Abstract
Objectives:
Patients with chronic kidney disease present a higher degree of left ventricular hypertrophy than expected for hypertension levels. In chronic kidney disease the plot between the quotient extracellular water/total body water and aldosterone is shifted up and to the right. There are few studies that verified the role of aldosterone in cardiac remodeling in this set of patients. The aim of this study was to evaluate the relationship between serum aldosterone and left ventricular mass index in patients with chronic kidney disease on hemodialysis.
Methods:
The patients were submitted to clinical and laboratory evaluation, bioelectrical impedance, echocardiography and ambulatory blood pressure monitoring. The 27 patients included were divided into two groups according to aldosterone level and compared with each other.
Results:
The group of patients with higher aldosterone levels had higher left ventricular mass index. These groups were heterogeneous with regard to ambulatory systolic blood pressure, body mass index, and aldosterone levels and homogeneous with regard to the quotient extracellular water/total body water, renin–angiotensin–aldosterone system blockers, beta blocker use and other clinical characteristics. The association between aldosterone levels and left ventricular mass index was adjusted to confounding variables by a multiple linear regression analysis in which aldosterone was independently associated with left ventricular mass index.
Conclusion:
The data presented are consistent with a pathogenic role of aldosterone in left ventricular hypertrophy in patients with chronic kidney dialysis in dialysis patients.
Trial registration:
ClinicalTrials.gov identifier: NCT01128101.
Keywords: aldosterone, hemodialysis, left ventricular hypertrophy
Introduction
Left ventricular hypertrophy (LVH) is a major cardiovascular risk factor and a powerful predictor of mortality in chronic kidney disease (CKD) patients [Martin et al. 2004]. In CKD, there is a higher degree of ventricular hypertrophy than expected for the hypertension levels, thus the relationship between ventricular mass and blood pressure is higher than that presented in essential hypertension itself, and has been called inappropriate hypertrophy [London et al. 2001; Martin et al. 2004; Olsen et al. 2004; Cerasola et al. 2011].
Several factors may be considered in the genesis of inappropriate hypertrophy in CKD. Among these factors are fluid and salt excess [Cailar et al. 2010], increase of the parathyroid hormone (PTH) [Custódio et al. 2012], anemia [Levin et al. 1999], arteriovenous fistula [Covic et al. 2010], sympathetic hyperactivity [Rump et al. 2000], increased activity of the renin–angiotensin–aldosterone system (RAAS) [Bhattacharya et al. 2009], and other unidentified factors.
It has been known that aldosterone is associated with abnormal myocardial growth [Matsubara et al. 2010]. Besides sodium retention, aldosterone also reduces myocardial norepinephrine uptake and sensitivity of baroreceptors, promotes myocardial fibrosis, fibroblast proliferation, and changes in sodium channel expression [Rocco and Fang, 2006]. These latter effects stimulate cardiac remodeling [Weber, 2001].
In edematous patients with heart failure or renal failure, aldosterone promotes an increase of extracellular volume, increasing plasma volume and a continuous retaining of sodium by the kidneys [Lee et al. 2008]. Furthermore, the relationship between aldosterone and extracellular volume is clearly shifted to the right in patients with CKD in hemodialysis (HD) [Bomback et al. 2009].
In this context, the effect of drugs which reduce serum concentration of angiotensin and its effect, which is the most potent stimulus for aldosterone secretion, might be supplanted, which leads to a new state of excessive aldosterone: a phenomenon called breakthrough of aldosterone [Schrier, 2010].
In summary: there is a very probable major role of aldosterone in the high prevalence of LVH of CKD patients but there are few studies that indicated the role of aldosterone in cardiac remodeling in this set of patients [Sato et al. 1999; Steigerwalt et al. 2007]. Because of the need for more information about the potential role of aldosterone in the progression of LVH in patients’ HD, the objective of this study is to determine whether the inappropriate elevation of the levels of this hormone may be associated with ventricular hypertrophy in these patients.
Methods
Study design
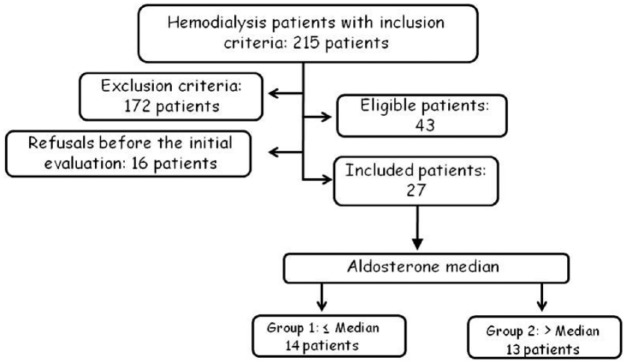
It constitutes a prospective observational trial to evaluate the association between aldosterone levels and left ventricular mass of HD patients (Figure 1). This protocol follows the 196/96 Resolution of National Health Council [Conselho Nacional De Saúde, 1996] and was approved by the local Ethics Committee Protocol (CEP 3439-2010). All patients gave their written informed consent.
Figure 1.
Flow chart of the selection process for inclusion and exclusion of patients with chronic kidney disease in hemodialysis in the clinical study.
Inclusion criteria
Patients with CKD on HD aged at least 18 years, who presented with left ventricular mass index (LVMI) greater than 51 g/m2.7 [Zoccali et al. 2001], indexed for height, with stable antihypertensive treatment in the last 6 months.
Exclusion criteria
Dialysis dose measured by Kt/V [Daugirdas, 1995] less than 1.2; history or evidence of angina or myocardial infarction, heart failure, peripheral vascular disease, previous hyperkalemia, valvular heart disease, atrial fibrillation, hemoglobin < 10g/dl, patients under treatment with spironolactone.
Groups
Patients were evaluated prior to inclusion in the study protocol and then divided into two groups according to the value of the median levels of all patients (Figure 1). Group 1 comprised patients who had lower aldosterone levels and Group 2, higher aldosterone levels. These groups were compared for the studied variables.
Variables
Blood samples were collected immediately prior to the start of HD for routine hematology (red blood cells, white blood count and platelet count), and biochemistry (urea, creatinine, potassium, calcium, phosphorus, alanine amino transferase, total proteins and fractions, alkaline phosphatase, transferrin, serum iron, ferritin, C-reactive protein (CRP), PTH, lipid profile and glycated hemoglobin). Samples were collected pre- and post-dialysis for urea. The dose of dialysis was determined by Daugirdas’ formula [Daugirdas, 1995]. For measurement of aldosterone levels the blood samples were collected after the patient remained supine for 30 minutes (for the stabilization of plasma levels). The samples were centrifuged at 2500 rpm, 4°C, and the plasmas were stored at -80°C for later immunoradiometric assay.
Echocardiography (ECHO)
Echocardiograms were performed at the Center for Diagnostic Imaging (Univertsity Hospital of Botucatu (SP) - Brazil) by a single examiner. The equipment used was the Vivid S6 GE (General Electric) equipped with multifrequency ultrasonic transducer (2.0 to 3.5 MHz) and record system. Images were obtained and analyzed following the recommendations of the American Society of Echocardiography [Chamber Quantification Writing Group, 2005] on the interdialytic day [Martin et al. 2003]. The following data were recorded: heart rate, systolic and diastolic dimensions of the left ventricle, diastolic thickness of the posterior wall and septum. These data were used to calculate ventricular mass, using the following formula: left ventricular mass (g) = 0.8 × {1:04 [(LVDD + EDPW + EDS)³ - (LVDD) ³]} + 0.6, where LVDD, left ventricular diastolic dimension; and EDPW and EDS represent the thickness of the end-diastolic posterior wall and interventricular septum respectively. The left ventricular mass was normalized for height to the power 2.7 [Zoccali et al. 2001].
Ambulatory blood pressure monitoring
Ambulatory blood pressure monitoring (ABPM) was performed with a SpaceLabs 90202 monitor (SpaceLabs, Redmond, WA, USA) in an inter-HD period that was 24 hours before the beginning of the next HD (24-h ABPM). Examinations were considered valid if they fulfilled the following criteria: minimum of recording 21 h, and minimum number of valid measurements per hour: three during waking hours and two during sleep hours [Sociedade Brasileira De Cardiologia, 2011]. The following parameters were considered for interpretation: 24 h, wake and sleep mean systolic blood pressure (SBP) and diastolic blood pressure (DBP) and percentage of nocturnal dipping for SBP and DBP.
Bioelectrical impedance
We used a monofrequencial bioimpedance device (BIA) of Biodynamics®, model 450 (Seattle, Washington, USA). The analysis of BIA was based on the measurement of total body resistance to the passage of an electric current of 800 μA and 50 kHz. The measurement was performed on the contralateral side of vascular access, with the patient in supine position on a nonconductive surface, with a check to make sure their legs did not touch one another and their arms did not touch their trunk. Weight, height, sex and age were recorded on the device. The values determined by the device are based on the calculation of equations proposed by Kushner and Schoeller [Kushner and Schoeller, 1986] and Cohn and colleagues [Cohn et al. 1985]. Resistance and reactance were determined to calculate total body water (TBW), and intracellular and extracellular water values (ECW).
Statistical analysis
The data were expressed as mean ± standard deviation for normally distributed variables and median (first and third quartiles) for the variables of nonparametric distribution. The groups were compared by a Student’s t-test, Mann–Whitney test, Chi-square test or Fisher test when appropriate. A multiple linear regression analysis was performed between aldosterone level and variables with p < 0.1 between groups, namely: LVMI as a dependent variable and adjusted to potential confounding factors including: SBP, albumin, body mass index (BMI) and aldosterone (model 1) and model 2: the same independent variables but left ventricular mass without indexation as an independent variable. CRP was forced in both models. Differences were considered statistically significant at p < 0.05.
Results
The flowchart of patient inclusion is depicted in Figure 1. From 215 patients, 172 presented exclusion criteria: 10 patients by inadequate dialysis dose; 32 by history or evidence of angina or myocardial infarction, 19 patients by heart failure, 15 by peripheral vascular disease, 22 by previous hyperkalemia, 4 by valvular heart disease, 6 by atrial fibrillation, 16 by hemoglobin < 10g/dl, 5 patients under treatment with spironolactone, and 43 patients fulfilled more than one exclusion criterion.
The clinical characteristics are presented in Table 1. The groups did not differ with regard to age, gender, height, interdialytic weight gain, dialysis vintage, ambulatory DBP, relationship between ECW/TBW, RAAS blockers use or beta-blockers. All other relevant medications were retrieved from medical records and depicted in this table. Only insulin use demonstrated a statistical significance between groups. No patient was taking oral antidiabetic medications. Body mass, BMI, TBW, ECW, awake SBP, left ventricular mass and LVMI were different between groups. With regard to urine volume, all patients of both groups were anuric (<100ml/24 h). Ethnicity was homogeneous between groups; only one patient of African descent participated in the lower aldosterone group. In regard of vascular accesses, in group 1 there were two patients with proximal fistulas, seven with distal fistulas and five with catheters; in group 2 there were no patients with proximal fistulas, eight with distal fistulas and five with catheters; p = 0.362.
Table 1.
Clinical characteristics of patients with chronic kidney disease in hemodialysis.
| Group 1 (n = 14) | Group 2 (n = 13) | p | |
|---|---|---|---|
| Age (years) | 50 ± 16.3 | 55 ± 16.2 | 0.445 |
| Gender/sex# | |||
| Male | 6 | 8 | 0.351 |
| Female | 8 | 5 | |
| Height (cm) | 160 ± 7.2 | 161 ± 8.7 | 0.745 |
| Body mass (kg) | 61 ± 9.7 | 72 ± 12.5 | 0.024 |
| Body mass index | 24 ± 3.7 | 28 ± 5.6 | 0.039 |
| Dialysis vintage (months)* | 31 (15–36) | 13 (9–29) | 0.244 |
| Diabetes mellitus | 4 | 6 | 0.196 |
| Total body water (l) | 31 ± 4.7 | 36 ± 5.0† | 0.010 |
| Extracellular water (l) | 14 ± 2.6 | 17 ± 3.3† | 0.017 |
| ECW/TBW (%) | 0.46 ± 0.042 | 0.47 ± 0.05 | 0.407 |
| Interdialytic weight gain (kg) | 2.1 ± 1.26 | 2.4 ± 2.05 | 0.655 |
| Left ventricular mass index (g/cm2,7) | 62 ± 14.7 | 74 ± 12.2† | 0.026 |
| Left ventricular mass (g) | 218 ± 45.8 | 268 ± 45.7† | 0.009 |
| Ambulatory blood pressure monitoring | |||
| 24 h systolic blood pressure (mm Hg) | 127 ± 14.2 | 140 ± 19.1† | 0.065 |
| 24 h diastolic blood pressure (mm Hg) | 78 ± 8.9 | 83 ± 12.3 | 0.224 |
| Wake systolic blood pressure (mm Hg) | 125 ± 15.3 | 141 ± 19.4† | 0.046 |
| Wake diastolic blood pressure (mm Hg) | 78 ± 9.8 | 84 ± 11.7 | 0.191 |
| Sleep systolic blood pressure (mm Hg) | 126 ± 18.8 | 138 ± 22.7 | 0.161 |
| Sleep diastolic blood pressure (mm Hg) | 73 ± 10.3 | 70 ± 18.7 | 0.650 |
| Systolic dipping (mm Hg) | 0.4 ± 11.00 | −1.9 ± 7.5 | 0.565 |
| Diastolic dipping (mm Hg) | −5.9 ± 11.8 | −14 ± 23.3 | 0.282 |
| Use of # | |||
| Angiotensin receptor blockers | 4 | 5 | 0.128 |
| Angiotensin-converting enzyme inhibitors | 4 | 4 | 0.603 |
| β-blockers | 7 | 7 | 0.849 |
| Calcium channel blockers | 3 | 6 | 0.173 |
| Sympatholytics | 2 | 5 | 0.152 |
| Diuretics | 3 | 6 | 0.173 |
| Insulin | 1 | 6 | 0.021 |
| Statins | 13 | 9 | 0.114 |
| Erythropoietin | 12 | 12 | 0.586 |
| Calcitriol | 9 | 9 | 0.785 |
| Intravenous iron | 6 | 8 | 0.332 |
| Phosphorus binders | 13 | 9 | 0.114 |
Data expressed in mean ± standard deviation, except when flagged with *: median (first quartile–third quartile); #: absolute number. †: selected to multiple analysis.
Table 2 shows laboratory data. The groups were homogeneous with regard to potassium, PTH, CRP, calcium, phosphorus, urea, creatinine, hemoglobin, iron, ferritin and glycated hemoglobin. Albumin approached statistical significance (p < 0.1). Group 2 presented aldosterone levels above the normal values median (Figure 2). The patients included in this study were anuric and not able to measure sodium excretion, furthermore ECW/TBW was used to determine the volume status of these patients (Figure 2). Group 2 presented higher LVMI (Figure 3). The groups were heterogeneous with regard to interventricular septum thickness (group 1: 12 ± 1.6 mm versus group 2: 13 ± 1.5 mm, p = 0.048) and LVDD (group 1: 45 ± 3.4 mm versus group 2: 48 ± 3.2 mm, p = 0.041).
Table 2.
Laboratory data of patients with chronic kidney disease in hemodialysis.
| Group 1 (n = 14) | Group 2 (n = 13) | p | |
|---|---|---|---|
| Aldosterone (ng/dl) | 12 ± 5.0 | 35.5 ± 18.5† | − |
| Parathyroid hormone (pg/mol) | 457 ± 265.2 | 465 ± 298.0 | 0.943 |
| C-reactive protein (mg/dl)* | 0.5 (0.5–1.1) | 0.5 (0.5–0.7) | 0.511 |
| Serum potassium (mmol/L) | 5 ± 0.7 | 5 ± 0.6 | 0.854 |
| Serum calcium (mg/dl) | 9 ± 0.8 | 9 ± 0.8 | 0.324 |
| Serum phosphorus (mg/dl) | 5 ± 0.8 | 5 ± 1.1 | 0.883 |
| Serum iron (mg/dl) | 88 ± 25.9 | 68.0 ± 35.7 | 0.103 |
| Ferritin (mg/mol) | 860 ± 577.1 | 834 ± 757.7 | 0.923 |
| Glucose (mg/dl)* | 84 (80–156) | 117 (99–146) | 0.198 |
| Glycated hemoglobin (%) | 6.0 ± 1.29 | 6.6 ± 1.89 | 0.343 |
| Hemoglobin (g/%) | 11 ± 1.6 | 12 ± 1.7 | 0.604 |
| Urea pre (mg/dl) | 112 ± 22.0 | 103 ± 30.6 | 0.373 |
| Creatinine (mg/dl) | 9 ± 1.8 | 9 ± 2.3 | 0.762 |
| Albumin (g/dl) | 4.1 ± 0.4 | 4.0 ± 0.4† | 0.063 |
Data expressed in mean ± standard deviation, except when flagged with *: median (first quartile–third quartile). †: selected to multiple analysis.
Figure 2.
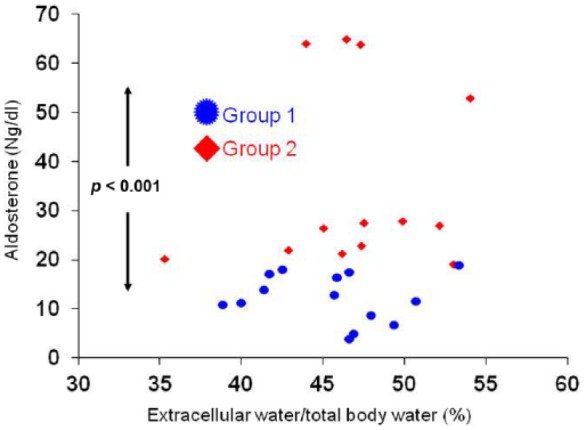
Relationship between aldosterone and volume status in patients with chronic kidney disease in hemodialysis.
Figure 3.
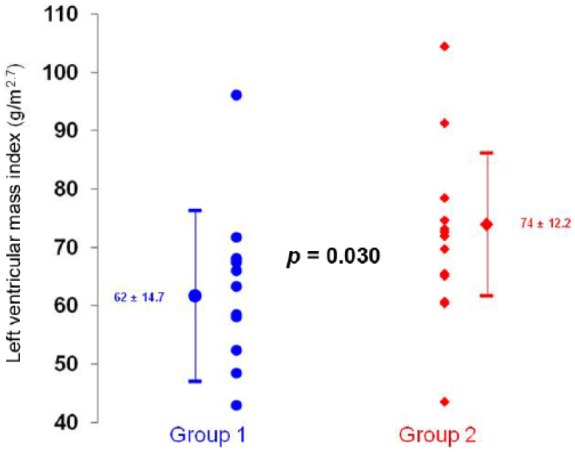
Left ventricular mass index in relation to plasma aldosterone in patients with chronic kidney disease in hemodialysis. Group 1: patients with an aldosterone equal to or below the median. Group 2: patients with aldosterone above the median, p = 0.030.
Figure 4.
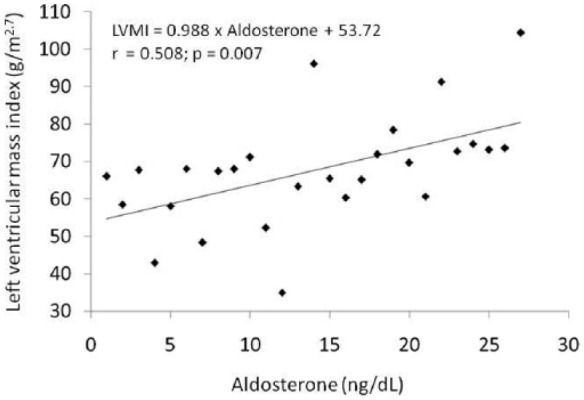
Regression between left ventricular mass index in relation to plasma aldosterone in patients with chronic kidney disease in hemodialysis.
LVMI, left ventricular mass index.
Table 3 shows p-values and univariate correlation coefficients between tested variables and LVMI. Table 4 shows the same data in relation to left ventricular mass. These variables were chosen to compose the multiple regression analysis in which aldosterone levels and BMI were independent variables associated with LVMI (aldosterone: r = 0.679, p = 0.018 and BMI: r = 0.492, p = 0.019). CRP was used in the model to assess the influence of a microinflammatory state (r = 2.569, p = 0.028) (Table 3). To rule out the possibility of mathematical coupling in relation to LVMI and BMI, multiple regression analysis was repeated using left ventricular mass as the dependent variable. In this analysis, it correlated with albumin, aldosterone and CRP (albumin: r = 0.632, p = 0.035; aldosterone: r = 0.975, p = 0.007 and CRP: r = 0.560, p = 0.023) (Table 4).
Table 3.
Univariate and multiple linear regression analysis between aldosterone level and left ventricular mass index as dependent variable adjusted to potential confounding factors (model 1) in patients with chronic kidney disease in hemodialysis.
| Univariate |
Multiple |
|||
|---|---|---|---|---|
| r | p | r | p | |
| ABPM SBP (mmHg) | 0.407 | 0.049 | −0.105 | 0.567 |
| Body mass index (kg/m2) | 0.570 | 0.002 | 0.492 | 0.019 |
| Albumin g/dl | −0.343 | 0.080 | 0.330 | 0.163 |
| C-reactive protein (mg/dl) | 0.085 | 0.672 | 0.451 | 0.028 |
| Aldosterone (ng/dl) | 0.508 | 0.007 | 0.679 | 0.018 |
Variable dependent: Left ventricular mass index.
ABPM SBP, 24 hours ambulatory systolic blood pressure.
Table 4.
Univariate and multiple linear regression analysis between aldosterone level and left ventricular mass as dependent variable adjusted to potential confounding factors (model 2) in patients with chronic kidney disease in hemodialysis.
| Univariate |
Multiple |
|||
|---|---|---|---|---|
| r | p | r | p | |
| ABPM SBP (mmHg) | 0.330 | 0.116 | −0.060 | 0.780 |
| Body Mass Index (kg/m2) | 0.377 | 0.053 | 0.069 | 0.747 |
| Albumin g/dl | −0.191 | 0.340 | 0.632 | 0.035 |
| C-reactive protein (mg/dl) | 0.163 | 0.418 | 0.560 | 0.023 |
| Aldosterone (ng/dl) | 0.505 | 0.007 | 0.975 | 0.007 |
Variable dependent: Left ventricular mass.
ABPM SBP, 24 hours ambulatory systolic blood pressure.
Discussion
Aldosterone breakthrough is defined as the inappropriate increase of aldosterone levels beyond physiological controllers, namely, stimulation by angiotensin II or potassium excess and inhibition by volume excess [Bomback and Klemmer, 2007; Schrier et al. 2010]. In patients with CKD, aldosterone levels are inappropriately high, even for a greater extracellular volume of these patients when compared to a control group [Bomback et al. 2009]. In other words, aldosterone is involved in the pathogenesis of LVH in other subsets of patients. The relationship between left ventricular mass and aldosterone is well stated among hypertensive and diabetic patients but not in CKD, particularly among dialysis patients, therefore, this premise was not evaluated in CKD patients. Therefore, the aim of this study was to determine whether aldosterone might be associated with inappropriate ventricular hypertrophy in HD patients. In the current study we found that LVH was associated with aldosterone levels independently of confounding factors, in a subset of patients not previously evaluated for this issue.
Interestingly, aldosterone breakthrough occurs despite treatment with angiotensin-converting-enzyme inhibitors, angiotensin receptor blockers or beta blockers [Schrier, 2010]. In the present study lower levels of aldosterone in group 1 cannot be explained by inhibition of the RAAS, since these patients used RAAS blockers or beta-blockers less frequently. It should be noted that group 2, which showed the higher serum aldosterone levels, was composed of patients with a higher BMI. This fact can constitute an alternative explanation for the autonomous and inappropriate secretion of aldosterone, since adipocytes can stimulate aldosterone secretion by a soluble factor [Miki et al. 2006].
Recently, it has been shown that there is cross-regulation between the RAAS and adipose tissue [Calhoun and Sharma, 2010; Flynn and Backris, 2011; Aghamohammadzadeh and Heagerty, 2012; Anagnostis et al. 2014]. Adipose tissue is a producer of adiponectin, an adipokine associated with an improvement in cardiorenal diseases in obesity. However, in situations in which dysfunction occurs in adipose tissue, as in diabetes mellitus, adiponectin levels are decreased, reducing the stimulation of adiponectin receptors in the adrenal cortex, generating an increase in plasma aldosterone [Flynn and Backris, 2011].
LVH occurs due to the growth of cardiomyocytes, and may be accompanied by other changes in tissue structure. Aldosterone stimulates cardiac remodeling [Weber, 2001], but it may also stimulate the production of reactive oxygen species and transforming growth factor beta (TGFb), via the mineralocorticoid receptor (MR) [Calhoun and Sharma, 2010]. The MR response in various organs (heart, blood vessels, liver, pancreatic cells and glomerular mesangial cells) has been regarded as responsible for nongenomic effects of aldosterone. The transcription factor NF-kB may also be involved in these effects, leading to inflammation, oxidative stress, apoptosis and fibrosis [Schrier et al. 2010; Leroy et al. 2009]. In the current study, we used CRP in the multiple regression models to assess the impact of inflammation in these patients, and there was a correlation between CRP and LVMI, confirming the literature data.
The lower albumin level in group 2 (which had higher left ventricular mass and LVMI) can also be explained by the fact that the average normal plasma aldosterone concentration (free and protein bound) is 6 ng/dl and a free fraction constitutes 30–40% of the total [McPhee, 2011]. However, the decreased serum albumin levels cause a shift in the balance between bound fraction and free fraction of aldosterone, increasing the latter; a fact consistent with the highest serum aldosterone values found in this group.
The group with aldosterone levels excess has more diabetic patients; however, without reaching statistically significant difference, so the higher degree of LVH in this group could be eventually explained by diabetes. But other authors have documented that diabetics have more aldosterone [De Oliveira et al. 1997], and more LVH [Rao et al. 2013]. So, it is a good premise that excess LVH in diabetes could be explained by aldosterone excess [Buglioni et al. 2015]. In a post hoc analysis of this study, a multiple logistic regression analysis, to include the presence of diabetes (a binary variable) as a confounding variable, with outcome variable LVMI categorized as above or below median, showed an odds ratio to association of aldosterone with greater LVMI of 1.96 CI 95% (1.007–1.42); p = 0.041 (not shown in results). Moreover, if we stratify the patients, removing all diabetic patients from both groups, the difference in left ventricular mass is maintained between high and low aldosterone groups. This association of aldosterone independent of diabetes presence points favorably to the premise mentioned above. Only insulin use demonstrated a statistical significance between groups. Insulin levels are not necessarily higher in insulin users because our diabetic patients have late-phase diabetes and probably less endogenous insulin. On the other hand, it is important to highlight that if we eliminate diabetics from the study, as demonstrated above, left ventricular mass continues to be more elevated in the high aldosterone group.
Patients of both groups were anuric and showed no difference in weight gain between interdialytic both groups. In terms of aldosterone and race we had only one Afro-American, and this patient was in the low aldosterone group. Although we are in Brazil, our city population is mostly of Italian origin.
Regarding blood pressure, we cannot exclude a role of this important factor; we only adjusted the association of left ventricular mass and aldosterone to blood pressure. We recognize the role that PTH and its relationship with the Klotho/FGF23 plays in the genesis of LVH (Leifheit-Nestler et al. 2015), but we were unable to effectuate studies in this way in the current study; we emphasize however, that PTH and phosphate values were homogeneous among our groups.
Some limitations must be recognized. It is a cross-sectional study with the characteristics inherent of this design, but the observed association remained statistically significant even when submitted to multiple analyses. Moreover, despite the limited number of patients, each patient underwent a complete evaluation of their cardiovascular and hormonal condition and the sample size was sufficient to detect statistically significant differences. These limitations require the confirmation of the present study in a work with a higher number of patients. Importantly, few studies evaluate the possible association between aldosterone levels and left ventricular mass in patients with CKD in HD.
In conclusion, patients with CKD in HD who demonstrate high aldosterone levels generally are diabetics with elevated BMI, resulting in lipid changes and increased LVMI. In these patients a direct association was observed between aldosterone levels and ventricular mass, independent of the evaluated confounding factors. This association had been demonstrated previously in hypertensive and diabetic patients, but not in dialysis.
Footnotes
Funding: We thank the financial support of FUNDUNESP (Foundation for the Development of UNESP, Process 0090910) and FAPESP (Foundation for Research Support of São Paulo, Process 2010/10439-1).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Greicy Mara Mengue Feniman De Stefano, Departamento de Clínica Médica da Faculdade de Medicina de Botucatu – UNESP, Curso de Farmácia da Faculdade Sudoeste Paulista – FSP, Rua João Miguel Rafael. 440, CEP 18602-220 – Botucatu – SP, Brazil.
Silméia Garcia Zanati-Basan, Departamento de Clínica Médica da Faculdade de Medicina de Botucatu – UNESP, Botucatu – SP, Brazil.
Laercio Martins De Stefano, Departamento de Clínica Médica da Faculdade de Medicina de Botucatu – UNESP, Botucatu – SP, Brazil.
Viviana Rugolo Oliveira e Silva, Departamento de Clínica Médica da Faculdade de Medicina de Botucatu – UNESP, Botucatu – SP, Brazil.
Patrícia Santi Xavier, Departamento de Clínica Médica da Faculdade de Medicina de Botucatu – UNESP, Botucatu – SP, Brazil.
Pasqual Barretti, Departamento de Clínica Médica da Faculdade de Medicina de Botucatu – UNESP, Botucatu – SP, Brazil.
Roberto Jorge da Silva Franco, Departamento de Clínica Médica da Faculdade de Medicina de Botucatu – UNESP, Botucatu – SP, Brazil.
Jacqueline Costa Teixeira Caramori, Departamento de Clínica Médica da Faculdade de Medicina de Botucatu – UNESP, Botucatu – SP, Brazil.
Luis Cuadrado Martin, Departamento de Clínica Médica da Faculdade de Medicina de Botucatu – UNESP, Botucatu – SP, Brazil.
References
- Aghamohammadzadeh R., Heagerty A. (2012) Obesity-related hypertension: epidemiology, pathophysiology treatments, and the contribution of perivascular adipose tissue. Ann Med 44: 74–S84. [DOI] [PubMed] [Google Scholar]
- Anagnostis P., Niki K., Vasilios G., Karagiannis A., Karagiannis A. (2014) Adiponectin and Aldosterone in Left Ventricular Hypertrophy: Na Intriguing Interplay. Angiology 3: 1–4. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S., Gandhi M., Kamalov G., Ahokas R., Sun Y., Gerling I., et al. (2009) Myocardial remodeling in low-renin hypertension. Molecular pathways to cellular injury in relative aldosteronism. Curr Hypertens Report 11: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomback A., Klemmer P. (2007). The incidence and implications of aldosterone breakthrough. Nature Nat Clin Pract Nephr 3: 486–492. [DOI] [PubMed] [Google Scholar]
- Bomback A., Kshirsagar A., Ferris M., Klemmer P. (2009) Disordered aldosterone-volume relationship in end-stage kidney disease. J Renin Angiotensin Aldosterone Sys 10: 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buglioni A., Cannone V., Cataliotti A., Sangaralingham S., Heublein D., Scott C., et al. (2015) Circulating aldosterone and natriuretic peptides in the general community: relationship to cardiorenal and metabolic disease. Hypertension 65: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailar G., Fesler P., Ribstein J., Mimran A. (2010) Dietary sodium, aldosterone, and left ventricular mass changes during long-term inhibition of the renin–angiotensin system. Hypertension 56: 865–870. [DOI] [PubMed] [Google Scholar]
- Calhoun D., Sharma K. (2010) The role of aldosteronism in causing obesity-related cardiovascular risk. Clin Cardiol 28: 517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasola G., Nardi E., Palermo A., Mulè G., Cottone S. (2011) Epidemiology and pathophysiology of left ventricular abnormalities in chronic kidney disease: a review. J Nephrol 24: 1–10. [DOI] [PubMed] [Google Scholar]
- Chamber Quantification Writing Group (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiographym a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463. [DOI] [PubMed] [Google Scholar]
- Cohn S., Vaswani A., Yasumura S., Yuen K., Ellis K. (1985) Assessment of cellular mass and lean body mass by noninvasive nuclear techniques. J Lab Clin Med 105: 305–311. [PubMed] [Google Scholar]
- Conselho Nacional de Saúde (1996) Resolução no.196, de 10 de outubro de 1996. Aprova as Diretrizes e Normas Regulamentadoras de Pesquisas Envolvendo Seres Humanos. DOU Brasília, 16 De Outubro De 1996. [Google Scholar]
- Covic A., Voroneanu L., Goldsmith D. (2010) The effects of vitamin D therapy on left ventricular structure and function - are these the underlying explanations for improved CKD patient survival? Nephron Clin Prac 116: 187–195. [DOI] [PubMed] [Google Scholar]
- Custódio M., Koike M., Neves K., Reis L., Graciolli F., Neves C., et al. (2012) Parathyroid hormone and phosphorus overload in uremia: impact on cardiovascular system. Nephrol Dialysis Transpl 27: 1437–1445. [DOI] [PubMed] [Google Scholar]
- Daugirdas J. (1995) Simplified equations for monitoring Kt/V, n, eKt/V, and en. Renal. Replacement Ther 2: 295–304. [DOI] [PubMed] [Google Scholar]
- De’Oliveira J., Price D., Fischer N., Allan D., McKnight J., Williams G., Hollenberg N. (1997) Autonomy of the renin system in type II diabetes mellitus: dietary sodium and renal hemodynamic responses to ACE inhibition. Kidney Int 52: 771–777. [DOI] [PubMed] [Google Scholar]
- Flynn C., Bakris G. (2011) Interaction between adiponectin and aldosterone. Cardio Renal Med 1: 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner R., Schoeller D. (1986) Estimation of total body water by bioelectrical impedance analysis. Am J Cli Nutr 44: 417–424. [DOI] [PubMed] [Google Scholar]
- Lee Y., Chiu H., Su H., Voon W., Lin T., Lai W., et al. (2008) Presence of chronic kidney disease and subsequent changes of left ventricular geometry over 4 years in an apparently healthy population aged 60 and older. Hypertens Res 31: 913–920. [DOI] [PubMed] [Google Scholar]
- Leifheit-Nestler M., Große Siemer R., Flasbart K., Richter B., Kirchhoff F., Ziegler W., et al. (2015) Induction of cardiac FGF23/FGFR4 expression is associated with left ventricular hypertrophy in patients with chronic kidney disease. Nephrol Dial Transpl 2015. December 17. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy V., De Seigneux S., Agassiz V., Hasler U., Rafestin-Oblin M., Vinciguerra M., et al. (2009) Aldosterone activates NF-kappaB in the collecting duct. J Am Soc Nephrol 10: 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin A., Thompson C., Ethier J., Carlissle E., Tobe S., Mendelssohn D., et al. (1999) Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis 34: 125–134. [DOI] [PubMed] [Google Scholar]
- London G., Pannier B., Guerin A., Blacher J., Marchais S., Darne B., et al. (2001) Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow-up of an interventional study. J Am Soc Nephrol 12: 2759–2767. [DOI] [PubMed] [Google Scholar]
- Martin L., Barretti P., Cornejo I., Felipe M., Forti A., Matsubara B., et al. (2003) Influence of fluid volume variations on the calculated value of the left ventricular mass measured by echocardiogram in patients submitted to hemodialysis. Renal Fail 25: 43–53. [DOI] [PubMed] [Google Scholar]
- Martin L., Franco R., Gavras I., Matsubara B., Garcia S., Caramori J., et al. (2004) Association between hypervolemia and ventricular hypertrophy in hemodialysis patients. Am J Hypertens 17: 1163–1169. [DOI] [PubMed] [Google Scholar]
- Matsubara B., Franco M., Janicki J., Matsubara L. (2010) Effect of felodipine on myocardial and renal injury induced by aldosterone-high salt hypertension in uninephrectomized rats. Brazilian J Med Biol Res 43: 506–514. [DOI] [PubMed] [Google Scholar]
- McPhee S. (2011) Distúrbios do Córtex Supra-renal. In: McPhee S., Ganong W. (eds), Fisiopatologia da Doença - Uma Introdução à Medicina Clínica, 5ª ed, Porto Alegre: AMGH, pp. 503–532. [Google Scholar]
- Miki N., Shigetaka Y., Shigeru S., Takashi N., Takanari G., Katsuyuki A., et al. (2006) Enhanced aldosterone signaling in the early nephropathy of rats with metabolic sybdrome: possible contribution of fat-derived factors. Am Soc Nephrol 17: 3438–3446. [DOI] [PubMed] [Google Scholar]
- Olsen M., Wachtell K., de Simone G., Palmieri V., Dige-Petersen H., Devereux R., et al. (2004) Is inappropriate left ventricular mass related to neurohormonal factors and/or arterial changes in hypertension? A LIFE substudy. J Hum Hypertens 18: 437–443. [DOI] [PubMed] [Google Scholar]
- Rao A., Shah R., Garg R., Abbasi S., Neilan T., Perlstein T., et al. (2013) Aldosterone and myocardial extracellular matrix expansion in type 2 diabetes mellitus. Am J Cardiol 112: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco T., Fang J. (2006) Farmacoterapia da insuficiência cardíaca congestiva. In: Hardman J. (ed), Goodman & Gilman: As Bases Farmacológicas da Terapêutica. 11nd edition, Rio de Janeiro: McGraw-Hill Interamericana do Brasil, pp. 679–702. [Google Scholar]
- Rump L., Amann K., Orth S., Ritz E. (2000) Sympathetic overactivity in renal disease: a window to understand progression and cardiovascular complications of uraemia? Nephrol Dialysis Transpl 15: 1735–1738. [DOI] [PubMed] [Google Scholar]
- Sato A., Funder J., Saruta T. (1999) Involvement of aldosterone in left ventricular hypertrophy of patients with end-stage renal failure treated with hemodialysis. Am J Hypertens 12: 867–873. [DOI] [PubMed] [Google Scholar]
- Schrier R. (2010) Aldosterone ‘escape’ vs ‘breakthrough’. Nat Rev Nephrol 6: 61. [DOI] [PubMed] [Google Scholar]
- Schrier R., Masoumi A., Elhassan E. (2010) Aldosterone: role in edematous disorders, hypertension, chronic renal failure, and metabolic syndrome. Clin J Am Soc Nephrol 5: 1132–1140. [DOI] [PubMed] [Google Scholar]
- Sociedade Brasileira de Cardiologia (2011). V Diretrizes Brasileiras de Monitorização Ambulatorial da Pressão Arterial (MAPA) e III Diretrizes Brasileiras de Monitorização Residencial da Pressão Arterial (MRPA). Arq Bras Cardiol 97: 1–40. [Google Scholar]
- Steigerwalt S., Zafar A., Mesiha N., Gardin J., Provenzano R. (2007) Role of aldosterone in left ventricular hypertrophy among African-American patients with end-stage renal disease on hemodialysis. Am J Nephrol 27: 159–163. [DOI] [PubMed] [Google Scholar]
- Weber K. (2001) Cardioreparation in hypertensive heart disease. Hypertension 38: 588–591. [DOI] [PubMed] [Google Scholar]
- Zoccali C., Benedetto F., Mallamaci F., Tripepi G., Giacone G., Cataliotti A., et al. (2001) Prognosis impact of the indexation of left-ventricular mass in patients undergoing dialysis. J Am Soc Nephrol 12: 2768–2774. [DOI] [PubMed] [Google Scholar]