Abstract
Asthma and chronic obstructive pulmonary disease (COPD) are major causes of global morbidity and mortality worldwide. The clinical course of both asthma and COPD are punctuated by the occurrence of exacerbations, acute events characterized by increased symptoms and airflow obstruction. Exacerbations contribute most of the morbidity, mortality and excess healthcare costs associated with both asthma and COPD. COPD and asthma exacerbations are frequently associated with respiratory virus infections and this has led to an intense research focus into the mechanisms of virus-induced exacerbations over the past decade. Current therapies are effective in reducing chronic symptoms but are less effective in preventing exacerbations, particularly in COPD. Understanding the mechanisms of virus-induced exacerbation will lead to the development of new targeted therapies that can reduce the burden of virus-induced exacerbations. In this review we discuss current knowledge of virus-induced exacerbations of asthma and COPD with a particular focus on mechanisms, human studies, virus–bacteria interactions and therapeutic advances.
Keywords: COPD, asthma, respiratory viruses, bacteria
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are chronic respiratory diseases that are a major cause of morbidity and mortality worldwide. Although the aetiology and pathogenesis of the two diseases are very different they share some common features including the occurrence of acute exacerbations. Respiratory virus infections are a major cause of exacerbations of COPD and asthma and this has led to an intense research focus into the mechanisms of virus-induced exacerbations with a view to developing new therapies to reduce the burden of virus-induced exacerbations.
Respiratory virus infections and asthma and COPD exacerbations
The Global Initiative for Asthma defined asthma as “a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role”. The chronic inflammation is associated with airway hyperresponsiveness that leads to recurrent episodes of wheezing, breathlessness, chest tightness, and coughing, particularly at night or in the early morning. These episodes are usually associated with widespread, but variable, airflow obstruction within the lung that is often reversible either spontaneously or with treatment [Bateman et al. 2008]. The Global Initiative for Obstructive Lung Disease defines COPD as “a common preventable and treatable disease, is characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases. Exacerbations and comorbidities contribute to the overall severity in individual patients”. It has long been recognized that viral respiratory-tract infections trigger exacerbations of asthma in both adults and children, whereas for many years bacterial infection was thought to be the main trigger of COPD exacerbations. Early studies reported low detection rates of viruses in both asthma and COPD exacerbations casting doubt on their role as exacerbation triggers [Buscho et al. 1978; Mitchell et al. 1978; Beasley et al. 1988]. The development of highly sensitive and specific molecular diagnostic techniques using polymerase chain reaction (PCR) technology led to a revolution in viral diagnostics. This is particularly relevant for human rhinoviruses (HRVs) that are difficult to culture and for which serology is impractical due to the large number of serotypes. Studies using PCR demonstrated the presence of viruses in 80–85% of asthma exacerbations in children and 60–80% of exacerbations in adults [Johnston et al. 1995; Wark et al. 2002; Grissell et al. 2005; Arden et al. 2010]. The most commonly identified viruses include HRV, influenza viruses, respiratory syncytial virus (RSV) and coronaviruses. Detection rates of respiratory viruses in COPD exacerbations are more variable than in asthma, varying from 22% to 64% of exacerbations [Seemungal et al. 2001; Bandi et al. 2003; Qiu et al. 2003; Rohde et al. 2003; Tan et al. 2003; Beckham et al. 2005; Cameron et al. 2006; Papi et al. 2006; Hutchinson et al. 2007; Ko et al. 2007; Bozinovski et al. 2008; Camargo et al. 2008; Daubin et al. 2008; McManus et al. 2008; Minosse et al. 2008; De Serres et al. 2009; Pant et al. 2009; Kherad et al. 2010; Singh et al. 2010; Almansa et al. 2011; Bafadhel et al. 2011; Dimopoulos et al. 2012; Perotin et al. 2013] (Table 1). A number of factors could account for this including variations in time from exacerbation onset to presentation, number of viruses tested for, length and season of study period, site of recruitment of the COPD population and type of samples collected. Whilst data are lacking, there is a suggestion that nasopharyngeal swabs, high influenza vaccination rates and studies confined to the winter are associated with lower virus detection (the latter possibly because of a lengthier delay to presentation in colder weather). However, differences in the number of viruses tested for and the population studied (community versus inpatient) do not seem to affect the virus-detection rate.
Table 1.
Characteristics of studies of viral infections in COPD exacerbations.
| Study | Year | % exacerbations where virus detected | Number of viruses tested | Study period | Population | Sample | Influenza vaccine uptake |
|---|---|---|---|---|---|---|---|
| Ko et al. | 2007 | 22% | 17 | 1 year | Hospital | NP swab | 40% |
| Hutchinson et al. | 2007 | 22% | 8 | 99 weeks | Community | NP swab | 87% |
| Daubin et al. | 2008 | 23% | 13 | 1 year | Hospital | Nasal swab or trachea-bronchial aspirate | NS |
| Camargo et al. | 2008 | 25% | 9 | Winter | Hospital | Nasal swab | 87% |
| Bozinovski et al. | 2008 | 26% | 6 | NS | Community | NP swab | NS |
| Almansa et al. | 2011 | 28% | 17 | Winter | Hospital | Pharyngeal swab | NS |
| Pant et al. | 2009 | 29% | 7 | Winter | Hospital | Sputum | NS |
| Bafadhel et al. | 2011 | 29% | NS | 1 year | Community | Sputum | NS |
| Singh et al. | 2010 | 30% | 17 | 2 years | Community | NP swab | NS |
| De Serres et al. | 2009 | 31% | 14 | Winter | Hospital | NP swab | 83% |
| McManus et al. | 2008 | 37% | 12 | 2 years | Hospital | Sputum | NS |
| Seemungal et al. | 2001 | 39% | 10 | 1 year | Community | Nasal aspirate +/- pharyngeal swab | 74% |
| Beckham et al. | 2005 | 42% | 11 | 4 years | Hospital | Nasal wash +/- pharyngeal swab | 73% |
| Cameron et al. | 2006 | 43% | 12 | Winter | Intensive care unit | NP aspirate + pharnygeal swab | NS |
| Perotin et al. | 2013 | 44% | 20 | 2 years | Community | Sputum | 72% |
| Bandi et al. | 2003 | 46% | 8 | NS | Community | Nasal wash + pharyngeal swab | NS |
| Qiu et al. | 2003 | 47% | NS | NS | Intensive care unit | NP secretions | NS |
| Hosseini et al. | 2015 | 47.6% | 16 | 3 years | Hospital | Unknown | |
| Papi et al. | 2006 | 48% | 12 | 2 years | Community | Sputum | NS |
| Kherad et al. | 2010 | 51% | 14 | 18 months | Hospital | NP swab | 75% |
| Dimopoulos et al. | 2012 | 54% | 17 | 2 years | Hospital | Sputum + oropharyngeal samples | 45% |
| Rohde et al. | 2003 | 56% | 6 | 1 year | Hospital | Nasal lavage + sputum | NS |
| Minosse et al. | 2008 | 57% | 12 | 14 months | Hospital | Sputum +/- ET aspirate +/- BAL | NS |
| Tan et al. | 2003 | 64% | 6 | NS | Hospital | Sputum | NS |
BAL, bronchoalveolar lavage; ET, endotracheal; NP, nasopharyngeal; NS, not specified.
Experimental rhinovirus infection
Although these studies have demonstrated an association between respiratory virus infection and exacerbations, they do not prove a causal link. Studies sampling patients at different time points (before [Singh et al. 2010; Bafadhel et al. 2011] or after [Seemungal et al. 2001; Papi et al. 2006; Kherad et al. 2010] an exacerbation) or using time-matched controls [Rohde et al. 2003; Hutchinson et al. 2007; McManus et al. 2008; Almansa et al. 2011] confirm that detection rates are higher in patients with an exacerbation, with low rates of infection in the same patients when clinically stable and in seasonal controls. However, as PCR detects viral nucleic acid it cannot prove the presence of live, replicating virus and therefore cannot exclude secondary causation.
Studies carrying out experimental HRV infections in subjects with asthma and COPD have been pivotal in demonstrating a causal relationship between respiratory virus infection and exacerbations, especially in COPD. Experimental HRV infection in asthma induces the typical clinical features of an exacerbation [Message et al. 2008; Jackson et al. 2014], and studies have successfully demonstrated that, following nasal inoculation, HRV can infect bronchial epithelium providing a plausible explanation for lower respiratory-tract symptoms observed in asthma [Papadopoulos et al. 2000; Mosser et al. 2005].
Our group has carried out experimental HRV infection in patients with COPD, successfully demonstrating in three separate cohorts that HRV infection induces exacerbations in more than 90% of infected patients [Mallia et al. 2006, 2011; Footitt et al. 2015]. Virus was detected prior to the onset of symptoms and the viral load increased during the course of infection, indicating that active viral replication was occurring. Viral clearance was followed by symptomatic resolution and virus load correlated with inflammatory markers and oxidative stress [Mallia et al. 2011; Footitt et al. 2015]. These data provide novel evidence of a causal relationship between respiratory virus infection and exacerbations in patients with COPD.
Newly discovered respiratory viruses
Even studies using PCR consistently fail to identify viral pathogens in a significant proportion of patients with viral-like illnesses. Recently a number of respiratory viruses have been identified and their role investigated in asthma and COPD exacerbations.
Human rhinovirus C
HRVs form a genus of positive-sense, single-stranded RNA viruses called Enterovirus, within the Picornavirus family. Initially divided into two distinct species, HRV-A and -B, development of improved PCR techniques has allowed the identification of a third species, HRV-C. HRV-C has been frequently detected in patients with respiratory-tract illness, particularly in children, and analysis of stored samples has determined that these are not newly emerged viruses as they can be identified in samples collected up to three decades ago [Linder et al. 2013]. The prevalence of HRV-C in children appears particularly high and appears to be disproportionately represented in children with asthma and children hospitalized with lower respiratory-tract illness [Miller et al. 2009; Calvo et al. 2010; Cox et al. 2013]. Detection rates of HRV-C in asthma exacerbations in the paediatric population are high [Arden et al. 2010; Bizzintino et al. 2011], but it is less clear whether severity of disease is related to virus type. Bizzintino and colleagues reported in children with acute asthma that HRV-C was associated with more severe exacerbations, whereas in adult patients with HRV, there was no significant difference in severity of respiratory illness between the different HRV species [McCulloch et al. 2014]. This variation in results suggests that further research is required to better quantify the impact of HRV-C on the severity of acute asthma.
The role of HRV-C in COPD is less well defined with few studies analysing the contribution of different HRV species. Where this has been carried out, HRV-A has consistently arisen as the most common HRV species associated with COPD exacerbations [Dimopoulos et al. 2012; Gandhi et al. 2012; Wark et al. 2013], suggesting a less important role for HRV-C in COPD exacerbations compared with asthma.
Human metapneumovirus and human bocavirus
Human metapneumovirus (MPV) is a single-stranded RNA virus belonging to the family Paramyxoviridae and human bocavirus (HBoV) is a human parvovirus. Both were first identified in samples from children with lower respiratory illness. As with HRV-C, analysis of stored samples revealed that MPV has been present for decades but was not previously recognized, and since its identification it has been isolated in all age groups and in a range of disease syndromes including upper respiratory-tract infections, pneumonia and exacerbations of asthma and COPD [Jartti et al. 2012]. Despite being frequently detected in children with respiratory illness, MPV appears not to be common in asthma exacerbations in children and adults [Williams et al. 2005; Khetsuriani et al. 2007]. The role of MPV in COPD exacerbations varies in different studies with some suggesting a minor role [Beckham et al. 2005; Rohde et al. 2005; McManus et al. 2008], whereas in other studies it is the second most common viral pathogen after HRV [Martinello et al. 2006; Perotin et al. 2013]. The role of HBoV has been less well studied but it has been detected in only a small proportion of asthma and COPD exacerbations [Ringshausen et al. 2009; Vallet et al. 2009; Hosseini et al. 2015].
Pandemic influenza
First reported in Mexico in 2009, a novel H1N1 influenza A virus containing swine, avian and human genes disseminated to World Health Organization confirmed pandemic levels. While those with underlying chronic lung diseases, such as asthma and COPD, together with the elderly, form a well-known high risk group for seasonal influenza, this novel pandemic strain displayed a propensity to affect young adults. Large, national studies in both the UK and Spain retrospectively reviewed epidemiological data obtained during the pandemic and consistently demonstrated that asthma was the most common underlying comorbidity in confirmed cases of H1N1 [Nguyen-Van-Tam et al. 2010; Rodriguez-Rieiro et al. 2012]. Indeed, asthma rates of 44% have been reported in children hospitalized with pandemic influenza [Dawood et al. 2011].
The data regarding the severity of outcomes in asthmatic patients are more conflicting. Multiple studies have specifically compared the severity of outcomes between asthmatics and non-asthmatics using markers such as intensive care unit (ICU) admission, need for invasive mechanical ventilation and death. Mortensen and colleagues reported that a third of hospitalized, asthmatic patients had severe outcomes [Mortensen et al. 2013]. However, other studies reported less severe outcomes with influenza infection in asthmatics. In a large, UK study of 1520 patients admitted with H1N1, Myles and colleagues demonstrated that asthma was associated with a reduced likelihood of severe outcome [Myles et al. 2013], and similar findings have been reported from the USA [Bramley et al. 2012]. This is in line with other studies citing lower requirements for mechanical ventilation and reduced in-hospital mortality in H1N1-infected asthmatic patients [De Miguel-Diez et al. 2012; McKenna et al. 2013]. Earlier presentations, lower threshold for admission and use of corticosteroid therapy have been hypothesized to account for this reduced severity.
There is much data supporting the association between COPD and seasonal influenza, while the pandemic generated interest in the severity of outcomes with H1N1 in this patient group. Gerke and colleagues used data from the pandemic to demonstrate an association between COPD admission rates and influenza activity [Gerke et al. 2013].
In a large study of 3025 patients with PCR-confirmed pandemic influenza, Santa-Olalla Peralta and colleagues demonstrated that COPD was significantly associated with the most severe outcomes, namely ICU admission and death [Santa-Olalla Peralta et al. 2010]. This is supported by other studies with significantly greater in-hospital mortality, length of stay and costs in patients with COPD [De Miguel-Diez et al. 2012]. The importance of these findings lies in the early initiation of antivirals and targeted vaccination of patients with underlying chronic lung disease.
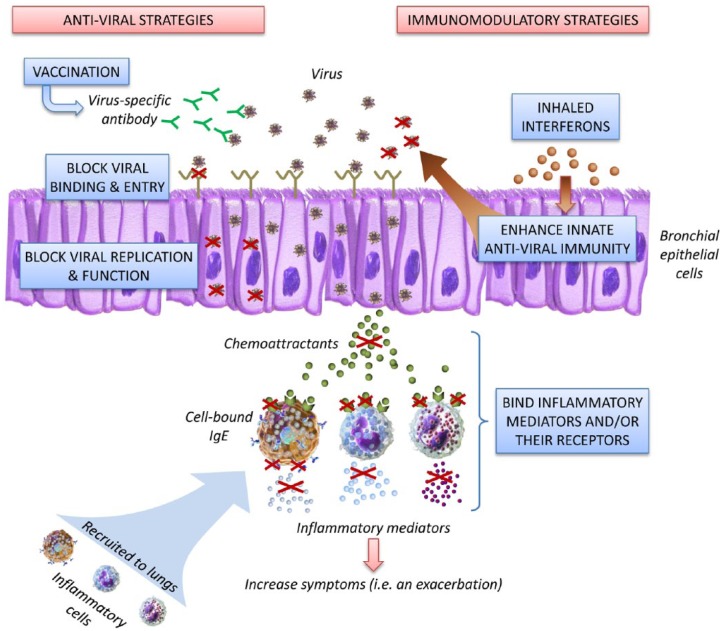
Figure 1.
Novel approaches to treating virus-induced exacerbations of asthma and COPD.
Mechanisms of virus-induced exacerbations in asthma and COPD
It has become clear that HRVs are the most common viral aetiological agents of exacerbations in both asthma and COPD. In healthy adults HRV causes a self-limiting syndrome with predominantly upper respiratory-tract manifestations. From epidemiological studies it is unclear whether patients with asthma and COPD are more susceptible to HRV infection, have more severe clinical illness with viral infections or are preferentially infected with different viruses (e.g. HRV-C).
Deficient innate antiviral immunity
Experimental HRV infection studies have proved invaluable in understanding the mechanisms of virus-induced exacerbations in both asthma and COPD. In these studies subjects with the disease and a control group of healthy subjects are experimentally infected with an HRV inoculum and studied intensively during the infection period. Both groups are inoculated with the same virus type at the same dose and via the same route and have the same assessments at the same time points. Therefore any differences seen between patients and controls can be attributed to differences in host, and not viral, factors. In both asthma and COPD virus loads are higher following virus infection compared with healthy controls [Mallia et al. 2011; Jackson et al. 2014; Footitt et al. 2015]. As both the subjects and controls received the same dose this implies that the host antiviral immunity is impaired and unable to control viral replication. Rhinoviruses enter and replicate predominantly within bronchial epithelial cells, activating a cascade of immune, inflammatory and cytotoxic responses [Papadopoulos et al. 2000; Bossios et al. 2005; Laza-Stanca et al. 2006]. Other cells, including airway smooth muscle cells [Oliver et al. 2006], fibroblasts [Ghildyal et al. 2005], and alveolar macrophages [Message et al. 2008], may also be important sites of infection, inducing inflammatory mediators [Hall et al. 2005; Message et al. 2008; Oliver et al. 2008; Nagarkar et al. 2010]. During infection, the viral RNA generated is recognized by innate immune receptors and triggers a signalling cascade, which leads to the activation of transcription factors, induction of type I interferon (IFN-α/-β) and type III interferon (IFN-λ), and pro-inflammatory cytokines and chemokines [Zhu et al. 1996, 1997; Johnston et al. 1998; Papadopoulos et al. 2000; Sheppard et al. 2003; Ieki et al. 2004; Spurrell et al. 2005; Contoli et al. 2006; Korpi-Steiner et al. 2006; Laza-Stanca et al. 2006; Edwards et al. 2007; Bossios et al. 2008].
IFNs are of upmost importance in the innate immune response to viral infection as they stimulate multiple antiviral pathways [Isaacs and Lindenmann, 1957]. Their antiviral effects occur directly through inhibition of viral replication in cells, and indirectly through stimulation of adaptive immune responses. The direct antiviral activity of type I IFNs is mediated by a number of mechanisms including blocking viral entry into cells, control of viral transcription, cleavage of RNA, blocking translation and induction of apoptosis [Fensterl and Sen, 2009]. Therefore failure to induce a robust IFN response following virus infection would be expected to lead to uncontrolled viral replication and increased inflammatory responses, and this could be a mechanism of virus-induced exacerbations in asthma and COPD. The first report that indicated that innate immune responses to virus infection may be impaired in asthma emerged in 2005 when Wark and colleagues investigated responses to HRV infection in bronchial epithelial cells [Wark et al. 2005]. HRV replication was increased in the cells from asthmatics compared with those from non-asthmatic subjects, and induction of IFN-β was both delayed and deficient in asthmatics. Administration of exogenous IFN-β resulted in reduced virus replication and the observation of a restored antiviral response with administration of IFN was validated in a subsequent study [Cakebread et al. 2011]. Other studies have reported IFN deficiency both in vitro and in vivo in asthma [Baraldo et al. 2012]. Our group has demonstrated that production of IFN-α, IFN-β and IFN-λ by alveolar macrophages is also impaired in asthma [Contoli et al. 2006; Sykes et al. 2012], and that exacerbation severity is inversely proportional to IFN-λ production.
However, other studies have not reported deficient IFN responses in asthmatics [Lopez-Souza et al. 2009; Bochkov et al. 2010; Sykes et al. 2013, 2014]. The reasons for this are unclear but possible reasons include different experimental conditions, different cell types, and different virus types and doses. As we will discuss later it may also be that IFN deficiency is limited to a subset of asthma patients. Thus although IFN deficiency is an interesting and biologically plausible possible mechanism underlying increased severity of virus infection in asthma, it has not been universally demonstrated.
It is only recently that it has been widely accepted that respiratory viruses are a major contributor to COPD exacerbations and research has focused predominantly on the role of bacteria in exacerbations. Therefore there are fewer studies investigating the mechanisms of virus-induced exacerbations in COPD when compared with asthma, and subsequently our knowledge base is much smaller.
Older in vitro studies showed that exposure of cell cultures to cigarette smoke results in significant reductions in IFN-α and IFN-β production [Sonnenfeld and Hudgens, 1986], suggesting that IFN deficiency may play a role in smoking-induced COPD. The first investigation of IFN in COPD was a study of airway epithelial cells from patients with COPD infected with HRV. This study reported that production of IFN-λ1 and IFN-λ2 was increased compared with cells from the control subjects [Schneider et al. 2010]. However paradoxically there were significantly increased viral titres in the COPD cells, suggesting other antiviral mediators may be impaired. A similar in vitro study also reported increased IFNs, but with no difference in virus load between COPD and non-COPD subjects [Baines et al. 2013]. Conversely in bronchoalveolar lavage (BAL) cells from subjects with COPD and nonobstructed smoker controls infected with HRV ex vivo IFN-β responses were significantly impaired [Mallia et al. 2011]. There was also impaired production of INF-α and IFN-λ although this did not reach statistical significance. In a murine COPD model infected with HRV-1B, IFN-α and IFN-β responses were deficient and viral clearance impaired [Sajjan et al. 2009], whereas another study using a murine model showed reduced IFN-λ but no change in IFN-β or virus load [Singanayagam et al. 2015]. Another potential mechanism underlying increased severity of HRV infection in COPD is increased surface expression of intracellular adhesion molecule (ICAM)-1. The major group of HRVs enter cells by binding to ICAM-1 and this is upregulated on epithelial cells from bronchial biopsies of patients with COPD [Di Stefano et al. 1994].
Evidence for a susceptibility phenotype in asthma and COPD
It is increasingly well recognized that asthma and COPD are heterogeneous diseases with a number of subtypes or phenotypes and among the phenotypes described are patients that have more frequent exacerbations. This suggests that some patients are either susceptible to infection or have more severe clinical disease following infection. Therefore impaired antiviral immunity may not be present in all patients and this may account for some of the discrepant results regarding IFN deficiency obtained from different studies.
Asthma
A proportion of asthma patients experience more frequent exacerbations despite treatment. The mechanisms of this remain undetermined but it is linked to poorer asthma control, higher sputum eosinophils and higher exhaled nitric oxide [Kupczyk et al. 2014]. In experimental HRV infection exacerbation patients with poorly controlled asthma at baseline display more symptoms, reduced production of antiviral T helper cell 1 (Th1) cytokines and higher virus load [Jackson et al. 2015]. Therefore poor asthma control and eosinophilic inflammation are related to exacerbation frequency but the mechanistic link between these has yet to be determined. Several studies have reported relationships between markers of asthma/allergy severity and IFN responses [Contoli et al. 2015]. In children HRV-induced IFN-λ and IFN-β induction correlated inversely with airway T helper cell 2 (Th2) markers and serum immunoglobulin (Ig) E levels [Baraldo et al. 2012]. Induction of IFN-α by dendritic cells from asthmatic patients infected with influenza A virus inversely correlated with serum IgE levels [Gill et al. 2010], and IgE levels correlated inversely with toll-like receptor 7 induction of IFN-γ-induced protein 10 in peripheral blood mononuclear cells (PBMCs) from asthmatic adolescents [Roponen et al. 2010]. In a group of adult subjects with mild-to-moderate asthma there was a significant correlation between type I interferon levels produced by PBMCs and BAL cells, and skin prick tests [Sykes et al. 2012].
A recent report has proposed that increased expression of suppressor of cytokine signalling (SOCS) 1 may link Th2 inflammation with IFN deficiency. SOCS proteins suppress cytokine-receptor signalling and virus-induced IFN-β promoter activation. SOCS1 levels were increased in epithelial cells from children with severe asthma and SOCS1 mRNA levels were significantly inversely correlated with induction of type I and type III interferons by HRV [Gielen et al. 2015]. In bronchial biopsy specimens from patients with mild-to-moderate asthma nuclear SOCS1 staining was significantly higher in asthmatic patients compared with healthy subjects, and numbers of nuclear SOCS1-positive cells correlated with serum total IgE levels, but not with exacerbations.
COPD
Similar to asthma not all COPD patients experience the same frequency of exacerbations. The Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) cohort study identified a distinct ‘frequent exacerbator’ phenotype. This was a group who, irrespective of disease severity, were more susceptible to exacerbations and could be identified by a previous history of two or more exacerbations per year [Hurst et al. 2010]. The mechanisms of frequent exacerbations remains unknown, but as virus infections are a major cause of exacerbations this may reflect a defect in innate host defence to viral pathogens.
There is some indirect evidence that an increased susceptibility to virus infection may be present in frequent exacerbators. In studies of naturally acquired virus-induced COPD exacerbations, virus infection was detected more commonly in frequent exacerbators [Seemungal et al. 2001; George et al. 2014]. However, the description of frequent exacerbators remains essentially clinical and there are as yet no studies investigating the underlying mechanisms.
In summary, there is evidence that antiviral immune responses are delayed and deficient in asthmatics and this is a plausible rationale for observed increased disease severity following virus infection in asthma. This is less well established in COPD. Further work is required to determine whether these impairments are common to all asthmatics and how Th2 inflammation is linked to impaired IFN responses.
Virus–bacterial co-infections in asthma and COPD
The majority of studies into the role of infection in asthma and COPD have focused on either viruses or bacteria with few studies examining the potential role of dual infection. Virus–bacterial co-infection is well recognized with influenza virus but it is unclear whether other respiratory viruses such as HRV result in secondary bacterial infections. Epidemiological studies appear to indicate that dual virus–bacterial infection does not play a major role in COPD exacerbations with low detection rates ranging from 6.5% [Cameron et al. 2006] to at most 27% [Papi et al. 2006; Perotin et al. 2013]. In experimental HRV infections in COPD, bacteria were detected in 60% of virus-induced exacerbations [Mallia et al. 2012]. Viruses and bacteria were detected at different time points with virus infection occurring first and bacterial infection at later time points. This suggests that studies of naturally occurring exacerbations that collect samples at a single time point significantly underestimate the true incidence of dual infection. A recent study in which patients were sampled at two time points during an exacerbation has confirmed this: 73% of patients positive for HRV but negative for bacteria at presentation were positive for bacteria when sampled again at day 14 [George et al. 2014]. An association between HRV infection and bacteria has also been reported in asthma [Kloepfer et al. 2014]
The effect of dual infection on outcomes in exacerbations has not been studied in detail but would appear to be associated with increased severity. The presence of a symptomatic cold in patients with COPD infected with Haemophilus influenzae is associated with higher bacterial loads [Wilkinson et al. 2006], and in a study of hospitalized patients with COPD exacerbations in Italy, co-infection was associated with impaired lung function and longer hospital stay [Papi et al. 2006]. In our experimental HRV infection studies, co-infection was associated with prolonged lower respiratory symptoms [Mallia et al. 2012]. In asthma exacerbations co-infection with HRV and Streptococcus pneumoniae or Moraxella cattarhalis was associated with more severe exacerbations [Kloepfer et al. 2014]. Therefore the prevalence of dual virus–bacterial infections in asthma and COPD exacerbations is likely to have been significantly underestimated and this has implications for designing new therapies for exacerbations.
Mechanisms of virus–bacterial co-infection
The mechanisms whereby viruses may increase susceptibility to bacterial infection remain poorly characterized and there is a dearth of in vivo studies in humans. In vitro studies have demonstrated that HRV and RSV can induce changes in airway epithelial cells that promote bacterial adhesion and invasion [Ishizuka et al. 2003; Sajjan et al. 2008; Wang et al. 2009]. HRV infection promotes internalization of Staphylococcus aureus into epithelial cells [Passariello et al. 2006], and, by disrupting the epithelial tight junctions, facilitates bacterial transmigration across the epithelium [Sajjan et al. 2008]. In a murine model mice co-infected with HRV and Haemophilus influenzae demonstrated reduced neutrophil recruitment and delayed bacterial clearance [Unger et al. 2012].
In addition, viruses may directly impair the host immune response to bacterial infection. HRV infection reduces macrophage cytokine release in response to bacterial products [Oliver et al. 2008], and degradation of antimicrobial peptides. In animal models of postinfluenza bacterial pneumonia, there are impairments in macrophage [Jakab, 1982] and neutrophil responses [McNamee and Harmsen, 2006], and IFN-γ signalling [Sun and Metzger, 2008]. In the human experimental infection model reduced airway levels of the antimicrobial peptide elafin and secretory leucoprotease inhibitor were associated with increased risk of secondary bacterial infections [Mallia et al. 2012]. Initial bacterial infection may also influence immune responses to viral infection. In vitro epithelial cell infection with Haemophilus influenzae increases rhinovirus binding, cytokine release and virus load [Sajjan et al. 2006; Gulraiz et al. 2015]. Pretreating peripheral blood monocytes or alveolar macrophages with the bacterial product lipopolysaccharide inhibits rhinovirus-induced secretion of C-X-C motif chemokine 10 (CXCL10) and chemokine (C-X-C motif) ligand 11, which are chemotactic for T cells [Karta et al. 2014].
Therapies
Therapies in asthma and COPD include both long-term preventive therapies to reduce the frequency of exacerbations, and treatments commenced after the onset of an exacerbation. A comprehensive description of treatments is beyond the scope of this review and therefore we will focus on preventive treatments that reduce exacerbations, particularly novel treatments, and how these may relate to the postulated mechanisms of increased susceptibility to virus infection. Inhaled corticosteroids (ICSs), inhaled bronchodilators including long-acting beta agonists (LABAs) and long-acting muscarinic receptor antagonists (LAMAs), and combinations of these, form the core of ‘preventive’ asthma and COPD therapies, with use dictated by individual disease severity [Bateman et al. 2008 Vestbo et al., 2013]. It is well established that these therapies reduce exacerbations but patients taking maximum inhaled therapy continue to experience exacerbations and therefore new therapeutic approaches are needed.
Asthma
ICSs have been the mainstay of anti-inflammatory treatment in asthma for many years and exert their beneficial effects by modulating the host adaptive immune response that drives the pathophysiology of asthma. In clinical trials ICSs are effective in reducing exacerbations but these studies are in highly selected patients and surveys of ‘real-life’ asthma patients have shown that almost half of asthma patients continue to experience exacerbations [Rabe et al. 2000; Price et al. 2014]. It is unclear whether virus-induced asthma exacerbations are characterized by a Th1 or Th2 predominant inflammatory response and therefore the effects of ICSs on virus-induced exacerbations are unclear. In atopic asthmatics experimentally infected with rhinovirus 16, pretreatment with the ICS budesonide was shown to improve airway hyperresponsiveness and eosinophilic inflammation, but did not prevent the inflammatory changes associated with HRV infection [Grunberg et al. 2001].
The Th2 cytokines interleukin-4 (IL-4), interleukin-5 (IL-5) and interleukin-13 (IL-13), and IgE are recognized to be central mediators in asthma. A number of new monoclonal antibodies have recently become available that target these mediators. These therapies have a number of beneficial effects including improvements in lung function, reduction in symptoms and reduced requirements for oral corticosteroids, and a significant reduction in exacerbations.
Omalizumab, a recombinant humanized monoclonal antibody against IgE, which reduces circulating serum levels of IgE, demonstrated a 58% reduction in asthma exacerbations in atopic patients with serum IgE levels greater than 30 IU/ml already receiving ICSs [Soler et al. 2001]. Similarly in patients with severe, eosinophilic asthma the monoclonal antibodies against IL-5 mepolizumab and reslizumab, reduced the rate of exacerbations by approximately 50% compared with placebo [Ortega et al. 2014; Castro et al. 2015]. Two studies of anti-IL-13 monoclonal antibodies lebrikizumab and tralokinumab in patients with uncontrolled asthma showed that they did not reduce the exacerbation rate [Corren et al. 2011; Brightling et al., 2015]. IL-13 induces secretion of a matricellular protein called periostin from bronchial epithelial cells, which may play a role in subepithelial fibrosis and periostin has been used as a biomarker to predict anti-IL-13 treatment effects. Pooled analysis of data from two randomized controlled trials stratifying the treated population by baseline serum periostin levels showed that lebrikizumab reduced exacerbations by 60% (95% confidence interval [CI] 18–80%) compared with placebo in ‘periostin-high’ patients, but only by 5% (95% CI -81– 47%) in ‘periostin-low’ patients [Hanania et al. 2015]. A small study has investigated the use of dupilumab, a monoclonal antibody that targets the IL-4 receptor α subunit, inhibiting both IL-4 and IL-13 signalling. Dupilumab reduced exacerbation rates by 87% compared with placebo (odds ratio 0.08; 95% CI 0.02–0.28; p < 0.001) in patients with moderate-to-severe asthma on combination treatment [Wenzel et al. 2013].
Following the description of impaired antiviral innate immunity in asthmatics, the possibility of augmenting deficient interferon responses has been explored as a therapeutic option. A randomized, placebo-controlled trial of inhaled IFN-β commenced at the onset of an asthma exacerbation did not change asthma symptoms, but did improve morning peak expiratory flow (p = 0.033), and enhanced innate immune responses as determined by serum biomarkers of antiviral activity including CXCL10 [Djukanovic et al. 2014]. Subanalysis of moderate-to-severe asthmatics showed a significant reduction in asthma control questionnaire-6 score (p = 0.004) compared with placebo, suggesting it may be a more effective treatment in those with more severe disease.
COPD
In contrast to asthma few new agents have been approved for use in COPD and those that have consist mainly of the same drug classes of ICSs, LABAs and LAMAs. Long-term use of the macrolide antibiotic azithromycin has been shown to reduce exacerbations in COPD [Albert et al. 2011]. Although it has been assumed that this is due to its antimicrobial action, macrolides have a number of additional mechanisms of action including anti-inflammatory effects. A recent publication reported that azithromycin has antiviral effects and stimulates IFN production in vitro [Gielen et al. 2010]. Use of azithromycin in COPD is not widespread due to concern about the emergence of bacterial resistance. If the anti-inflammatory and antiviral effects of azithromycin mediate some of the reduction in exacerbations then macrolides that retain these mechanisms but are not antibacterial may overcome this hurdle.
Antiviral agents
Another approach to preventing virus-induced exacerbations is to target the infecting viral agent. Antiviral therapy is most well established for influenza but discussion of this is beyond the scope of this review. We will briefly describe here agents that have been developed for treatment of HRV infections. Most serotypes of HRV attach to cells via ICAM-1, therefore blocking this receptor may halt viral infection. In a randomized, placebo-controlled trial in experimental HRV infections, tremacamra, a recombinant soluble ICAM-1 molecule introduced nasally, was effective in reducing symptoms compared with placebo (p < 0.001), whether used before or after inoculation of virus [Turner et al. 1999]. A human ICAM-1 antibody, 14C11, has been shown in a mouse model to inhibit HRV-induced asthma exacerbations [Traub et al. 2013]. Importantly 14C11 did not prevent cell-trafficking via lymphocyte function-associated antigen 1/ICAM-1 interactions, which are important for host defence. ICAM-1 receptor blockers may have a role in preventing viral exacerbations of asthma and COPD and require further evaluation.
Pleconaril is an oral capsid-function inhibitor, which was demonstrated in a large randomized, placebo-controlled trial of healthy adults with picornavirus infections to shorten the duration of illness by 1 day compared with placebo [Hayden et al. 2003]. However, these benefits were outweighed by concerns regarding the risks of drug interactions, particularly with oral contraceptives, and pleconaril was not approved for treatment of the common cold by the US Food and Drug Administration [Senior, 2002]. No studies of pleconaril or other antiviral drugs have been carried out in patients with asthma or COPD. The results from experimental infection studies provide a theoretical basis that antiviral agents may be of clinical benefit in treating asthma and COPD exacerbations. Studies in these specific groups are needed to demonstrate this.
Vaccination is a highly effective means of preventing viral infections and influenza vaccination is recommended for all patients with asthma and COPD. However, effective vaccines against other common respiratory pathogens such as HRV, RSV and coronaviruses are not available. Given the diversity in HRV serotypes, developing an effective vaccine with cross-reactivity against over 100 strains is a considerable challenge, further complicated by the recent recognition of HRV-C species. Highly conserved regions of the VP0 (VP4 and VP2) capsid protein have been identified and immunization with recombinant HRV/VP0 protein promoted airway CD4+ T-cell responses in a mouse model, with more rapid viral RNA clearance from the lungs of mice after subsequent infection [Glanville et al. 2013]. Antibodies to the VP1 protein may also induce antibodies active against different serotypes [Edlmayr et al. 2011], although others have disputed this [Papi and Contoli, 2011]. It is clear that a vaccine protective against HRV remains highly problematic.
Conclusion
It is now well established that respiratory virus infections are a major cause of acute exacerbations in both COPD and asthma, although there remain many unanswered questions and areas for further research. Experimental infection studies have clearly demonstrated increased severity of clinical illness with HRV infection in asthma and COPD, and determining the mechanisms of this offer an attractive target for new therapies. Interferon deficiency may be one such mechanism and has already led to the development of a new treatment. However, it remains unclear whether IFN deficiency is present in all asthmatic patients and whether it is also relevant to COPD. The roles of newly discovered viruses and of dual virus–bacterial infections are other areas where further research is needed. The demonstration that secondary bacterial infection is common following HRV infection in COPD holds out the prospect that antiviral treatments may reduce bacterial infection and reduce antibiotic use. However, this awaits the development of new, more effective antiviral agents.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Richard Hewitt, National Heart and Lung Institute, Imperial College London, UK.
Hugo Farne, National Heart and Lung Institute, Imperial College London, UK.
Andrew Ritchie, National Heart and Lung Institute, Imperial College London, UK.
Emma Luke, Imperial Healthcare NHS Trust, London, UK.
Sebastian L. Johnston, National Heart and Lung Institute, Imperial College London, UK
Patrick Mallia, Faculty of Medicine, National Heart and Lung Institute, Imperial College London, London SW7 2AZ, UK.
References
- Albert R., Connett J., Bailey W., Casaburi R., Cooper J., Jr, Criner G., et al. (2011) Azithromycin for prevention of exacerbations of COPD. N Engl J Med 365: 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almansa R., Sanchez-Garcia M., Herrero A., Calzada S., Roig V., Barbado J., et al. (2011) Host response cytokine signatures in viral and nonviral acute exacerbations of chronic obstructive pulmonary disease. J Interferon Cytokine Res 31: 409–413. [DOI] [PubMed] [Google Scholar]
- Arden K., Chang A., Lambert S., Nissen M., Sloots T., Mackay I. (2010) Newly identified respiratory viruses in children with asthma exacerbation not requiring admission to hospital. J Med Virol 82: 1458–1461. [DOI] [PubMed] [Google Scholar]
- Bafadhel M., McKenna S., Terry S., Mistry V., Reid C., Haldar P., et al. (2011) Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 184: 662–671. [DOI] [PubMed] [Google Scholar]
- Baines K., Hsu A., Tooze M., Gunawardhana L., Gibson P., Wark P. (2013) Novel immune genes associated with excessive inflammatory and antiviral responses to rhinovirus in COPD. Respir Res 14: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandi V., Jakubowycz M., Kinyon C., Mason E., Atmar R., Greenberg S., et al. (2003) Infectious exacerbations of chronic obstructive pulmonary disease associated with respiratory viruses and non-typeable Haemophilus influenzae. FEMS Immunol Med Microbiol 37: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraldo S., Contoli M., Bazzan E., Turato G., Padovani A., Marku B., et al. (2012) Deficient antiviral immune responses in childhood: distinct roles of atopy and asthma. J Allergy Clin Immunol 130: 1307–1314. [DOI] [PubMed] [Google Scholar]
- Bateman E., Hurd S., Barnes P., Bousquet J., Drazen J., Fitzgerald M., et al. (2008) Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J 31: 143–178. [DOI] [PubMed] [Google Scholar]
- Beasley R., Coleman E., Hermon Y., Holst P., O’Donnell T., Tobias M. (1988) Viral respiratory tract infection and exacerbations of asthma in adult patients. Thorax 43: 679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham J., Cadena A., Lin J., Piedra P., Glezen W., Greenberg S., et al. (2005) Respiratory viral infections in patients with chronic, obstructive pulmonary disease. J Infect 50: 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzintino J., Lee W., Laing I., Vang F., Pappas T., Zhang G., et al. (2011) Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J 37: 1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkov Y., Hanson K., Keles S., Brockman-Schneider R., Jarjour N., Gern J. (2010) Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol 3: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossios A., Gourgiotis D., Skevaki C., Saxoni-Papageorgiou P., Lotvall J., Psarras S., et al. (2008) Rhinovirus infection and house dust mite exposure synergize in inducing bronchial epithelial cell interleukin-8 release. Clin Exp Allergy 38: 1615–1626. [DOI] [PubMed] [Google Scholar]
- Bossios A., Psarras S., Gourgiotis D., Skevaki C., Constantopoulos A., Saxoni-Papageorgiou P., et al. (2005) Rhinovirus infection induces cytotoxicity and delays wound healing in bronchial epithelial cells. Respir Res 6: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozinovski S., Hutchinson A., Thompson M., MacGregor L., Black J., Giannakis E., et al. (2008) Serum amyloid A is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 177: 269–278. [DOI] [PubMed] [Google Scholar]
- Bramley A., Dasgupta S., Skarbinski J., Kamimoto L., Fry A., Finelli L., et al. (2012) Intensive care unit patients with 2009 pandemic influenza A (H1N1pdm09) virus infection – United States, 2009. Influenza Other Respir Viruses 6: e134–e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightling C., Chanez P., Leigh R., O’Byrne P., Korn S., She D., et al. (2015) Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med 3: 692–701. [DOI] [PubMed] [Google Scholar]
- Buscho R., Saxtan D., Shultz P., Finch E., Mufson M. (1978) Infections with viruses and Mycoplasma pneumoniae during exacerbations of chronic bronchitis. J Infect Dis 137: 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakebread J., Xu Y., Grainge C., Kehagia V., Howarth P., Holgate S., et al. (2011) Exogenous IFN-beta has antiviral and anti-inflammatory properties in primary bronchial epithelial cells from asthmatic subjects exposed to rhinovirus. J Allergy Clin Immunol 127: 1148–1154. [DOI] [PubMed] [Google Scholar]
- Calvo C., Casas I., Garcia-Garcia M., Pozo F., Reyes N., Cruz N., et al. (2010) Role of rhinovirus C respiratory infections in sick and healthy children in Spain. Pediatr Infect Dis J 29: 717–720. [DOI] [PubMed] [Google Scholar]
- Camargo C., Jr, Ginde A., Clark S., Cartwright C., Falsey A., Niewoehner D. (2008) Viral pathogens in acute exacerbations of chronic obstructive pulmonary disease. Intern Emerg Med 3: 355–359. [DOI] [PubMed] [Google Scholar]
- Cameron R., De Wit D., Welsh T., Ferguson J., Grissell T., Rye P. (2006) Virus infection in exacerbations of chronic obstructive pulmonary disease requiring ventilation. Intensive Care Med 32: 1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro M., Zangrilli J., Wechsler M., Bateman E., Brusselle G., Bardin P., et al. (2015) Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 3: 355–366. [DOI] [PubMed] [Google Scholar]
- Contoli M., Ito K., Padovani A., Poletti D., Marku B., Edwards M., et al. (2015) Th2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy 70: 910–920. [DOI] [PubMed] [Google Scholar]
- Contoli M., Message S., Laza-Stanca V., Edwards M., Wark P., Bartlett N., et al. (2006) Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med 12: 1023–1026. [DOI] [PubMed] [Google Scholar]
- Corren J., Lemanske R., Hanania N., Korenblat P., Parsey M., Arron J., et al. (2011) Lebrikizumab treatment in adults with asthma. N Engl J Med 365: 1088–1098. [DOI] [PubMed] [Google Scholar]
- Cox D., Bizzintino J., Ferrari G., Khoo S., Zhang G., Whelan S., et al. (2013) Human rhinovirus species C infection in young children with acute wheeze is associated with increased acute respiratory hospital admissions. Am J Respir Crit Care Med 188: 1358–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubin C., Parienti J., Vabret A., Ramakers M., Fradin S., Terzi N., et al. (2008) Procalcitonin levels in acute exacerbation of COPD admitted in ICU: a prospective cohort study. BMC Infect Dis 8: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood F., Kamimoto L., D’Mello T., Reingold A., Gershman K., Meek J., et al. (2011) Children with asthma hospitalized with seasonal or pandemic influenza, 2003–2009. Pediatrics 128: e27–e32. [DOI] [PubMed] [Google Scholar]
- De Miguel-Diez J., Carrasco-Garrido P., Hernandez-Barrera V., Rodriguez-Rodriguez P., Puente-Maestu L., De Miguel A., et al. (2012) Hospitalizations from pandemic influenza (pH1N1) infections among patients with asthma or COPD in Spain. J Infect 65: 95–98. [DOI] [PubMed] [Google Scholar]
- De Serres G., Lampron N., La Forge J., Rouleau I., Bourbeau J., Weiss K., et al. (2009) Importance of viral and bacterial infections in chronic obstructive pulmonary disease exacerbations. J Clin Virol 46: 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stefano A., Maestrelli P., Roggeri A., Turato G., Calabro S., Potena A., et al. (1994) Upregulation of adhesion molecules in the bronchial mucosa of subjects with chronic obstructive bronchitis. Am J Respir Crit Care Med 149: 803–810. [DOI] [PubMed] [Google Scholar]
- Dimopoulos G., Lerikou M., Tsiodras S., Chranioti A., Perros E., Anagnostopoulou U., et al. (2012) Viral epidemiology of acute exacerbations of chronic obstructive pulmonary disease. Pulm Pharmacol Ther 25: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djukanovic R., Harrison T., Johnston S., Gabbay F., Wark P., Thomson N., et al. (2014) The effect of inhaled IFN-beta on worsening of asthma symptoms caused by viral infections. A randomized trial. Am J Respir Crit Care Med 190: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlmayr J., Niespodziana K., Popow-Kraupp T., Krzyzanek V., Focke-Tejkl M., Blaas D., et al. (2011) Antibodies induced with recombinant VP1 from human rhinovirus exhibit cross-neutralisation. Eur Respir J 37: 44–52. [DOI] [PubMed] [Google Scholar]
- Edwards M., Haas J., Panettieri R., Jr, Johnson M., Johnston S. (2007) Corticosteroids and beta2 agonists differentially regulate rhinovirus-induced interleukin-6 via distinct cis-acting elements. J Biol Chem 282: 15366–15375. [DOI] [PubMed] [Google Scholar]
- Fensterl V., Sen G. (2009) Interferons and viral infections. Biofactors 35: 14–20. [DOI] [PubMed] [Google Scholar]
- Footitt J., Mallia P., Durham A., Ho W., Trujillo-Torralbo M., Telcian A., et al. (2015) Oxidative and nitrosative stress and histone deacetylase-2 activity in exacerbations of chronic obstructive pulmonary disease. Chest DOI: 10.1378/chest.14-2637. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi A., Walsh E., Formica M., Hennessey P., Criddle M., Peterson D., et al. (2012) Factors associated with symptomatic rhinovirus infection in patients with COPD. J Clin Virol 55: 343–347. [DOI] [PubMed] [Google Scholar]
- George S., Garcha D., Mackay A., Patel A., Singh R., Sapsford R., et al. (2014) Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J 44: 87–96. [DOI] [PubMed] [Google Scholar]
- Gerke A., Tang F., Yang M., Foster E., Cavanaugh J., Polgreen P. (2013) Predicting chronic obstructive pulmonary disease hospitalizations based on concurrent influenza activity. COPD 10: 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghildyal R., Dagher H., Donninger H., De Silva D., Li X., Freezer N., et al. (2005) Rhinovirus infects primary human airway fibroblasts and induces a neutrophil chemokine and a permeability factor. J Med Virol 75: 608–615. [DOI] [PubMed] [Google Scholar]
- Gielen V., Johnston S., Edwards M. (2010) Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur Respir J 36: 646–654. [DOI] [PubMed] [Google Scholar]
- Gielen V., Sykes A., Zhu J., Chan B., Macintyre J., Regamey N., et al. (2015) Increased nuclear suppressor of cytokine signaling 1 in asthmatic bronchial epithelium suppresses rhinovirus induction of innate interferons. J Allergy Clin Immunol 136: 177–188 e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill M., Bajwa G., George T., Dong C., Dougherty I., Jiang N., et al. (2010) Counterregulation between the FcεRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol 184: 5999–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville N., Mclean G., Guy B., Lecouturier V., Berry C., Girerd Y., et al. (2013) Cross-serotype immunity induced by immunization with a conserved rhinovirus capsid protein. PLoS Pathog 9: e1003669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissell T., Powell H., Shafren D., Boyle M., Hensley M., Jones P., et al. (2005) Interleukin-10 gene expression in acute virus-induced asthma. Am J Respir Crit Care Med 172: 433–439. [DOI] [PubMed] [Google Scholar]
- Grunberg K., Sharon R., Sont J., In ‘T, Veen J., Van Schadewijk W., De Klerk E., et al. (2001) Rhinovirus-induced airway inflammation in asthma: effect of treatment with inhaled corticosteroids before and during experimental infection. Am J Respir Crit Care Med 164: 1816–1822. [DOI] [PubMed] [Google Scholar]
- Gulraiz F., Bellinghausen C., Bruggeman C., Stassen F. (2015) Haemophilus influenzae increases the susceptibility and inflammatory response of airway epithelial cells to viral infections. FASEB J 29: 849–858. [DOI] [PubMed] [Google Scholar]
- Hall D., Bates M., Guar L., Cronan M., Korpi N., Bertics P. (2005) The role of p38 MAPK in rhinovirus-induced monocyte chemoattractant protein-1 production by monocytic-lineage cells. J Immunol 174: 8056–8063. [DOI] [PubMed] [Google Scholar]
- Hanania N., Noonan M., Corren J., Korenblat P., Zheng Y., Fischer S., et al. (2015) Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax 70: 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F., Herrington D., Coats T., Kim K., Cooper E., Villano S., et al. (2003) Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin Infect Dis 36: 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini S., Ghasemian E., Jamaati H., Tabaraie B., Amini Z., Cox K. (2015) Association between respiratory viruses and exacerbation of COPD: a case-control study. Infect Dis (Lond) 47: 523–529. [DOI] [PubMed] [Google Scholar]
- Hurst J., Vestbo J., Anzueto A., Locantore N., Mullerova H., Tal-Singer R., et al. (2010) Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 363: 1128–1138. [DOI] [PubMed] [Google Scholar]
- Hutchinson A., Ghimire A., Thompson M., Black J., Brand C., Lowe A., et al. (2007) A community-based, time-matched, case-control study of respiratory viruses and exacerbations of COPD. Respir Med 101: 2472–2481. [DOI] [PubMed] [Google Scholar]
- Ieki K., Matsukura S., Kokubu F., Kimura T., Kuga H., Kawaguchi M., et al. (2004) Double-stranded RNA activates RANTES gene transcription through co-operation of nuclear factor-kappaB and interferon regulatory factors in human airway epithelial cells. Clin Exp Allergy 34: 745–752. [DOI] [PubMed] [Google Scholar]
- Isaacs A., Lindenmann J. (1957) Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci 147: 258–267. [PubMed] [Google Scholar]
- Ishizuka S., Yamaya M., Suzuki T., Takahashi H., Ida S., Sasaki T., et al. (2003) Effects of rhinovirus infection on the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells. J Infect Dis 188: 1928–1939. [DOI] [PubMed] [Google Scholar]
- Jackson D., Makrinioti H., Rana B., Shamji B., Trujillo-Torralbo M., Footitt J., et al. (2014) IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med 190: 1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D., Trujillo-Torralbo M., Del-Rosario J., Bartlett N., Edwards M., Mallia P., et al. (2015) The influence of asthma control on the severity of virus-induced asthma exacerbations. J Allergy Clin Immunol 136: 497. [DOI] [PubMed] [Google Scholar]
- Jakab G. (1982) Immune impairment of alveolar macrophage phagocytosis during influenza virus pneumonia. Am Rev Respir Dis 126: 778–782. [DOI] [PubMed] [Google Scholar]
- Jartti T., Jartti L., Ruuskanen O., Soderlund-Venermo M. (2012) New respiratory viral infections. Curr Opin Pulm Med 18: 271–278. [DOI] [PubMed] [Google Scholar]
- Johnston S., Papi A., Bates P., Mastronarde J., Monick M., Hunninghake G. (1998) Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J Immunol 160: 6172–6181. [PubMed] [Google Scholar]
- Johnston S., Pattemore P., Sanderson G., Smith S., Lampe F., Josephs L., et al. (1995) Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ 310: 1225–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karta M., Gavala M., Curran C., Wickert L., Keely P., Gern J., et al. (2014) LPS modulates rhinovirus-induced chemokine secretion in monocytes and macrophages. Am J Respir Cell Mol Biol 51: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kherad O., Kaiser L., Bridevaux P., Sarasin F., Thomas Y., Janssens J., et al. (2010) Upper-respiratory viral infection, biomarkers, and COPD exacerbations. Chest 138: 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khetsuriani N., Kazerouni N., Erdman D., Lu X., Redd S., Anderson L., et al. (2007) Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol 119: 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloepfer K., Lee W., Pappas T., Kang T., Vrtis R., Evans M., et al. (2014) Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol 133: 1301–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko F., Ip M., Chan P., Chan M., To K., Ng S., et al. (2007) Viral etiology of acute exacerbations of COPD in Hong Kong. Chest 132: 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi-Steiner N., Bates M., Lee W., Hall D., Bertics P. (2006) Human rhinovirus induces robust IP-10 release by monocytic cells, which is independent of viral replication but linked to type I interferon receptor ligation and STAT1 activation. J Leukoc Biol 80: 1364–1374. [DOI] [PubMed] [Google Scholar]
- Kupczyk M., Ten Brinke A., Sterk P., Bel E., Papi A., Chanez P., et al. (2014) Frequent exacerbators – a distinct phenotype of severe asthma. Clin Exp Allergy 44: 212–221. [DOI] [PubMed] [Google Scholar]
- Laza-Stanca V., Stanciu L., Message S., Edwards M., Gern J., Johnston S. (2006) Rhinovirus replication in human macrophages induces NF-kappaB-dependent tumor necrosis factor alpha production. J Virol 80: 8248–8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder J., Kraft D., Mohamed Y., Lu Z., Heil L., Tollefson S., et al. (2013) Human rhinovirus C: age, season, and lower respiratory illness over the past 3 decades. J Allergy Clin Immunol 131: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Souza N., Favoreto S., Wong H., Ward T., Yagi S., Schnurr D., et al. (2009) In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol 123: 1384–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallia P., Footitt J., Sotero R., Jepson A., Contoli M., Trujillo-Torralbo M., et al. (2012) Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 186: 1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallia P., Message S., Gielen V., Contoli M., Gray K., Kebadze T., et al. (2011) Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med 183: 734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallia P., Message S., Kebadze T., Parker H., Kon O., Johnston S. (2006) An experimental model of rhinovirus induced chronic obstructive pulmonary disease exacerbations: a pilot study. Respir Res 7: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinello R., Esper F., Weibel C., Ferguson D., Landry M., Kahn J. (2006) Human metapneumovirus and exacerbations of chronic obstructive pulmonary disease. J Infect 53: 248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch D., Sears M., Jacob J., Lyon G., Burd E., Caliendo A., et al. (2014) Severity of rhinovirus infection in hospitalized adults is unrelated to genotype. Am J Clin Pathol 142: 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna J., Bramley A., Skarbinski J., Fry A., Finelli L., Jain S. (2013) Asthma in patients hospitalized with pandemic influenza A (H1N1)pdm09 virus infection – United States, 2009. BMC Infect Dis 13: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus T., Marley A., Baxter N., Christie S., O’Neill H., Elborn J., et al. (2008) Respiratory viral infection in exacerbations of COPD. Respir Med 102: 1575–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee L., Harmsen A. (2006) Both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. Infect Immun 74: 6707–6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Message S., Laza-Stanca V., Mallia P., Parker H., Zhu J., Kebadze T., et al. (2008) Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A 105: 13562–13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E., Edwards K., Weinberg G., Iwane M., Griffin M., Hall C., et al. (2009) A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol 123: 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minosse C., Selleri M., Zaniratti M., Cappiello G., Longo R., Schifano E., et al. (2008) Frequency of detection of respiratory viruses in the lower respiratory tract of hospitalized adults. J Clin Virol 42: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell I., Inglis J., Simpson H. (1978) Viral infection as a precipitant of wheeze in children. Combined home and hospital study. Arch Dis Child 53: 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen E., Louie J., Pertowski C., Cadwell B., Weiss E., Acosta M., et al. (2013) Epidemiology and outcomes of adults with asthma who were hospitalized or died with 2009 pandemic influenza A (H1N1) – California, 2009. Influenza Other Respir Viruses 7: 1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser A., Vrtis R., Burchell L., Lee W., Dick C., Weisshaar E., et al. (2005) Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med 171: 645–651. [DOI] [PubMed] [Google Scholar]
- Myles P., Nguyen-Van-Tam J., Semple M., Brett S., Bannister B., Read R., et al. (2013) Differences between asthmatics and nonasthmatics hospitalised with influenza A infection. Eur Respir J 41: 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkar D., Bowman E., Schneider D., Wang Q., Shim J., Zhao Y., et al. (2010) Rhinovirus infection of allergen-sensitized and -challenged mice induces eotaxin release from functionally polarized macrophages. J Immunol 185: 2525–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Van-Tam J., Openshaw P., Hashim A., Gadd E., Lim W., Semple M., et al. (2010) Risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May–September 2009). Thorax 65: 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver B., Johnston S., Baraket M., Burgess J., King N., Roth M., et al. (2006) Increased proinflammatory responses from asthmatic human airway smooth muscle cells in response to rhinovirus infection. Respir Res 7: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver B., Lim S., Wark P., Laza-Stanca V., King N., Black J., et al. (2008) Rhinovirus exposure impairs immune responses to bacterial products in human alveolar macrophages. Thorax 63: 519–525. [DOI] [PubMed] [Google Scholar]
- Ortega H., Liu M., Pavord I., Brusselle G., Fitzgerald J., Chetta A., et al. (2014) Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 371: 1198–1207. [DOI] [PubMed] [Google Scholar]
- Pant S., Walters E., Griffiths A., Wood-Baker R., Johns D., Reid D. (2009) Airway inflammation and anti-protease defences rapidly improve during treatment of an acute exacerbation of COPD. Respirology 14: 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos N., Bates P., Bardin P., Papi A., Leir S., Fraenkel D., et al. (2000) Rhinoviruses infect the lower airways. J Infect Dis 181: 1875–1884. [DOI] [PubMed] [Google Scholar]
- Papi A., Bellettato C., Braccioni F., Romagnoli M., Casolari P., Caramori G., et al. (2006) Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 173: 1114–1121. [DOI] [PubMed] [Google Scholar]
- Papi A., Contoli M. (2011) Rhinovirus vaccination: the case against. Eur Respir J 37: 5–7. [DOI] [PubMed] [Google Scholar]
- Passariello C., Schippa S., Conti C., Russo P., Poggiali F., Garaci E., et al. (2006) Rhinoviruses promote internalisation of Staphylococcus aureus into non-fully permissive cultured pneumocytes. Microbes Infect 8: 758–766. [DOI] [PubMed] [Google Scholar]
- Perotin J., Dury S., Renois F., Deslee G., Wolak A., Duval V., et al. (2013) Detection of multiple viral and bacterial infections in acute exacerbation of chronic obstructive pulmonary disease: a pilot prospective study. J Med Virol 85: 866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D., Fletcher M., Van Der Molen T. (2014) Asthma control and management in 8,000 European patients: the recognise asthma and link to symptoms and experience (REALISE) survey. NPJ Prim Care Respir Med 24: 14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Zhu J., Bandi V., Atmar R., Hattotuwa K., Guntupalli K., et al. (2003) Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 168: 968–975. [DOI] [PubMed] [Google Scholar]
- Rabe K., Vermeire P., Soriano J., Maier W. (2000) Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) study. Eur Respir J 16: 802–807. [DOI] [PubMed] [Google Scholar]
- Ringshausen F., Tan A., Allander T., Borg I., Arinir U., Kronsbein J., et al. (2009) Frequency and clinical relevance of human bocavirus infection in acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 4: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Rieiro C., Carrasco-Garrido P., Hernandez-Barrera V., Lopez De Andres A., Jimenez-Trujillo I., Gil De, Miguel A., et al. (2012) Pandemic influenza hospitalization in spain (2009): incidence, in-hospital mortality, comorbidities and costs. Hum Vaccin Immunother 8: 443–447. [DOI] [PubMed] [Google Scholar]
- Rohde G., Borg I., Arinir U., Kronsbein J., Rausse R., Bauer T., et al. (2005) Relevance of human metapneumovirus in exacerbations of COPD. Respir Res 6: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde G., Wiethege A., Borg I., Kauth M., Bauer T., Gillissen A., et al. (2003) Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax 58: 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roponen M., Yerkovich S., Hollams E., Sly P., Holt P., Upham J. (2010) Toll-like receptor 7 function is reduced in adolescents with asthma. Eur Respir J 35: 64–71. [DOI] [PubMed] [Google Scholar]
- Sajjan U., Ganesan S., Comstock A., Shim J., Wang Q., Nagarkar D., et al. (2009) Elastase- and LPS-exposed mice display altered responses to rhinovirus infection. Am J Physiol Lung Cell Mol Physiol 297: L931–L944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan U., Jia Y., Newcomb D., Bentley J., Lukacs N., Lipuma J., et al. (2006) H. influenzae potentiates airway epithelial cell responses to rhinovirus by increasing ICAM-1 and TLR3 expression. FASEB J 20: 2121–2123. [DOI] [PubMed] [Google Scholar]
- Sajjan U., Wang Q., Zhao Y., Gruenert D., Hershenson M. (2008) Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am J Respir Crit Care Med 178: 1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa-Olalla Peralta P., Cortes-Garcia M., Vicente-Herrero M., Castrillo-Villamandos C., Arias-Bohigas P., Pachon-Del Amo I., et al. (2010) Risk factors for disease severity among hospitalised patients with 2009 pandemic influenza A (H1N1) in Spain, April–December 2009. Euro Surveill 15: pii=19667. [DOI] [PubMed] [Google Scholar]
- Schneider D., Ganesan S., Comstock A., Meldrum C., Mahidhara R., Goldsmith A., et al. (2010) Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182: 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemungal T., Harper-Owen R., Bhowmik A., Moric I., Sanderson G., Message S., et al. (2001) Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 164: 1618–1623. [DOI] [PubMed] [Google Scholar]
- Senior K. (2002) FDA panel rejects common cold treatment. Lancet Infect Dis 2: 264. [DOI] [PubMed] [Google Scholar]
- Sheppard P., Kindsvogel W., Xu W., Henderson K., Schlutsmeyer S., Whitmore T., et al. (2003) IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol 4: 63–68. [DOI] [PubMed] [Google Scholar]
- Singanayagam A., Glanville N., Walton R., Aniscenko J., Pearson R., Pinkerton J., et al. (2015) A short-term mouse model that reproduces the immunopathological features of rhinovirus-induced exacerbation of COPD. Clin Sci (Lond) 129: 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Lee S., Porter P., Xu C., Ohno A., Atmar R., et al. (2010) Human rhinovirus proteinase 2A induces Th1 and Th2 immunity in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 125: 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler M., Matz J., Townley R., Buhl R., O’Brien J., Fox H., et al. (2001) The anti-IGE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J 18: 254–261. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld G., Hudgens R. (1986) Effect of sidestream and mainstream smoke exposure on in vitro interferon-alpha/beta production by L-929 cells. Cancer Res 46: 2779–2783. [PubMed] [Google Scholar]
- Spurrell J., Wiehler S., Zaheer R., Sanders S., Proud D. (2005) Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am J Physiol Lung Cell Mol Physiol 289: L85–L95. [DOI] [PubMed] [Google Scholar]
- Sun K., Metzger D. (2008) Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med 14: 558–564. [DOI] [PubMed] [Google Scholar]
- Sykes A., Edwards M., Macintyre J., Del Rosario A., Bakhsoliani E., Trujillo-Torralbo M., et al. (2012) Rhinovirus 16-induced IFN-alpha and IFN-beta are deficient in bronchoalveolar lavage cells in asthmatic patients. J Allergy Clin Immunol 129: 1506–1514. [DOI] [PubMed] [Google Scholar]
- Sykes A., Edwards M., Macintyre J., Del Rosario A., Gielen V., Haas J., et al. (2013) TLR3, TLR4 and TLRs7-9 induced interferons are not impaired in airway and blood cells in well controlled asthma. PLoS One 8: e65921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes A., Macintyre J., Edwards M., Del Rosario A., Haas J., Gielen V., et al. (2014) Rhinovirus-induced interferon production is not deficient in well controlled asthma. Thorax 69: 240–246. [DOI] [PubMed] [Google Scholar]
- Tan W., Xiang X., Qiu D., Ng T., Lam S., Hegele R. (2003) Epidemiology of respiratory viruses in patients hospitalized with near-fatal asthma, acute exacerbations of asthma, or chronic obstructive pulmonary disease. Am J Med 115: 272–277. [DOI] [PubMed] [Google Scholar]
- Traub S., Nikonova A., Carruthers A., Dunmore R., Vousden K., Gogsadze L., et al. (2013) An anti-human ICAM-1 antibody inhibits rhinovirus-induced exacerbations of lung inflammation. PLoS Pathog 9: e1003520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R., Wecker M., Pohl G., Witek T., McNally E., St George R., et al. (1999) Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection: a randomized clinical trial. JAMA 281: 1797–1804. [DOI] [PubMed] [Google Scholar]
- Unger B., Faris A., Ganesan S., Comstock A., Hershenson M., Sajjan U. (2012) Rhinovirus attenuates non-typeable Hemophilus influenzae-stimulated IL-8 responses via TLR2-dependent degradation of Irak-1. PLoS Pathog 8: e1002969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet C., Pons-Catalano C., Mandelcwajg A., Wang A., Raymond J., Lebon P., et al. (2009) Human bocavirus: a cause of severe asthma exacerbation in children. J Pediatr 155: 286–288. [DOI] [PubMed] [Google Scholar]
- Vestbo J., Hurd S., Agusti A., Jones P., Vogelmeier C., Anzueto A., et al. (2013) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 187: 347–365. [DOI] [PubMed] [Google Scholar]
- Wang J., Kwon H., Jang Y. (2009) Rhinovirus enhances various bacterial adhesions to nasal epithelial cells simultaneously. Laryngoscope 119: 1406–1411. [DOI] [PubMed] [Google Scholar]
- Wark P., Johnston S., Bucchieri F., Powell R., Puddicombe S., Laza-Stanca V., et al. (2005) Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 201: 937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wark P., Johnston S., Moric I., Simpson J., Hensley M., Gibson P. (2002) Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur Respir J 19: 68–75. [DOI] [PubMed] [Google Scholar]
- Wark P., Tooze M., Powell H., Parsons K. (2013) Viral and bacterial infection in acute asthma and chronic obstructive pulmonary disease increases the risk of readmission. Respirology 18: 996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel S., Ford L., Pearlman D., Spector S., Sher L., Skobieranda F., et al. (2013) Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med 368: 2455–2466. [DOI] [PubMed] [Google Scholar]
- Wilkinson T., Hurst J., Perera W., Wilks M., Donaldson G., Wedzicha J. (2006) Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest 129: 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J., Crowe J., Jr, Enriquez R., Minton P., Peebles R., Jr, Hamilton R., et al. (2005) Human metapneumovirus infection plays an etiologic role in acute asthma exacerbations requiring hospitalization in adults. J Infect Dis 192: 1149–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Tang W., Gwaltney J., Jr, Wu Y., Elias J. (1997) Rhinovirus stimulation of interleukin-8 in vivo and in vitro: role of NF-kappaB. Am J Physiol 273: L814–L824. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Tang W., Ray A., Wu Y., Einarsson O., Landry M., et al. (1996) Rhinovirus stimulation of interleukin-6 in vivo and in vitro. Evidence for nuclear factor kappa B-dependent transcriptional activation. J Clin Invest 97: 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]