Abstract
Immune checkpoint inhibitors (ICPIs) are considered one of the most important breakthroughs in cancer treatment of the past decade; notably, different studies of programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) inhibitors have reported impressive clinical activity and durable responses in patients with advanced non-small cell lung cancer (NSCLC). These findings have led to the changing of the current therapeutic algorithm of advanced NSCLC, adding a new standard first-line treatment option for patients with PD-L1-positive tumors. Pembrolizumab, a highly selective anti-PD-1 humanized monoclonal antibody, was approved by the United States Food and Drug Administration (US FDA) in October 2016 for previously untreated metastatic NSCLC patients whose tumors have high PD-L1 expression, tumor proportion score (TPS) ⩾ 50%, as well as for metastatic NSCLC patients whose tumors express PD-L1 with TPS ⩾ 1% progressing on or after platinum-based chemotherapy. However, many issues remain outstanding, mainly regarding the identification of an optimal biomarker which can help selecting patients more likely to respond to ICPIs. In this review, we discuss the clinical results obtained so far with the anti-PD-1 pembrolizumab in advanced NSCLC, commenting on the role of PD-L1 as a predictive factor and providing an update of the future perspectives.
Keywords: immune checkpoint, immunotherapy, NSCLC, PD-L1, pembrolizumab
Introduction
Lung cancer is the leading cause of cancer-related death worldwide.1 Most lung cancer patients present with an already inoperable advanced disease at the time of diagnosis. Platinum-based chemotherapy remains the first-line standard therapy in unselected advanced non-small cell lung cancer (NSCLC) patients having showed an improvement in survival, quality of life and symptom control when compared with best supportive care. However, median overall survival (OS) is still poor at approximately 1 year.2–4 Furthermore, the important side effects related to such therapies allow their use only in fit patients with limited comorbidities. The identification of different subsets of NSCLC, each characterized by specific oncogenic driver alterations, such as epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) or ROS-1 rearrangements, has led to the development of small molecule tyrosine kinase inhibitors (TKIs) which have drastically changed the treatment scenario for these patients. Despite the relevant improvement in response rate and time-to-progression achieved with TKIs, tumor control is only temporary because of the onset of drug resistance mechanisms, prompting the development of second and third-generation TKIs.5–7 However, as of today, the majority of NSCLC patients still lack druggable genetic alterations and more effective therapies are still needed. In second-line treatment, until very recently, docetaxel, pemetrexed, and erlotinib8 have been the standard of care (SOC) for patients with nonsquamous histology and wildtype molecular status, while in squamous NSCLC only docetaxel and erlotinib were the only treatment options. Recently, the combination of docetaxel with new antiangiogenic agents, such as nintedanib or ramucirumab, showed higher efficacy compared with single agent docetaxel, though associated with a greater toxicity.9–11 In addition, based on recent clinical trial results, immunotherapy and, particularly, newly developed immune checkpoint inhibitors (ICPIs) are challenging current treatment paradigms. Over the last few decades, the improved understanding of cancer biology showed that there is a strict interaction between system and tumor progression.
As a matter of fact, it has been extensively demonstrated that lung cancer can evade immune surveillance using different immunosuppressive mechanisms, including ‘immune check points’ which are receptors expressed on T cells regulating the immune response. The first immune checkpoints described were cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and the programmed cell death protein 1 (PD-1).12,13 T cells express CTLA-4 on their surface which regulates the amplitude of T cell activation, down-modulates T helper cell activity and enhances regulatory T cell (Treg) immunosuppressive activity.14
PD-1 receptor is one of the most important inhibitory receptors which is expressed by T activated cells, B cells, monocytes, and natural killer cells and binds to two specific ligands, programmed death-ligand (PD-L)1 (or B7-H1 or CD274) and PD-L2 (or B7 DC or CD273). Such ligands are usually found in tumor cells and antigen-presenting cells and the interaction with their receptor leads to the inhibition of cytotoxic T lymphocyte proliferation as well as to the apoptosis of infiltrative T cells and the increase of regulatory T cells in the tumor microenvironment.12,15
Under normal physiological conditions, PD-1 regulates the activity of effector T cells in response to infection, thus limiting the potential tissue damage and protecting the human body against the activation of immune system. Cancer cells are able to escape immune response through different mechanisms including overexpression of PD-L1 and PD-L2 which bind to PD-1 receptors on T cells leading to their suppression.
PD-L1 has been found to be overexpressed in different types of tumors including melanoma, glioblastoma, NSCLC, renal cell, hepatocellular, gastric and cutaneous cancer. Moreover, its expression can not only be induced by a variety of proinflammatory molecules including gamma interferon (INF-γ), tumor necrosis factor (TNF)-α and vascular endothelial growth factor (VEGF).16 The tumor microenvironment promotes the secretion of these proinflammatory molecules leading to an upregulation of PD-L1 expression in tumor cells, thus facilitating immune suppression. It is also important to consider that some other inhibitory cells infiltrate tumors, like regulatory T CD4+ cells FOXP3+ (Tregs), cancer-associated fibroblasts, myeloid-derived suppressor cells and tumor-associated macrophages.12,17 These cells generate an immunosuppressive microenvironment by several mechanisms, such as transforming growth factor (TGF)-β and interleukin-10 secretion, secretion of platelet-derived growth factor and VEGF. Tumor cells, on the other hand, produce down-regulation of the major histocompatibility complex (MHC)-I and antigen expression and increase PD-L1 expression in tissue. As a result, solid tumors attain an immunological response insufficient to eliminate cancer cells, which is the reason why enhancing the function and quantity of cytotoxic T cells may be of clinical benefit.12,18,19
To overcome these immune suppression mechanisms and increase antitumor immunity through T cell re-engagement, clinical research in recent years has focused on targeting these immune checkpoints using monoclonal antibodies like ipilimumab and tremelimumab (anti-CTLA-4), pembrolizumab and nivolumab (anti-PD-1) and atezolizumab, avelumab and durvalumab (anti-PD-L1).
Anti-PD-1 and anti-PD-L1 antibodies block the binding between PD-1 and PD-L1/PD-L2, thereby restoring T cell activity in peripheral tissues.20 In clinical trials, PD-1 and PD-L1 inhibitors produce durable responses in approximately 20% of unselected patients with advanced NSCLC.21–24 Various fully human or humanized immunoglobulin (Ig)G anti-PD-L1 antibodies, that specifically inhibit the binding of PD-1 to its ligand PD-L1, are currently being investigated in NSCLC patients.
Nivolumab, a fully humanized IgG4 anti-PD-1 monoclonal antibody, has been the first ICPI to receive United States Food and Drug Administration (US FDA) and European Medicines Agency (EMA) approval for pretreated advanced NSCLC patients, based on the results of the phase III ‘twin trials’ CheckMate 017 and CheckMate 057, which enrolled advanced squamous and nonsquamous NSCLC patients, respectively, who had disease progression after one prior platinum-containing regimen, regardless of PD-L1 status.25,26
Based on the results of two randomized clinical trials (phase II trial POPLAR and phase III trial OAK), atezolizumab, an anti-PD-L1 antibody that had previously received US FDA accelerated approval for the treatment of locally advanced or metastatic urothelial carcinoma that has progressed after platinum-containing chemotherapy, has also been recently approved by the US FDA for metastatic NSCLC patients progressing during or following platinum-containing chemotherapy, regardless of tumor PD-L1 status.27–29
Durvalumab and avelumab, both anti-PD-L1 antibodies, are currently being evaluated within clinical trials, based on the promising results achieved in early clinical studies.30–32
Pembrolizumab (MK-3475) is a highly selective anti-PD-1 humanized monoclonal IgG4 kappa isotype antibody which can disrupt the interaction between PD-1 and PD-L1 leading to the recognition of cancer cells by cytotoxic T cells. On October 2016, it was approved by the US FDA in the first-line setting for metastatic NSCLC patients whose tumors have high PD-L1 expression, tumor proportion score (TPS) ⩾ 50% and with no EGFR or ALK genomic aberrations, and for metastatic NSCLC patients whose tumors express PD-L1 with TPS ⩾ 1% progressing on or after platinum-based chemotherapy.
The aim of this review is to present and discuss the clinical results obtained so far with the anti-PD-1 pembrolizumab in advanced NSCLC.
Clinical data from phase I/II trials
KEYNOTE-001
KEYNOTE-001 was a large, international, phase I study designed to evaluate safety, pharmacokinetics, pharmacodynamics and antitumor activity of pembrolizumab in advanced melanoma and NSCLC patients. For NSCLC cohorts (parts C and F), 1143 patients were screened with 495 receiving at least one dose of pembrolizumab intravenously at different doses (2 mg per kilogram every 3 weeks, 10 mg per kilogram every 2 or 3 weeks) until intolerable toxicity, progression or investigator’s decision.33 Tumor characteristics included a prevalence of nonsquamous histology (81%) and the presence of EGFR and KRAS mutations in 15.5% and 26.1% of tumors, respectively; the presence of ALK gene rearrangement was detected in 2% of cases. In the overall population, objective response rate (ORR) was 19.4% with disease stabilization rate reaching 21.8%. According to previous therapies, ORR was 18% and 24.8% in previously treated and treatment-naïve patients, respectively and it did not differ significantly among patients treated with different dose/schedules and histologic subtypes, whereas response was more likely in smokers (22.5% versus 10.3% in never smokers). Median progression-free survival (PFS) was 3.7 [95% confidence interval (CI) 2.9–4.1] months, 3.0 (2.2–4.0) months and 6.0 (4.1–8.6) months in the overall population, pretreated and untreated patients, respectively. Median OS was 12.0 (9.3–14.7) months, 9.3 (8.4–12.4) months and 16.2 (16.2–not reached) months for the overall, pretreated and untreated patients, respectively. Furthermore, at the time of data cutoff analysis, 84.4% of responding patients had not progressed, with a median duration of response of 12.5 (1.0–23.3) months in the total population, 10.4 (1.0–10.4) months in the previously treated patients and 23.3 (1.0–23.3) months in untreated patients. Among 1143 screened patients, 824 were evaluable for PD-L1 expression and its positivity (TPS ⩾ 1%) was detected in 60.8% of them with a strong positivity (TPS ⩾ 50%) observed in 23.2% of all patients (22.7% in pretreated patients and 24.9% in treatment-naïve patients). Interestingly, a significant correlation between TPS and treatment outcome was observed, specifically in terms of ORR, PFS and OS, in patients whose tumors showed a TPS ⩾ 50% when compared with those expressing a TPS of 1–49% or <1% (Table 1).
Table 1.
Updated analysis of clinical outcomes for patients with advanced NSCLC enrolled in the KEYNOTE-001 study.
| Treatment-naïve (n = 101) |
Previously treated (n = 449) |
|
|---|---|---|
| Median OS (95% CI), months | ||
| PD-L1 TPS ⩾ 1% | 22.1 (16.7–27.2) | 11.3 (8.3–14.0) |
| TPS ⩾ 50% | NR (22.1–NR) | 15.4 (10.6–18.5) |
| TPS 1–49% | 19.5 (10.7–22.2) | 8.2 (6.0–12.7) |
| TPS < 1% | 14.7 (3.4–NR) | 8.6 (5.5–12.0) |
| Squamous | 15.6 (6.0–21.0) | 14.7 (10.4–18.4) |
| TPS ⩾ 50% | // | 14.0 (8.0–NR) |
| TPS ⩾ 1% | // | 14.0 (8.3–17.9) |
| TPS < 1% | // | 14.7 (1.2–18.4) |
| Nonsquamous | 26.3 (22.0–NR) | 9.4 (7.3–12.6) |
| TPS ⩾ 50% | // | 15.4 (9.9–18.8) |
| TPS ⩾ 1% | // | 10.5 (7.1–13.7) |
| TPS < 1% | // | 8.6 (5.5–10.6) |
| Current/former smoker | 22.0 (16.7–27.2) | 12.2 (9.2–14.3) |
| TPS ⩾ 50% | // | 15.7 (11.1–NR) |
| TPS ⩾ 1% | // | 13.2 (9.4–15.6) |
| TPS < 1% | // | 8.6 (4.9–13.3) |
| Never smoker | NR (16.2–NR) | 7.6 (5.9–12.1) |
| TPS ⩾ 50% | // | 8.2 (4.9–17.3) |
| TPS ⩾ 1% | // | 7.3 (5.1–13.7) |
| TPS < 1% | // | 9.1 (4.2–21.3) |
| EGFR wildtype | // | 12.1 (9.1–14.3) |
| TPS ⩾ 50% | 15.7 (11.1–NR) | |
| TPS ⩾ 1% | 13.2 (9.2–15.4) | |
| TPS < 1% | 9.1 (5.8–13.6) | |
| EGFR mutant | // | 6.0 (4.6–9.9) |
| TPS ⩾ 50% | 6.5 (2.0–13.7) | |
| TPS ⩾ 1% | 6.5 (4.4–12.6) | |
| TPS < 1% | 5.7 (2.2–NR) | |
| ORR (%) | ||
| TPS ⩾ 50% | 58.3 | 38.3 |
| TPS 1–49% | 17.4 | 12.9 |
| TPS < 1% | 10 | 9.9 |
| Median PFS (95% CI), months | ||
| TPS ⩾ 50% | 12.5 (6.2–NR) | 4.3 (2.3–8.3) |
| TPS 1–49% | 4.2 (3.1–6.4) | 2.4 (2.1–3.4) |
| TPS < 1% | 3.5 (2.1–19.0) | 2.1 (2.0–3.0) |
CI, confidence interval; EGFR, epidermal growth factor receptor; NR, not reached; NSCLC, non-small cell lung cancer; ORR, objective response rate; OS, overall survival; PD-L1, programmed death-ligand 1; PFS, progression-free survival; TPS, tumor proportion score.
The most common treatment-related adverse events (AEs) were fatigue (19.4%), pruritus (10.7%), decreased appetite (10.5%), rash (9.7%), arthralgia (9.1%), diarrhea (8.1%), nausea (7.5%), hypothyroidism (6.9%). A total of 47 (9.5%) patients developed treatment-related AEs of grade 3–5, including pneumonitis (9 cases, 1.8%) with 1 fatal event. Based on these results, on October 2015 the US FDA granted accelerated approval for pembrolizumab for the treatment of patients with advanced NSCLC expressing PD-L1 and progressed after a previous platinum-based chemotherapy or targeted therapy, if appropriate. The approved dose schedule is 2 mg per kilogram of body weight every 3 weeks. The decision to approve this dose was based on several considerations. First, there are no significant differences in terms of outcome between the different doses and schedules investigated in KEYNOTE-001 trial, confirmed also from KEYNOTE-010 trial later. Second, it is plausible that a lower dose has the same antitumor activity, due to mechanism of action of the drug. Third, the exposure-response relationships for efficacy and safety for pembrolizumab across doses of 2 mg/kg to 10 mg/kg do not differ, as recently reported.34
Long-term OS data of the KEYNOTE-001 study have been presented at the 2016 American Society of Clinical Oncology (ASCO) Annual Meeting (Table 1).35 At a median follow up of 23.1 months, it was confirmed an impressive benefit in OS from pembrolizumab among PD-L1-positive advanced NSCLC patients. Median OS in the overall population was 22.1 months for treatment-naïve patients and 10.6 months for pretreated patients, with a 2-year OS rate of 44.5% and 30.4%, respectively. Patients with PD-L1 strongly positive tumors (TPS ⩾ 50%) had the longest survival, with a median OS in treatment-naïve patients not reached and of 15.4 months in the previously treated patients. In this subgroup, the 2-year survival rate was 60.6% for treatment-naïve and 38% for pretreated patients. This analysis confirmed that the lack of PD-L1 expression was predictive of a poor benefit from pembrolizumab. Treatment outcomes in different subgroups are reported in Table 1.
KEYNOTE-010
KEYNOTE-010 was a randomized, open-label, phase II/III trial which enrolled 1034 patients with pretreated advanced NSCLC with PD-L1 expression ⩾1% of tumor cells. Patients were assigned to receive pembrolizumab 2 mg/kg (n = 345, arm A) or 10 mg/kg (n = 346, arm B) every 3 weeks or docetaxel 75 mg/sqm (n = 343, arm C) every 3 weeks (Table 2).36 Primary endpoints were OS and PFS both in the overall population and in patients whose tumors expressed a PD-L1 TPS ⩾ 50%. Tumor characteristics included a prevalence of nonsquamous histology (70%) and the presence of an EGFR mutation and ALK gene translocation in 8.3% and 0.7% of tumors, respectively. Out of 1034 enrolled patients, 442 (42.7%) had tumors with PD-L1 TPS ⩾ 50% and of these, 139 were assigned to pembrolizumab 2 mg/kg, 151 to pembrolizumab 10 mg/kg and the remaining 152 to docetaxel.
Table 2.
KEYNOTE-010 study: clinical outcomes of pembrolizumab versus docetaxel.
| Total population | Pembro 2 mg/kg n = 345 |
Pembro 10 mg/kg n = 346 |
Docetaxel n = 343 |
|---|---|---|---|
| mOS (95% CI), months | 10.4 (9.4–11.9) | 12.7 (10.0–17.3) | 8.5 (7.5–9.8) |
| HR (95% CI) | 0.71 (0.58–0.88) | 0.61 (0.49–0.75) | – |
| mPFS (95% CI), months | 3.9 (3.1–4.1) | 4.0 (2.7–4.3) | 4.0 (3.1–4.2) |
| HR (95% CI) | 0.88 (0.74–1.05) | 0.79 (0.66–0.94) | – |
| ORR, % (95% CI) | 18 (14.1–22.5) | 18.5 (14.5–23.0) | 9.3 (6.5–12.9) |
| mDOR (range), months | NR (4.2–10.5) | NR (4.2–12.5) | 6 (2.7–6.1) |
| Ongoing response (%) | 80.6 | 75 | 59.4 |
| TPS ⩾ 50% population | Pembro 2 mg/kg n = 139 |
Pembro 10 mg/kg n = 151 |
Docetaxel n = 152 |
| mOS (95% CI), months | 14.9 (10.4–NR) | 17.3 (11.8–NR) | 8.2 (6.4–10.7) |
| HR (95% CI) | 0.54 (0.38–0.77) | 0.50 (0.36–0.70) | – |
| mPFS (95% CI), months | 5.0 (4.0–6.5) | 5.2 (4.1–8.1) | 4.1 (3.6–4.3) |
| HR (95% CI) | 0.59 (0.44–0.78) | 0.59 (0.45–0.78) | – |
| ORR, % (95% CI) | 30.2 (22.7–38.6) | 29.1 (22.0–37.1) | 7.9 (4.1–13.4) |
| mDOR (range), months | NR (4.2–10.4) | NR (4.4–12.6) | 8 (2.6–8.3) |
| Ongoing response (%) | 88.1 | 79.5 | 58.3 |
| TPS 1–49% population | Pembro 2 mg/kg n = 205 |
Pembro 10 mg/kg n = 195 |
Docetaxel n = 191 |
| mOS (95% CI), months | 9.4 (8.7–10.5) | 10.8 (8.9–13.3) | 8.6 (7.8–9.9) |
| HR (95% CI) | 0.79 (0.61–1.04) | 0.71 (0.53–0.94) | – |
| mPFS (95% CI), months | 3.1 (2.1–3.8) | 2.3 (2.1–4.0) | 3.9 (2.5–4.3) |
| HR (95% CI) | 1.07 (0.85–1.34) | 0.99 (0.78–1.25) | – |
| ORR, % (95% CI) | 10 (6.0–15.0) | 10 (6.0–15.0) | 10 (6.0–16.0) |
| mDOR (range), months | 46 (9+ to 87+) | 45 (13+ to 74+) | 26 (6+ to 31) |
| Ongoing response (%) | 65 | 65 | 35 |
CI, confidence interval; HR, hazard ratio; mDOR, median duration of response; mOS, median overall survival; mPFS, median progression-free survival; NR, not reached; ORR, objective response rate; Pembro, pembrolizumab; TPS, tumor proportion score.
In the overall population, median OS was 10.4 months for patients in arm A, 12.7 months in arm B and 8.5 months for patients enrolled in the docetaxel control arm. In patients with a PD-L1 TPS ⩾ 50% of tumor cells treated with pembrolizumab, median OS was significantly longer when compared with those treated with docetaxel (Table 2). OS benefit was similar between the two arms with pembrolizumab, both in PD-L1 TPS ⩾ 50% [hazard ratio (HR) 1.12, 95% CI 0.77–1.62] and in the overall population (HR 1.17, 95% CI 0.94–1.45). Conversely, OS for pembrolizumab, regardless of the dose, was significantly higher than that obtained in docetaxel arm (Table 2). In the subgroup OS analysis, pembrolizumab provided a statistically significant benefit over docetaxel across all patient subgroups, with the exception of EGFR mutation and squamous subgroups.
In the overall population, there was no statistically significant difference in terms of median PFS among the three arms, whereas in patients with a PD-L1 TPS ⩾ 50% of tumor cells, median PFS was significantly longer for arm A (HR 0.59, p = 0.0001) and arm B (HR 0.59, p < 0.0001). PFS benefit was similar between the two arms with pembrolizumab, both in PD-L1 TPS ⩾ 50% (HR 1.01, 95% CI 0.75–1.36) and in the overall population (HR 1.09, 95% CI 0.92–1.30).
ORR was significantly higher for patients treated with pembrolizumab (arms A and B) compared with docetaxel (arm C), both in the overall population and in the PD-L1 TPS ⩾ 50% subgroup. In this regard, in the overall population, ORR was higher in both pembrolizumab arms over the docetaxel arm (p = 0.005 and 0.002 for arm A and B, respectively versus docetaxel). The highest objective responses were seen in PD-L1 TPS ⩾ 50% subgroup treated with pembrolizumab (p < 0.0001 for each arm versus docetaxel). Treatment with pembrolizumab was overall well tolerated and toxicity was that expected, as well as manageable. Treatment-related AEs, occurring in at least 10% of patients, were registered in 65% of all patients treated with pembrolizumab, with an overlapping incidence using the two different doses, and 81.2% with docetaxel. AEs of grade 3–5 had a higher incidence in the docetaxel arm (35%) when compared with pembrolizumab 2 mg/kg (13%) and 10 mg/kg (16%). Immune-related AEs were reported in 19.5% of all patients treated with pembrolizumab and the most relevant were hypothyroidism (Table 3). A post hoc analysis assessed the efficacy of pembrolizumab in patients with PD-L1 TPS of 1–49% enrolled in KEYNOTE-010 and results were presented at the 2016 ASCO Annual Meeting.37 A total of 591 (57.2%) out of 1034 enrolled patients had tumors expressing a TPS of 1–49%: in this population, pembrolizumab provided a significant prolonged survival when compared with docetaxel. Median OS was 9.4 months (arm A), 10.8 (arm B) and 8.6 months with docetaxel (arm C), with no clear difference between the two pembrolizumab arms (HR 1.15, 95% CI 0.88–1.52). No difference was reported in terms of PFS and ORR across all treatment arms, whereas median duration of response (DOR) was longer for patients treated with pembrolizumab over docetaxel (Table 2). Furthermore, pembrolizumab improved OS also in nonresponding patients and this benefit seemed to be restricted to patients who remained on study for at least 18 weeks.
Table 3.
Drug-related AEs that occurred in ⩾2% of patients enrolled in the KEYNOTE-001, 010 and 024 studies.
| AEs | KEYNOTE-001 n = 495 pts |
KEYNOTE-010 n = 682 pts |
KEYNOTE-024 n = 154 pts |
|||
|---|---|---|---|---|---|---|
| Any grade (%) | Grade 3–5 (%) | Any grade (%) | Grade 3–5 (%) | Any grade (%) | Grade 3–5 (%) | |
| Fatigue | 96 (19.4) | 4 (0.8) | 95 (13.9) | 10 (1.4) | 16 (10.4) | 2 (1.3) |
| Pruritus | 53 (10.7) | 0 | 57 (8.3) | 0 | – | – |
| Decreased appetite | 52 (10.5) | 5 (1.0) | 79 (11.5) | 4 (0.5) | 14 (9.1) | 0 |
| Rash | 67 (13.5) | 1 (0.2) | 87 (12.7) | 3 (0.4) | 6 (3.9) | 6 (3.9) |
| Arthralgia | 45 (9.1) | 2 (0.4) | 32 (4.7) | 2 (0.3) | – | – |
| Diarrhea | 40 (8.1) | 3 (0.6) | 46 (6.7) | 2 (0.3) | 22 (14.3) | 6 (3.9) |
| Nausea | 37 (7.5) | 4 (0.8) | 68 (9.9) | 3 (0.4) | 15 (9.7) | 0 |
| Hypothyroidism | 34 (6.9) | 1 (0.2) | 48 (7.0) | 0 | 14 (9.1) | 0 |
| AST/ALT increased | 26 (5.2) | 5 (1.0) | 41 (6.0) | 5 (0.7) | – | – |
| Asthenia | 24 (4.8) | 5 (1.0) | 39 (5.7) | 3 (0.4) | – | – |
| Anemia | 21 (4.2) | 0 | 24 (3.5) | 4 (0.5) | 8 (5.2) | 3 (1.9) |
| Dyspnoea | 21 (4.2) | 19 (3.8) | 21 (3.0) | 4 (0.5) | – | – |
| Pyrexia | 21 (4.2) | 3 (0.6) | 24 (3.5) | 1 (0.1) | 16 (10.4) | 0 |
| Weight decreased | 19 (3.8) | 2 (0.4) | 15 (2.1) | 1 (0.1) | – | – |
| Dry skin | 18 (3.6) | 0 | 18 (2.6) | 0 | – | – |
| Pneumonitis | 18 (3.6) | 9 (1.8) | 26 (3.8) | 12 (1.7) | 9 (5.8) | 4 (2.6) |
| Vomiting | 14 (2.8) | 3 (0.6) | 25 (3.6) | 1 (0.1) | 4 (2.6) | 1 (0.6) |
| Myalgia | 13 (2.6) | 0 | 19 (2.7) | 0 | 3 (1.9) | 0 |
| Constipation | 10 (2.0) | 2 (0.4) | 23 (3.3) | 0 | 6 (3.9) | 0 |
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
KEYNOTE-021
KEYNOTE-021 was a multicohort phase I/II trial combining pembrolizumab with platinum-doublet chemotherapy, immunotherapy or EGFR-targeted therapy in patients with locally advanced or metastatic NSCLC. Cohorts A, B and C evaluated the combination of pembrolizumab with platinum-doublet chemotherapy with the aim to identify recommended doses for subsequent trials and preliminary results were presented at the 2015 ASCO Annual Meeting and updated in 2016.38 Overall, 74 patients (25 in cohort A, 25 in B and 24 in C) have been treated, of whom 50% were male, 90.5% current or former smokers, 71.6% with adenocarcinoma. PD-L1 tumor positivity (TPS ⩾ 1%) was detected in 68.9% of patients, of whom 33.7% with a TPS ⩾ 50%, whereas PD-L1 was negative in 29.7% of patients. In the whole population ORR was 57%. In cohort A (pembrolizumab 2 mg/kg or 10 mg/kg + carboplatin AUC 6 + paclitaxel 200 mg/m2 every 3 weeks for four courses followed by pembrolizumab 2 mg/kg or 10 mg/kg every 3 weeks up to 2 years) 13 out of 25 patients obtained a partial response with an ORR of 52%. In cohort B (pembrolizumab 2 mg/kg or 10 mg/kg + carboplatin AUC 6 + paclitaxel 200 mg/m2 + bevacizumab every 3 week up to 4 courses followed by maintenance with pembrolizumab 2 mg/kg or 10 mg/kg plus bevacizumab 15 mg/kg every 3 weeks) 12 out of 25 patients had a partial response with an ORR of 48%. In cohort C (pembrolizumab 2 mg/kg or 10 mg/kg + carboplatin AUC 6 + pemetrexed 500 mg/m2 every 3 weeks up to 4 cycles followed by maintenance with pemetrexed 500 mg/m2 + pembrolizumab 2 mg/kg or 10 mg/kg every 3 weeks) there was 1 complete response and 16 partial responses with an ORR reaching 71%. According to PD-L1 status, for tumors with TPS ⩾ 50% ORR was 60% (56% in cohort A, 50% in cohort B and 75% in cohort C); for TPS ⩾ 1% 57% (53% in cohort A, 50% in cohort B and 69% in cohort C), whereas for TPS < 1% ORR was 54% (44% in cohort A, 40% in cohort B and 75% in cohort C). With a median follow-up duration of 12 months, median PFS was 10.3 (3.7–not reached) months in cohort A, not reached (4.1– not reached) in cohort B and 10.2 (6.3–15.2) months in cohort C.
One dose-limiting toxicity of grade 3 was reported in cohort C with pembrolizumab at the dose of 10 mg/kg, leading to treatment discontinuation. AEs of grade 3–4 were reported in 56% of patients in cohort A and 67% of patients in cohort C, without correlation between pembrolizumab doses. Cohort B seemed to be associated with more toxicity than the other combinations (AEs of grade ⩾3 were 71%), including three patients who discontinued treatment due to a drug-related AE of grade 3 (pneumonitis, drug hypersensitivity and autoimmune colitis). Immune-related AEs were reported in four (16%) patients in cohort A (one case of rash popular of grade 3), nine (38%) patients in cohort B (one case each of pneumonitis and pancreatitis of grade 3) and seven (29%) patients in cohort C (one case each of colitis and toxic epidermal necrolysis of grade 3).
The combination of pembrolizumab and ipilimumab was investigated in cohort D (dose finding) and cohort H (dose expansion) of the KEYNOTE-021 trial.39 In cohort D doses of pembrolizumab and ipilimumab were reduced to 2 mg/kg and 1 mg/kg, respectively, based on emerging toxicity data of ipilimumab at higher doses from other studies. In cohort H, ipilimumab and pembrolizumab doses were set at 1 mg/kg and 2 mg/kg, respectively, administered every 3 weeks for 4 cycles followed by pembrolizumab 2 mg/kg every 3 weeks until disease progression or up to 2 years. At the time of data analysis, 51 patients received the combination: 45 patients were treated with pembrolizumab 2 mg/kg plus ipilimumab 1 mg/kg in cohort D (12 patients) and cohort H (33 patients), whereas the first 6 patients in cohort D received pembrolizumab 10 mg/kg plus ipilimumab 3 mg/kg or pembrolizumab 10 mg/kg plus ipilimumab 1 mg/kg (3 for each dose combination). Among the 51 patients, 51% were male, 74.5% current or former smokers, 70.5% receiving more than 1 previous therapy and 80.4% had adenocarcinoma. PD-L1 TPS positivity (⩾1%) was detected in 29 (56.8%) cases, of which 10 (19.6%) with strong TPS (⩾50%), whereas the remaining 22 (43.1%) cases were negative for PD-L1 expression. Among 44 patients evaluable for response, 11 had a response (ORR 25%, similar to that of pembrolizumab alone) with a disease control rate (DCR) reaching 63.6% and a median DOR of 13.8 months. There was no correlation between PD-L1 status and outcome. With a median follow up of 7 months, median PFS and OS was 6.1 (95% CI 1.5–16.6) and 16.6 (6.1–not reached) months, respectively. The combination treatment showed a significant toxicity profile; treatment-related AEs were reported in 42 (93%) of the 45 patients receiving pembrolizumab 2 mg/kg and ipilimumab 1 mg/kg every 3 weeks. Serious AEs were reported in 49% of the patients, leading to a treatment discontinuation in 9% of them. Immune-related AEs of any grade were described in 40% of patients (one case each of colitis and pneumonitis of grade 3).
KEYNOTE-024
Results of the KEYNOTE-024 trial have been recently published.40 KEYNOTE-024 was a randomized, open-label, phase III study comparing pembrolizumab with SOC platinum-based chemotherapy in patients with previously untreated metastatic NSCLC with PD-L1 TPS ⩾ 50%. The primary objective of this study was to demonstrate a superiority of pembrolizumab in terms of PFS when compared with SOC. Secondary endpoints were OS, ORR and safety. Patients were assigned, in a 1:1 ratio, to receive pembrolizumab 200 mg at fixed dose intravenously every 3 weeks until disease progression or up to 2 years or standard chemotherapy (carboplatin + paclitaxel, pemetrexed + carboplatin/cisplatin, gemcitabine + carboplatin/cisplatin up to 4–6 cycles, followed by maintenance with pemetrexed, for nonsquamous histology only). For all patients allocated in the arms of SOC, crossover to pembrolizumab was allowed at the time of documented progressive disease. Overall, two interim analyses were planned to evaluate the superiority of pembrolizumab over SOC with respect to ORR and PFS. At the time of the second interim analysis, the primary objective of the study was met, with pembrolizumab being significantly superior for both PFS and OS compared with standard chemotherapy. For this reason, the trial was stopped early to allow patients receiving chemotherapy to cross over to the pembrolizumab arm. Tumor samples from 1653 patients were evaluable for PD-L1 assessment with 500 (30.2%) being strongly positive for PD-L1 expression (TPS ⩾ 50%). Out of 500 patients, 305 were randomly allocated to receive pembrolizumab (n = 154) or platinum-based chemotherapy (n = 151). The median duration of the treatment was 7.0 and 3.5 months in the pembrolizumab arm and chemotherapy arm, respectively. A total of 43.7% of patients receiving chemotherapy crossed over to pembrolizumab at the time of disease progression. The difference in terms of PFS and OS in favor of pembrolizumab was statistically and clinically significant. The median PFS was longer in the pembrolizumab arm than in the chemotherapy arm (10.3 versus 6.0 months, respectively; HR 0.50, p < 0.001) and this benefit was seen in all subgroups of patients. Similarly, patients in the pembrolizumab arm had longer survival (HR 0.60, p = 0.005), higher response rates than chemotherapy (44.8% versus 27.8%, respectively) and longer DOR (median DOR: not reached versus 6.3 months; Table 4). In addition, pembrolizumab demonstrated a better toxicity profile. Treatment-related AEs of all grades were reported in 73.4% of patients in the pembrolizumab arm and in 90% of those enrolled in the chemotherapy group, whereas AEs of grade ⩾3 were 26.6% and 53.3%, respectively. The most common treatment-related AEs (of all grades) in the pembrolizumab arm were diarrhea (14.3%), pyrexia (10.4%) and fatigue (10.4%); those reported in the chemotherapy arm were anemia (44%), nausea (43.3%), fatigue (28.7%), decreased appetite (26%), neutropenia (22.7%) and vomiting (20%). Immune-related AEs were prevalent in the pembrolizumab arm (29.2% versus 4.7% in the control arm) and included thyroid dysfunction (16.8%), pneumonitis (5.8%) and infusion reaction (4.5%).
Table 4.
KEYNOTE-024 study: clinical outcomes of pembrolizumab versus first-line chemotherapy.
| Total population (TPS ⩾ 50%) |
Pembro 200 mg n = 154 |
First-line
chemotherapy n = 151 |
p |
|---|---|---|---|
| mPFS (95% CI), months | 10.3 (6.7–NR) | 6.0 (4.2–6.2) | <0.001 |
| HR (95% CI) | 0.50 (0.37–0.68) | ||
| mOS (95% CI), months | NR | NR | 0.005 |
| HR (95% CI) | 0.60 (0.41–0.89) | ||
| 6-month OS rate (%) (95% CI) |
80.2 (72.9–85.7) |
72.4 (64.5–78.9) |
|
| ORR, % (95% CI) | 44.8 (36.8–53.0) | 27.8 (20.8–35.7) | |
| mDOR (range), months | NR (1.9+ to 14.5+) | 6.3 (2.1+ to 12.6+) | |
+, ongoing response; CI, confidence interval; HR, hazard ratio; mDOR, median duration of response; mOS, median overall survival; mPFS, median progression-free survival; NR, not reached; ORR, objective response rate; Pembro, pembrolizumab; TPS, tumor proportion score.
Brain metastases and pembrolizumab activity
To date, the majority of clinical trials with immune-oncology agents do not allow enrollment of patients with brain metastases, unless previously treated, radiologically stable and not requiring a high dose of corticosteroids. Preliminary results of a nonrandomized, open-label, phase II trial of pembrolizumab for patients with untreated or progressive brain metastases from melanoma and NSCLC have been published.41 This trial screened 52 patients with untreated or progressive brain metastases (including metastases that were asymptomatic, of a maximum diameter ranging from 5 to 20 mm and not requiring corticosteroids) from melanoma (18 patients) and NSCLC (34 patients). A total of 36 patients were enrolled, of whom 18 with PD-L1 positive NSCLC. Of this cohort, 12 were female (67%), with adenocarcinoma (78%), who had received at least one previous systemic therapy (72%) and any locoregional therapy (56%) for central nervous system (CNS) disease. At a median follow-up time of 6.8 months, 6 (33%) out of 18 NSCLC patients had a response on brain metastases, including 4 complete responses and 2 partial responses (1 of these not confirmed). CNS radiological responses were durable (up to 7 months in one case) and concordant with a confirmed systemic response, whereas none of the patients who had lesions pretreated with whole brain radiotherapy or stereotactic radiosurgery showed a CNS response. AEs were consistent with those reported in previous trials with pembrolizumab and neurological AEs were uncommon and manageable.
Ongoing trials
KEYNOTE-042
KEYNOTE-042 is a randomized, open-label, phase III study comparing pembrolizumab with SOC platinum-based chemotherapy in patients with previously untreated, advanced or metastatic NSCLC with PD-L1 positive expression (>1%) (Table 5). The primary objective is OS in the PD-L1 TPS ⩾ 50% and all population groups. Secondary endpoints are PFS in the PD-L1 TPS ⩾ 50% subgroup and, safety and tolerability in the all population group. Patients will be randomized in a 1:1 ratio to receive pembrolizumab 200 mg every 3 weeks until disease progression or up to 2 years or investigator’s choice of SOC (carboplatin + paclitaxel or carboplatin + pemetrexed for a maximum of 6 cycles, followed by maintenance with pemetrexed, for nonsquamous histology only). Enrollment is ongoing and it will continue until approximately 1240 patients are included.
Table 5.
Active ongoing trials of pembrolizumab in NSCLC.
| ClinicalTrials.gov ID | Phase | Treatment arm(s) | Description and objectives |
|---|---|---|---|
| First-line treatment | |||
| NCT01840579 | 1 | – Pembrolizumab – Pembrolizumab + platinum/pemetrexed – Pembrolizumab + CBDCA/paclitaxel – Pembrolizumab + CBDCA/nab-paclitaxel |
Advanced solid tumors and NSCLC – DLT |
| NCT02511184 | 1 | – Pembrolizumab + crizotinib | Advanced ALK+ nonsquamous NSCLC – DLT – ORR, DOR, TTR, PFS, PK |
| NCT02382406 | 1/2 | – Pembrolizumab + CBDCA/nab-paclitaxel | Stage IIIb/IV NSCLC – Safety, tolerability, RP2D, PFS, ORR – OS, PD-L1 expression |
| NCT02039674 | 1/2 | – Pembrolizumab + CBDCA/paclitaxel – Pembrolizumab + CBDCA/paclitaxel/bevacizumab – Pembrolizumab + CBDCA/pemetrexed – Pembrolizumab + ipilimumab – Pembrolizumab + erlotinib/gefitinib |
Stage IIIb/IV NSCLC – ORR, RP2D – OS, PFS, DOR |
| NCT02581943 | 2 | – Pembrolizumab – Pembrolizumab + low-dose CBDCA/paclitaxel |
Stage IIIb/IV NSCLC; PS 2; up to 2 prior
therapies – DOR, ORR, PFS, OS, immune markers |
| NCT02591615 | 2 | – CBDCA/paclitaxel or pemetrexed – Pembrolizumab CBDCA/paclitaxel or pemetrexed |
Stage IV NSCLC – ORR – PFS, safety and tolerability |
| NCT02578680 | 3 | – Platinum/pemetrexed – Pembrolizumab + platinum/pemetrexed |
Advanced nonsquamous NSCLC – PFS per RECIST 1.1 – ORR, OS, PFS per irRECIST |
| NCT02775435 | 3 | – CBDCA + paclitaxel/nab-paclitaxel – Pembrolizumab → CBDCA + paclitaxel/nab-paclitaxel |
Advanced squamous NSCLC – PFS, OS – ORR |
| NCT02220894 | 3 | – Pembrolizumab – SOC (platinum-based chemotherapy) |
PD-L1-positive, stage IIIb/IV
NSCLC – OS – PFS |
| Maintenance treatment | |||
| NCT02564380 | 2 | – Pembrolizumab – Placebo |
Stage IV squamous NSCLC after platinum-based
CT – PFS – ORR, OS, PD-L1 expression, safety, QoL |
| >First-line treatment | |||
| NCT02364609 | 1 | – Pembrolizumab + afatinib | Advanced/metastatic NSCLC with EGFR activating mutations
after PD on erlotinib – Safety and tolerability – ORR, DCR, PFS |
| NCT02475213 | 1 | – Pembrolizumab + MGA271 | Advanced solid tumors and NSCLC expressing
B7-H3 – Toxicity – PK, antitumor activity |
| NCT02443324 | 1 | – Pembrolizumab + ramucirumab | Advanced NSCLC treated with 0–3 prior lines of
therapy – DLT – ORR, DOR, TTR, PFS, OS, PK |
| NCT02451930 | 1 | – Pembrolizumab + necitumumab | Stage IV NSCLC pretreated with a previous platinum-based
CT – DLT, ORR – PK, DCR, DOR, PFS, OS |
| NCT02437136 | ½ | – Pembrolizumab + entinostat | Advanced NSCLC previously untreated and treated with
PD-1/PD-L1 antibodies – AE, ORR – CBR, PFS, OS, DOR, TTR |
| NCT02422381 | ½ | – Pembrolizumab + gemcitabine | Advanced NSCLC – Toxicity – PFS, OS, response |
| NCT02638090 | 1/2 | – Pembrolizumab – Pembrolizumab + vorinostat |
Advanced/metastatic pretreated NSCLC but immunotherapy
naïve – MTD – PFS, ORR |
| NCT02178722 | ½ | – Pembrolizumab + epacadostat | Advanced NSCLC after at least one platinum-based
CT – DLT, ORR – PFS, safety and tolerability, OS |
| NCT02501096 | ½ | – Pembrolizumab + lenvatinib | Advanced NSCLC – MTD, ORR, DLT – PFS, OS, DOR, CBR, PK |
| NCT02574598 | 2 | – Docetaxel – Docetaxel + pembrolizumab |
Advanced PD-L1+ NSCLC after 1 platinum-based
CT – ORR |
| NCT02546986 | 2 | – Pembrolizumab + placebo – Pembrolizumab + CC-486 |
Stage IIIb/IV NSCLC after 1 prior platinum-based
CT – PFS – ORR, DCR, OS, PK, AE |
| NCT02492568 | 2 | – Pembrolizumab – SBRT→pembrolizumab |
Stage IV NSCLC after at least 1 prior platinum-based
CT – ORR – DCR, PFS, OS, toxicity |
| NCT02085070 | 2 | – Pembrolizumab | Untreated brain metastasis in metastatic
NSCLC – ORR – Brain response rate |
| NCT02681549 | 2 | – Pembrolizumab + bevacizumab | Untreated brain metastasis in metastatic
NSCLC – Brain metastasis response rate – ORR, PFS, safety and toxicity |
AE, adverse event; ALK, anaplastic lymphoma kinase; CBDCA, carboplatin; CBR, clinical benefit rate; CT, chemotherapy; DLT, dose-limiting toxicities; DOR, duration of response; EGFR, epidermal growth factor receptor; irRECIST, immune-related Response Evaluation Criteria in Solid Tumors; MTD, maximum tolerated dose; NSCLC, non-small cell lung cancer; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PK, pharmacokinetics; QoL, quality of life; RECIST, Response Evaluation Criteria in Solid Tumors; RP2D, recommended phase 2 dose; SBRT, stereotactic body radiation therapy; SOC, standard of care; TTR, time to response.
KEYNOTE-189
KEYNOTE-189 is a randomized, double-blind, phase III study designed to compare the efficacy and safety of pembrolizumab plus platinum-doublet chemotherapy versus platinum-doublet chemotherapy alone as first-line in patients with advanced or metastatic nonsquamous NSCLC, regardless of PD-L1 expression (Table 5). The primary endpoint is PFS. Secondary endpoints are OS, PFS in PD-L1 TPS ⩾ 1% population, ORR, DOR, safety and tolerability. The study will enroll approximately 570 patients in a 2:1 ratio to receive pembrolizumab 200 mg intravenously every 3 weeks plus pemetrexed and carboplatin/cisplatin for 4 cycles followed by pembrolizumab plus pemetrexed as maintenance every 3 weeks or placebo in combination with the same chemotherapy regimens. Patients will be stratified according to smoking status (current/former versus never), platinum compound (cisplatin versus carboplatin) and PD-L1 TPS (⩾1% versus <1%). Pembrolizumab will be continued until disease progression, intolerable toxicity, investigator’s decision or up to 2 years; whereas pemetrexed will be continued until intolerable toxicity, disease progression or investigator’s decision. For all patients allocated in the arms of SOC, crossover to pembrolizumab is allowed at the time of documented progressive disease. This study is currently recruiting participants.
KEYNOTE-407
KEYNOTE-407 is a randomized, double-blind, phase III study designed to compare the efficacy and safety of pembrolizumab plus platinum-doublet chemotherapy with platinum-doublet chemotherapy alone as first-line in patients with advanced or metastatic squamous NSCLC, regardless of PD-L1 expression (Table 5).
The primary endpoint is PFS. Secondary endpoints are OS, PFS in PD-L1 TPS ⩾ 1% population, ORR, DOR, safety and tolerability. The study will enroll approximately 560 patients who will be randomized in a 2:1 ratio to receive pembrolizumab 200 mg intravenously every 3 weeks in combination with standard chemotherapy (carboplatin plus nab-paclitaxel) versus chemotherapy alone (carboplatin plus nab-paclitaxel). This study is currently recruiting participants.
KEYNOTE-598
KEYNOTE-598 is a phase III randomized trial comparing pembrolizumab 200 mg flat dose every 3 weeks with pembrolizumab in combination with ipilimumab 1 mg/kg every 6 weeks in NSCLC (any histology) patients with TPS ⩾ 50%. The trial is about to start and it is aiming to enroll 542 patients.
Predictive factors
Despite the impressive clinical activity and durable responses seen in patients with advanced NSCLC treated with ICPIs,42 a substantial proportion of patients do not respond, showing primary resistance to these drugs.43 As such, the selection of patients most likely to benefit from immunotherapy is crucial in order to avoid exposure to potentially toxic and ineffective drugs as well as to prevent inappropriate allocation of health resources. The identification of predictive biomarkers in order to identify potential responders is currently one of the most active research area. Given the mechanism of action of ICPIs, PD-L1 expression, explored in the majority of cases by immunohistochemistry (IHC), has emerged as the logical biomarker to adopt for the molecular selection of patients with advanced NSCLC receiving PD-1/PD-L1 inhibitors (Figures 1 and 2). Different studies have tried to determine whether the efficacy of these antibodies correlated with PD-L1 expression in the tumor. In the phase I trial with nivolumab given across various solid tumors, including advanced NSCLC, only patients with PD-L1 positive tumor samples on immunohistochemical analysis (⩾5% tumor cells with PD-L1 expression) responded, whereas no objective response was observed in patients with PD-L1 negative tumor.22 Likewise, in the phase I KEYNOTE-001 trial, assessing the safety and antitumor activity of pembrolizumab in advanced NSCLC patients, responses correlated with PD-L1 expression by tumor cells.33 The trial in fact was also designed to evaluate and validate a PD-L1 expression cutoff within the tumor or inflammatory T cells in the tumor stroma as predictive biomarker of tumor response to pembrolizumab. PD-L1 status was initially tested using a prototype IHC assay for study enrollment and its positivity was defined as membranous staining in at least 1% of tumor cells, infiltrating tumor inflammatory cells or positive staining of immune cells in the tumor stroma. Subsequently, a companion diagnostic assay was developed using a modified version of the prototype assay (DAKO EnVision FLEX + HRP-Polymer kit and 22C3 antibody clone, Dako Carpinteria, CA) to determine the optimal PD-L1 expression level cutoff. A TPS indicated the percentage of tumor cells expressing any membranous staining and the optimal cutoff, set at ⩾50% of tumor cells positive for PD-L1 expression, defined PD-L1 strongly positive tumors. A TPS ranging from 1% to 49% of tumor cell positivity defined PD-L1 weakly positive tumors, whereas a TPS < 1% designated tumors as PD-L1 negative. Among 1143 patients screened, 824 were evaluable for PD-L1 expression and its positivity (TPS ⩾ 1%) was detected in 60.8% of them. Furthermore, a PD-L1 strong positivity (TPS ⩾ 50%) was observed in 23.2% of all patients (22.7% in pretreated patients and 24.9% in treatment-naïve patients), 37.7% of tumor samples showed a PD-L1 weak positivity (TPS ranging 1–49%), whereas the prevalence of PD-L1 negative tumors (TPS < 1%) was 39.2%. There was a significant correlation between TPS and treatment outcome. Indeed, significant differences were demonstrated in terms of ORR, PFS and OS in patients whose tumors showed a TPS ⩾ 50% when compared with those expressing a TPS ranging 1–49% or <1%. In the validation cohort, patients with PD-L1 strong positivity had an ORR of 45.2% (43.9% in previously treated versus 50% in untreated patients) with a median PFS of 6.3 (95% CI 4.2–not reached) months (6.1 months in previously treated and 12.5 months in untreated patients) and OS not reached in overall subgroup population. In patients whose tumors showed a proportion score of 1–49%, ORR was 16.5%, with a median PFS of 4.1 (2.3–4.4) months and a median OS of 10.6 (7.3–not reached) months. Finally, ORR, PFS and OS were 10.7%, 4.0 (2.1–6.2) months and 10.4 (5.8–not reached) months, respectively, in patients with a proportion score <1%.
Figure 1.
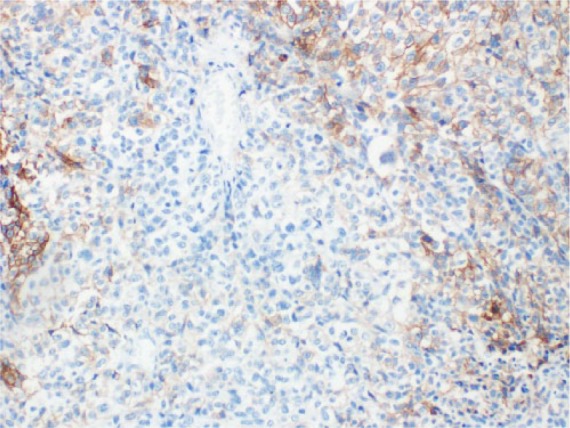
PD-L1 NSCLC IHC staining.
IHC, immunohistochemistry; NSCLC, non-small cell lung cancer; PD-L1, programmed death-ligand 1.
Figure 2.
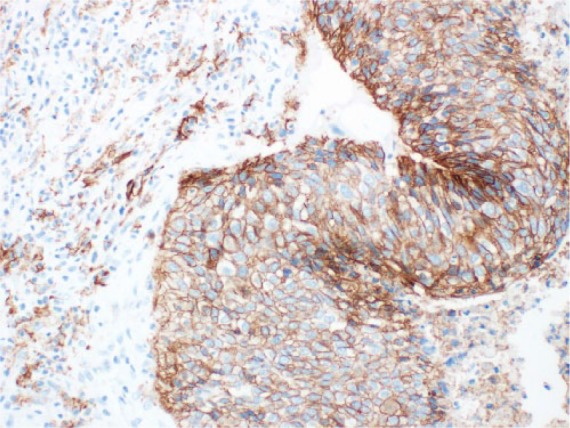
PD-L1 NSCLC IHC staining.
IHC, immunohistochemistry; NSCLC, non-small cell lung cancer; PD-L1, programmed death-ligand 1.
Finally, another phase I trial with atezolizumab involving different types of cancer showed a correlation between the level of PD-L1 expressed by the intratumoral immune infiltrate and clinical response.24 In a meta-analysis including 1475 patients treated with pembrolizumab, nivolumab or atezolizumab, a relevant differential effect in terms of activity according to PD-L1 expression on tumor cells status was observed, with PD-L1 positive tumors showing a significantly higher response rate (34% versus 19.9%).43 Nonetheless, such findings were not confirmed in other studies, with clinical activity being detected also in patients with PD-L1 negative tumors.44–49 Based on these controversial results, the potential predictive role of PD-L1 IHC assays is still an open issue for clinical research. Multiple factors have been considered in order to explain these inconsistent findings. First, a number of issues related to the standardization of PD-L1 testing have been raised, including the standardization of the laboratory method of PD-L1: various staining techniques are in fact available, using different antibodies for IHC and different levels of positivity.50 At least four monoclonal antibodies (namely clones 22-C3, 28-8, SP142 and SP263) have been developed as companion diagnostics of different PD-1 or PD-L1 inhibitors. The blueprint mixed industrial-academic project has compared these four antibodies on different staining platforms showing that three of the four reagents are actually comparable in terms of sensitivity, specificity and reproducibility.51 Moreover, the cutoff value used across the different studies in order to classify a tumor as PD-L1 positive has been extremely variable, with some studies using 1%, 5%, 10% or 25%.24,52 Thus, it may be difficult to determine a cutoff that defines a clinically significant positive and predictive value. In a recent meta-analysis of clinical trials with anti-PD-1/PD-L1 drugs, presented at the ASCO 2016 Annual Meeting by Khunger and colleagues, a 5% cutoff appeared to have maximum discrimination (OR 2.72, p = .01).53 PD-L1 expression between surgical samples and matched biopsy specimens can also be discordant: Ilie and colleagues showed an overall discordance rate of 48%, with biopsy specimens underscoring the PD-L1 expression in almost all cases compared with the surgical samples.54 This suggests that PD-L1 is heterogeneously expressed in tumor cells and examination of tissue microarrays may not be able to accurately describe the complex and various PD-L1 pattern of expression of the whole tumor.55 Another potential discrepancy is that some studies looked at PD-L1 expression by tumor cells, whereas others considered PD-L1 expressed by cells of the microenvironment, which could also be an important determinant of response: most of the results of the clinical trials have considered PD-L1 immunostaining just in cancer cells but there are no definitive data on the predictive role of PD-L1-positive macrophages in predicting response to ICPIs.56 Finally, PD-L1 testing is not a validated biomarker as yet because of its dynamic status: PD-L1 is in fact inducible, notably by interferon (IFN)-γ exposure.16 Therefore tumors that do not express this marker at baseline may become PD-L1 positive as a result of an inflammatory background. Consistent with this hypothesis, basal expression of PD-L1 does not seem to have a predictive role on response when anti-PD-1 is combined with anti-CTLA-4 antibody in melanoma patients. This could be explained by the inflammation induced by anti-CTLA-4 antibody which can induce PD-L1 expression in previously negative tumors.57 Changes in the PD-L1 level have also been observed across the clinical course of NSCLC treatment, raising the question whether it is necessary to repeat a biopsy before commencing an anti-PD-1/PD-L1 treatment.58 In addition, it seems that the IHC PD-L1 expression in tumor cells can fade over time, with older archival tissue showing a significant reduction in the percentage of immunoreactive cells for PD-L1. Such a finding, though it needs to be confirmed by other prospective studies, was limited to tumor cells whereas the immunoreactivity of tumor-infiltrating lymphocytes in paraffin tissue blocks seemed to be maintained over the time.59
PD-L1 expression remains a potential predictive biomarker for ICPIs but efforts are still needed to standardize its assessment and to identify a shared cutoff to define positivity. In addition, its clinical validation as a predictor of response requires large prospective studies stratified according to PD-L1 status. It is possible that IHC analysis of PD-L1 will not remain the gold-standard biomarker for response to PD-1/PD-L1 inhibitors in the future.
Other potential biomarkers however are now emerging. As ICPIs are active in tumors typically associated with high somatic mutation rates such as NSCLC, bladder cancer and melanoma,60,61 it has been suggested that the mutational landscape can play a key role in the response to PD-1/PD-L1 inhibitors. Rizvi and colleagues showed a correlation between mutation burden in NSCLC and response to PD-1 inhibition.56 Whole-exome sequencing of NSCLCs treated with pembrolizumab was performed, showing that a high somatic nonsynonymous mutation burden was associated with greater durable clinical benefit (defined as partial response or stable disease of at least 6 months), longer PFS and higher ORR. Moreover, patients who had a durable clinical benefit from pembrolizumab had also a higher neoantigen burden, formed as a consequence of somatic mutations. This is therefore in line with the hypothesis that recognition of neoantigens is important for the activity of anti-PD-1 therapy, allowing the tumor to become ‘immunogenic’; in other words, the presence of mutations in the tumor leads to the generation of neoantigens, which are not expressed by normal cells and that are likely to be recognized by the immune system. Finally, the observation that T cell response against a mutation-associated neoantigen can be detected in peripheral blood lymphocytes may be useful in order to develop blood-based assay to monitor the response to anti-PD-1/PD-L1 treatment. However, large-scale studies are required in order to establish a correlation between mutation load and ICPI activity, and to assess its potential role as predictive biomarker. Whole-exome sequencing however may not be routinely available in clinical practice and it is a high-cost and time-consuming analysis. A recent study has evaluated the potential role of comprehensive cancer-gene panels (CGPs) to estimate tumor mutational load, showing that the association between mutational load and clinical benefit to PD-1 inhibition is also observed when CGPs are used to estimate mutation burden. Of note, comprehensive gene panels including >300 cancer genes should be used, as predictive accuracy was proved to be lost when CGPs with less than 150 cancer genes were employed.62
Further studies are clearly needed to better understand the mechanism of action of ICPIs in vivo thus allowing the identification of other predictive biomarkers. So far, the patients who seem to benefit the most from anti-PD-1/PD-L1 agents are those with high PD-L1 expression or with immunogenic tumors or with a pre-existing immune response, that is, intratumoral immune infiltrate.
Discussion
The improved understanding of the role played by the immune system in tumor immunosurveillance has led to the discovery that tumors can escape immune response through dysregulation of coinhibitory or checkpoint signals; as such, lung cancer and its progression are no longer thought to be dependent only on molecular alterations within cancer cells but it is now clear that they are related also to the interaction between cancer cells and immune system.
ICPIs represent a significant breakthrough in the treatment of NSCLC. Durable responses have been reported in patients with NSCLC with pembrolizumab showing impressive results in terms of ORR and OS in both a large basket phase I trial and a randomized, controlled, phase II/III clinical trial.
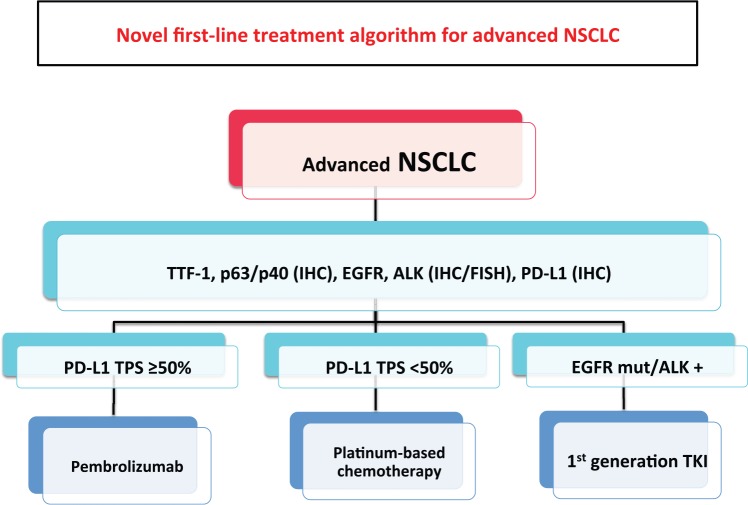
In the randomized phase III KEYNOTE-024 trial,40 pembrolizumab was superior to standard platinum-based chemotherapy in terms of both PFS and OS in previously untreated metastatic NSCLC with PD-L1 TPS ⩾ 50%. Moreover, pembrolizumab conferred higher response rates than chemotherapy (44.8% versus 27.8%, respectively) and longer DOR (median DOR: not reached versus 6.3 months) with also a better toxicity profile. The relevance of this study is such that these results are changing the current therapeutic algorithm of advanced NSCLC, adding a new standard first-line treatment option for patients with PD-L1 positive tumors (Figure 3). The identification of driver mutations such as EGFR mutation or ALK rearrangement dramatically changed the treatment scenario for patients whose tumor harbor these molecular aberrations; likewise, it is likely that in the near future metastatic NSCLC patients with PD-L1 overexpression will represent a new subgroup of patients with better outcomes.
Figure 3.
New first-line treatment algorithm for advanced NSCLC.
ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; FISH, fluorescent in situ hybridization; IHC, immunohistochemistry; NSCLC, non-small cell lung cancer; PD-L1, programmed death-ligand 1; TTF, thyroid transcription factor.
Different results however were obtained when the role of nivolumab was assessed in first-line treatment. The CheckMate-026 trial investigated the efficacy of first-line treatment with nivolumab compared with platinum-based doublet chemotherapy in patients with advanced NSCLC and PD-L1 positive tumors (defined as present in 1% or more tumor cells).63 The primary endpoint was PFS in patients with PD-L1 in 5% or more tumor cells.
The trial randomized 541 patients to receive either nivolumab 3 mg/kg intravenously every 2 weeks or the investigator’s choice of chemotherapy. The latter was chosen based on the histology; gemcitabine plus cisplatin or gemcitabine plus carboplatin or paclitaxel plus carboplatin for patients with squamous tumors and pemetrexed plus cisplatin or pemetrexed with carboplatin for those with nonsquamous histology. Nivolumab, however, did not meet its primary endpoint of PFS showing no benefit of nivolumab over standard chemotherapy.
The discordant results of KEYNOTE-024 and CheckMate-026 studies may reflect the different study design and, particularly, the selection in the first study only of patients with highly PD-L1 expressing tumors, as well as the differences in IHC PD-L1 analyses.
Overall, data from these studies assessing the role of ICPIs in NSCLC patients seem to confirm that there is a correlation between the degree of PD-L1 expression and the amount of clinical benefit. It is therefore now essential to evaluate newly diagnosed advanced NSCLC patients not only for driver mutations such as EGFR, ALK and ROS-1 but also for PD-L1 expression. However, there are still some outstanding questions that need to be further addressed. The first concern is about PD-L1 testing. To date, PD-L1 is the best studied immuno-oncology biomarker; however, there are many variables in the IHC assays including: (1) the time between sample collection and treatment with a PD-1 or PD-L1 inhibitor; (2) tumor cells, immune cells, as well as stroma cells can express PD-L1 with considerable heterogeneity within the tumor microenvironment itself; (3) PD-L1 expression can be induced by IFN-γ during disease progression and treatment, thus a tumor which is originally PD-L1 negative or with low levels of expression, can eventually become PD-L1 positive; (4) different PD-L1 antibodies have been used so far in each clinical trial and currently there is no validated antibody for IHC. At present, PD-L1 IHC using 22C3 antibody is the only US FDA-approved companion diagnostic for selecting NSCLC patients for pembrolizumab.
Another concern is related to the amount of histological tissue required in order to molecularly characterize the tumor, including PD-L1 assessment. As a matter of fact, in most of the patients the diagnosis is determined on cytological samples, obtained by transthoracic needle aspiration, transbronchial needle aspiration or thoracentesis, with no histological samples available. It is clear that such issues can affect the possibility of achieving a detailed molecular characterization which is currently based on the frequency of a specific mutation, with EGFR, KRAS and ALK being the most frequent aberrations tested. However, it is likely that PD-L1 assessment will have to be implemented, considered its incidence and its relevance in clinical practice, thus increasing the need for adequate and representative tissue samples. In addition, this issue is also exacerbated by the fact that PD-L1 testing has not been validated on cytological samples but on histological specimens.
Pembrolizumab proved to be active also in second-line treatment, based on the results of KEYNOTE-010:36 in this trial, pembrolizumab showed activity regardless of PD-L1 expression, but the median OS was significantly longer in patients with a PD-L1 TPS ⩾ 50% of tumor cells. These results seem to suggest that the lack of PD-L1 expression predicts for a poor benefit from pembrolizumab. On the contrary, nivolumab as well as atezolizumab are active in the second-line setting, regardless of PD-L1 expression. Indeed, the results of CheckMate-017 showed no statistically significant difference in ORR between PD-L1-positive and PD-L1-negative patients treated with nivolumab.25 However, in CheckMate-057 a significant difference in ORR between the two cohorts was observed (31% versus 9%, PD-L1 positive and PD-L1, respectively).26 The reason for such discordant results is to be sought in the different population enrolled in both trials, with CheckMate-057 recruiting patients with nonsquamous histology and CheckMate-017 trial enrolling patients with squamous histology. As a result, nivolumab has now been approved by the US FDA and EMA in all advanced pretreated NSCLC patient subgroups, regardless of PD-L1 expression while pembrolizumab received US FDA approval for pretreated patients, whose tumors express PD-L1 ⩾ 50% and by the EMA for patients whose tumors express PD-L1 > 1%. The US FDA recommended dose and schedule of pembrolizumab for NSCLC is 200 mg intravenously every 3 weeks.
The phase III OAK trial enrolled 1225 pretreated NSCLC who were stratified according to PD-L1 status, number of prior chemotherapy regimens and histology and randomized to atezolizumab (1200 mg every 3 weeks) or docetaxel (75 mg/m2 every 3 weeks).29 A preliminary analysis based on the data from 850 patients, showed a 27% improvement in OS in patients receiving atezolizumab (p = 0.0003), regardless of the PD-L1 expression levels and including patients with PD-L1 expression <1%. When patients were stratified according to their level of PD-L1 expression, the OS was 59% greater among patients in the highest tertile of PD-L1 expression who were treated with atezolizumab, compared with the same group treated with docetaxel (p < 0.0001). The results of this study are in line with those reported with nivolumab and pembrolizumab, showing that atezolizumab can have some degree of activity also in the subgroup of patients with low expression of PD-L1; however, to what extent patients with low PD-L1 expression can benefit from ICPIs remains unclear, arising the concern once again of excluding potentially responder patients whose tumor although do not express high levels of PD-L1 from immunotherapy.
Patients with oncogene-addicted NSCLC, such as those with activating EGFR mutations or ALK rearrangements are those who, according to subgroup analyses, appear to derive little or no benefit from ICPIs. A recent meta-analysis included three studies comparing ICPIs (nivolumab/pembrolizumab/atezolizumab) with docetaxel as second-line treatment in advanced NSCLC patients. The results showed that ICPIs prolonged OS in the overall study population and in the subgroup of EGFR wildtype patients but not in EGFR-mutant patients.64
Currently, there are many ongoing phase I studies evaluating the combination of anti-PD-1 and EGFR inhibitors, in particular pembrolizumab and afatinib in EGFR-mutated patients progressing on previous line treatment with erlotinib, pembrolizumab in combination with crizotinib in ALK-translocated NSCLC patients and nivolumab plus ceritinib in NSCLC ALK-translocated patients who progressed on crizotinib. The results of these studies are eagerly awaited as they will help elucidate the role of ICPIs in NSCLC patients harboring targetable genetic alterations such as EGFR mutations or ALK rearrangement.
Overall, only a minority of NSCLC patients have high levels of PD-L1 expression; in the attempt of increasing the immunogenicity of the so-called ‘immune-ignorant’ tumors, combinations of different immunotherapy strategies have been employed, associating PD-1/PD-L1 inhibitors with CTLA-4 inhibitors or vaccines, and trials assessing such combinations are currently ongoing. The results of the CheckMate-067 trial showed the such approach may be useful; in this double-blind, phase III study, patients with previously untreated advanced melanoma were randomly assigned to receive either nivolumab 3 mg/kg every 2 weeks or nivolumab 1 mg/kg every 3 weeks plus ipilimumab 3 mg/kg per every 3 weeks for four doses, followed by nivolumab 3 mg/kg every 2 weeks or ipilimumab 3 mg/kg every 3 weeks for four doses. The results of subgroup analyses showed that PD-L1-negative patients benefitted the most from the combination of nivolumab plus ipilimumab with a median PFS of 11.2 months compared with the 5.3 months of the nivolumab arm and the 2.8 months of the ipilimumab arm.55
Finally, immunotherapy approaches have been hypothesized to work best in the context of minimal residual disease, making the adjuvant setting the ideal clinical scenario, potentially improving the cure rate after surgery. As such, it will be interesting to analyze the results of the randomized phase III trial KEYNOTE-091/PEARLS in which patients with early stage NSCLC after resection and completion of standard adjuvant therapy are randomized to pembrolizumab versus placebo.
Conclusion
Pembrolizumab is an effective and well tolerated treatment for advanced NSCLC patients, showing durable responses and prolonged OS especially in patients with high expression of PD-L1, both when compared with first-line platinum-based chemotherapy and with docetaxel in the second-line setting and, therefore, will become the SOC best option for the treatment of these patients.
To date, PD-L1 expression is the only validated predictive biomarker for selecting pembrolizumab treatment. However, it is far from being the ideal biomarker and its role in predicting efficacy from ICPIs remains undefined due to conflicting results from randomized clinical trials. Better candidate biomarkers are therefore needed for the future.
Footnotes
Funding: This study was partially supported by an AIRC 2016 Investigator Grant #14214. Tissue samples were provided by the Cooperative Human Tissue Network which is funded by the National Cancer Institute. Other investigators may have received specimens from the same patients. Partially supported by AIRC Investigator Grant 2016 #19026.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Karim Rihawi, Policlinico S. Orsola-Malpighi, Bologna, Italy.
Francesco Gelsomino, Policlinico S. Orsola - Malpighi, Via Albertoni 15, 40138 Bologna (BO), Italy.
Francesca Sperandi, Policlinico S. Orsola-Malpighi, Bologna, Italy.
Barbara Melotti, Policlinico S. Orsola-Malpighi, Bologna, Italy.
Michelangelo Fiorentino, Policlinico S. Orsola-Malpighi, Bologna, Italy.
Laura Casolari, University of Parma, Parma.
Andrea Ardizzoni, Policlinico S. Orsola-Malpighi, Bologna, Italy.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 2. Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008; 26: 3543–3551. [DOI] [PubMed] [Google Scholar]
- 3. Delbaldo C, Michiels S, Syz N, et al. Benefits of adding a drug to a single-agent or a 2-agent chemotherapy regimen in advanced non-small-cell lung cancer: a meta-analysis. JAMA 2004; 292: 470–484. [DOI] [PubMed] [Google Scholar]
- 4. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomized phase 3 trial. Lancet Oncol 2012; 13: 239–246. [DOI] [PubMed] [Google Scholar]
- 5. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011; 12: 735–742. [DOI] [PubMed] [Google Scholar]
- 6. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013; 368: 2385–2394. [DOI] [PubMed] [Google Scholar]
- 7. Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014; 370: 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Passaro A, Cortesi E, de Marinis F. Second-line treatment of non-small-cell lung cancer: chemotherapy or tyrosine kinase inhibitors? Expert Rev Anticancer Ther 2011; 11: 1587–1597. [DOI] [PubMed] [Google Scholar]
- 9. Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-celllung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014; 384: 665–673. [DOI] [PubMed] [Google Scholar]
- 10. Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014; 15: 143–155. [DOI] [PubMed] [Google Scholar]
- 11. Claudia SP, Paolo M, Antonio R, et al. New antiangiogenetic therapy beyond bevacizumab in the treatment of advanced non-small cell lung cancer. Curr Pharm Des 2015; 21: 4763–4772. [DOI] [PubMed] [Google Scholar]
- 12. Bustamante Alvarez JG, Gonzalez-Cao M, Karachaliou N, et al. Advances in immunotherapy for treatment of lung cancer. Cancer Biol Med 2015; 12: 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anagnostou VK, Brahmer JR. Cancer immunotherapy: a future paradigm shift in the treatment of non-small cell lung cancer. Clin Cancer Res 2015; 21: 976–984. [DOI] [PubMed] [Google Scholar]
- 14. Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8: 793–800. [DOI] [PubMed] [Google Scholar]
- 15. Karwacz K, Bricogne C, MacDonald D, et al. PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8+ T cells. EMBO Mol Med 2011; 3: 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fridman WH, Pages F, Sautes-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12: 298–306. [DOI] [PubMed] [Google Scholar]
- 18. Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348: 203–213. [DOI] [PubMed] [Google Scholar]
- 19. Sakuishi K, Apetoh L, Sullivan JM, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 2010; 207: 2187–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harvey RD. Immunologic and clinical effects of targeting PD-1 in lung cancer. Clin Pharmacol Ther 2014; 96: 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28: 3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366: 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gettinger S, Herbst RS. B7-H1/PD-1 blockade therapy in non-small cell lung cancer: current status and future direction. Cancer J 2014; 20: 281–289. [DOI] [PubMed] [Google Scholar]
- 24. Herbst RS, Soria J-C, Kowanetz M, et al. Predictive correlates of response to the anti- PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837–1846. [DOI] [PubMed] [Google Scholar]
- 28. Spigel DR, Chaft JE, Gettinger SN, et al. Clinical activity and safety from a phase II study (FIR) of MPDL3280A (anti-PDL1) in PD-L1–selected patients with non-small cell lung cancer (NSCLC). J Clin Oncol 2015; 33(Suppl. 15): 8028. [Google Scholar]
- 29. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomized controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rizvi NA, Brahmer JR, Ou SHI, et al. Safety and clinical activity of MEDI4736, an antiprogrammed cell death-ligand 1 (PDL1) antibody, in patients with non-small cell lung cancer (NSCLC). J Clin Oncol 2015; 33(Suppl. 15): 8032. [Google Scholar]
- 31. Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 2016; 17: 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gulley JL, Spigel D, Kelly K, et al. Avelumab (MSB0010718C), an anti-PDL1 antibody, in advanced NSCLC patients: a phase 1b, open-label expansion trial in patients progressing after platinum-based chemotherapy. J Clin Oncol 2015; 33(Suppl. 15): 8034. [Google Scholar]
- 33. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- 34. Chatterjee M, Turner DC, Felip E, et al. Systematic evaluation of pembrolizumab in patients with advanced non-small-cell lung cancer. Ann Oncol 2016; 27: 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hui R, Ghandi L, Carcereny Costa E, et al. Long-term OS for patients with advanced NSCLC enrolled in the KEYNOTE-001 study of pembrlizumab (pembro). J Clin Oncol 2016; 34(Suppl. 15): 9026. [Google Scholar]
- 36. Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 37. Garon EB, Herbst RS, Kim D-W, et al. Pembrolizumab vs docetaxel for previously treated advanced NSCLC with a PD-L1 tumor proportion score (TPS) 1%-49%: results from KEYNOTE-010. J Clin Oncol 2016; 34(Suppl. 15): 9024. [Google Scholar]
- 38. Gadgeel SM, Stevenson J, Langer CJ, et al. Pembrolizumab (pembro) plus chemotherapy as front-line therapy for advanced NSCLC: KEYNOTE-021 cohorts A-C. J Clin Oncol 2016; 34(Suppl. 15): 9016. [Google Scholar]
- 39. Gubens MA, Sequist LV, Stevenson J, et al. Phase I/II study of pembrolizumab (pembro) plus ipilimumab (ipi) as second-line therapy for NSCLC: KEYNOTE-021 cohorts D and H. J Clin Oncol 2016; 34(Suppl. 15): 9027. [Google Scholar]
- 40. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. Epub ahead of print 8 October 2016. [DOI] [PubMed] [Google Scholar]
- 41. Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016; 17: 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rahman OA. Correlation between PD-L1 expression and outcome of NSCLC patients treated with anti-PD-1/PD-L1 agents: a meta-analysis. Crit Rev Onc Hem 2016; 101: 75–85. [DOI] [PubMed] [Google Scholar]
- 43. Carbognin L, Pilotto S, Milella M, et al. Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS One 2015; 10: e0130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372: 320–330. [DOI] [PubMed] [Google Scholar]
- 45. Kefford R, Ribas A, Hamid O, et al. Clinical efficacy and correlation with tumor PD-L1 expression in patients (pts) with melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. J Clin Oncol 2014; 32(Suppl. 15): 3005.25024073 [Google Scholar]
- 46. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372: 2521–2532. [DOI] [PubMed] [Google Scholar]
- 47. Postow MA, Cardona DM, Taube JM, et al. Peripheral and tumor immune correlates in patients with advanced melanoma treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) monotherapy or in combination with ipilimumab. J Transl Med 2014; 12(Suppl. 1): O8. [Google Scholar]
- 48. Daud A, Ribas A, Robert C, et al. Long-term efficacy of pembrolizumab (pembro; MK-3475) in a pooled analysis of 655 patients (pts) with advanced melanoma (MEL) enrolled in KEYNOTE-001. ASCO Meet Abstr 2015; 33: 9005. [Google Scholar]
- 49. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol 2014; 33: 1430–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Manson G, Norwood J, Marabelle A, et al. Biomarkers associated with checkpoint inhibitors. Ann Oncol 2016; 27: 1199–1206. [DOI] [PubMed] [Google Scholar]
- 51. Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol 2017; 12: 208–222. [DOI] [PubMed] [Google Scholar]
- 52. Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2015; 33: 2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Khunger M, Rakshit S, Schalper KA, et al. Meta-analysis of tumor PD-L1 expression as a predictive biomarker of benefit from PD-1/PD-L1 axis inhibitors in solid tumors. J Clin Oncol 2016; 34(Suppl. 15): 11603. [DOI] [PubMed] [Google Scholar]
- 54. Ilie M, Long-Mira E, Bence C, et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol 2016; 27: 147–153. [DOI] [PubMed] [Google Scholar]
- 55. Chakravarti N, Prieto VG. Predictive factors of activity of anti-programmed death-1/programmed death ligand-1 drugs: immunohistochemistry analysis. Transl Lung Cancer Res 2015; 4: 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 1270–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gainor JF, Sequist LV, Shaw AT, et al. Clinical correlation and frequency of programmed death ligand-1 (PD-L1) expression in EGFR-mutant and ALK-rearranged non-small cell lung cancer (NSCLC). J Clin Oncol 2016; 33(Suppl. 15): 8012. [Google Scholar]
- 59. Giunchi F, Degiovanni A, Daddi N, et al. Fading with time of PD-L1 immunoreactivity in non-small-cell lung cancer tissues: a methodological study. Appl Immunoistochemi Mol Morphol. Epub ahead of print 31 October 2016. [DOI] [PubMed] [Google Scholar]
- 60. Soo RA. Shedding light on the molecular determinants of response to anti-PD-1 therapy. Transl Lung Cancer Res 2015; 4: 816–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013; 499: 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Campesato LS, Barroso-Sousa R, Jimenez L, et al. Comprehensive cancer-gene panels cane be used to estimate mutational load and predict clinical benefit to PD-1 blockade in clinical practice. Oncotarget 2015; 6: 34221–34227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Socinsky M, Creelan B, Horn L, et al. CheckMate 026: a phase 3 trial of nivolumab vs investigator’s choice (IC) of platinum-based doublet chemotherapy (PT-DC) as first-line therapy for stage IV/recurrent programmed death ligand 1 (PD-L1) − positive NSCLC. Ann Oncol 2016; 27(Suppl. 6). [Google Scholar]
- 64. Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer: a meta-analysis. J Thorac Oncol 2017; 12: 403–407. [DOI] [PubMed] [Google Scholar]