB are the two primary modalities available to the bronchoscopist for the evaluation of peripheral lung lesions. Prior to discussion of peripheral nodule biopsy, it is important to stress that any planned peripheral lung biopsy procedures be preceded by thoughtful consideration of other potential lesions (hepatic, brain, LN etc.), the presence of cancer in which would result in significant upstaging. There may be minimal benefit in performing either rEBUS or NB for a peripheral lung lesion when metastatic sites are present. As such, biopsy targets should be chosen with an eye to obtaining staging and diagnostic information with a minimal number of procedures for the patient.2,3
Guided bronchoscopic approaches are an attractive alternative to CTGBx due to a potentially lower complication rate and ability to incorporate mediastinal and hilar nodule staging. That said, the diagnostic yield of guided bronchoscopic biopsy of peripheral lung lesions remains lower than that of CTGBx despite recent advances.
rEBUS
rEBUS use was first reported in 2002.36 rEBUS utilizes a flexible probe with an ultrasound unit mounted on the end. The probe generates a 360° ultrasound field of view, which allows for accurate real-time detection of solid lesions. Until recently, ground glass lesions (GGOs) were thought to be poorly defined using rEBUS, however recent data suggest that GGOs have an associated ‘blizzard’ ultrasound pattern.37 (Figure 3).
Figure 3.
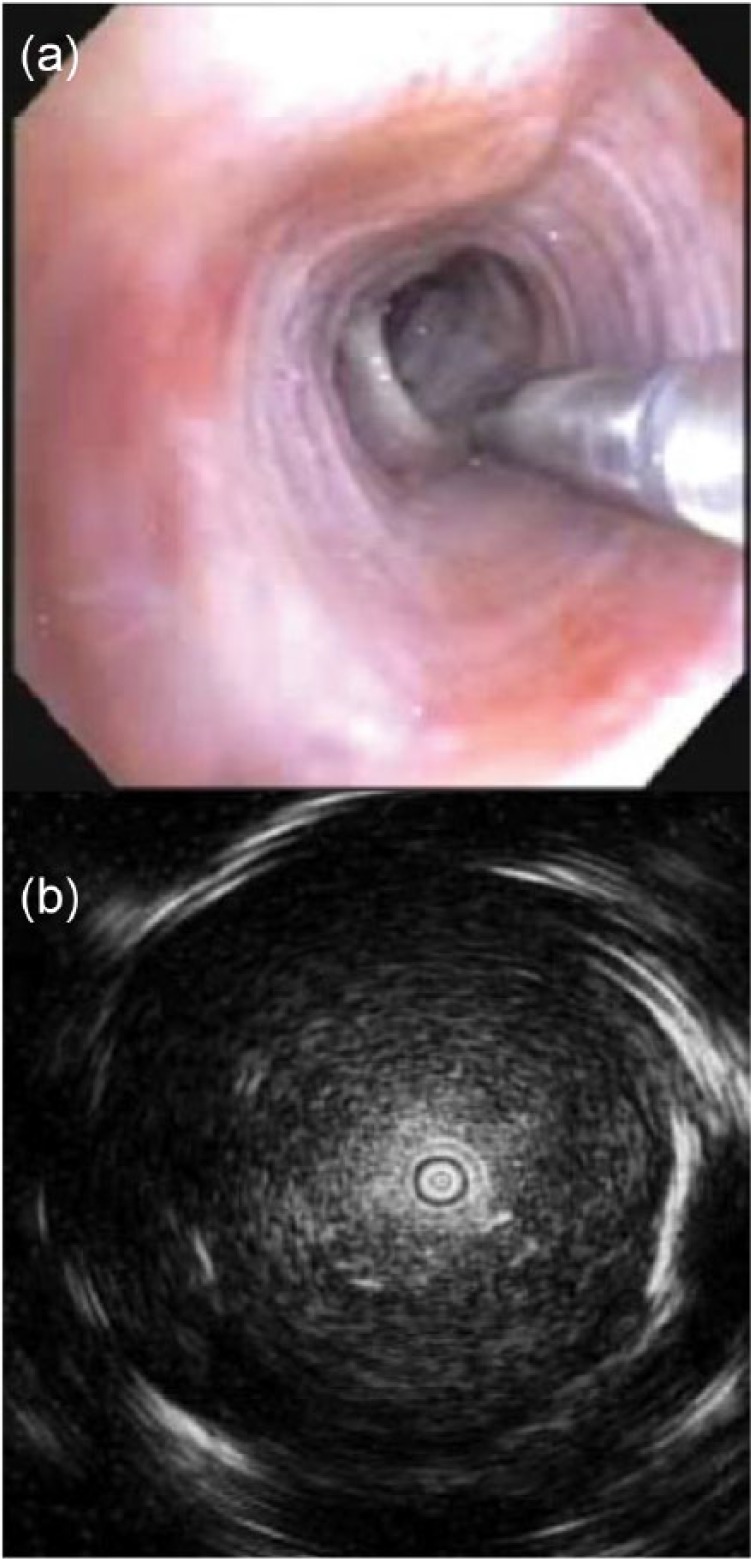
Radial endobronchial ultrasound. (a) Intraprocedural view of the radial probe being passed into a distal bronchus. (b) Typical appearance of a solid lesion with a concentric view. Images © Georg Thieme Verlag KG.
The bronchoscope and probe with or without a guide sheath are navigated through lobar, segmental, and sub-segmental airways until the lesion is located. Once located, the probe is removed and biopsy instruments are passed into the lesion.38 Multiple biopsy methods are available during the performance of rEBUS procedures. TBNA has been shown to be associated with the highest yield, followed by transbronchial biopsies; it is important to note that size constraints preclude the use of TBNA with a guide sheath.39
Multiple studies have evaluated the performance of rEBUS-guided biopsies. In 2011, a meta-analysis evaluated the results of 16 studies which included over 1400 patients. The meta-analysis included studies published in English in which the radial probe was used in the diagnosis of peripheral nodules. All study diagnoses were confirmed either histologically or by follow-up for at least 6 months. The combined point specificity was 100% and the point sensitivity was 73%. When stratified by lesion sizes of up to 20 mm and over 20 mm, the overall diagnostic yield was 56% and 78%, respectively. They reported a complication rate of 0–7.4% and a pooled pneumothorax rate of 1.0%, with 0.4% requiring tube thoracostomy.40
Another large retrospective study was reported in 2014 comprising a single center’s experience with 467 patients. They included cases in which rEBUS was used for diagnostic purposes; positive results were defined as a definitive diagnosis or inflammation if the lesion resolved on surveillance imaging. The diagnostic yield of lesions up to 20 mm and over 20 mm was 58% and 78%, respectively. Ultrasound probe position in relation to the lesion was shown to be a significant factor in regards to diagnostic yield, with concentric and eccentric views of the lesion being associated with diagnostic yields of 84% and 48%, respectively. The pneumothorax rate was 2.8% with a little over half of those patients with pneumothorax requiring tube thoracostomy. No episodes of significant bleeding were reported.41
Two recent publications have focused on the yield of rEBUS in GGOs. The first study examined 40 patients with a mean lesion diameter of 22 mm, a majority of which were classified as mixed ground glass or semi-solid. The authors were able to visualize 60% of the lesions by rEBUS. Biopsy of these lesions led to a diagnostic yield of 65%.42 The second study retrospectively examined 67 patients who underwent rEBUS under fluoroscopic guidance. The authors were able to visualize 79% of mixed GGOs and 55% of pure GGOs. As with the previous study, the majority of the lesions were mixed GGOs and biopsy of them led to a reported diagnostic yield of 57%. Increasing lesion size was associated with improved yield.42,43
These data suggest that rEBUS is a safe and effective modality for the sampling of peripheral lung nodules. Factors associated with higher diagnostic yields were nodules over 2 cm in diameter and a concentric orientation lung lesion to the rEBUS probe.
NB
There are two major types of NB, ENB and VNB. In ENB, externally generated magnetic fields are used to guide the bronchoscope and instruments to the target lesion. In VNB the software recognizes patterns from the white light images and integrates the information into a virtual map in which targets and other structures can be superimposed.
ENB
ENB was first described in 1998 by Solomon and colleagues. They used a technique in which a navigation device designed for use in other procedures was secured to a bronchoscope and used to biopsy artificially created targets in pigs.44 Currently, there are two commercially available systems in the United States: the SuperDimension System (Medtronic, Minneapolis, MN, USA) and the SPiNDrive/Perc System (Veran Medical Technologies, St Louis, MO, USA).
In order to perform ENB, a CT scan is obtained using specific slice thickness parameters and is loaded into the navigation software where a 4-D map is created. Following creation of the reconstructed image, the bronchoscopist plans their approach. The SuperDimension System uses a full inspiration only scan while the SPiNDrive System uses both inspiratory and expiratory scans to account for nodule movement during the respiratory cycle. A magnetic field is generated either by a board placed under the patient in the case of the SuperDimension System or by a hovering pad by the SPiNDrive System. The bronchoscopist then fine tunes the registration of the system and begins navigation. In the case of SuperDimension, the navigation system tracks a locatable guide inserted into a catheter. The locatable guide is removed once the lesion is located in order to pass instruments through the catheter to the target. In the case of the SPiNDrive System the biopsy instruments are tip tracked and used to sample the lesion in real time. While many factors appear to be involved in determining procedure success, use of needle aspiration appears to be superior to forceps biopsy45 (Figure 4).
Figure 4.
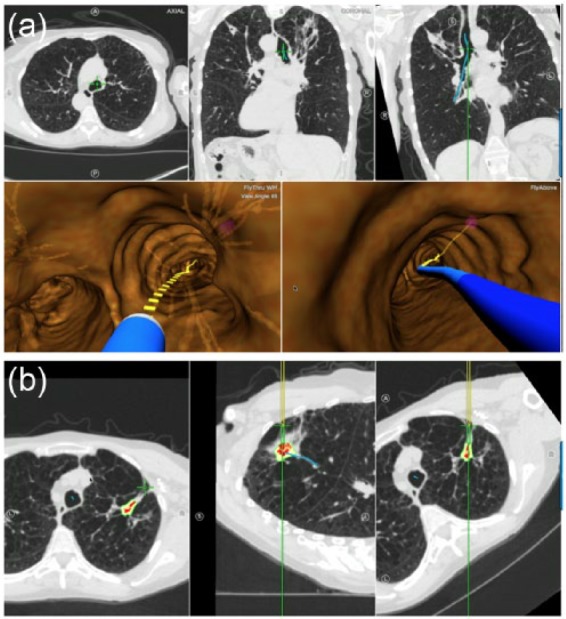
Electromagnetic navigational bronchoscopy. (a) Navigational bronchoscopy views. The top row is the real-time computed tomography (CT) scan views. The bottom row is the real-time virtual bronchoscopic images. (b) Real-time CT scan views guiding an electromagnetic-guided transthoracic needle biopsy procedure. Images courtesy of Veran Medical Technologies, St Louis, MO, USA.
In 2014, Gex and colleagues published a meta-analysis of ENB that included 15 studies and 1033 lung nodules, of which all but one trial used the SuperDimension System. They reported a diagnostic yield of 65%, a sensitivity of 71%, and a NPV of 52%. Interestingly, they reported that navigation to the lesion of interest was successful in 97% of cases. The studies variably used additional guidance modalities such as rEBUS and fluoroscopy in order to add real-time confirmation to the virtual navigation map. In multivariate analysis, the only factor independently associated with an increase in bronchoscopic yield was the presence of a bronchus sign. At the individual study level, the use of general anesthesia and ROSE were associated with better performance of ENB, while the use of fluoroscopy was inversely related. The pneumothorax rate was 3%, in which about half required a chest tube. The ‘minor or moderate’ bleeding rate was 1%.46
Several studies provide a possible explanation for the discrepancy between the ability to navigate to the lesion and the diagnostic yield by looking to the motion of lung nodules during inspiration and expiration. Chen and colleagues examined the relative motion of 46 nodules ranging in size from 6 to 42 mm between inspiratory and expiratory CT scans. They found an average motion of 18 mm with more motion in lower lobes.47 In addition, Leira and colleagues demonstrated in pig models that a wedged bronchoscope causes significant nodule displacement, likely due to the forces exerted by the bronchoscopist to maintain the wedged position.48
Few data are available specific to the Veran system. Yarmus and colleagues studied 24 patients with a solitary pulmonary nodule and radiographic N0 disease. All patients underwent EBUS-TBNA LN evaluation, followed by ENB to the target lesion, and then electromagnetic transthoracic needle aspiration (EMTTNA). EMTTNA is a technology specific to the VERAN system in which the navigation software is used to guide a needle through the chest wall into the target lesion. The combined diagnostic yield of all three sampling modalities was 92%, with ENB + EMTTNA having a diagnostic yield of 87%. Interestingly, ENB was diagnostic in only 33% of patients, highlighting the limitation of an endobronchial approach when attempting to biopsy lung nodules that are not associated with a bronchus. Five patients (24%) developed a pneumothorax, two of whom (8%) required a chest tube. Of note, four of the five patients who developed a pneumothorax were diagnosed via EMTTNA.49
VNB
VNB guidance for the biopsy of peripheral nodules was first described in a case report in 2002 by Asano and colleagues.50 At the time this manuscript was drafted, only one commercially available VNB system was available in the United States, the Bronchus LungPoint system (Bronchus, San Jose, CA, USA). Worldwide, the Bf-NAVI System (Olympus, Tokyo, Japan) is the most commonly used system. A new system, the Archimedes system (Bronchus) will likely be available shortly; it features the ability to perform bronchoscopic transparenchymal needle access (BTPNA).
To perform VNB, a preprocedure CT scan is obtained using the requisite protocol. The software then constructs a model of the patient’s airways, the user identifies the target lesions, and the system constructs pathways to the lesion. The target is then displayed on the virtual bronchoscopic view during the procedure, facilitating biopsy. The LungPoint system features pattern recognition software which recognizes and labels the subsegmental bronchi and superimposes other structures onto the image. Frequently, thin and ultrathin bronchoscopes are used during the procedure.51
Few data are available specific to the LungPoint system. Eberhardt and colleagues reported their experience with 25 patients with lesions less than 42 mm. They used both a conventional and an ultrathin bronchoscope. A diagnosis was obtained in 80% of the patients. Only one pneumothorax occurred which did not require intervention.52
A majority of the data surrounding VNB have been obtained using the Bf-NAVI System, which is not available in the United States. As of 2013, the diagnostic yields reported in studies containing more than 10 patients have ranged from 63% to 84%, with a weighted average yield for all lesions of 74%. The weighted average yield for lesions less than 2 cm was 67%. Of note, several studies used adjunct methods for real-time identification of the lesion, including rEBUS and radiographic guidance. The complication rates reported ranged from 0% to 4% with a weighted average yield of 1%. The complications reported included pneumothoraxes, hemorrhages, and bradycardic episodes.51
The two randomized clinical trials concerning the utility of VNB conducted to date, performed by Ishida and colleagues and Asano and colleagues, have produced somewhat contradictory results. In 2011, Ishida and colleagues performed a prospective, multicenter study in which they randomized 199 patients with an undiagnosed pulmonary nodule undergoing rEBUS to VBN-assisted EBUS or non-VBN-assisted groups. The diagnostic yield of the VNB group was 80% versus 67% for the group without VNB; the benefit trended towards being greater in smaller lesions.53 Asano and colleagues performed a prospective, multicenter study in which 334 patients were randomized to VNB and non-VNB groups; both patient groups used concurrent radiographic confirmation. The diagnostic yield of the VNB group was 67% and for the non-VNB group it was 60%.54
VNB has been shown to positively affect the diagnostic yield of lesions in the right upper lobe, lesions not visible on fluoroscopy, lesions in the peripheral outer third of the lungs, and lesions which surround the bronchus on rEBUS.51,55 Factors associated with decreased yields include location within the superior segment of the left lower lobe and nonsolid lesions.51
BTPNA
BTPNA is an emerging technique in which tools are guided by a VNB system from the bronchus to a lesion through the parenchyma in a tunnel created during the procedure.56 This technique has recently been tested in humans in small pilot studies. The first report, published in 2015, reported on the experience with 12 patients who underwent the technique and then went on to have a surgical resection. The target nodules had to be located at least 10 mm from the pleural surface. The procedure was performed under general anesthesia in an operating theater. Successful transparenchymal nodule access was possible in 10 of the 12 patients enrolled. All 10 biopsies obtained by BTPNA were diagnostic. Both patients in whom the procedure could not be completed had lesions located in the apical segment of the left upper lobe; two other patients with left upper lobe nodules were able to undergo the procedure. One patient had elevated troponins postprocedure; no other adverse reactions were noted. Postresection evaluation of the lung through which the transparenchymal access was performed showed no hemorrhage or parenchymal lacerations.57
The results of a second study have recently been reported in which six patients underwent the procedure in the bronchoscopy suite, also under general anesthesia but without subsequent surgical lung resection. A successful biopsy was obtained in five of the six patients. Again, the patient in whom the investigators were unable to obtain a specimen had a left upper lobe lesion, however this was due to a registration error. Another patient in the study had a left upper lobe lesion and was able to successfully undergo biopsy. The patients were observed for 72 h following the procedure. Two patients developed postoperative pneumothorax, one of whom required tube thoracostomy.45
Comparison of guided bronchoscopy methods
It is difficult to parse out the relative contributions of the various methods of guided bronchoscopy to overall yield given the overlapping use of several modalities in many studies. In 2012, Wang-Memoli and colleagues published the results of a meta-analysis addressing this topic. They analyzed the results of 39 studies in which 3052 individual nodules were evaluated. The pooled diagnostic yield of all guided bronchoscopy modalities was 70%. The contributions of the various analyzed techniques, ranked from highest to lowest estimated yield, were guide sheath guidance, VNB, rEBUS, ultrathin bronchoscopy, and ENB. The yield estimate ranged from 67% to 73%. Lesion size had a significant impact on overall yield, with an estimated yield of 61% and 83% for lesions up to 2 cm and over 2 cm, respectively. They found a pneumothorax rate of 1.5% and a chest tube insertion rate of 0.6%. One patient had severe hypoxemic respiratory failure.58
Recently, the results of the AQuIRE registry have been published in which the sequential results of 581 patients were entered into a registry and analyzed. The overall yield was 54% with the use of ENB and rEBUS associated with worsened yields of 57% and 39%, respectively when compared to a yield of 64% when neither technique was used. When the two guidance techniques were combined, the yield was 47%. The complications rate was 2%, almost all of which were pneumothorax driven. Two episodes of hypoxemic respiratory failure occurred and one episode of hemorrhage occurred. Again, the authors found that TBNA improved yield overall.59
The discrepancy between the two studies is confusing, particularly in light of earlier reports which indicated a synergistic effect of using ENB and rEBUS.60 The authors of the AQuiRE registry report note that there are several factors which can explain the low yield found in their publication compared with previous data. These proposed differences include publication bias in favor of small positive trials, a higher prevalence of cancer in the research setting, and the standardization of definitions for the study. In addition, the authors proposed that the study design may have resulted in the shifting of more challenging cases into guided bronchoscopy and in centers with both modalities and shifting of the most difficult cases into the less used modality.59 Others have noted that the bulk of cases in the AQuiRE registry utilized the SuperDimension System for EMN.61
The landscape for peripheral lung nodule evaluation and sampling remains complex, with mixed data and new technologies being introduced rapidly as lung cancer screening increases interest. Additional data and rigorous evaluation of current and novel technologies will be needed in order to continue to define the optimum approach and improve patient care.
Conclusion
The role of bronchoscopy in the evaluation and staging of lung cancer continues to rapidly expand. Introduction of EBUS and its increasing ubiquity have led to improvements in LN staging via ultrasound-guided needle biopsy of mediastinal and hilar LNs. In addition, renewed interest in peripheral lung nodule evaluation via existing and novel guided biopsy techniques has been driven by the recent publication of the National Lung Screening Trial (NSLT) and the subsequent United States Preventative Services Task Force (USPSTF) recommendation for lung cancer screening in high risk individuals.62,63 These and future advances have paved the way for what can only be considered an exciting future for advanced diagnostic bronchoscopy in the evaluation and staging of lung cancer.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Adam R. Belanger, Division of Pulmonary and Critical Care, Section of Interventional Pulmonology, University of North Carolina at Chapel Hill, NC, USA
Jason A. Akulian, Assistant Professor of Medicine, Director, Section of Interventional Pulmonology, Division of Pulmonary and Critical Care, University of North Carolina at Chapel Hill, 8007 Burnett Womack Bldg., CB 7219, Chapel Hill, NC 27713, USA.
References
- 1. National Cancer Institute. SEER cancer statistics factsheets: lung and bronchus cancer, http://seer.cancer.gov/statfacts/html/lungb.html. 2016
- 2. Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e142S–e165S. [DOI] [PubMed] [Google Scholar]
- 3. Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e211S–e250S. [DOI] [PubMed] [Google Scholar]
- 4. Herth FJ, Becker HD, Ernst A. Ultrasound-guided transbronchial needle aspiration: an experience in 242 patients. Chest 2003; 123: 604–607. [DOI] [PubMed] [Google Scholar]
- 5. Rusch VW, Crowley J, Giroux DJ, et al. The IASLC lung cancer staging project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2007; 2: 603–612. [DOI] [PubMed] [Google Scholar]
- 6. Gilbert C, Yarmus L, Feller-Kopman D. Use of endobronchial ultrasound and endoscopic ultrasound to stage the mediastinum in early-stage lung cancer. J Natl Compr Canc Netw 2012; 10: 1277–1282. [DOI] [PubMed] [Google Scholar]
- 7. Nakajima T, Yasufuku K. The techniques of endobronchial ultrasound-guided transbronchial needle aspiration. Innovations (Phila) 2011; 6: 57–64. [DOI] [PubMed] [Google Scholar]
- 8. Chandra S, Nehra M, Agarwal D, et al. Diagnostic accuracy of endobronchial ultrasound-guided transbronchial needle biopsy in mediastinal lymphadenopathy: a systematic review and meta-analysis. Respir Care 2012; 57: 384–391. [DOI] [PubMed] [Google Scholar]
- 9. Ost DE, Ernst A, Lei X, et al. Diagnostic yield of endobronchial ultrasound-guided transbronchial needle aspiration: results of the AQuIRE Bronchoscopy Registry. Chest 2011; 140: 1557–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eapen GA, Shah AM, Lei X, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration: results of the AQuIRE registry. Chest 2013; 143: 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herth F, Becker HD, Ernst A. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest 2004; 125: 322–325. [DOI] [PubMed] [Google Scholar]
- 12. Ernst A, Anantham D, Eberhardt R, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008; 3: 577–582. [DOI] [PubMed] [Google Scholar]
- 13. Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. J Am Med Assoc 2010; 304: 2245–2252. [DOI] [PubMed] [Google Scholar]
- 14. Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thora Cardiovasc Surg 2011; 142: 1393–1400. e1. [DOI] [PubMed] [Google Scholar]
- 15. Um SW, Kim HK, Jung SH, et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small-cell lung cancer. J Thorac Oncol 2015; 10: 331–337. [DOI] [PubMed] [Google Scholar]
- 16. Herth FJ, Annema JT, Eberhardt R, et al. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol 2008; 26: 3346–3350. [DOI] [PubMed] [Google Scholar]
- 17. Bonta PI, Crombag L, Annema JT. Linear endobronchial and endoesophageal ultrasound: a practice change in thoracic medicine. Curr Opin Pulm Med 2016; 22: 281–288. [DOI] [PubMed] [Google Scholar]
- 18. Zhang R, Ying K, Shi L, et al. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal lymph node staging of lung cancer: a meta-analysis. Eur J Cancer 2013; 49: 1860–1867. [DOI] [PubMed] [Google Scholar]
- 19. Xiang Y, Zhang F, Akulian J, et al. EBUS-TBNA by a new Fuji EBUS scope (with video). J Thorac Dis 2013; 5: 36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yarmus L, Akulian J, Ortiz R, et al. A randomized controlled trial evaluating airway inspection effectiveness during endobronchial ultrasound bronchoscopy. J Thorac Dis 2015; 7: 1825–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wada H, Hirohashi K, Nakajima T, et al. Assessment of the new thin convex probe endobronchial ultrasound bronchoscope and the dedicated aspiration needle: a preliminary study in the porcine lung. J Bronchology Interv Pulmonol 2015; 22: 20–27. [DOI] [PubMed] [Google Scholar]
- 22. Gimeno-Garcia AZ, Elwassief A, Paquin SC, et al. Randomized controlled trial comparing stylet-free endoscopic ultrasound-guided fine-needle aspiration with 22-G and 25-G needles. Dig Endosc 2014; 26: 467–473. [DOI] [PubMed] [Google Scholar]
- 23. Yang MJ, Yim H, Hwang JC, et al. Endoscopic ultrasound-guided sampling of solid pancreatic masses: 22-gauge aspiration versus 25-gauge biopsy needles. BMC Gastroenterol 2015; 15: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel P, Wada H, Kato T, et al. First evaluation of the new thin convex probe endobronchial ultrasound (TCP-EBUS) in human ex-vivo lungs. Chest 2015; 148: 814A. [Google Scholar]
- 25. Trosini-Désert V, Jeny F, Taillade L, et al. Bronchial endoscopic ultrasound elastography: preliminary feasibility data. Eur Respir J 2013; 41: 477–479. [DOI] [PubMed] [Google Scholar]
- 26. Izumo T, Sasada S, Chavez C, et al. Endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Jpn J Clin Oncol 2014; 44: 956–962. [DOI] [PubMed] [Google Scholar]
- 27. Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med 2003; 348: 2535–2542. [DOI] [PubMed] [Google Scholar]
- 28. Gould MK, Tang T, Liu IL, et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med 2015; 192: 1208–1214. [DOI] [PubMed] [Google Scholar]
- 29. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e93S–120S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geraghty PR, Kee ST, McFarlane G, et al. CT-guided transthoracic needle aspiration biopsy of pulmonary nodules: needle size and pneumothorax rate. Radiology 2003; 229: 475–481. [DOI] [PubMed] [Google Scholar]
- 31. Wallace MJ, Krishnamurthy S, Broemeling LD, et al. CT-guided percutaneous fine-needle aspiration biopsy of small (< or =1-cm) pulmonary lesions. Radiology 2002; 225: 823–828. [DOI] [PubMed] [Google Scholar]
- 32. Ohno Y, Hatabu H, Takenaka D, et al. CT-guided transthoracic needle aspiration biopsy of small (< or = 20 mm) solitary pulmonary nodules. AJR Am J Roentgenol 2003; 180: 1665–1669. [DOI] [PubMed] [Google Scholar]
- 33. Kothary N, Lock L, Sze DY, et al. Computed tomography-guided percutaneous needle biopsy of pulmonary nodules: impact of nodule size on diagnostic accuracy. Clin Lung Cancer 2009; 10: 360–363. [DOI] [PubMed] [Google Scholar]
- 34. Kazerooni EA, Lim FT, Mikhail A, et al. Risk of pneumothorax in CT-guided transthoracic needle aspiration biopsy of the lung. Radiology 1996; 198: 371–375. [DOI] [PubMed] [Google Scholar]
- 35. Yeow KM, Su IH, Pan KT, et al. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest 2004; 126: 748–754. [DOI] [PubMed] [Google Scholar]
- 36. Herth FJF, Ernst A, Becker HD. Endobronchial ultrasound-guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J 2002; 20: 972–974. [DOI] [PubMed] [Google Scholar]
- 37. Izumo T, Sasada S, Chavez C, et al. Radial endobronchial ultrasound images for ground-glass opacity pulmonary lesions. Eur Respir J 2015; 45: 1661–1668. [DOI] [PubMed] [Google Scholar]
- 38. Kikuchi E, Yamazaki K, Sukoh N, et al. Endobronchial ultrasonography with guide-sheath for peripheral pulmonary lesions. Eur Respir J 2004; 24: 533–537. [DOI] [PubMed] [Google Scholar]
- 39. Chao TY, Chien MT, Lie CH, et al. Endobronchial ultrasonography-guided transbronchial needle aspiration increases the diagnostic yield of peripheral pulmonary lesions: a randomized trial. Chest 2009; 136: 229–236. [DOI] [PubMed] [Google Scholar]
- 40. Steinfort DP, Khor YH, Manser RL, et al. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J 2011; 37: 902–910. [DOI] [PubMed] [Google Scholar]
- 41. Chen A, Chenna P, Loiselle A, et al. Radial probe endobronchial ultrasound for peripheral pulmonary lesions. A 5-year institutional experience. Ann Am Thor Soc 2014; 11: 578–582. [DOI] [PubMed] [Google Scholar]
- 42. Izumo T, Sasada S, Chavez C, et al. The diagnostic utility of endobronchial ultrasonography with a guide sheath and tomosynthesis images for ground glass opacity pulmonary lesions. J Thorac Dis 2013; 5: 745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ikezawa Y, Sukoh N, Shinagawa N, et al. Endobronchial ultrasonography with a guide sheath for pure or mixed ground-glass opacity lesions. Respiration 2014; 88: 137–143. [DOI] [PubMed] [Google Scholar]
- 44. Solomon SB, White P, Jr, Acker DE, et al. Real-time bronchoscope tip localization enables three-dimensional CT image guidance for transbronchial needle aspiration in swine. Chest 1998; 114: 1405–1410. [DOI] [PubMed] [Google Scholar]
- 45. Harzheim D, Sterman D, Shah PL, et al. Bronchoscopic transparenchymal nodule access: feasibility and safety in an endoscopic unit. Respiration 2016; 91: 302–306. [DOI] [PubMed] [Google Scholar]
- 46. Gex G, Pralong JA, Combescure C, et al. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration 2014; 87: 165–176. [DOI] [PubMed] [Google Scholar]
- 47. Chen A, Pastis N, Furukawa B, et al. The effect of respiratory motion on pulmonary nodule location during electromagnetic navigation bronchoscopy. Chest 2015; 147: 1275–1281. [DOI] [PubMed] [Google Scholar]
- 48. Leira HO, Lango T, Sorger H, et al. Bronchoscope-induced displacement of lung targets: first in vivo demonstration of effect from wedging maneuver in navigated bronchoscopy. J Bronchology Interv Pulmonol 2013; 20: 206–212. [DOI] [PubMed] [Google Scholar]
- 49. Yarmus LB, Arias S, Feller-Kopman D, et al. Electromagnetic navigation transthoracic needle aspiration for the diagnosis of pulmonary nodules: a safety and feasibility pilot study. J Thorac Dis 2016; 8: 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Asano F, Matsuno Y, Matsushita T, et al. Transbronchial diagnosis of a pulmonary peripheral small lesion using an ultrathin bronchoscope with virtual bronchoscopic navigation. J Bronchol 2002; 9: 108–111. [Google Scholar]
- 51. Asano F, Eberhardt R, Herth FJ. Virtual bronchoscopic navigation for peripheral pulmonary lesions. Respiration 2014; 88: 430–440. [DOI] [PubMed] [Google Scholar]
- 52. Eberhardt R, Kahn N, Gompelmann D, et al. LungPoint – a new approach to peripheral lesions. J Thorac Oncol 2010; 5: 1559–1563. [DOI] [PubMed] [Google Scholar]
- 53. Ishida T, Asano F, Yamazaki K, et al. Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: a randomised trial. Thorax 2011; 66: 1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Asano F, Shinagawa N, Ishida T, et al. Virtual bronchoscopic navigation combined with ultrathin bronchoscopy. A randomized clinical trial. Am J Respir Crit Care Med 2013; 188: 327–333. [DOI] [PubMed] [Google Scholar]
- 55. Asano F, Matsuno Y, Shinagawa N, et al. A virtual bronchoscopic navigation system for pulmonary peripheral lesions. Chest 2006; 130: 559–566. [DOI] [PubMed] [Google Scholar]
- 56. Silvestri GA, Herth FJ, Keast T, et al. Feasibility and safety of bronchoscopic transparenchymal nodule access in canines: a new real-time image-guided approach to lung lesions. Chest 2014; 145: 833–838. [DOI] [PubMed] [Google Scholar]
- 57. Herth FJ, Eberhardt R, Sterman D, et al. Bronchoscopic transparenchymal nodule access (BTPNA): first in human trial of a novel procedure for sampling solitary pulmonary nodules. Thorax 2015; 70: 326–332. [DOI] [PubMed] [Google Scholar]
- 58. Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012; 142: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ost DE, Ernst A, Lei X, et al. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. Results of the AQuIRE registry. Am J Respir Crit Care Med 2016; 193: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med 2007; 176: 36–41. [DOI] [PubMed] [Google Scholar]
- 61. Semaan RW, Lerner AD, Lee HJ, et al. Electromagnetic guidance for the diagnosis of pulmonary nodules: don’t put the nail in the Coffin. Am J Respir Crit Care Med 2016; 194: 121. [DOI] [PubMed] [Google Scholar]
- 62. National Lung Screening Trial Research Team,Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365: 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moyer VA and. U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160: 330–338. [DOI] [PubMed] [Google Scholar]


