Abstract
Background:
Surgeons employ the latissimus dorsi flap (LDF) for reconstruction of a large variety of breast cancer surgery defects, including quadrantectomy, lumpectomy, modified radical mastectomy, and others. The LDF may be used in delayed or immediate reconstruction, in combination with tissue expanders for a staged reconstruction, with implant-based immediate reconstruction, or alone as an autogenous flap.
Methods:
The authors discuss the historical uses and more recent developments in the LDF. More recent advancements, including the “scarless” approach and augmentation with the thoracodorsal artery perforator flap, are discussed.
Results:
The LDF is a reliable means for soft tissue coverage providing form and function during breast reconstruction with acceptable perioperative and long-term morbidities.
Conclusions:
When there is a paucity of tissue, the LDF can provide tissue volume in autologous reconstruction, as well as a reliable vascular pedicle for implant-based reconstruction as in the setting of irradiated tissue.
Keywords: breast cancer, breast reconstruction, latissimus dorsi
Historical Uses of Latissimus Dorsi Flap
Iginio Tansini1 first described the latissimus dorsi muscle flap in 1906, but the technique did not gain popularity in breast reconstruction until the 1970s.2 In the interim, William Halsted’s radical mastectomy procedure, with skin grafting or closure by secondary intention of the resulting defect, defined the gold standard for breast surgery and reconstruction.3 In 1977, Schneider et al4 described the anatomy of the latissimus dorsi flap (LDF) and its use with implant-based reconstruction in a 31-year-old woman who underwent radical mastectomy 4 years prior. The latissimus dorsi helped to restore form and function by providing muscle coverage over the implant, replacing the breast skin and creating a natural ptosis.
In subsequent years, numerous variations of the LDF were described for breast reconstruction. In 1978, Bostwick et al5 described the use of a skin island over the muscle to replace defects of the skin in reconstruction after radical mastectomy. These techniques required an implant to replace volume, with the latissimus flap providing muscle coverage of the silicone implant and breast skin replacement. Papp and McCraw6 developed a de-epithelialized latissimus flap as a volume replacement technique in 1983.
Various techniques were designed to create an “extended” LDF, with the aim of bringing additional tissue to circumvent implant use. The first such flap was described in 1983 by Hokin and Silfverskiold, who included lumbar fat extensions.7 In 1985, Papp and McCraw6,8 modified the design to carry fat on the surface of the latissimus muscle, thus creating the total autogenous latissimus breast reconstruction.
Concurrent to these developments in the LDF, the transverse rectus abdominis muscle (TRAM) flap was being developed for autologous breast reconstruction. Described in 1982 by Hartrampf et al,9 the TRAM overtook the LDF as the primary modality for autologous breast reconstruction. A decade later, Allen and Treece described the first successful distal inferior epigastric perforator (DIEP) flap breast reconstruction, adding another technique to the autogenous breast reconstruction armament.10 However, the LDF offers a reliable alternative for autologous breast reconstruction and remains a mainstay of breast surgery in several specific situations.
Latissimus Dorsi Flap and Indications in Breast Surgery
There are several specific indications for the LDF.3 For autogenous breast cancer reconstruction, the LDF is first line for patients who are not candidates for the TRAM flap, due to previous abdominoplasty, prior TRAM, insufficient abdominal skin or fat, and high-risk comorbidities such as diabetes, obesity, or tobacco use. In patients whose breasts have been radiated, the LDF can be used to provide well-vascularized tissue to the ischemic chest wall. The LDF can also provide tissue to correct partial mastectomy or lumpectomy defects, to augment thin or unreliable skin flaps over an implant, or to maximize aesthetic outcome of a prophylactic mastectomy. Relative contraindications to the use of the latissimus muscle are a posterior lateral thoracotomy where the muscle and its blood supply was previously divided or division of the thoracodorsal nerve during an axillary node dissection, resulting in an atrophic muscle.
Latissimus Dorsi Flap Anatomy
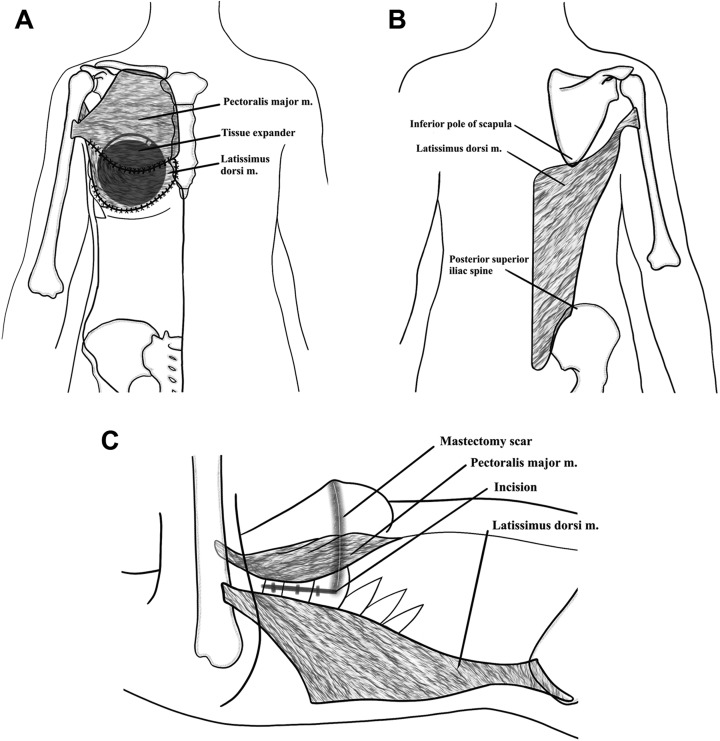
The latissimus dorsi muscle is a flat, triangular muscle that covers the posterior trunk, with its superior medial portion resting deep to the trapezius muscle and its remainder directly under subcutaneous tissue. The muscle origins include the external surface of the 3rd or 4th most inferior ribs, the iliac crest, the spinous processes of the lower 6th or 7th thoracic, lumbar, and superior sacral vertebrae, as well as the inferior angle of the scapula. The muscle fibers run toward the axilla, where they insert as the broad tendon into the intertubercular groove of the humerus.11 Of note, the latissimus dorsi muscle fibers form an aponeurotic attachment with the lower border of the serratus anterior and superiorly converge with fibers of the teres major to form the posterior axillary fold. The latissimus dorsi functions to adduct, extend, and medially rotate the humerus, as well as secure the tip of the scapula against the posterior chest wall.3 The muscle is expendable; its functions are preserved in its absence by the shoulder girdle muscles (Figure 1).
Figure 1.
Schematic of relevant anatomy of the latissimus dorsi flap for breast reconstruction: (A) anterior, (B) posterior, and (C) lateral views.
Mathes and Nahai classified the latissimus dorsi muscle as type V12; its dominant pedicle is the thoracodorsal artery, and the muscle receives segmental circulation from perforators off of the posterior intercostal arteries and the lumbar artery.3,12 (What part of statement is from Ref 2 as that publication is not by Mathes and Nahai.) With a large diameter and minimal anatomic variation, the thoracodorsal artery provides a highly reliable blood supply.3,12,13 The vessel enters the underside of the latissimus in the posterior axilla, giving off a branch to the serratus muscle, continues into the muscle and bifurcates into a large lateral descending branch and small transverse branch.3 In addition, numerous musculocutaneous perforators allow for skin island design anywhere on the muscle.
Operative Technique
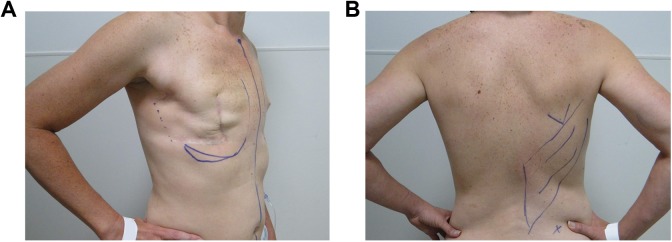
The goal of optimal operative technique is to maximize the soft tissue coverage provided by the flap, while minimizing the magnitude of donor site defect and donor site complications. Markings are performed preoperatively with the patient in the upright position and anteriorly include the midline, inframammary fold and lateral edge of breast tissue and posteriorly include lateral margin of the latissimus along the posterior axillary line, superior margin at the tip of the scapula, and inferior margin at the iliac crest (Figure 2). The skin paddle may be designed transversely, obliquely, or vertically; each orientation carries advantages and disadvantages for dissection, tissue harvest, and ultimate scar.
Figure 2.
Preoperative markings. A patient after bilateral mastectomies and right chest wall radiation presents for a right latissimus dorsi flap and bilateral tissue expander placement. A, Midline, inframammary fold, medial and lateral extents of breast mound. Note prior mastectomy scar and proposed extension of incision for posterior access to latissimus dorsi. B, Tip of scapula, extent of latissimus dissection and iliac crest.
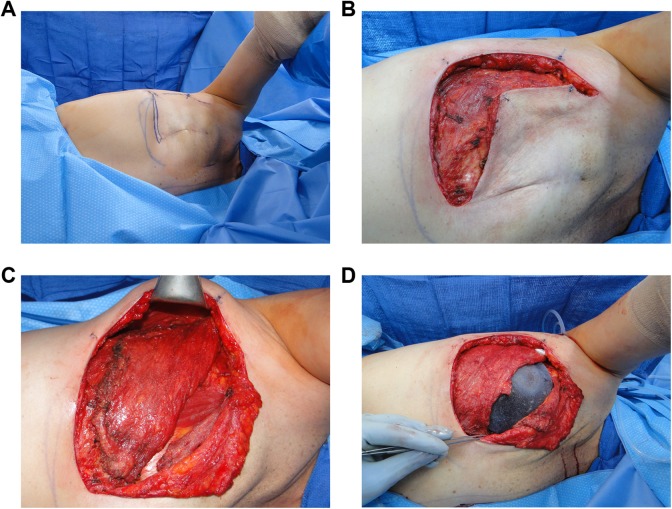
In the operating room, the patient is placed in the lateral decubitus position for unilateral or prone position for bilateral flap elevation. Dissection is carried out beneath the thoracolumbar fascia, leaving the deep fat attached to the back skin flaps. The latissimus is separated from the serratus anterior at the lateral border; from the paraspinous muscle fascia, lumbosacral fascia, and vertebral column; from the trapezius fibers superomedially; and from the teres major fibers in the axilla. After identification of the thoracodorsal vessels, the latissimus is divided near its attachment to the humerus. The myocutaneous or myofascial flap is then transferred to the mastectomy defect through a subcutaneous tunnel in the axilla.3 (Figure 3)
Figure 3.
Intraoperative photos. A, Patient in the left lateral decubitus position for access to latissimus dorsi flap dissection. B, Initial dissection raising prior mastectomy flap off of the pectoralis major. C, Latissimus dorsi flap rotated anteriorly to the chest wall into proposed position. D, Tissue expander placed within latissimus sling. Next pectoralis major (retracted cephalad) will be sutured to latissimus to provide complete coverage of the expander.
The patient is then placed in the supine position, and the surgeon proceeds with flap placement according to the type of reconstruction. When a tissue expander is to be used in a 2-stage reconstruction, the expander can be placed between the latissimus and the pectoralis major or deep to both muscles. The latter placement can allow additional aesthetic freedom. For example, the pectoralis major can provide upper pole coverage and the latissimus placed inferiorly can create a natural ptosis. The latissimus is then sutured medially and inferiorly to the underlying muscle and fascia. Additional sutures placed along the anterior axillary line aid in preventing flap or implant migration as well as protect the pedicle from excess tension. In a total autogenous LDF, the cutaneous paddle is molded into the form of an asymmetric U, with the distal fat and muscle folded under to create the desired volume and projection of the breast.3
Recent Innovations
This paper’s senior author (Z.P.) as well as others14,15 have developed a “skinless” approach, which avoids taking a skin paddle to complete staged reconstruction with a muscle flap alone. This method is ideal for relatively thin mastectomy patients who are unsuitable for abdominal tissue transfer but have had radiation to the chest wall. The procedure is usually combined with a tissue expander but may also be used in single stage with an implant.
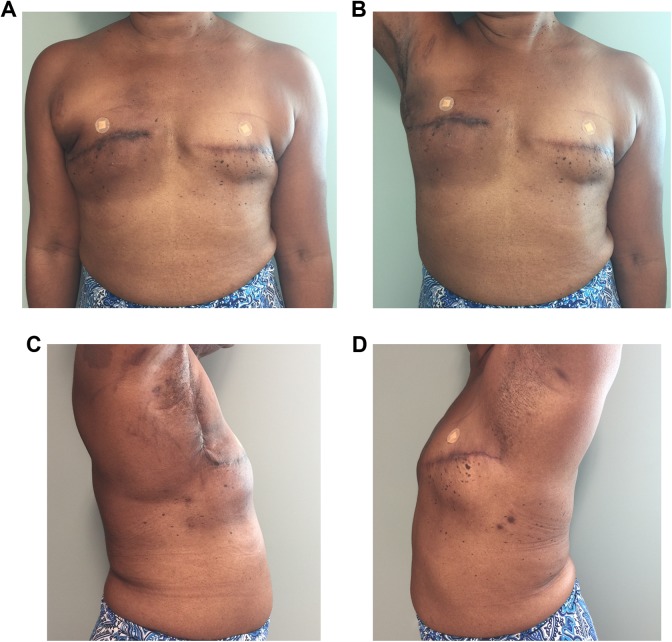
This “skinless” approach has many benefits. First, the technique removes the need for a posterior donor site scar through a small lateral extension of the mastectomy incision (Figure 4). In addition, there is no skin mismatch from the donor to the recipient site. In the advent of skin-sparing and nipple-sparing mastectomies combined with effective submuscular tissue expansion, this “skinless” approach serves as a beneficial alternative in breast reconstruction.14,15
Figure 4.
Postoperative photos. A patient after a right “scarless” latissimus dorsi flap and bilateral tissue expander placement. A, Frontal view. B, Frontal view with right arm raised. C, Right lateral view. D, Left lateral view. This technique removes the need for a posterior donor site scar through a small lateral extension of the mastectomy incision.
A retrospective chart review of our institution’s patients who underwent 2-stage reconstruction using the scarless and skinless LDF between 2004 and 2011 was conducted. We reviewed a total of 23 procedures in 18 patients. Our results are notable for overall excellent patient satisfaction and low donor site morbidity. Our cohort had 2 postoperative donor site seromas, both of which were managed nonoperatively with serial aspiration and one instance of failed reconstruction with expander exposure secondary to infection. These results are comparable to a 32.8% postoperative complication rate described in a French cohort of 121 patients undergoing 2-stage scarless LDF and tissue expander reconstruction following prior radiotherapy.16
One of the shortcomings and criticisms of the traditional LDF is that the skin island overlying the flap is difficult to orient properly for successful breast volume replacement in reconstruction while providing good muscle coverage from the latissimus muscle. More recently, the thoracodorsal artery perforator (TDAP) tissue has been described, with or without the latissimus muscle for volume replacement of either partial or total breast tissue defects.17,18 The TDAP flap utilizes the residual lateral lipodystrophy tissue often present after a mastectomy as autologous tissue for breast cancer reconstruction. This not only results in volume augmentation for breast reconstruction but also removes the dystrophic fat below the axilla. The senior author (Z.P.) has performed 14 such procedures in 11 patients with good results. The procedure may be combined with expanders or implants or may be used independently as another alternative for autologous reconstruction.
Published literature describes additional variations of the TDAP, termed the muscle-sparing latissimus dorsi (MSLD) flap, as it carries a large skin paddle and spares variable portions of the latissimus dorsi muscle.18,19 Similar to our center’s experience, these flaps deliver excellent outcomes in a variety of applications. A series of 126 MSLD flaps used in 83 patients for immediate primary, delayed primary, and salvage breast reconstruction reported only 30% minor flap-related complications, with no cases of complete flap loss, and excellent aesthetic results.20
Complications
The most common complication in breast reconstruction with the LDF is donor site seroma at the harvest site.3,21,22 Seromas are treated with prolonged suction drainage or outpatient aspiration, if the surgical drain has already been removed. To prevent this morbidity, the surgeon may perform quilting sutures or use a fibrin sealant at the donor site defect at the time of wound closure and encourage the patient to avoid excessive upper extremity use resulting in shearing forces during the postoperative period.23,24
Ischemic complications are uncommon, due to the reliable vascular supply of the thoracodorsal artery to the LDF. Even in patients with diabetes or tobacco use, there is minimal risk of flap necrosis. Hokin and Silfverskiold7 reported a 7% rate of partial flap necrosis. Significant flap necrosis is uncommon and usually secondary to vascular pedicle injury during the operative dissection or pedicle thrombosis from twisting of the flap on its pedicle.
Additional donor site morbidity includes dorsal hernia, loss of shoulder mobility, shoulder weakness, hollowness at the harvest site, and winged scapula.25,26 In a literature review comprising 11 studies, Smith found that LDF reconstruction does cause impaired shoulder range of motion, strength, and functioning generally resolves by 12 months postoperatively; this finding was supported by additional studies that showed similar improvement in shoulder function.27-29
If a tissue expander or implant is used, the patient may experience device migration, device extrusion, or periprosthetic infection. Capsular contracture was initially described in high rates and a cause of criticism for the LDF. However, more recent cases series report lower rates of contracture that lead to an unexpected aesthetic result, which can be reduced when tissue expanders are utilized prior to placement of permanent implants.30-32
Patient Counseling
Patients are generally counseled that the surgery will take 3 to 4 hours for a typical latissimus breast reconstruction. Postoperatively, they have 2 donor site drains and 1 to 2 breast drains. These remain until outputs are below 30 mL/d, or generally up to 3 or 4 weeks.3 In select patients who undergo immediate reconstruction with tissue expander, the donor site drain may remain for up to 6 weeks.
On average, patients remain in the hospital for 3 days. They may start upper extremity and range of motion exercises 2 weeks after surgery and can anticipate regaining normal function for activities such as driving and returning to work in 3 to 6 weeks.3
For patients who received a tissue expander, expansion may begin 1 to 3 weeks postoperatively or after all drains are removed, depending on the surgeon’s preference. Serial expansion continues every 1 to 2 weeks until the desired volume is achieved. Implant exchange is performed 4 to 6 weeks after the last expansion, which generally occurs 4 to 6 months after the initial surgery.3,22
Conclusion
Among the plethora of breast reconstruction techniques, the LDF is a versatile, reliable means for soft tissue coverage, providing form and function with acceptable perioperative and long-term morbidities for a variety of breast defects. Although eclipsed by the TRAM and DIEP flaps for primary autogenous breast reconstruction in the 1980s and 1990s, the LDF continues to be used in autogenous and implant-based breast reconstruction, both immediate and delayed. Indeed, the LDF offers solutions for a variety of patients; when there is a paucity of tissue, the LDF can provide tissue volume in autologous reconstruction, as well as a reliable vascular pedicle for implant-based reconstruction. A query of the Nationwide Inpatient Sample data set revealed 2304 patient admissions nationwide for LDF breast reconstruction between 2008 and 2010; among these patients, the LDF was used most commonly for delayed reconstruction, in patients with previous radiation, or as a salvage procedure in patients with prior failed reconstruction.33
In addition to its versatility, the LDF provides reliable results. Although reported, donor site morbidity is uncommon (donor site hernia), may be prevented or treated (donor site seroma), or resolves with time (shoulder function). Rates of additional postoperative complications are acceptable when compared to the TRAM flap, DIEP flap, and other breast reconstruction modalities.34-36 A series of 277 patients demonstrated acceptable flap and donor site complications with the LDF in obese and overweight patients as well, further adding to the versatility of this workhorse flap.37
A number of innovations to the traditional LDF—including the extended LDF, the scarless approach, and the muscle-sparing LDF or TDAP flap—further increase the applicability of this technique to variable patient scenarios. These innovations also allow flexibility in aesthetic outcome, as the surgeon can manipulate the volume carried by the flap, provide definition to the inframammary fold and create ptosis, and minimize donor site scarring.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Tansini I. Sopra il mio nuovo processo di amputazione della mamella [in Italian]. Riforma Med. 1906;12:757. [Google Scholar]
- 2. Maxwell GP. Iginio Tansini and the origin of the latissimus dorsi musculocutaneous flap. Plast Reconstr Surg. 1980;65(5):686–692. [DOI] [PubMed] [Google Scholar]
- 3. Spear SL, Clemens MW. Latissimus dorsi flap breast reconstruction In: Neligan PC, Grotting JC, Ed. Plastic Surgery. 3rd ed Philadelphia, PA: Saunders (Elsevier); 2012:370–392. [Google Scholar]
- 4. Schneider WJ, Hill HL, Jr, Brown RG. Latissimus dorsi myocutaneous flap for breast reconstruction. Br J Plast Surg. 1977;30(4):277–281. [DOI] [PubMed] [Google Scholar]
- 5. Bostwick J, III, Vasconez LO, Jurkiewicz MJ. Breast reconstruction after a radical mastectomy. Plast Reconstr Surg. 1978;61(5):682–693. [DOI] [PubMed] [Google Scholar]
- 6. Papp C, McCraw JB. Autogenous latissimus breast reconstruction. Clin Plast Surg. 1998;25(2):261–266. [PubMed] [Google Scholar]
- 7. Hokin JA, Silfverskiold KL. Breast reconstruction without an implant: results and complications using an extended latissimus dorsi flap. Plast Reconstr Surg. 1987;79(1):58–66. [PubMed] [Google Scholar]
- 8. McCraw JB, Papp C, Edwards A, McMellin A. The autogenous latissimus breast reconstruction. Clin Plast Surg. 1994;21(2):279–288. [PubMed] [Google Scholar]
- 9. Hartrampf CR, Scheflan M, Black P. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg. 1982;69(2):216–224. [DOI] [PubMed] [Google Scholar]
- 10. Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg. 1994;32(1):32–38. [DOI] [PubMed] [Google Scholar]
- 11. Bartlett SP, May JW, Jr, Yaremchuk MJ. The latissimus dorsi muscle: a fresh cadaver study of the primary neurovascular pedicle. Plast Reconstr Surg. 1981;67(5):631–636. [PubMed] [Google Scholar]
- 12. Mathes SJ, Nahai F. Classification of the vascular anatomy of muscles: experimental and clinical correlation. Plast Reconstr Surg. 1981;67(2):177–187. [PubMed] [Google Scholar]
- 13. Chang DW, Youssef A, Cha S, Reece GP. Autologous breast reconstruction with the extended latissimus dorsi flap. Plast Reconstr Surg. 2002;110(3):751–759. [DOI] [PubMed] [Google Scholar]
- 14. Elliott LF, Ghazi BH, Otterburn DM. The scarless latissimus dorsi flap for full muscle coverage in device-based immediate breast reconstruction: an autologous alternative to acellular dermal matrix. Plast Reconstr Surg. 2011;128(1):71–79. [DOI] [PubMed] [Google Scholar]
- 15. Lee MA, Miteff KG. The scarless latissimus dorsi flap provides effective lower pole prosthetic coverage in breast reconstruction. Plast Reconstr Surg Global Open. 2014;2(5):e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Runz A, Boccara D, Bekara F, Chaouat M, Mimoun M. Outcome of 122 delayed breast reconstruction following post-mastectomy radiotherapy: the scarless latissimus dorsi flap with tissue expansion technique. Ann Chir Plast Esthet. 2017;62(1):23–30. [DOI] [PubMed] [Google Scholar]
- 17. Angrigiani C, Grilli D, Siebert J. Latissimus dorsi musculocutaneous flap without muscle. Plast Reconstr Surg. 1995;96(7):1608–1614. [DOI] [PubMed] [Google Scholar]
- 18. Schwabegger AH, Harpf C, Rainer C. Muscle-sparing latissimus dorsi myocutaneous flap with maintenance of muscle innervation, function, and aesthetic appearance of the donor site. Plast Reconstr Surg. 2003;111(4):1407–1411.. [DOI] [PubMed] [Google Scholar]
- 19. Saint-Cyr M, Nagarkar P, Schaverien M, Dauwe P, Wong C, Rohrich RJ. The pedicled descending branch muscle-sparing latissimus dorsi flap for breast reconstruction. Plast Reconstr Surg. 2009;123(1):13–24. [DOI] [PubMed] [Google Scholar]
- 20. Cook J, Waughtel J, Brooks C, Hardin D, Hwee YK, Barnavon Y. The Muscle-sparing latissimus dorsi flap for breast reconstruction. Ann Plast Surg. 2017;78(5):S263–S268. [DOI] [PubMed] [Google Scholar]
- 21. Hammond DC. Latissimus dorsi flap breast reconstruction. Clin Plast Surg. 2007;34(1):75–82. [DOI] [PubMed] [Google Scholar]
- 22. Menke H, Erkens M, Olbrisch RR. Evolving concepts in breast reconstruction with latissimus dorsi flaps: results and follow-up of 121 consecutive patients. Ann Plast Surg. 2001;47(2):107–114. [DOI] [PubMed] [Google Scholar]
- 23. Bailey SH, Oni G, Guevara R, Wong C, Saint-Cyr M. Latissimus dorsi donor-site morbidity: the combination of quilting and fibrin sealant reduce length of drain placement and seroma rate. Ann Plast Surg. 2012;68(6):555–558. [DOI] [PubMed] [Google Scholar]
- 24. Hart AM, Duggal C, Pinell-White X, Losken A. A prospective randomized trial of the efficacy of fibrin glue, triamcinolone acetonide, and quilting sutures in seroma prevention after latissimus dorsi breast reconstruction. Plast Reconstr Surg. 2017;139(4):854e–863e. [DOI] [PubMed] [Google Scholar]
- 25. Obregón L, Ruiz-Castilla M, Binimelis MM, et al. Laparoscopic repair of non-complicated lumbar hernia secondary to a latissimus dorsi flap. J Plast Reconstr Surg. 2014;67(3):407–410. [DOI] [PubMed] [Google Scholar]
- 26. Fraser SM, Fatayer H, Achuthan R. Lumbar herniation following extended autologous latissimus dorsi breast reconstruction. BMC Surg. 2013;13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith S. functional morbidity following latissimus dorsi flap breast reconstruction. J Adv Pract Oncol. 2014;5(3):181–187. [PMC free article] [PubMed] [Google Scholar]
- 28. Glassey N, Perks GB, McCulley SJ. A prospective assessment of shoulder morbidity and recovery time scales following latissimus dorsi breast reconstruction. Plast Reconstr Surg. 2008;122(5):1334–1340. [DOI] [PubMed] [Google Scholar]
- 29. Garusi C, Manconi A, Lanni G, et al. Shoulder function after breast reconstruction with the latissimus dorsi flap: a prospective cohort study—combining DASH score and objective evaluation. Breast. 2016;27:78–86. [DOI] [PubMed] [Google Scholar]
- 30. Ignazio T, Andrej B, Fischer T. Evaluation of late results in breast reconstruction by latissimus dorsi flap and prosthesis implantation. Plast Reconstr Surg. 2006;117(5):1387–1394. [DOI] [PubMed] [Google Scholar]
- 31. Perdikis G, Koonce S, Collis G, Eck D. Latissimus dorsi myocutaneous flap for breast reconstruction: bad rap or good flap? Eplasty. 2011;11:e39. [PMC free article] [PubMed] [Google Scholar]
- 32. Hardwicke JT, Prinsloo D. An analysis of 277 consecutive latissimus dorsi breast reconstructions: a focus on capsular contracture. Plast Reconstr Surg. 2011;128(1):63–70. [DOI] [PubMed] [Google Scholar]
- 33. DeLong MR, Tandon VJ, Rudkin GH, Da Lio AL. Latissimus dorsi flap breast reconstruction—a Nationwide Inpatient Sample review. Ann Plast Surg. 2017;78(5 suppl 4):S185–S188. [DOI] [PubMed] [Google Scholar]
- 34. Teisch LF, Gerth DJ, Tashiro J, Golpanian S, Thaller SR. Latissimus dorsi flap versus pedicled transverse rectus abdominis myocutaneous breast reconstruction: outcomes. J Surg Res. 2015;199(1):274–279. [DOI] [PubMed] [Google Scholar]
- 35. Lindegren A, Halle M, Docherty Skogh AC. Postmastectomy breast reconstruction in the irradiated breast: a comparative study of DIEP and latissimus dorsi flap outcome. Plast Reconstr Surg. 2012;130(1):10–18. [DOI] [PubMed] [Google Scholar]
- 36. Fischer JP, Nelson JA, Au A, Tuggle CT, III, Serletti JM, Wu LC. Complications and morbidity following breast reconstruction—a review of 16,063 cases from the 2005-2010 NSQIP datasets. J Plast Surg Hand Surg. 2014;48(2):104–114. [DOI] [PubMed] [Google Scholar]
- 37. Yezhelyev M, Duggal CS, Carlson GW, Losken A. Complications of latissimus dorsi flap breast reconstruction in overweight and obese patients. Ann Plast Surg. 2013;70(5):557–562. [DOI] [PubMed] [Google Scholar]