Abstract
For a long time, vitamin D was regarded as an essential component for the maintenance of appropriate calcium metabolism. Indeed, the calcium-related functions were broadly studied and validated in numerous clinical and epidemiologic studies. All of these vitamin D effects are mediated by a specific receptor. Remarkably, recent investigations show that the vitamin D receptor (VDR) also affects autoimmunity and by these means, the course of neoplasias and tissue inflammation. Moreover, the VDR regulates genes that affect cellular activity including cell differentiation and apoptosis and, by these means, angiogenesis. Actually, vitamin D deficiency has been associated with structural and functional cardiovascular changes that can be reversed by receptor stimulation. In this regard, some of the injurious effects of vitamin D deficiency such as myocardial hypertrophy and high blood pressure seem linked to increased renin-angiotensin activity. Interestingly, chronic renal disease, a condition often associated with greater cardiovascular risk, high blood pressure, myocardial hypertrophy and inappropriate stimulation of the renin angiotensin system, is also tied to inadequate vitamin D activity. In fact, studies in several animal models such as the rat ureteral obstruction model, the 5/6 nephrectomy model and others, clearly show that VDR stimulation prevents both structural and functional changes in the heart and the kidney. Clinical trials are needed to validate the vitamin D potential benefits in chronic kidney disease and its associated cardiovascular risk.
Keywords: cardiovascular risk, chronic kidney disease, vitamin D receptor
Introduction
Recent investigations have deeply changed our notions regarding vitamin D involvement on fundamental metabolic functions. Indeed, this was a compound seen at the beginning of the last century as a miracle substance that could reverse rickets, an ominous and mysterious disease, rampant among poor children living in polluted cities around the world. In fact, because early notions saw vitamin D as a compound essential to prevent the bony abnormalities seen in animal models and in clinical situations, the concept was tightly attached to calcium and bone metabolism. Certainly, without vitamin D, only 10–15% of the calcium would be absorbed in the gastrointestinal tract. It is the interaction of vitamin D with its receptor that increases intestinal calcium absorption by about 30–40% and phosphorus absorption by nearly 80% [Holick and Garabedian, 2006; Bouillon, 2001; de Luca, 2004; Heaney et al. 2003]. Unfortunately, despite the remarkable advances in the knowledge and understanding of the nature and mechanisms of action of vitamin D, rickets continues to be diagnosed. Moreover, the US Food and Drug Administration, recommends the addition of vitamin D to milk and cereals hoping for a reduction in the incidence of rickets in children and osteoporosis in adults.
Be that as it may, modern literature shows that vitamin D also regulates gene expression associated with autoimmunity, tumours and infections and it does so by controlling the activation of a specific receptor, the vitamin D receptor (VDR), a type 1 nuclear receptor and a DNA transcription factor. Moreover, the traditional notion limiting the conversion of circulating 25-hydroxy vitamin D to the active 1,25-dihydroxy vitamin D only in the kidney, has been changed as most tissues and cells in the body have not only VDRs but also the enzymatic arrangement to generate the active form. In addition, newer investigations are showing an assortment of critical functions played by the vitamin D–VDR interactions. Certainly the VDR is present in intestinal cells, the central nervous system, prostate, and other tissues [de Luca, 2004; Holick, 2006a; Dusso et al. 2005].
Thus, the previous notion limiting vitamin D actions purely to calcium and phosphate metabolism has been changed. For instance, individuals with 25-hydroxy vitamin D blood levels lower than 20 ng/ml have a 30–50% higher incidence of breast, prostate, colon and other malignancies [Luscombe et al. 2001; Gorham et al. 2005; Giovannucci et al. 2006; Ahonen et al. 2000; Holick 2006b; Nagpal and Rathnachalam, 2005].
VDR effects unrelated to calcium-phosphorus metabolism
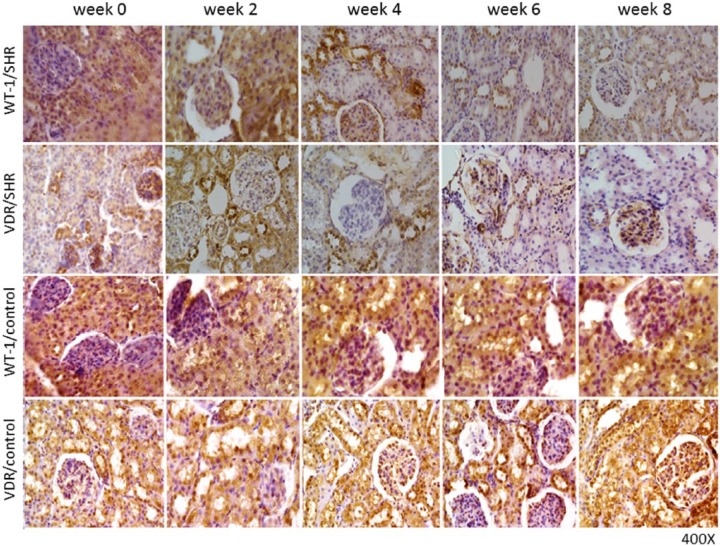
Associations between circulating vitamin D levels and disorders as diverse as osteoarthritis, rheumatoid arthritis, type 1 diabetes, inadequate insulin synthesis and schizophrenia have been described. Although much work is needed to confirm and understand all these associations, current evidences seem to indicate that vitamin D exerts crucial cellular functions. Indeed, directly or indirectly, 1,25-dihydroxy vitamin D regulates many genes, including those responsible for the regulation of cellular proliferation, differentiation, apoptosis, and angiogenesis [Holick, 2006a; Krause et al. 1998; Thomas et al. 1998]. Moreover, detecting the VDR in most body cells suggested a role for vitamin D in many body systems and thereby, not limiting its impact only to calcium metabolism. In this regard, we recently showed that changes in the expression pattern of the WT-1 transcription factor, a key mediator of nephrogenesis, could contribute to anatomical and functional kidney disorders linked to hypertension development. These changes could respond to VDR modulation (Figure 1) [Mazzei et al. 2016].
Figure 1.
Histological sections of neonatal SHR group and the control group Wistar Kyoto rats (WKYs) kidney cortices.
Newborn male SHRs and WKYs, were evaluated during their first 8 weeks of life (from birth, i.e. week 0–8).
After 4 weeks of hypertension evolution, decreased WT-1 and VDR immunostaining levels were seen in cortex tubule cells. Moreover, after 8 weeks of hypertension development, a greater decrease in WT-1 and VDR immunostaining levels were seen in cortex tubule cells, compared with what was seen in newborn SHRs. Contrary, in WKYs, WT-1 and VDR immunostaining was relatively high in the epithelial duct segments. Magnification: ×400.
SHR, spontaneously hypertensive rat; VDR, vitamin D receptor; WKY, Wistar Kyoto rats.
Cardiovascular effects
The vitamin D cell effects recently described may explain the increased risk of hypertension and cardiovascular illness as described in individuals living in areas where sun exposure is lower. In these populations, hypertensive patients were exposed to ultraviolet B radiation three times a week for 3 months. As a result, 25-hydroxy vitamin D levels rose by about 180%, and blood pressure normalized [Krause et al. 1998; Thomas et al. 1998]. This is consistent with epidemiological studies linking lower levels of cholecalciferol to cardiovascular diseases.
Moreover, vitamin D deficiency has been associated with congestive heart failure and increased levels of inflammatory factors such as interleukin and C-reactive protein [Kong and Li, 2003]. This seems particularly important, as vitamin D could then be a natural vascular protector factor. In fact, experimental vitamin D deficiency is accompanied by increased renin expression and angiotensin II concentration, high blood pressure and cardiac hypertrophy [Li et al. 2002; Xiang et al. 2005; Kong et al. 2010; Ferder et al. 2013]. In agreement with these findings, VDR-knockout mice develop high blood pressure and left ventricular hypertrophy [Xiang et al. 2005], whereas vitamin D analogues improve left ventricular hypertrophy and diastolic function in the spontaneously hypertensive rat (SHR) [Ferder et al. 2013].
Moreover, combined paricalcitol and enalapril therapy ameliorated the cardiac oxidative injury occurring in a rat uremic model [Husain et al. 2009], and protected from the inflammatory and oxidative endothelial damage observed during the development of atherosclerosis [Husain et al. 2010]. In this respect, atherosclerosis is regarded as a chronic inflammatory condition that precedes clinical situations such as cardiovascular dysfunction, myocardial infarction, unstable angina, sudden cardiac death, stroke and peripheral thromboses. Moreover, it has been proposed that the renin-angiotensin system (RAS), through its main mediator, angiotensin II, also has a direct influence on the progression of the atherosclerotic process via effects on endothelial function, inflammation, fibrinolytic balance, and plaque stability [Husain et al. 2015]. Preclinical as well as clinical studies have provided evidence clearly demonstrating that vitamin D triggers vascular benefits. Indeed, vitamin D reduces the progression of coronary atherosclerosis in patients with stable angina pectoris, decreases vascular inflammatory markers and improves common carotid intima-media thickness and plaque volume in patients with diagnosed atherosclerosis [Artaza et al. 2009; Gunta et al. 2013; Norman and Powell, 2014; Panizo et al. 2013]. Moreover, a recent study on an older Australian population not only showed the beneficial effects of vitamin D on systemic arterial inflammation but also on circulating lipids levels and on biomarkers of endothelial cell activation. The authors concluded that higher vitamin D status might protect the endothelium [Alyami et al. 2016].
Interestingly, a recent investigation in an Italian population showed that a genetic variation of the VDR was associated with type 2 diabetes and impaired insulin secretion in adults, and increased cardio-metabolic risk in children [Sentinelli et al. 2016]. Notwithstanding a continued appreciation of the potential role of vitamin D in the prevention of cardiovascular disease, we are still missing a whole large-scale, randomized trial testing this agent for the primary prevention of cardiovascular disease. In this line of investigation, the VITamin D and OmegA-3 TriaL (VITAL), a randomized, double-blind, placebo-controlled trial is currently searching for benefits and risks of vitamin D and marine omega-3 fatty acids in the primary prevention of cancer and cardiovascular disease among 25,875 patients [Pradhan and Manson, 2016; Bassuk et al. 2016]. Be that as it may, current data are inconclusive as to whether supplementation with these agents reduces cardiovascular disease risks, and other nonskeletal illnesses in the general population. The results of VITAL are expected to help in individual decisions, in clinical recommendations, and in public health guidelines.
These novel notions on the beneficial cardiovascular effects of vitamin D are being expanded by several lines of investigation that show that the vitamin D protecting actions could be extended not only to the heart and the brain but also to the kidney [Levey et al. 2003].
Renal effects of vitamin D
This area of information is particularly important as, regardless its nature, chronic kidney disease increases cardiovascular risk [Sarnak et al. 2003; Holick, 2007]. Thus, vitamin D-induced renal protection could indirectly reduce cardiovascular risk by reducing renal disease progression. If so, protecting the diseased kidney with vitamin D could become a therapeutic goal not only to prevent cardiovascular or renal disease progression, but also to satisfy the multiple vitamin D needs. Indeed, the kidney, although not the sole regulator of 1,25-dihydroxy cholecalciferol, continues to be a major producer of this most active vitamin D metabolite and as a result, advanced kidney injury could have significant consequences not only on bone metabolism (rickets, osteomalacia, secondary hyperparathyroidism) but also in other organ systems, including cellular proliferation, differentiation and angiogenesis [Holick,2007; Branisteanu et al. 1993].
This is why much attention is being placed on the vitamin D effects on the kidney. Indeed, vitamin D has been shown to decrease protein excretion rate in Heymann nephritis, an experimental model that replicates membranous nephropathy in the human [Lemire et al. 1992], and in other nephropathy models [Lillevang et al. 1992; Schwarz et al. 1998]. These studies in animal models suggest that vitamin D exerts a protective effect on the diseased kidney as suggested by the reduction in albumin excretion rate.
In this respect, we have addressed this notion in the obstructive uropathy rat model. Indeed, VDR activation through paricalcitol prevents fibrosis and decreases the number of TUNEL-positive apoptotic cells in this ureteral obstruction rat model. By electron microscopy, these changes are attended by mitochondrial expansion, dilated crests and wider gaps. Interestingly, all these changes are inhibited by paricalcitol. Moreover, the increased AT1-receptor mRNA and nicotinamide adenine dinucleotide phosphate (NADPH) activity observed in untreated rats with neonatal obstructive uropathy are reversed in mitochondrial fractions from paricalcitol-treated animals in the same rat model. This notion strongly suggests that the paricalcitol-induced rise in VDR expression exerts beneficial effects that are mediated at least in part by protective effects on the mitochondria (Figure 2) [García et al. 2012].
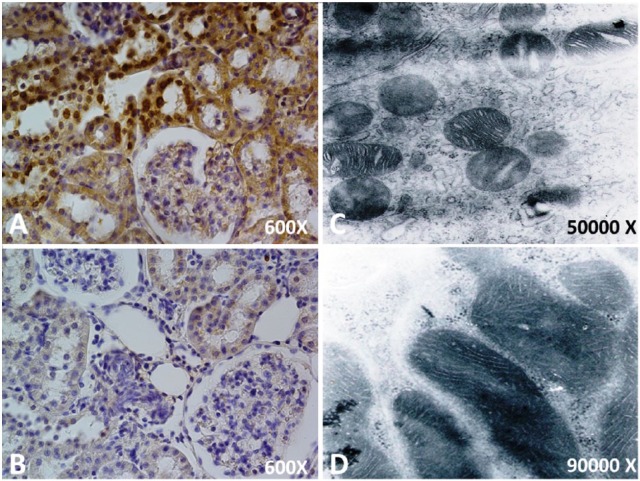
Figure 2.
Kidney cortices histological sections following unilateral obstruction for 15 days. The cytoprotective effect of paricalcitol is shown.
(A) Localization of apoptotic nuclei by TUNEL technique. Apoptotic nuclei appear as heavy brown-stained nuclei in tubule epithelial cells. After 15 days of ipsilateral obstruction in the renal cortex, apoptotic nuclei appear as heavy brown-stained nuclei in dilated collecting ducts and in lesser proportions in proximal tubules.
(B) There is a slight increase in apoptotic cells in epithelium from dilated collecting ducts and proximal tubules in the obstructed kidney cortices of the paricalcitol treatment.
Electron microscopy study of the cortex of the kidney following unilateral obstruction for 15 days. Effects of paricalcitol at the mitochondrial level are shown.
(C) Electron microscopy obtained from the cortical cortex of an obstructed kidney without treatment. Note that the mitochondria are present in the space between the dilated mitochondrial crestae in nontreated tubules (C). Presumably, both pictures (C and D) correspond to convoluted distal tubules, but, because of the specific experimental conditions, most of the ultrastructural characteristics were modified.
(D) Electron microscopy obtained from the cortical cortex of an obstructed kidney treated with paricalcitol.
Remarkable, previous studies by Khong and colleagues have shown that low levels of vitamin D are associated with elevated activity of the RAS and hypertension [Kong et al. 2010]. For these reasons, SHRs were treated with paricalcitol. Although blood pressure was not strikingly reduced in the paricalcitol-treated group, fibrosis, apoptosis, mitochondrial damage, and NADPH oxidase activity were reduced in the paricalcitol-treated SHRs. Additionally, high AT1 receptor expression, like low heat shock protein 70 (Hsp70) expression, were reversed in the renal cortices of paricalcitol-treated animals. The recovery parameters were consistent with an improvement in VDR expression. These data suggested that VDR-modulated Hsp70/AT1 is involved in the mechanism by which paricalcitol provides renal protection in SHRs [García et al. 2014; Manucha, 2014].
This is particularly relevant because it is known that Hsp70 regulates signalling pathways for cellular oxidative stress responses. Hsp70 has been shown to protect against angiotensin II-induced hypertension and to exert a cytoprotective effect [Molina et al. 2016; Rodríguez-Iturbe et al. 2012; Bocanegra et al. 2010; Nishiyama and Hitomi, 2010].
Notably, cardiovascular disease is often associated with chronic kidney disease and vice versa. Thus, it seems feasible that myocardial VDRs are among the likely links between the two disorders. Unfortunately, the structural and electrophysiological effects of myocardial VDR modification and its impact on the response to ischaemia-reperfusion are currently unknown. In this regard, we have studied the myocardial changes linked to VDR deficiency in Langendorff-perfused hearts in the obstructive nephropathy rat model. Ureteral obstructed rats showed a reduction in VDR and increase angiotensin II type 1 receptor expression. These rats showed fibrosis and myofibril reduction with an increase in mitochondrial size and dilated crests. These changes were reversed by paricalcitol. In addition, during ischemia-reperfusion, paricalcitol-treated ureteral-obstructed rats had a lower incidence of ventricular fibrillation compared with untreated ureteral-obstructed rats. The action potential duration was prolonged throughout the experiment in paricalcitol-treated rats. Therefore, the reduction in myocardial VDR expression in this rat model seems linked to myocardial remodelling and increase arrhythmogenesis, as paricalcitol protects against these changes by restoring myocardial VDR levels and prolonging action potentials [Diez et al. 2015].
In agreement with these concepts, we have recently expanded these notions in 5/6 nephrectomy rats using calcitriol (1,25-dihydroxy vitamin D3). Indeed, calcitriol not only lowered blood pressure in this salt-sensitive hypertension model but also lessened renal function changes, left ventricular hypertrophy, and myocardial AT1 receptor-mediated oxidative stress [Bordcoch et al. 2014].
In brief, activation of the VDR seems to protect the kidney from disease progression through intracellular effects that affect mitochondrial structure and function. These effects seem to extend to the heart where hypertrophy and remodelling are inhibited by activation of the VDR, either from paricalcitol or from calcitriol. Whether the beneficial events on the heart are direct effects from receptor activation or secondary to renal protection is yet to be defined. Nevertheless, clinical trials are needed to confirm the vitamin D beneficial effects on the heart and the kidney. Up to now, randomized clinical trials have yielded encouraging though indefinite results [Shoben et al. 2008; Thadhani et al. 2011; Agarwal et al. 2011]. Certainly, conclusions such as these should open up a novel and interesting therapeutic approach for the treatment of chronic kidney disease and its associated cardiovascular risk.
Acknowledgments
Both authors contributed to conception and design of the review, with substantial contribution to data, analysis and interpretation of the data, drafting of the article, and critical revision of the article for intellectual content.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, or publication of this article: This work was supported by grants from the Research and Technology Council of Cuyo University (SECyT), Mendoza, Argentina, and from the National Council of Scientific and Technical Research (CONICET) PIP 2010-2012, both of which were awarded to Walter Manucha. Grant no. PICT 2012-0234 Préstamo BID 2777 OC/AR.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Walter Manucha, Instituto de Medicina y Biología Experimental de Cuyo (IMBECU), Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), Mendoza, Argentina Área de Farmacología, Departamento de Patología, Facultad de Ciencias Médicas, Universidad Nacional de Cuyo, Mendoza, Argentina.
Luis I. Juncos, Fundación J. Robert Cade, Pedro de Oñate 253-Córdoba 5003, Argentina.
References
- Agarwal R., Hynson J., Hecht T., Light R., Sinha A. (2011) Short-term vitamin D receptor activation increases serum creatinine due to increased production with no effect on the glomerular filtration rate. Kidney Int 80: 1073–1079. [DOI] [PubMed] [Google Scholar]
- Ahonen M., Tenkanen L., Teppo L., Hakama M., Tuohimaa P. (2000) Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control 11: 847–852. [DOI] [PubMed] [Google Scholar]
- Alyami A., Lam V., Soares M., Zhao Y., Sherriff J., Mamo J., et al. (2016) The association of vitamin D status with dyslipidaemia and biomarkers of endothelial cell activation in older Australians. Nutrients 8 pii: E457. DOI: 10.3390/nu8080457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artaza J., Mehrotra R., Norris K. (2009) Vitamin D and the cardiovascular system. Clin J Am Soc Nephrol 4: 1515–1522. [DOI] [PubMed] [Google Scholar]
- Bassuk S., Manson J., Lee I., Cook N., Christen W., Bubes V., et al. (2016) Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials 47: 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocanegra V., Manucha W., Peña M., Cacciamani V., Vallés P. (2010) Caveolin-1 and Hsp70 interaction in microdissected proximal tubules from spontaneously hypertensive rats as an effect of Losartan. J Hypertens 28: 143–155. [DOI] [PubMed] [Google Scholar]
- Bordcoch G., Masjoan Juncos J., Manucha W., Juncos L. (2014) La vitamina D disminuye la síntesis de superóxido y la hipertrofia ventricular inducida por angiotensina II en ratas con nefrectomía 5/6. Abstract from XXXV Annual Meeting of the Argentine Council for High Blood Pressure. Puerto Madero, Buenos Aires, Argentina. [Google Scholar]
- Bouillon R. (2001) Vitamin, D: from photosynthesis, metabolism, and action to clinical applications. In: DeGroot L., Jameson J. (eds), Endocrinology, Philadelphia, US: W.B Saunders, pp. 1009–1028. [Google Scholar]
- Branisteanu D., Leenaerts P., van Damme B., Bouillon R. (1993) Partial prevention of active Heymann nephritis by 1 alpha, 25 dihydroxyvitamin D3. Clin Exp Immunol 94: 412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca H. (2004) Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 80(Suppl. 6): 1689S–1696S. [DOI] [PubMed] [Google Scholar]
- Diez E., Altamirano L., García I., Mazzei L., Prado N., Fornes M., et al. (2015) Heart remodeling and ischemia-reperfusion arrhythmias linked to myocardial vitamin d receptors deficiency in obstructive nephropathy are reversed by paricalcitol. J Cardiovasc Pharmacol Ther 20: 211–220. [DOI] [PubMed] [Google Scholar]
- Dusso A., Brown A., Slatopolsky E. (2005) Vitamin D. Am J Physiol Renal Physiol 289: F8–F28. [DOI] [PubMed] [Google Scholar]
- Ferder M., Inserra F., Manucha W., Ferder L. (2013) The world pandemic of vitamin D deficiency could possibly be explained by cellular inflammatory response activity induced by the renin-angiotensin system. Am J Physiol Cell Physiol 304: C1027–C1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García I., Altamirano L., Mazzei L., Fornés M., Molina M., Ferder L., et al. (2012) Role of mitochondria in paricalcitol-mediated cytoprotection during obstructive nephropathy. Am J Physiol Renal Physiol 302: F1595–F1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García I., Altamirano L., Mazzei L., Fornés M., Cuello-Carrión F., Ferder L., et al. (2014) Vitamin D receptor-modulated Hsp70/AT1 expression may protect the kidneys of SHRs at the structural and functional levels. Cell Stress Chaperones 19: 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E., Liu Y., Rimm E., Hollis B., Fuchs C., Stampfer M., et al. (2006) Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst 98: 451–459. [DOI] [PubMed] [Google Scholar]
- Gorham E., Garland C., Garland F., Grant W., Mohr S., Lipkin M., et al. (2005) Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol 97: 179–194. [DOI] [PubMed] [Google Scholar]
- Gunta S., Thadhani R., Mak R. (2013) The effect of vitamin D status on risk factors for cardiovascular disease. Nat Rev Nephrol 9: 337–347. [DOI] [PubMed] [Google Scholar]
- Heaney R., Dowell M., Hale C., Bendich A. (2003) Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 22: 142–146. [DOI] [PubMed] [Google Scholar]
- Holick M. (2006a) Calcium plus vitamin D and the risk of colorectal cancer. N Engl J Med 354: 2287–2288. [DOI] [PubMed] [Google Scholar]
- Holick M. (2006b) Resurrection of vitamin D deficiency and rickets. J Clin Invest 116: 2062–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M. (2007) Vitamin D deficiency. N Engl J Med 357: 266–281. [DOI] [PubMed] [Google Scholar]
- Holick M., Garabedian M. (2006) Vitamin D: photobiology, metabolism, mechanism of action, and clinical applications. In: Favus M. (ed.), Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 6th ed. Washington, DC: American Society for Bone and Mineral Research, pp. 129–137. [Google Scholar]
- Husain K., Ferder L., Mizobuchi M., Finch J., Slatopolsky E. (2009) Combination therapy with paricalcitol and enalapril ameliorates cardiac oxidative injury in uremic rats. Am J Nephrol 29: 465–472. [DOI] [PubMed] [Google Scholar]
- Husain K., Hernandez W., Ansari R., Ferder L. (2015) Inflammation, oxidative stress and renin angiotensin system in atherosclerosis. World J Biol Chem 6: 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain K., Suarez E., Isidro A., Ferder L. (2010) Effects of paricalcitol and enalapril on atherosclerotic injury in mouse aortas. Am J Nephrol 32: 296–304. [DOI] [PubMed] [Google Scholar]
- Krause R., Buhring M., Hopfenmuller W., Holick M., Sharma A. (1998) Ultraviolet B and blood pressure. Lancet 352: 709–710. [DOI] [PubMed] [Google Scholar]
- Kong J., Kim G., Wei M., Sun T., Li G., Liu S., et al. (2010) Therapeutic effects of vitamin D analogs on cardiac hypertrophy in spontaneously hypertensive rats. Am J Pathol 177: 622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., Li Y. (2003) Effect of ANG II type I receptor antagonist and ACE inhibitor on vitamin D receptor-null mice. Am J Physiol Regul Integr Comp Physiol 285: R255–R261. [DOI] [PubMed] [Google Scholar]
- Lemire J., Ince A., Takashima M. (1992) 1,25-Dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/l mice. Autoimmunity 12: 143–148. [DOI] [PubMed] [Google Scholar]
- Levey A., Schoolwerth A., Coresh J., Culleton B., Hamm L., McCullough P., et al. (2003) Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology. Hypertension 42: 1050–1065. [DOI] [PubMed] [Google Scholar]
- Li Y., Kong J., Wei M., Chen Z., Liu S., Cao L. (2002) 1-25 Dihydroxivitamin D3 is a negative endocrine regulator of the renin angiotensin system. J Clin Invest 110: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillevang S., Rosenkvist J., Andersen C., Larsen S., Kemp E., Kristensen T. (1992) Single and combined effects of the vitamin D analogue KH1060 and cyclosporin A on mercuric-chloride-induced autoimmune disease in the BN rat. Clin Exp Immunol 88: 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscombe C., Fryer A., French M., Liu S., Saxby M., Jones P., et al. (2001) Exposure to ultraviolet radiation: association with susceptibility and age at presentation with prostate cancer. Lancet 358: 641–642. [DOI] [PubMed] [Google Scholar]
- Manucha W. (2014) HSP70 family in the renal inflammatory response. Inflamm Allergy Drug Targets 13: 235–240. [DOI] [PubMed] [Google Scholar]
- Mazzei L., García M., Calvo J., Casarotto M., Fornés M., Abud M., et al. (2016) Changes in renal WT-1 expression preceding hypertension development. BMC Nephrol 17: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina M., Ferder L., Manucha W. (2016) Emerging role of nitric oxide and heat shock proteins in insulin resistance. Curr Hypertens Rep 18: 1. doi: 10.1007/s11906-015-0615-4 [DOI] [PubMed] [Google Scholar]
- Nagpal S., Rathnachalam R. (2005) Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 26: 662–687 [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Hitomi H. (2010) Role of caveolin and heat shock protein 70 interaction in the antioxidative effects of an angiotensin II type 1 receptor blocker in spontaneously hypertensive rats proximal tubules. J Hypertens 28: 9–12. [DOI] [PubMed] [Google Scholar]
- Norman P., Powell J. (2014) Vitamin D and cardiovascular disease. Circ Res 114: 379–393. [DOI] [PubMed] [Google Scholar]
- Panizo S., Barrio-Vázquez S., Naves-Díaz M., Carrillo-López N., Rodríguez I., Fernández-Vázquez A., et al. (2013) Vitamin D receptor activation, left ventricular hypertrophy and myocardial fibrosis. Nephrol Dial Transplant 28: 2735–2744. [DOI] [PubMed] [Google Scholar]
- Pradhan A., Manson J. (2016) Update on the Vitamin D and OmegA-3 trial (VITAL). J Steroid Biochem Mol Biol 155(Pt B): 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Iturbe B., Franco M., Tapia E., Quiroz Y., Johnson R. (2012) Renal inflammation, autoimmunity and salt-sensitive hypertension. Clin Exp Pharmacol Physiol 39: 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnak M., Levey A., Schoolwerth A., Coresh J., Culleton B., Lee Hamm L., et al. (2003) Kidney disease as a risk factor for development of cardiovascular disease. A statement from the American Heart Association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation 108: 2154–2169. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Amann K., Orth S., Simonaviciene A., Wessels S., Ritz E. (1998) Effect of 1,25 (OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int 53: 1696–1705. [DOI] [PubMed] [Google Scholar]
- Sentinelli F., Bertoccini L., Barchetta I., Capoccia D., Incani M., Pani M., et al. (2016) The vitamin D receptor (VDR) gene rs11568820 variant is associated with type 2 diabetes and impaired insulin secretion in Italian adult subjects, and associates with increased cardio-metabolic risk in children. Nutr Metab Cardiovasc Dis 26: 407–413. [DOI] [PubMed] [Google Scholar]
- Shoben A., Rudser K., de Boer I., Young B., Kestenbaum B. (2008) Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol 19: 1613–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thadhani R., Appelbaum E., Chang Y., Pritchett Y., Bhan I., Agarwal R., et al. (2011) Vitamin D receptor activation and left ventricular hypertrophy in advanced kidney disease. Am J Nephrol 33: 139–149. [DOI] [PubMed] [Google Scholar]
- Thomas K., Lloyd-Jones D., Thadhani R., Shaw A., Deraska D., Kitch B., et al. (1998) Hypovitaminosis D in medical inpatients. N Engl J Med 338: 777–783. [DOI] [PubMed] [Google Scholar]
- Xiang W., Kong J., Chen S., Cao L., Qiao G., Zheng W., et al. (2005) Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems.Am J Physiol Endocrinol Metab 288: e125–e132. [DOI] [PubMed] [Google Scholar]