Abstract
Primary liver cancer—including hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC)—incidence is increasing and is an important source of cancer-related mortality worldwide. Management of these cancers, even when localized, is challenging due to the association with underlying liver disease and the complex anatomy of the liver. Although for ICC, surgical resection provides the only potential cure, for HCC, the risks and benefits of the multiple curative intent options must be considered to individualize treatment based upon tumor factors, baseline liver function, and the functional status of the patient. The principles of surgical resection for both HCC and ICC include margin-negative resections with preservation of adequate function of the residual liver. As the safety of surgical resection has improved in recent years, the role of liver resection for HCC has expanded to include selected patients with preserved liver function and small tumors (ablation as an alternative), tumors within Milan criteria (transplant as an alternative), and patients with large (>5 cm) and giant (>10 cm) HCC or with poor prognostic features (for whom surgery is infrequently offered) due to a survival benefit with resection for selected patients. An important surgical consideration specifically for ICC includes the high risk of nodal metastasis, for which portal lymphadenectomy is recommended at the time of hepatectomy for staging. For both diseases, onco-surgical strategies including portal vein embolization and parenchymal-sparing resections have increased the number of patients eligible for curative liver resection by improving patient outcomes. Multidisciplinary evaluation is critical in the management of patients with primary liver cancer to provide and coordinate the best treatments possible for these patients.
Keywords: hepatocellular carcinoma, intrahepatic cholangiocarcinoma, liver neoplasms, hepatectomy
Introduction
Primary liver cancer is the fifth most common cancer in the world and the second leading cause of cancer deaths.1 Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, followed by intrahepatic cholangiocarcinoma (ICC)—together representing over 95% of primary liver malignancies. Despite both cancer types arising from primary liver cells, the natural history and patterns of failure of each significantly differs. With the improvement in safety of surgical techniques for hepatectomy, as well as advancements in systemic therapy and the greater availability of local therapies, more patients are eligible for treatments that significantly improve overall survival (OS) and, in some cases, provide the potential for cure. A comprehensive understanding of the nature of each disease, the treatment options available, and the importance of multidisciplinary management is critical to provide optimal care for these patients and for appropriate selection of surgical candidates.
Hepatocellular Carcinoma
Epidemiology and Risk Factors
Hepatocellular carcinoma represents 80% to 85% of primary liver cancers.1.2 The incidence varies widely, with the majority of new cases arising in east Asia (with China representing half of the world’s cases of HCC) and sub-Saharan Africa.1,2 Hepatocellular carcinoma occurs predominantly in patients with chronic liver disease, with differences in the geographic distribution of risk factors, such as viral hepatitis, contributing to the worldwide variability in overall incidence. In addition, HCC is 2 to 3 times more common in men than in women, and in some areas at a rate of 4 to 1,2 which has also been attributed partly to gender-based differences in risk factors.
The most common risk factors for HCC include chronic viral hepatitis B and C, aflatoxin exposure, alcohol, and nonalcohol fatty liver disease or steatohepatitis.2,3 Globally, hepatitis B is the most common cause of HCC (especially in China), whereas hepatitis C and alcohol are the most common causes in the United States. In addition, genetic diseases that cause chronic liver disease can also increase the risk of HCC, such as hemochromatosis and α-1 antitrypsin deficiency. Hepatitis B vaccination has resulted in an overall drop of hepatitis B and an expected decrease of HCC in countries where hepatitis B infection is endemic. On the other hand, in the United States, the rate of HCC related to hepatitis C has become the fastest-rising cause of cancer-related death.3 This rate is expected to continue to increase over time, as there is a 20-year delay in HCC formation from the time of acquiring hepatitis C.1 The OS of patients with HCC is poor at less than 15% at 5 years3 and less than 20% when examining cancer-specific survival.4
Diagnosis
Hepatocellular carcinoma can often be diagnosed noninvasively with specific imaging characteristics in the correct setting. Typical features noted on a liver protocol (dynamic—3-phase) computed tomography (CT) scan or magnetic resonance imaging (MRI) include early arterial enhancement and delayed washout in lesions over 2 cm, enhancing capsule, and growth over time, all in the context of underlying liver disease.5 In smaller lesions, the imaging characteristics may be less obvious but similar findings can be seen and considered diagnostic in conjunction with an elevated α-fetoprotein.3 If the imaging is not consistent with HCC and/or for those patients presenting with de novo lesions in the absence of underlying liver disease, a tissue biopsy can be obtained to help confirm the diagnosis.
Staging
There are several staging systems that have been developed for HCC using patient and tumor factors to try to best stratify by prognosis and guide treatment based upon stage. An important consideration as to why there are multiple staging systems is that the management of HCC and overall prognosis are distinct from other cancers in that both are dependent not only on characteristics of the tumor itself but also on the underlying function of the liver and the functional status of the patient. The Barcelona Clinic Liver Cancer (BCLC) staging system is widely accepted in clinical practice and considered by many to be the gold standard staging system. It utilizes the patient’s functional status, Child-Turcotte-Pugh (CTP) score, and tumor characteristics (number and size of nodules, vascular invasion) to stage patients as very early (0), early (A), intermediate (B), advanced (C), or terminal (D).5 Although the BCLC system is commonly used and cited in most international guidelines, it has a number of shortcomings, in particular as it relates to its applicability for patients who may benefit from surgical strategies including resection. As such, a number of other staging systems are available (discussed in another manuscript of this issue) that can be more helpful for guiding surgical treatment and can better inform patients and providers regarding prognosis. Among the most important features of any given staging system is the inclusion of tumor-related factors as well as liver function and patient’s performance status. These are critical components when selecting the appropriate treatment, in particular for patients being considered for surgical management, as the ability to withstand surgery and derive benefits in long-term outcomes is determined in great part by the baseline liver function and ability of the liver to recover following a major resection.
Surgical Management—Indications and Outcomes
A number of different treatment options are currently available for patients with HCC. Treatment should be individualized based on disease stage, liver function, and patient’s performance status. Curative intent options should be offered for eligible patients, including ablation (surgical or percutaneous) for small tumors typically <2 cm, liver resection, and orthotopic liver transplantation (OLT). Unfortunately, 30% to 66% of patients do not receive any treatment during the course of their disease, primarily due to lack of referral to appropriate specialist/care team.6,7 Further, treatment approach is often determined by the treating provider’s choice, which varies by specialty, and is not necessarily evidence-based—resulting in significant disparities among treated patients as well.6–8 Based on this, current recommendations include multidisciplinary evaluation of all patients,9 with recent studies showing improved process of care and overall better outcomes with implementation of this standard.10,11
The role of liver resection for HCC has continued to expand over the last decade. In general, liver resection should be considered for patients with nonmetastatic disease and normal underlying liver function or with compensated cirrhosis and no evidence of portal hypertension. Patients with known liver disease must have the liver function evaluated by a validated system (Table 1). Our practice is to classify patients based on CTP criteria,12 with only those patients with CTP class A considered for major resection. An alternative measure is the Model For End-Stage Liver Disease (MELD) score, with a threshold of <10 points as the cutoff for safe liver resection.13 Recent studies have emphasized the additional discriminative function of the Albumin-Bilirrubin (ALBI) score,14 even within CTP A category,15 although its role for improving patient selection has not been examined. Portal hypertension is evaluated through clinical parameters (ie, ascites, abdominal wall varices, history of upper gastrointestinal variceal bleeding) and indirect laboratory (ie, thrombocytopenia) and imaging (ie, splenomegaly, recanalized umbilical vein, gastric/esophageal varices) surrogates. Occasionally, there may be patients with contradictory findings in whom direct hepatic vein–portal vein gradient can be measured to rule out portal hypertension (<10 mm Hg), prior to proceeding with liver resection.5
Table 1.
Most Commonly Used Validated Tools to Stratify Patients Based on Liver Function and Selection for Surgical Management Hepatectomy.
| Tool | Description Variables | |||||
|---|---|---|---|---|---|---|
| Child-Turcotte-Pugh12 | Parameter | Points Based on Findings | Class by Points Score | |||
| 1 | 2 | 3 | ||||
| Encephalopathy (grade) | None | 1-2 | 3-4 | Class A = 5-6 Class B = 7-9 Class C = 10-15 | ||
| Ascitis | None | Mild/moderate | Severe | |||
| Albumin (g/dL) | >3.5 | 2.8-3.5 | <2.8 | |||
| INR | <1.7 | 1.7-2.3 | >2.3 | |||
| Bilirubin (mg/dL) | <2 | 2-3 | >3 | |||
| MELDa, 13 | Parameters used | MELD scoreb | ||||
| Bilirubin (mg/dL) | Serum Na (meq/L) | INR | Creatininec (mg/dL) | 6-40 | ||
| ALBI14 | Parameters used | ALBI scored | ||||
| Log10 bilirubin | Albumin (g/L) | A1, A2, A3 | ||||
Abbreviations: INR, international normalized ratio.
aThe MELD score is applicable for patients >12 years old.
bMELD score calculators: https://optn.transplant.hrsa.gov/resources/allocation-calculators/meld-calculator/
cAn additional dichotomous variable relates to patients having kidney replacement therapy within 1 week.
dThe ALBI score can easily be calculated using a nomogram-type tool and a heat map, both provided in the original publication.
Among patients meeting the described criteria, the role of resection should include consideration of other potentially curative competing strategies (ie, ablation and OLT) and the corresponding outcomes based on an intention-to-treat analysis. Three randomized controlled trials16–18 and at least 3 meta-analyses19–21 have examined the comparative efficacy of liver resection versus ablation for early-stage disease. The trials varied in their selection criteria, 1 including solitary lesions <5 cm,16 and the other 2 with similar features as those described for the Milan criteria.17,18 Each of the trials had important methodologic flaws limiting the interpretation of results. Nonetheless, 1 of the trials and all meta-analysis found percutaneous radiofrequency ablation (RFA) to be inferior to liver resection when comparing overall survival and recurrence-free survival, with the only advantage of RFA related to being a less invasive approach and hence associated with fewer complications and shorter hospitalizations (Table 2). Pooled results from 25 nonrandomized trials examined in the Cochrane meta-analysis found equivalent long-term outcomes for patients with very early-stage tumors (<2 cm) when comparing both approaches.21 Based on these data, our group’s preference is for liver resection of small HCC. However, when a patient presents with a small tumor (<2 cm) and borderline liver function, or with high burden of comorbidities, and/or for those in whom such tumor is located in a deep portion of the liver (thus requiring a major liver resection), ablation is an adequate treatment alternative. One important consideration regarding outcomes following percutaneous ablation is the inherent limitation of this technique for treating lesions in unfavorable locations—for example, close to hollow viscus, high in the dome by the diaphragm, or in close relation to hilar structures or major vessels. In these cases, a more appropriate approach may be surgical (laparoscopic or open) ablation, or liver resection even if it involves a more extensive operation.
Table 2.
Short- and Long-Term Outcomes Following Liver Resection (LR) Versus Radiofrequency Ablation (RFA) for Early-Stage HCC.a
| Study Author (year) | Inclusion Criteria | OS | DFS/RFS | Postprocedure Outcomes | Comments |
|---|---|---|---|---|---|
| Chen et al (2006)16 | Solitary <5 cm; N = 180 | 4-year OS: RFA = 67.9%; LR = 64% (P = ns) | 4-year DFS: RFA = 46.4%; LR = 51.6% (P = ns) | Mortality: RFA = 0%; LR = 1.1% (P = ns); major complications: RFA = 4%; LR = 55% (P < .05) | Authors emphasize equivalent long-term outcomes, as well as lower risk of complications (and shorter length of hospital stay) with RFA; notably, a number of patients withdrew from RFA arm and analysis was not on intention-to-treat basis |
| Huang et al (2010)17 | Milan criteria and amenable for both LR and RFA; N = 230 | 5-year OS: RFA = 54.7%; LR = 75.6% (P = .001) | 5-year RFS: RFA = 28.6%; LR = 51.3% (P = .01) | Mortality: 0% for both groups; adverse events: RFA: 4%; LR: 28% (P < .05) | Authors recommend LR for management of HCC within Milan criteria despite increased complications and length of hospital stay |
| Feng et al (2012)18 | Maximum number of tumors = 2 and maximum tumor size <4 cm; N = 168 | 3-year OS: RFA = 67.2%; LR = 74.8% (P = .34) | 3-year RFS: RFA = 49.6%; LR = 61.1% (P = .12) | Mortality: 0% for both groups; complications: RFA = 9.5%; LR = 21.4% (P = .01) | Authors emphasize overall similar long-term outcomes but caution the use of percutaneous ablation for lesions in difficult areas to get to, in which cases local recurrence is higher |
Abbreviations: DFS, disease-free survival; HCC, hepatocellular carcinoma; ns, not significant; OS, overall survival; RFS, recurrence-free survival.
aResults from available randomized controlled trials and systematic reviews/meta-analysis.
When considering liver resection for lesions >2 cm, the role of OLT should be also examined. Orthotopic liver transplantation has been considered the gold standard for treatment of patients within Milan criteria (1 tumor ≤5 cm or up to 3 tumors none >3 cm).22 Long-term outcomes following these criteria are in parallel to that of patients undergoing OLT for benign conditions, with 5-year OS of 65% to 78%,22,23 thus supporting the allocation of cadaveric livers for this population, regardless of organ shortage. Additionally, other groups have published “expanded” criteria—the University of California, San Francisco (UCSF) criteria being the most studied expansion, in which OLT is offered for those with a solitary tumor of up to 6.5 cm, or up to 3 tumors the largest ≤4.5 cm, and the total added diameter of the 3 tumors ≤8 cm. Five-year OS using these criteria reached 75% in the initial publication, although these results have not been universally reproduced.24 Nonetheless, based on these data, patients with evidence of advanced liver disease (as described earlier), who are not ideal candidates for liver resection due to increased risk of posthepatectomy liver failure (PHLF) and death, should be referred for liver transplantation, and bridging strategies must be implemented as per local protocols based on waiting times and risk of progression.5,9
Significant controversy exists, however, regarding the ideal treatment for patients within Milan and/or UCSF criteria with preserved liver function. Traditionally, OLT has been the preferred approach as it is considered to treat the tumor and the liver disease and has excellent long-term outcomes including overall and recurrence-free survival. Variation in the utilization of resection and transplantation can be influenced by shortage of organs,25 ineligibility for OLT due to other nonmedical transplant-related reasons (eg, poor social support),26 and by primary specialty of the treating surgeon,7 among others. Over the last decade, however, there have been a number of studies focused on better examining the best approach for these patients, with a higher level of evidence. At least 2 recent meta-analysis have evaluated this question.27,28 Notably, postoperative morbidity and mortality, and short-term (1-year) OS were worse for those treated with OLT. When comparing pooled 5-year OS, OLT fared better than liver resection (63% vs 58% and 61% vs 49%, respectively, for each study).27,28 However, when evaluating the risk of mortality using meta-analysis methodology, the studies had contradictory results. An important limitation of these 2 reports was the lack of adjustment based on important characteristics including patient’s age, donor’s age, waiting list time, and baseline liver function—all important determinants of overall outcomes for both procedures. The role of a more individualized approach was emphasized in a study by Cucchetti et al, in which both treatment strategies were compared using a Markov model simulation and included sensitivity analysis based on these features. The authors reported improved outcomes for those receiving OLT who had advanced liver disease (ie, MELD score ≥10) and/or portal hypertension. Interestingly, however, they also reported equivalent survival outcomes for patient with well-compensated cirrhosis (MELD score <10) and observed improved survival following liver resection for those with T1 lesions (solitary lesion without vascular invasion).29 These findings have been replicated by a number of studies.30,31 The group from University of Miami published a comparative intention-to-treat analysis of OLT (n = 257) versus resection (n = 106)—hence including survival outcomes for those on the waiting list but never making it to transplant (n = 37 [14%]). Notably, despite a median time to OLT of only 48 days, they reported equivalent 5-year OS for OLT and liver resection patients (52% and 53%, respectively). Further, when comparing those with a MELD score <10, 5-year OS was significantly better for those having liver resection as compared to OLT (63% vs 41%, respectively for those within Milan criteria and 62% vs 40%, respectively, for those within UCSF criteria). The survival advantage observed for liver resection is likely derived from lower postoperative and early (1 year) mortality and the variable but still significant dropout rate while on the waiting list.30 Other studies have examined the cost-effectiveness of different approaches for early HCC including ablation, resection, and OLT and have also reported findings supporting liver resection over transplantation as a first approach.32,33 Based on all these findings, our preferred approach is to treat patients within UCSF criteria and with compensated cirrhosis with liver resection, in the context of multidisciplinary discussion and appropriate patient counseling.
Despite recent data reporting improved survival following liver resection for this selected population, it should be noted that overall recurrence is high with 5-year recurrence rates ranging 18% to 72%.34–38 Although there are promising agents being developed,39–42 there are currently no effective adjuvant treatments to help reduce this risk.43 Among evolving data is the observed benefit in recurrence-free and OS associated with low viral load and overall treatment of hepatitis viral infections.44,45 Similarly, the role of salvage OLT following recurrence after liver resection is being defined. A number of studies have shown that liver recurrence often presents with disease within Milan criteria37,46 and hence making salvage OLT a treatment option. Studies comparing salvage and primary OLT have reported similar postoperative, early, and long-term outcomes and emphasized its role as a real option for this patient population,47 although there are still contradictory results and future studies are still warranted.34,48
Lastly, liver resection for patients presenting with HCC beyond 5 cm and/or with poor prognostic features (ie, vascular invasion) has an important role. Although these patients are at high risk of treatment failure, recurrence, and mortality, outcomes of liver resection should be examined and weighed against the lack of any other potentially curative option.49 Although a number of international guidelines have excluded the role of surgery for these patients,5,50 multiple studies have found a clear benefit for selected patients and have questioned the appropriateness of published recommendations, in particular in relation to the role of liver resection.51,52
The benefit of liver resection in large and giant (>10 cm) HCC has been well-documented. Postoperative outcomes have been found to be equivalent to those operated on for smaller HCC, and overall postoperative mortality remains low <3%.53,54 Similarly, when evaluating long-term outcomes, liver resection for giant HCC has been associated with a 5-year OS ranging 27% to 53%,49 with investigators supporting the role for liver resection based on more favorable outcomes as compared to other noncurative options (eg, Transarterial chemoembolization [TACE]).54,55 Furthermore, a recent study used propensity score analysis to compare outcomes of patients with solitary HCC >5 cm following liver resection versus TACE. The authors reported better 5-year OS with liver resection (41.3% vs 18.5%; P = .007) emphasizing the good results with resection as well as the favorable outcomes when compared to other more commonly recommended treatments (TACE).56 Based on these data and other similar studies, we offer liver resection to all patients with solitary tumors >5 cm, regardless of size, as long as the previously described selection criteria are met, and an oncologically sound resection (margin negative) can be accomplished.
Similarly, liver resection for HCC in the context of vascular invasion, although still controversial, has proven to have a clear benefit for well-selected patients. Vascular invasion, more commonly presenting as portal vein tumor thrombus (PVTT), is well known to be an ominous prognostic factor; it is often interpreted as metastatic disease, and hence most international guidelines recommend palliative treatment, most commonly sorafenib.5 Outcomes with liver resection vary, and a 5-year OS ranges from 10% to 41%.49 Over the last 5 years, there has been important advancements regarding the way to manage patients with HCC and PVTT. Three different classifications of PVTT have been published with each reporting worse OS with more extensive PVTT and with tumor thrombus located in the more proximal vessels (main portal vein or first-order branch-contralateral to the disease site).57 Based on published reports, a recent consensus statement and a systematic review,57,58 liver resection can be indicated for patients with preserved liver function and resectable disease with PVTT type I-II (Cheng’s classification) and VP 1-VP 3 (Japanese classification)—essentially, when the PVTT extends down to the right or left main PV branches on the ipsilateral side of disease (ie, all expected to be resected with planned hepatectomy) and not to the main PV or superior mesenteric vein (type III and IV, respectively and VP4). These recommendations are in great part derived from a meta-analysis comparing TACE to liver resection in patients with PVTT, which found improved survival in those meeting above criteria.59 Consensus recommendations recently highlighted the high risk of recurrence and consideration for adjuvant therapy with TACE and/or sorafenib, although these recommendations have not been validated with high-level data.
Other high-risk scenarios in which liver resection can play a limited role include patients with multifocal disease,60,61 those following rupture of HCC into the peritoneal cavity62 and those with periportal lymph node (LN) involvement.63,64 The data for these scenarios are more limited, and hepatectomy can only be recommended for individualized cases after thorough multidisciplinary evaluation.
Surgical and Technical Strategies
Liver resection has evolved significantly over the last few decades, making it a safe operation when performed in the appropriate context and with adequate patient selection.9 Cirrhosis is a known risk factor for higher risk of postoperative complications, including bile leak, PHLF, and death. When considering hepatectomy for treatment of HCC, consideration of baseline liver function is paramount; resection should not only follow general oncologic principles (complete R0 resection) but it must also be performed in a way to maximize recovery, minimize postoperative complications, and preserve adequate liver function. A number of different strategies have been studied that help guide liver resection principles for this population, while also improving long-term outcomes.
In addition to validated tools that stratify patients based on baseline liver function (Table 1) and help with selecting patients for resection (as previously described), among those treated with hepatectomy, the ability to recover and maintain adequate liver function postoperatively is also determined by the extent of liver resection—that is, volume of residual liver (future liver remnant [FLR]) and its function. Functional studies such as indocyanine green retention rate at 15 minutes are used (primarily in Asia) to help guide the extent of liver resection anticipated to be tolerated.65,66 In North America and Europe, as it is in our practice, the volume of the FLR in patients already selected for resection (and therefore with preserved liver function/compensated cirrhosis) is used to guide the extent of resection anticipated to be tolerated.9 For patients with compensated cirrhosis and no portal hypertension, an FLR ratio ≥40% is ideal and has been shown to be the threshold for safe resection in this population.67 Previous studies evaluating the contribution of different liver segments toward the total liver volume have shown the right lobe to represent 65% to 67% of the total liver volume, and thus following a right hepatectomy, the anticipated FLR ratio would be <40% threshold.68,69 A strategy to allow for safe major liver resection in patients who would otherwise be left with an FLR <40% is the systematic use of preoperative portal vein embolization (PVE).70 Portal vein embolization has been proven to be a safe and feasible procedure. It is most commonly performed via a percutaneous approach, and the portal vein branch ipsilateral to the tumor-bearing liver is embolized.71 This induces regeneration and hypertrophy of the contralateral lobe within 4 to 8 weeks after PVE. The success rate of PVE varies based on indication, tumor histology, baseline liver function, and starting FLR ratio—but overall has been reported in a range around 85%.72 The overall outcomes following major hepatectomy after PVE in the general population and for those with HCC specifically have been found to be equivalent to those having liver resection without PVE and adequate baseline FLR, hence confirming the benefit of PVE as a strategy to expand the pool of patients eligible for resection.72–74 Additionally, a prospective clinical trial comparing upfront surgery to PVE followed by surgery in patients having right hepatectomy found that PVE significantly reduced the postoperative complications in patients with liver disease, further confirming the added benefits of PVE for this population.75 Similarly, both OS and recurrence-free survival for patients with HCC resected after PVE has been shown to mirror or exceed in some cases, those of HCC patients treated without PVE.73
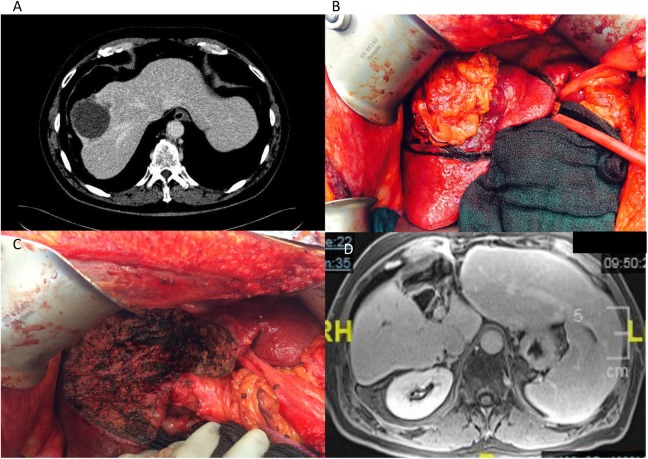
Limiting the extent of resection based on tumor location and with segment-oriented procedures can also allow for safe hepatectomy in patients who otherwise would need to rely on preoperative PVE—hypertrophy. Liver parenchyma-preserving resections that allow complete margin-negative resection and preserve a larger volume of liver are ideal for this population of patients and have been shown to be associated with equivalent long-term outcomes including overall and recurrence-free survival, while minimizing the risk of PHLF and death. A multi-institutional analysis comparing postoperative outcomes of right posterior sectionectomy (n = 100) to right hepatectomy (n = 480) for a variety of liver tumors revealed the former to present with significantly lower rate of PHLF (1% vs 8.5%; P = .005).76 Similarly, another study comparing right posterior sectionectomy to right hepatectomy for HCC specifically found a trend toward increased PHLF following right hepatectomy (9.4% vs 2%) and no statistical difference in 5-year OS (83% for right posterior sectionectomy vs 76% for right hepatectomy) and disease-free survival (52% for both).77 Based on these results, recommendations are for right posterior sectionectomy over right hepatectomy for patients in whom a complete resection can be accomplished. A similar dilemma presents for patients with centrally located tumors. The standard recommendation has been for extended right or left hepatectomy, with small residual liver, which in the setting of cirrhosis further increases the risk of PHLF and death. An alternative to this approach is central or mesohepatectomy (removal of Couinaud’s segments IV, V, and VIII and the middle hepatic vein at its origin), allowing for resection of the central tumor while preserving a significantly larger portion of uninvolved liver. A case-control study from Memorial Sloan Kettering Cancer Center revealed that postoperative bilirubin >4 mg/dL was significantly more common in those undergoing an extended hepatectomy as compared to central resection (39% vs 2%; P < .01).78 A similar study from China found that central hepatectomy as compared to extended right/left hepatectomy for centrally located HCC was associated with lower risk of PHLF (1.7% vs 10.6%), and 5-year overall and recurrence-free survival were equivalent for both groups.79 Furthermore, a recent systematic review comparing these 2 approaches supports the use of central liver resection over extended hepatectomies, with the caveat that central hepatectomy is a more complex and technically demanding procedure.80 Our practice is to perform parenchyma-preserving anatomic procedures including right posterior sectionectomy, central hepatectomy, and monosegmentectomies and bisegmentectomies whenever a margin-negative resection can be accomplished (Figures 1 and 2).
Figure 1.
Anatomic central hepatectomy (mesohepatectomy) for HCC. A, Venous phase of abdominal CT showing the centrally located tumor with involvement of the middle and right hepatic veins. The patient had a right inferior hepatic vein, which allowed the plans for a central hepatectomy. B, Intraoperative image of the tumor centrally located in the context of cirrhotic liver. C, Intraoperative image after central hepatectomy showing the central defect and spared right posterior and left lateral sections. D, Delayed phase of abdominal MRI 1 year after resection revealing central defect and enlarged right posterior and left lateral sections. CT indicates computed tomography; HCC, hepatocellular carcinoma; MRI, magnetic resonance imaging.
Figure 2.
Anatomic bisegmentectomy for centrally located HCC—accomplishing complete resection, using segment-oriented technique and able to preserve liver parenchyma. A, Venous phase of abdominal CT scan revealing a deep lesion close to the takeoff of the right anterior pedicle. B, Intraoperative imaging after transection of the right anterior pedicle, showing demarcated bisegment (segments 5 and 8). C, Postresection intraoperative imaging revealing the surgical defect following bisegmentectomy. D, Ex vivo image of surgical specimen revealing the 2 segments and the lesion deep in the area with adequate margins. CT indicates computed tomography; HCC, hepatocellular carcinoma.
In the context of parenchyma-preserving resections, and in particular when treating smaller lesions, there is controversy regarding the role of true anatomic (segment oriented) resections versus nonanatomical resections (wedge resections). Recently, however, at least 2 meta-analysis have examined this question and have arrived at the same conclusion regarding the superiority of anatomic resections from an oncologic standpoint. Both studies also found no differences in postoperative outcomes including complications, PHLF, and death.81,82 The most recent meta-analysis included analysis of 11 nonrandomized studies, with the primary outcome of early and late intrahepatic recurrence. Based on the biologic rationale that intrahepatic metastasis occur via vascular spread that follows anatomic portal distribution, these outcomes were relevant. The authors found that anatomic resections were associated with lower local recurrences and higher 5-year disease-free survival than nonanatomic resections (odds ratio [OR]: 0.27, 95% confidence interval [CI]: 0.17-0.43 and OR: 2.1, 95% CI: 1.41-3.12, respectively). Interestingly, the primary benefit was derived from an effect on early intrahepatic recurrences, with no difference in late or distant recurrence.82 Despite these results, it is difficult to support the use of anatomic resections in all cases, particularly since in some circumstances, this approach may result in a major resection with a small liver remnant that could otherwise be avoided. This was evaluated in a study from Taiwan in which the authors examined a 20-year experience including close to 400 patients. They found that extent of resection—minor or major (both with anatomic and nonanatomic resections)—was not a predictor of overall long-term outcomes, but rather the degree of cirrhosis and other validated tumor characteristics.83 After putting these data together, it is our common practice and recommendation to perform anatomic resections whenever possible, particularly when these also result in parenchyma-preserving procedures (see Figure 2)—however, for patients with peripheral tumors, our approach is for nonanatomic resections as we believe complete resection (margin negative) and sparing of the liver parenchyma provide the best long-term results for this population.
As discussed in the previous section, a number of patients present with larger than 5 to 10 cm tumors, often requiring major liver resections, including right hepatectomy and extended left and right hepatectomies. The role of these procedures has been studied as well, and extended resections performed at high-volume centers, and with appropriate selection criteria, have been associated with equivalent postoperative outcomes and survival.84 Different surgical approaches have been described including the conventional approach starting with mobilization of the liver, inflow/outflow control, and subsequent parenchymal transection. During the last 2 decades, a number of studies have helped define the anterior approach and its role for large HCC. In this approach, the liver and tumor are not mobilized/exposed until the inflow/outflow control and parenchymal transection have been completed. Since its initial description, different groups have found important benefits of this technique including lower intraoperative blood loss and need for transfusion.85,86 Further, a randomized clinical trial comparing anterior and conventional approach for right hepatectomy for HCC >5 cm in size found similar results regarding blood loss and need for transfusion and also presented compelling data regarding improved OS.87 Although there were methodologic issues with this trial, different groups have recently replicated these long-term results further supporting this technique for this population of patients.86,88 As an aid for parenchymal transection, Belghiti et al published a novel approach to facilitate transection—the Hanging Maneuver: a “blind” retrohepatic tunnel is dissected anterior to the inferior vena cava (IVC), and a Penrose drain or tape is used to hang the liver and guide the parenchymal transection.89 As these 2 techniques were being developed in parallel, many groups including ours now use a combination of the hanging maneuver to facilitate the anterior approach for right hepatectomy in all patients with HCC >5 cm (Figure 3).86
Figure 3.
Combination of “hanging maneuver” technique and anterior approach for resection of a large right-sided HCC (14 cm). A, Abdominal CT scan (axial image) revealing a giant right lobe HCC. B, Intraoperative image revealing a Penrose drain inserted along the retrohepatic space and anterior to the inferior vena cava (IVC) to allow to “hang” the liver while anterior transection is performed. C, Intraoperative image revealing post-transection aspect of the liver. Note that the right liver has not yet been mobilized. D, Intraoperative image revealing the last portion of the procedure, after transection of the right hepatic vein, and as the right lobe is being mobilized and once transection has been completed. Note the IVC completely exposed after the anterior approach, which is greatly facilitated by the hanging maneuver. CT indicates computed tomography; HCC, hepatocellular carcinoma.
Intrahepatic Cholangiocarcinoma
Epidemiology
Intrahepatic cholangiocarcinoma is a rare tumor but still represents the second most common primary liver cancer, following HCC. Cholangiocarcinomas (intrahepatic and extrahepatic) represent 3% of all gastrointestinal tumors.90,91 Intrahepatic cholangiocarcinoma is distinguished from extrahepatic cholangiocarcinoma by its location proximal to the second-order bile ducts92 and accounts for 20% to 25% of all cholangiocarcinomas,93 although the rates of ICC compared to extrahepatic cholangiocarcinoma have been increasing with time.92,94 Of all patients presenting with ICC, only about one-third of patients are eligible for curative treatment, and a 5-year survival is low at 18%.93
Intrahepatic cholangiocarcinoma has a slight male predominance, and the average age of diagnosis is 50 years.93 The majority of cholangiocarcinomas occur sporadically, although several risk factors for their development have been identified. These include factors that incite an inflammatory reaction in the liver, such as biliary duct disorders like primary sclerosing cholangitis and choledochal cysts, parasitic infections, toxin exposure, hepatitis B and C, and nonalcoholic steatohepatitis.92,95 Geographically, the incidence of cholangiocarcinoma varies greatly, with the incidence being higher in Asian countries than in Western countries,90 due to varying exposure to risk factors. Due to the lack of a consistent cause of cholangiocarcinoma and the inability to screen for it, the majority of patients with ICC are identified incidentally and in an advanced stage. As many patients have tumors in a background of liver dysfunction, this can have significant implications in terms of patient eligibility for surgical treatment.
Macroscopically, types of ICC include mass forming, periductal infiltrating, and intraductal growth types.96 The mass-forming type, as the name suggests, presents as a mass in the parenchyma that does not invade along the main ducts and is the most common type of ICC. The periductal-infiltrating type grows along the length of the bile duct and can result in peripheral ductal dilatation. The intraductal growth type, which is the rarest type, grows intraluminally. Intrahepatic cholangiocarcinoma can also macroscopically be a mix of the different types.96 The mass-forming and periductal-infiltrating mixed type tends to occur centrally and be more aggressive than other types.
Diagnosis
Tissue biopsy is the only way to diagnose ICC, as metastasis to the liver is more common than ICC. However, suspicion for this disease can be raised based upon clinical presentation, abnormal imaging findings, and abnormal laboratory values (such as elevated liver function tests including bilirubin and elevated CA 19-9). Clinical presentation is often vague, as patients with ICC typically present with subtle findings. Patients diagnosed with early-stage disease are often (12%-30% of the time) identified after imaging for unrelated reasons.93 Symptoms that may prompt imaging include abdominal pain or discomfort, weight loss, or an abdominal mass.97 Compared to patients with extrahepatic cholangiocarcinoma who may present with jaundice, patients with ICC typically do not.93,98 Often a distinct mass indicative of ICC is not seen on initial imaging; the presence of ICC is often suspected based upon secondary imaging characteristics, such as dilated intrahepatic bile ducts in a portion of the liver with associated atrophy of the parenchyma. Laboratory values are not diagnostic either; CA 19-9 is typically elevated in about 50% of patients, and CEA is elevated in 15% to 20%.97 Aside from these mentioned factors, the potential for metastasis must be ruled out, and work-up to identify a possible primary, including colonoscopy, esophagogastroduodenoscopy, mammogram, and chest imaging, should be performed as well.99
A histologic diagnosis can be difficult to obtain preoperatively. If a patient has findings on imaging that are indicative of an ICC that appears resectable, preoperative biopsy may be omitted. Imaging characteristics consistent with ICC on CT include a hypoechoic mass with thin rim-like enhancement, increasing contrast uptake in the mass in the delayed or venous phases, ductal dilatation, and hepatic atrophy.93,97 On MRI, imaging characteristics consistent with ICC include a hypodense mass on T1 which is hyperdense on T2 and peripheral enhancement with progressive concentric filling.93,97 If, however, the tumor is deemed unresectable and systemic treatment is planned histologic diagnosis is recommended for confirmation.97 Histologically, the biopsy demonstrates adenocarcinoma, and distinguishing from a metastasis can be aided with the use of immunohistochemical staining by the pathologist (including positive CK7 and negative TTF1, CK20, CDX2, and DPC4) and by additional testing in case a primary tumor with liver metastasis is suspected.97 The role of positron emission tomography scans in the management of patients with ICC is not well defined and is currently not routinely recommended.100
Surgical Management
Surgical resection remains the only potential cure for patients with ICC. However, most patients present with advanced disease and thus are not eligible for resection. Among resectable patients, roughly 75% of patients require a hemihepatectomy or extended hepatectomy to remove the tumor,98,100,101 and about 80% are resected with negative margins.99 Even with resection, the chance for long-term cure remains low.102 A population-level analysis using the Surveillance, Epidemiology, and End Results database demonstrated an improvement in recent years in survival after resection for ICC, although 5-year survival following resection remains low at about 20% to 30%.91,101
Resectability for ICC follows the same principles as that for HCC. An important difference is that resection is the only curative treatment for ICC, with ablation and OLT not currently indicated. For optimal local control, tumors should be completely excised with negative margins, while leaving behind an adequate FLR, with intact arterial, portal venous, and hepatic venous flow and biliary-enteric drainage.70,93,97 Similar tools used for HCC, such as PVE, can be used with cholangiocarcinoma to aid in improving the potential for resectability. Although data evaluating surgical strategies for ICC are lacking, the surgical principles are similar and are often interchangeable. While in the past staging for ICC was combined with HCC, separate staging systems were developed for each tumor in the seventh edition of the American Joint Committee on Cancer (AJCC) guidelines.103 The current staging guidelines use characteristics which have been demonstrated to be poor prognostic indicators, including the presence of multiple tumors, vascular invasion, regional lymph node metastasis, periductal invasion, and distant metastasis.102,104,105 Tumor size has not clearly been shown to be a significant prognostic indicator105,106 and thus is not included as part of the current staging system. A recent international and multi-institutional study98 of patients undergoing surgical resection for ICC in modern times described median survival as 27 months and 5-year OS as 31% after resection. The majority of patients presented with single tumors (73%) and no evidence of vascular invasion (69%), although close to 30% had lymph node metastasis confirmed after resection.
Preoperative staging can be performed with the use of imaging (CT or MRI). If there is evidence of multiple tumors, extrahepatic disease, or nodal disease, resection is not recommended with very few exceptions.97 Staging laparoscopy can be helpful for patients without clear imaging evidence demonstrating contraindications to resection, but in whom there is suspicion for metastasis from high tumor markers or imaging,93,97 although the sensitivity of this procedure is only about 55% and therefore is not recommended routinely.
An adequate surgical margin with complete microscopic negative resection (R0) is essential, although it is not clear if wider margins provide any added long-term benefit. A recent multi-institutional study found that R0 resections were significantly associated with improved survival and decreased local recurrence, and the width of the margin did not provide any added benefit.107 A second multicenter study confirmed that R1 resection is a predictor of poor outcome, for those with negative lymph node involvement.108 In terms of extent of resection, therefore, the goal of surgery should be complete surgical removal with negative margins while leaving behind an adequate liver remnant.93,100
The seventh edition of the AJCC guidelines103 was the first edition to separate ICC from HCC, bringing attention to the importance of lymph node assessment when treating ICC. Lymph node metastases are found in close to half of patients with ICC, and it is clear that the presence of lymph node metastasis portends a poor outcome for these patients.97 However, prospective trials have not addressed the need for lymphadenectomy. It is known that lymphadenectomy is critical for staging, but it is not clear if there is any therapeutic benefit to lymphadenectomy. A recent multicenter study noted that presently, only about half of patients with ICC undergo lymphadenectomy at the time of liver resection.98 The median number of nodes harvested is 3, and 30% of patients undergoing lymphadenectomy were found to have nodal metastasis.98 In this study, risk factors associated with nodal metastasis included vascular or biliary invasion. Of note, nodal disease itself is associated with decreased OS. In addition, in those patients with nodal metastasis, the number of tumors and the presence of vascular invasion no longer had a prognostic effect on survival, suggesting that the presence of nodal metastasis is one of the most important prognostic indicators in patients with ICC.
For this reason, in addition to resection of the involved liver, portal lymphadenectomy is recommended for ICC for the assessment of tumor metastasis to the adjacent lymph node basins, which is critical for staging and for prognostic information.97 The technique of portal lymphadenectomy involves the removal of all nodal tissue within the porta hepatis to skeletonize the remaining non-nodal structures of the bile duct, hepatic artery, and portal vein. In addition, studies of the lymphatic drainage of the liver have shown the left lobe of the liver drains toward the lesser curve of the stomach (left gastric nodal territory), and the right lobe of the liver drains toward the hepatoduodenal ligament93 and the retropancreatic area.97 Although this drainage is not consistent, lymph nodes in these areas should be removed as well for adequate lymphadenectomy of the corresponding tumor. Currently, there are no benchmarks to guide the ideal number of lymph nodes during portal lymphadenectomy.97
With improvements in anesthetic safety, surgical technique, and perioperative care in recent years, the safety of liver resections has improved over time. For ICC, postoperative mortality rates range from 1% to 14%, and morbidity rates range from 6% to 43%.93 As these improvements in safety have been made, the indications for resection have been expanding. As an example, prior studies have suggested that increasing age is negatively associated with survival of patients with ICC,105 although a recent multi-institutional study identified increased rates of complications but similar mortality.109 Thus, tumor characteristics are likely more relevant as prognostic indicators than age, and resection should be offered to well-selected elderly patients who otherwise have resectable disease.
Despite the improvements noted in survival among resected patients over time, over half of patients experience a recurrence in the liver within a year of surgery.97,110 Within 2 years after surgery, the majority of recurrences (over 80%) involve the liver, and after 2 years, the majority of recurrences (about 60%) are extrahepatic.111 Factors negatively associated with survival after resection include tumor size, lymph node metastasis, presence of vascular invasion, multiple tumors, and an incomplete resection.97,111 An international study assessed treatment for recurrent ICC after curative intent surgery. Predictors of intrahepatic recurrence included cirrhosis, multiple tumors, and larger tumors, whereas lymph node metastasis was more predictive of extrahepatic recurrence.112
Given the overall high recurrence rates and poor survival, a number of studies have evaluated the role of adjuvant therapies for resected ICC. Recurrence following surgery for ICC is both locoregional in the liver and lymph nodes as well as systemic, and hence both chemotherapy and radiation can play a role.113–115 A recent meta-analysis assessing the benefit of adjuvant therapy after resection (chemotherapy, chemoradiation, or radiation alone) compared to those patients undergoing resection alone in patients with biliary tract cancers (including extrahepatic and gallbladder) found there was a small but not statistically significant improvement in OS with adjuvant therapy (chemotherapy or chemoradiation), which was particularly beneficial for those with nodal involvement and margin-positive resections.116 The main limitation of this meta-analysis and other studies has been the heterogeneous population of patients included. More information on adjuvant therapies for ICC can be found in the corresponding manuscripts of this issue. Noteworthy though, are the results of the BILCAP study, recently presented in the 2017 ASCO meeting; the authors randomized 447 patients with biliary tract cancer to capecitabine versus observation following curative resection and found a 25% lower risk of mortality in the treatment arm with median survival extended from 36 months to 51 months following surgery.117
Lastly, the role for neoadjuvant therapy in high-risk patients with cholangiocarcinoma has been studied, and although phase III or high-level data are currently not available, studies have shown some encouraging results in selected populations. There is, though, encouraging data describing the use of chemotherapy for unresectable tumors that have a dramatic response, after which surgical resection has been considered. Reports from Japan described the use of gemcitabine and the combination of gemcitabine and cisplatin for locally advanced biliary tract tumors. These tumors were unresectable due to the size of the tumor requiring a major hepatic resection leaving an inadequate FLR, extensive vascular invasion, or extensive biliary invasion. Between one-quarter and one-third of patients were ultimately taken to surgery after demonstrating evidence of tumor downsizing, and these patients had long-term survival at similar rates as published for patients who were upfront resectable disease.118,119 Analysis of the use of intra-arterial therapies for unresectable ICC has also demonstrated that approximately 25% of patients can have a partial or complete response, with a concomitant improvement in long-term survival.120 Hepatic resection after intra-arterial therapies in conjunction with chemotherapy has also been shown to be feasible for selected populations.121
Presently, recommendations for use of any therapy in a standard neoadjuvant regimen cannot be given; patients who may be considered for this approach include those with unresectable disease due to size and location, as well as those with minimal or equivocal periportal LN involvement. For both scenarios, neoadjuvant therapy is used as a test of biologic behavior to ensure disease does not progress and in some cases to induce downsizing so as to allow for a complete margin-negative resection.122
Conclusions
Primary liver tumors are associated with significant morbidity and mortality worldwide. Treatment of these diseases is highly complex, as considerations include not only the tumor biology and anatomic considerations within the liver but also the underlying function of the liver and the patient’s functional status. Although there are a number of potentially curative treatment options available for HCC, including ablation, resection, and transplant, surgical resection for HCC has evolved and expanded over the last several years to be a very effective option for many patients with HCC and preserved liver function, with long-term survival similar if not better than traditionally offered therapies. In addition, as the safety of surgical resection has improved, the oncologic benefit of surgery for patients with poor prognostic factors, such as large HCC or vascular invasion, is also now being demonstrated, for well-selected patients. Of paramount importance when considering liver resection is knowledge of onco-surgical strategies that decrease the risk of PHLF, including PVE to hypertrophy the FLR and parenchymal-sparing resections with segmental-oriented procedures. These have been demonstrated to increase the number of eligible patients for resection without compromising oncologic outcomes.
For ICC, surgery remains the only potentially curative treatment. Principles of surgical resection are similar to HCC, with margin-negative resection and preservation of adequate function of the FLR. Similar onco-surgical strategies can be used for ICC as for HCC. However, secondary to the high rate of lymph node metastasis and that nodal disease portends a poor prognosis for patients with ICC, portal lymphadenectomy is recommended in addition to surgical resection for prognostic and staging information. Even with successful surgical resection, though, there are high rates of locoregional and systemic recurrence. Studies are increasingly demonstrating survival benefits with adjuvant therapy after resection for resected patients and that neoadjuvant therapy can be considered in an attempt to downsize tumors. For both HCC and ICC, as the indications for surgical resection continue to expand, so does the potential to offer cure. With the multitude of patient, tumor, and treatment factors involved in treating patients with primary liver tumors, it remains critically important to assess each patient in a multidisciplinary setting to best individualize the care for all patients.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am. 2015;24(1):1–17. [DOI] [PubMed] [Google Scholar]
- 2. McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19(2):223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127. [DOI] [PubMed] [Google Scholar]
- 4. Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992-2008. Hepatology. 2012;55(2):476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruix J, Sherman M; American Association for the Study of Liver Disease. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davila JA, Kramer JR, Duan Z, et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and nonpatient factors. Hepatology. 2013;57(5):1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hyder O, Dodson RM, Nathan H, et al. Referral patterns and treatment choices for patients with hepatocellular carcinoma: a United States population-based study. J Am Coll Surg. 2013;217(5):896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nathan H, Bridges JF, Schulick RD, et al. Understanding surgical decision making in early hepatocellular carcinoma. J Clin Oncol. 2011;29(6):619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benson AB, III, D’Angelica MI, Abbott DE, et al. NCCN guidelines insights: hepatobiliary cancers, version 1.2017. J Natl Compr Canc Netw. 2017;15(5):563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salami AC, Barden GM, Castillo DL, et al. Establishment of a regional virtual tumor board program to improve the process of care for patients with hepatocellular carcinoma. J Oncol Pract. 2015;11(1):e66–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lau K, Salami A, Barden G, et al. The effect of a regional hepatopancreaticobiliary surgical program on clinical volume, quality of cancer care, and outcomes in the Veterans Affairs System. JAMA Surg. 2014;149(11):1153–1161. [DOI] [PubMed] [Google Scholar]
- 12. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649. [DOI] [PubMed] [Google Scholar]
- 13. Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–871. [DOI] [PubMed] [Google Scholar]
- 14. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang YY, Zhong JH, Su ZY, et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg. 2016;103(6):725–734. [DOI] [PubMed] [Google Scholar]
- 16. Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243(3):321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252(6):903–912. [DOI] [PubMed] [Google Scholar]
- 18. Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57(4):794–802. [DOI] [PubMed] [Google Scholar]
- 19. Weis S, Franke A, Mossner J, Jakobsen JC, Schoppmeyer K. Radiofrequency (thermal) ablation versus no intervention or other interventions for hepatocellular carcinoma. Cochrane Database Syst Rev. 2013;(12):CD003046. [DOI] [PubMed] [Google Scholar]
- 20. Qi X, Tang Y, An D, et al. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: a meta-analysis of randomized controlled trials. J Clin Gastroenterol. 2014;48(5):450–457. [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, Luo Q, Li Y, Deng S, Wei S, Li X. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinomas: a meta-analysis of randomized and nonrandomized controlled trials. PLoS One. 2014;9(1):e84484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. [DOI] [PubMed] [Google Scholar]
- 23. Mazzaferro V, Bhoori S, Sposito C, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17(suppl 2):S44–S57. [DOI] [PubMed] [Google Scholar]
- 24. Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394–1403. [DOI] [PubMed] [Google Scholar]
- 25. Makuuchi M, Sano K. The surgical approach to HCC: our progress and results in Japan. Liver Transpl. 2004;10(2 suppl 1):S46–S52. [DOI] [PubMed] [Google Scholar]
- 26. Krahn LE, DiMartini A. Psychiatric and psychosocial aspects of liver transplantation. Liver Transpl. 2005;11(10):1157–1168. [DOI] [PubMed] [Google Scholar]
- 27. Xu XS, Liu C, Qu K, Song YZ, Zhang P, Zhang YL. Liver transplantation versus liver resection for hepatocellular carcinoma: a meta-analysis. Hepatobiliary Pancreat Dis Int. 2014;13(3):234–241. [DOI] [PubMed] [Google Scholar]
- 28. Proneth A, Zeman F, Schlitt HJ, Schnitzbauer AA. Is resection or transplantation the ideal treatment in patients with hepatocellular carcinoma in cirrhosis if both are possible? A systematic review and meta-analysis. Ann Surg Oncol. 2014;21(9):3096–3107. [DOI] [PubMed] [Google Scholar]
- 29. Cucchetti A, Cescon M, Ercolani G, et al. Comparison between observed survival after resection of transplantable hepatocellular carcinoma and predicted survival after listing through a Markov model simulation. Transpl Int. 2011;24(8):787–796. [DOI] [PubMed] [Google Scholar]
- 30. Koniaris LG, Levi DM, Pedroso FE, et al. Is surgical resection superior to transplantation in the treatment of hepatocellular carcinoma? Ann Surg. 2011;254(3):527–537; discussion 537-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vitale A, Huo TL, Cucchetti A, et al. Survival benefit of liver transplantation versus resection for hepatocellular carcinoma: impact of MELD score. Ann Surg Oncol. 2015;22(6):1901–1907. [DOI] [PubMed] [Google Scholar]
- 32. Spolverato G, Vitale A, Ejaz A, et al. The relative net health benefit of liver resection, ablation, and transplantation for early hepatocellular carcinoma. World J Surg. 2015;39(6):1474–1484. [DOI] [PubMed] [Google Scholar]
- 33. Lim KC, Wang VW, Siddiqui FJ, et al. Cost-effectiveness analysis of liver resection versus transplantation for early hepatocellular carcinoma within the Milan criteria. Hepatology. 2015;61(1):227–237. [DOI] [PubMed] [Google Scholar]
- 34. Adam R, Azoulay D, Castaing D, et al. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg. 2003;238(4):508–518; discussion 518-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tanaka S, Noguchi N, Ochiai T, et al. Outcomes and recurrence of initially resectable hepatocellular carcinoma meeting Milan criteria: rationale for partial hepatectomy as first strategy. J Am Coll Surg. 2007;204(1):1–6. [DOI] [PubMed] [Google Scholar]
- 36. Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235(3):373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cherqui D, Laurent A, Mocellin N, et al. Liver resection for transplantable hepatocellular carcinoma: long-term survival and role of secondary liver transplantation. Ann Surg. 2009;250(5):738–746. [DOI] [PubMed] [Google Scholar]
- 38. Ho CM, Lee PH, Chen CL, Ho MC, Wu YM, Hu RH. Long-term outcomes after resection versus transplantation for hepatocellular carcinoma within UCSF criteria. Ann Surg Oncol. 2012;19(3):826–833. [DOI] [PubMed] [Google Scholar]
- 39. Zhong JH, Li H, Li LQ, et al. Adjuvant therapy options following curative treatment of hepatocellular carcinoma: a systematic review of randomized trials. Eur J Surg Oncol. 2012;38(4):286–295. [DOI] [PubMed] [Google Scholar]
- 40. Furtado R, Crawford M, Sandroussi C. Systematic review and meta-analysis of adjuvant I (131) lipiodol after excision of hepatocellular carcinoma. Ann Surg Oncol. 2014;21(8):2700–2707. [DOI] [PubMed] [Google Scholar]
- 41. Xia Y, Qiu Y, Li J, et al. Adjuvant therapy with capecitabine postpones recurrence of hepatocellular carcinoma after curative resection: a randomized controlled trial. Ann Surg Oncol. 2010;17(12):3137–3144. [DOI] [PubMed] [Google Scholar]
- 42. Lee JH, Lee JH, Lim YS, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148(7):1383–1391.e6. [DOI] [PubMed] [Google Scholar]
- 43. Wang J, He XD, Yao N, Liang WJ, Zhang YC. A meta-analysis of adjuvant therapy after potentially curative treatment for hepatocellular carcinoma. Can J Gastroenterol. 2013;27(6):351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shindoh J, Hasegawa K, Matsuyama Y, et al. Low hepatitis C viral load predicts better long-term outcomes in patients undergoing resection of hepatocellular carcinoma irrespective of serologic eradication of hepatitis c virus. J Clin Oncol. 2013;31(6):766–773. [DOI] [PubMed] [Google Scholar]
- 45. Huang G, Lau WY, Wang ZG, et al. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg. 2015;261(1):56–66. [DOI] [PubMed] [Google Scholar]
- 46. Lee SY, Konstantinidis IT, Eaton AA, et al. Predicting recurrence patterns after resection of hepatocellular cancer. HPB (Oxford). 2014;16(10):943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhu Y, Dong J, Wang WL, Li MX, Lu Y. Short- and long-term outcomes after salvage liver transplantation versus primary liver transplantation for hepatocellular carcinoma: a meta-analysis. Transplant Proc. 2013;45(9):3329–3342. [DOI] [PubMed] [Google Scholar]
- 48. Landman MP, Feurer ID, Pinson CW, Moore DE. Which is more cost-effective under the MELD system: primary liver transplantation, or salvage transplantation after hepatic resection or after loco-regional therapy for hepatocellular carcinoma within Milan criteria? HPB (Oxford). 2011;13(11):783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guglielmi A, Ruzzenente A, Conci S, et al. Hepatocellular carcinoma: surgical perspectives beyond the Barcelona Clinic Liver Cancer recommendations. World J Gastroenterol. 2014;20(24):7525–7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EAST-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48(5):599–641. [DOI] [PubMed] [Google Scholar]
- 51. Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations? An observational study of the HCC east-west study group. Ann Surg. 2013;257(5):929–937. [DOI] [PubMed] [Google Scholar]
- 52. Gavriilidis P, Roberts KJ, Askari A, et al. Evaluation of the current guidelines for resection of hepatocellular carcinoma using the appraisal of guidelines for research and evaluation II instrument. J Hepatol. 2017;67(5):991–998. [DOI] [PubMed] [Google Scholar]
- 53. Pandey D, Lee KH, Wai CT, Wagholikar G, Tan KC. Long term outcome and prognostic factors for large hepatocellular carcinoma (10 cm or more) after surgical resection. Ann Surg Oncol. 2007;14(10):2817–2823. [DOI] [PubMed] [Google Scholar]
- 54. Ettorre GM, Levi Sandri GB, Colasanti M, et al. Liver resection for hepatocellular carcinoma ≥5 cm. Transl Gastroenterol Hepatol. 2017;2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pawlik TM, Poon RT, Abdalla EK, et al. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg. 2005;140(5):450–457; discussion 457-458. [DOI] [PubMed] [Google Scholar]
- 56. Zhu SL, Ke Y, Peng YC, et al. Comparison of long-term survival of patients with solitary large hepatocellular carcinoma of BCLC stage A after liver resection or transarterial chemoembolization: a propensity score analysis. PLoS One. 2014;9(12):e115834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jiang JF, Lao YC, Yuan BH, et al. Treatment of hepatocellular carcinoma with portal vein tumor thrombus: advances and challenges. Oncotarget. 2017;8(20):33911–33921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cheng S, Yang J, Shen F, et al. Multidisciplinary management of hepatocellular carcinoma with portal vein tumor thrombus—Eastern Hepatobiliary Surgical Hospital consensus statement. Oncotarget. 2016;7(26):40816–40829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xue TC, Xie XY, Zhang L, Yin X, Zhang BH, Ren ZG. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol. 2013;13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ng KK, Vauthey JN, Pawlik TM, et al. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? results from a multi-institutional database. Ann Surg Oncol. 2005;12(5):364–373. [DOI] [PubMed] [Google Scholar]
- 61. Ishizawa T, Hasegawa K, Aoki T, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterol. 2008;134(7):1908–1916. [DOI] [PubMed] [Google Scholar]
- 62. Orcutt ST, Sultenfuss MA, Anaya DA. A large liver mass with acute hemorrhage. JAMA Surg. 2016;151(1):83–84. [DOI] [PubMed] [Google Scholar]
- 63. Hasegawa K, Makuuchi M, Kokudo N, et al. Impact of histologically confirmed lymph node metastases on patient survival after surgical resection for hepatocellular carcinoma: report of a Japanese nationwide survey. Ann Surg. 2014;259(1):166–170. [DOI] [PubMed] [Google Scholar]
- 64. Ercolani G, Grazi GL, Ravaioli M, et al. The role of lymphadenectomy for liver tumors: further considerations on the appropriateness of treatment strategy. Ann Surg. 2004;239(2):202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the study of the liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4(2):439–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kudo M, Matsui O, Izumi N, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer. 2014;3(3-4):458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vauthey JN, Dixon E, Abdalla EK, et al. Pretreatment assessment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford). 2010;12(5):289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Abdalla EK, Denys A, Chevalier P, Nemr RA, Vauthey JN. Total and segmental liver volume variations: implications for liver surgery. Surgery. 2004;135(4):404–410. [DOI] [PubMed] [Google Scholar]
- 69. Leelaudomlipi S, Sugawara Y, Kaneko J, Matsui Y, Ohkubo T, Makuuchi M. Volumetric analysis of liver segments in 155 living donors. Liver Transpl. 2002;8(7):612–614. [DOI] [PubMed] [Google Scholar]
- 70. Anaya DA, Blazer DG, Abdalla EK. Strategies for resection using portal vein embolization: hepatocellular carcinoma and hilar cholangiocarcinoma. Semin Intervent Radiol. 2008;25(2):110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Orcutt ST, Kobayashi K, Sultenfuss M, et al. Portal vein embolization as an oncosurgical strategy prior to major hepatic resection: anatomic, surgical, and technical considerations. Front Surg. 2016;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Abulkhir A, Limongelli P, Healey AJ, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247(1):49–57. [DOI] [PubMed] [Google Scholar]
- 73. Palavecino M, Chun YS, Madoff DC, et al. Major hepatic resection for hepatocellular carcinoma with or without portal vein embolization: perioperative outcome and survival. Surgery. 2009;145(4):399–405. [DOI] [PubMed] [Google Scholar]
- 74. Shindoh J, Tzeng CW, Aloia TA, et al. Safety and efficacy of portal vein embolization before planned major or extended hepatectomy: an institutional experience of 358 patients. J Gastrointest Surg. 2014;18(1):45–51. [DOI] [PubMed] [Google Scholar]
- 75. Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: Prospective clinical trial. Ann Surg. 2003;237(2):208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fisher SB, Kneuertz PJ, Dodson RM, et al. A comparison of right posterior sectorectomy with formal right hepatectomy: a dual-institution study. HPB (Oxford). 2013;15(10):753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yip VS, Poon RT, Chok KS, et al. Comparison of survival outcomes between right posterior sectionectomy and right hepatectomy for hepatocellular carcinoma in cirrhotic liver: a single-centre experience. World J Surg. 2015;39(11):2764–2770. [DOI] [PubMed] [Google Scholar]
- 78. Lee SY, Sadot E, Chou JF, et al. Central hepatectomy versus extended hepatectomy for liver malignancy: a matched cohort comparison. HPB (Oxford). 2015;17(11):1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen X, Li B, He W, Wei YG, Du ZG, Jiang L. Mesohepatectomy versus extended hemihepatectomy for centrally located hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2014;13(3):264–270. [DOI] [PubMed] [Google Scholar]
- 80. Lee SY. Central hepatectomy for centrally located malignant liver tumors: a systematic review. World J Hepatol. 2014;6(5):347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhou Y, Xu D, Wu L, Li B. Meta-analysis of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Langenbecks Arch Surg. 2011;396(7):1109–1117. [DOI] [PubMed] [Google Scholar]
- 82. Ye JZ, Miao ZG, Wu FX, Zhao YN, Ye HH, Li LQ. Recurrence after anatomic resection versus nonanatomic resection for hepatocellular carcinoma: a meta-analysis. Asian Pac J Cancer Prev. 2012;13(5):1771–1777. [DOI] [PubMed] [Google Scholar]
- 83. Dahiya D, Wu TJ, Lee CF, Chan KM, Lee WC, Chen MF. Minor versus major hepatic resection for small hepatocellular carcinoma (HCC) in cirrhotic patients: a 20-year experience. Surgery. 2010;147(5):676–685. [DOI] [PubMed] [Google Scholar]
- 84. Poon RT, Fan ST, Lo CM, et al. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg. 2002;236(5):602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liu CL, Fan ST, Lo CM, Tung-Ping Poon R, Wong J. Anterior approach for major right hepatic resection for large hepatocellular carcinoma. Ann Surg. 2000;232(1):25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Beppu T, Imai K, Okuda K, et al. Anterior approach for right hepatectomy with hanging maneuver for hepatocellular carcinoma: a multi-institutional propensity score-matching study. J Hepatobiliary Pancreat Sci. 2017;24(3):127–136. [DOI] [PubMed] [Google Scholar]
- 87. Liu CL, Fan ST, Cheung ST, Lo CM, Ng IO, Wong J. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: a prospective randomized controlled study. Ann Surg. 2006;244(2):194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chan KM, Wang YC, Wu TH, et al. The preference for anterior approach major hepatectomy: experience over 3 decades and a propensity score-matching analysis in right hepatectomy for hepatocellular carcinoma. Medicine. 2015;94(34):e1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Belghiti J, Guevara OA, Noun R, Saldinger PF, Kianmanesh R. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg. 2001;193(1):109–111. [DOI] [PubMed] [Google Scholar]
- 90. Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54(1):173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nathan H, Pawlik TM, Wolfgang CL, Choti MA, Cameron JL, Schulick RD. Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based seer database analysis. J Gastrointest Surg. 2007;11(11):1488–1496; discussion 1496-1487. [DOI] [PubMed] [Google Scholar]
- 92. Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29(2):221–232. [DOI] [PubMed] [Google Scholar]
- 93. Brown KM, Parmar AD, Geller DA. Intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am. 2014;23(2):231–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40(3):472–477. [DOI] [PubMed] [Google Scholar]
- 95. Kinoshita M, Kubo S, Tanaka S, et al. The association between non-alcoholic steatohepatitis and intrahepatic cholangiocarcinoma: a hospital based case-control study. J Surg Oncol. 2016;113(7):779–783. [DOI] [PubMed] [Google Scholar]
- 96. Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg. 2003;10(4):288–291. [DOI] [PubMed] [Google Scholar]
- 97. Weber SM, Ribero D, O’Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17(8):669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29(23):3140–3145. [DOI] [PubMed] [Google Scholar]
- 99. Maithel SK, Gamblin TC, Kamel I, Corona-Villalobos CP, Thomas M, Pawlik TM. Multidisciplinary approaches to intrahepatic cholangiocarcinoma. Cancer. 2013;119(22):3929–3942. [DOI] [PubMed] [Google Scholar]
- 100. Carpizo DR, D’Angelica M. Management and extent of resection for intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am. 2009;18(2):289–305, viii-ix. [DOI] [PubMed] [Google Scholar]
- 101. Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149(6):565–574. [DOI] [PubMed] [Google Scholar]
- 102. Spolverato G, Vitale A, Cucchetti A, et al. Can hepatic resection provide a long-term cure for patients with intrahepatic cholangiocarcinoma? Cancer. 2015;121(22):3998–4006. [DOI] [PubMed] [Google Scholar]
- 103. Edge SB. American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7th ed New York, NY: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 104. Spolverato G, Kim Y, Ejaz A, et al. Conditional probability of long-term survival after liver resection for intrahepatic cholangiocarcinoma: a multi-institutional analysis of 535 patients. JAMA Surg. 2015;150(6):538–545. [DOI] [PubMed] [Google Scholar]
- 105. Hyder O, Marques H, Pulitano C, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an eastern and western experience. JAMA Surg. 2014;149(5):432–438. [DOI] [PubMed] [Google Scholar]
- 106. Nathan H, Aloia TA, Vauthey JN, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16(1):14–22. [DOI] [PubMed] [Google Scholar]
- 107. Ribero D, Pinna AD, Guglielmi A, et al. Surgical approach for long-term survival of patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis of 434 patients. Arch Surg. 2012;147(12):1107–1113. [DOI] [PubMed] [Google Scholar]
- 108. Farges O, Fuks D, Boleslawski E, et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg. 2011;254(5):824–829; discussion 830. [DOI] [PubMed] [Google Scholar]
- 109. Vitale A, Spolverato G, Bagante F, et al. A multi-institutional analysis of elderly patients undergoing a liver resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2016;113(4):420–426. [DOI] [PubMed] [Google Scholar]
- 110. Gil E, Joh JW, Park HC, Yu JI, Jung SH, Kim JM. Predictors and patterns of recurrence after curative liver resection in intrahepatic cholangiocarcinoma, for application of postoperative radiotherapy: a retrospective study. World J Surg Oncol. 2015;13:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Doussot A, Gonen M, Wiggers JK, et al. Recurrence patterns and disease-free survival after resection of intrahepatic cholangiocarcinoma: preoperative and postoperative prognostic models. J Am Coll Surg. 2016;223(3):493–505.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Spolverato G, Kim Y, Alexandrescu S, et al. Management and outcomes of patients with recurrent intrahepatic cholangiocarcinoma following previous curative-intent surgical resection. Ann Surg Oncol. 2016;23(1):235–243. [DOI] [PubMed] [Google Scholar]
- 113. Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery. 2013;153(6):811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Choi SB, Kim KS, Choi JY, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol. 2009;16(11):3048–3056. [DOI] [PubMed] [Google Scholar]
- 115. Yamamoto M, Takasaki K, Otsubo T, Katsuragawa H, Katagiri S. Recurrence after surgical resection of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2001;8(2):154–157. [DOI] [PubMed] [Google Scholar]
- 116. Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30(16):1934–1940. [DOI] [PubMed] [Google Scholar]
- 117. Cancer Discovery. Capecitabine extends survival for biliary tract cancer. http://cancerdiscovery.aacrjournals.org/content/early/2017/05/31/2159-8290.CD-NB2017-079. Accessed August 27, 2017. [DOI] [PubMed]
- 118. Kato A, Shimizu H, Ohtsuka M, et al. Surgical resection after downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer: a retrospective single-center study. Ann Surg Oncol. 2013;20(1):318–324. [DOI] [PubMed] [Google Scholar]
- 119. Kato A, Shimizu H, Ohtsuka M, et al. Downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer patients treated with gemcitabine plus cisplatin combination therapy followed by radical surgery. Ann Surg Oncol. 2015;22(suppl 3):S1093–S1099. [DOI] [PubMed] [Google Scholar]
- 120. Hyder O, Marsh JW, Salem R, et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann Surg Oncol. 2013;20(12):3779–3786. [DOI] [PubMed] [Google Scholar]
- 121. Rayar M, Sulpice L, Edeline J, et al. Intra-arterial yttrium-90 radioembolization combined with systemic chemotherapy is a promising method for downstaging unresectable huge intrahepatic cholangiocarcinoma to surgical treatment. Ann Surg Oncol. 2015;22(9):3102–3108. [DOI] [PubMed] [Google Scholar]
- 122. National Comprehensive Cancer Network. Hepatobiliary cancers 3. 2017. https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed August 27, 2017.