Abstract
Lung cancer is the leading cause of death due to cancer worldwide. It is estimated that approximately 1.2 million new cases of lung cancer are diagnosed each year. Early detection and treatment are crucial for improvements in both prognosis and quality of life of lung cancer patients. The ataxia telangiectasia mutated (ATM) gene is a cancer-susceptibility gene that encodes a key apical kinase in the DNA damage response pathway. It has recently been shown to play an important role in the development of lung cancer. The main functions of the ATM gene and protein includes participation in cell cycle regulation, and identification and repair of DNA damage. ATM gene mutation can lead to multiple system dysfunctions as well as a concomitant increase in tumor tendency. In recent years, many studies have indicated that single nucleotide polymorphism of the ATM gene is associated with increased incidence of lung cancer. At the same time, the ATM gene and its encoding product ATM protein predicts the response to radiotherapy, chemotherapy, and prognosis of lung cancer, thus suggesting that the ATM gene may be a new potential target for the diagnosis and treatment of lung cancer.
Keywords: ataxia telangiectasia mutated, ATM, lung cancer, treatment
Introduction
Morbidity and mortality from lung cancer are ranked highest among malignant tumors. The American Cancer Society (ACS) 2015 annual report on cancer screening pointed out that lung cancer is the leading cause of death from cancer in both males and females. In 2015, there were 222,000 new lung cancer cases, and 158,000 cases of lung-cancer-related deaths in the United States. Lung cancer accounted for 27% of all death due to cancer.
According to the 2015 Annual Report of China Cancer Registration, 733,000 new lung cancer cases were diagnosed in China in 2011, accounting for 17.08% of all new cases of malignant tumors. The number of deaths resulting from lung cancer was 600,200, which was 21.68% of all deaths from malignant tumors.
According to the 2012 Annual Report of China Cancer Registration, northeast China had the highest lung cancer morbidity. Furthermore, lung cancer (morbidity and mortality) is ranked highest among malignant tumors in China.1 During recent years, despite great advancements in precision medicine and molecular-targeted therapy, and constant improvements in lung cancer diagnoses and treatment methods, the 2012 Annual Report of China Cancer Registration still reported a 5-year survival rate of only 16.1% for lung cancer. Previous research found that the prognosis of lung cancer patients is dependent on the stage of lung cancer being diagnosed. If lung cancer is detected at pathological stage IB and IA, the 5-year survival rate can be up to 67% and 57%, respectively, with some patients even achieving complete remission.2
Earlier diagnosis can therefore lead to significant improvements in 5-year survival rate. Recently, people are beginning to appreciate the role of family genetic susceptibility. Many genes are found to be closely related to lung cancer susceptibility, such as Ras, Myc, c-erbB-1, c-erbB-2, c-Myb, c-raf-1, KRAS2 and other oncogenes, as well as Bcl-2, c-mmyc, p53, ataxia telangiectasia mutated (ATM) and other tumor suppressor genes. Variations in the ATM gene and its encoding product ATM protein is found to affect the pathogenesis, development, response to treatment, and prognosis of lung cancer (Figure 1). This article reviews recent research advances in the role of ATM in lung cancer.
Figure 1.
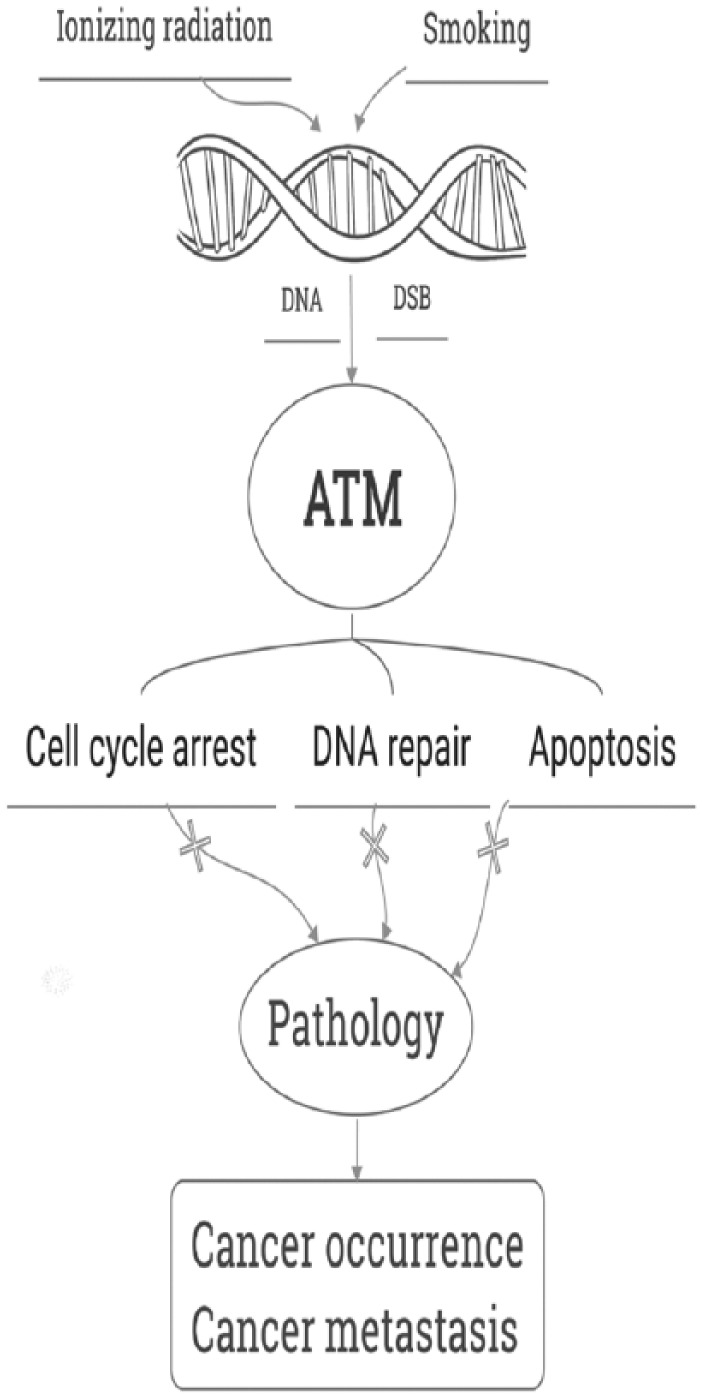
Overview of the role of ATM in lung cancer.
A brief overview of the ATM gene and protein
The ATM gene is the pathogenic mutated gene on chromosome 11q22.3 that leads to a rare disease, ataxia telangiectasia (AT). AT was first reported by Louis-Bar in 1941, and therefore is also known as Louis-Bar syndrome. It is an autosomal recessive disease that involves multiple organ systems and is characterized by neurodegeneration, immune deficiency, cancer predisposition, chromosomal instability, and radiation sensitivity. The morbidity of this disease is about 1/40,000–1/100,000; the pathogenesis is not yet fully understood, but it is characterized by its defective response to DNA double-strand break (DSB).3
The ATM gene open reading frame has 9168 nucleotides, and encodes a 350 kDa protein composed of 3056 amino acid residues, called the ATM protein.4 The ATM protein is a member of the PI3K-like protein kinase family, which is a family of large proteins involved in genome stability, cellular responses to DNA damage, and cell cycle control. More than 900 regulated phosphorylation sites encompassing over 700 proteins have been identified as targets for the ATM protein. Most of these proteins are found to be involved in or interact with DNA damage regulation.5
The main function of the ATM protein is to participate in the regulation of the cell cycle, and to identify and repair DNA damage.6–8 Ionizing radiation can induce DNA DSB and thereby lead to a cascade reaction of signaling pathways, including recruitment of the MRE11–RAD50–NBS1 (MRN) complex, which is essential for the optimal activation of ATM. ATM then becomes rapidly phosphorylated and induces apoptosis by MDM2 p53 and BAX.9,10
ATM also activates the ATM–AMPK–TSC2–mTORC1 pathway, inducing autophagy following exposure to reactive oxygen species (ROS). These divergent pathways (resulting from different ATM subcellular localization) may explain some of the pleiotropic phenotypes seen in AT patients.11
ATM gene and the occurrence of lung cancer
In recent years, more attention has been paid to the role of genetic and epigenetic changes in carcinogenesis. The pathogenesis of most tumors is multifactorial – a result of the interaction between genetic and environmental factors. However, genetic changes are the most direct cause of cancer at the molecular level. Many researchers have shown that mutation and inactivation of the ATM gene may increase genetic instability and lead to disorders in the DNA DSB repair pathway, which affect the sensitivity of cells to ionizing radiation and thus enhance susceptibility to the development of tumors.12–14
According to National Comprehensive Cancer Network (NCCN) guidelines, air pollution (especially indoor air pollution) is one of the most important risk factors for the development of lung cancer. Smoking history (both past and present) and exposure to second-hand smoke are also leading risk factors. However, not all smokers develop cancer. Indeed, it is estimated that only 5–10% of past or present smokers eventually develop lung cancer. On the other hand, up to 90% of lung cancers are attributable to smoking.15 It is speculated that individual variation in susceptibility to lung cancer might be caused by individual variations in the genes involved in the DNA repair process.16 There are at least 88 polymorphic loci on the ATM gene, with a high number of rare variants.17 Many studies have indicated that the single nucleotide polymorphism of the ATM gene affects the sensitivity of cells to radiation-induced DNA damage, which is closely related to cancer pathogenesis.12,13,18
Research evaluating the role of the ATM gene single nucleotide polymorphisms (SNPs) in lung cancer development has identified several ATM polymorphisms associated with increased lung cancer risk. For example, subjects with the A allele at the site (IVS62+60G>A) had significantly higher risk of lung cancer compared to those with the G allele. Different ATM gene haplotypes of the SNP sites was also associated with altered lung cancer risk. When the haplotypes of four ATM single nucleotide polymorphism sites (–4518A>G, IVS21–77C>T, IVS61–55T>C and IVS62+60G>A) were studied, GCCA (the most commonly found haplotype) was found to be associated with low risk of lung cancer, while the ATTA haplotype confers a higher risk. Furthermore, subjects that have the (NN)TA haplotype also had significantly increased risk of lung cancer compared with those without this haplotype.19
These results suggest that SNPs or haplotypes of the ATM gene play an important role in the development of lung cancer. A study in a Caucasian population20 found that homozygous variant genotypes of ATM08 (rs227060) and ATM10 (rs170548) were associated with elevated risk for non-small cell lung carcinoma (NSCLC) compared to the wild type. A matched case-control study of a Taiwanese population showed that nine ATM polymorphisms (rs189037, rs228597, rs228592, rs664677, rs609261, rs599558, rs609429, rs227062, and rs664982) were significantly associated with lung cancer among never-smokers, but not among smokers.21 Gene–lifestyle interaction was analyzed in another study of lung cancer risk, also in the Taiwanese population. Seven different SNPs of the ATM gene (rs600931, rs652311, rs227060, rs228589, rs227092, rs624366, and rs189037) were genotyped, and results showed that the ATM allele rs652311 may enhance the effect of smoking on lung cancer development and thereby increase lung cancer risk.22
Polymorphisms in the ATM allele rs189037 (G>A) have also been shown to be predictive of lung cancer risk in a Han Chinese population,23 and was associated with increased risk of lung adenocarcinoma in Chinese non-smoking females,24 as well as increased risk of head and neck cancer.25
However, not all studies support the association between ATM and lung cancer occurrence. In patients diagnosed with lung cancer before the age of 50, ATM variants in lung cancer patients did not differ significantly from the control subjects. Moreover, multiple analyses of lung cancer patients who carried at least one risk allele of the ATM did not show significantly altered risks.26
Based on the above-mentioned studies, the role of ATM in the occurrence of lung cancer is still not completely clear and more research is needed in this area. If ATM polymorphism can be proved to have a clear relationship with lung cancer, then it would open up the possibility of using ATM gene analysis as an important diagnostic tool in the future to predict individual susceptibility to lung cancer. At-risk patients can be followed up to make early diagnosis possible and thereby greatly improve prognosis in the disease.
ATM gene and metastasis of lung cancer
ATM not only is involved in the occurrence and development of lung cancer, but also plays a major role in cancer metastasis. ATM has been shown to be crucial in both TNF-α- and IL-6-induced lung cancer metastases. IL-6 can increase the expressions of MMP-3 and MMP-13 via phosphorylation of ATM. These metalloproteases (MMPs) facilitate cell migration and thereby promote lung cancer metastasis. Inhibition of ATM abrogates the effect of IL-6 on lung cancer metastasis in vivo.27
ATM has also been suggested to be an upstream regulator of the TNF-α activated ERK/p38-NF-κB pathway. Activation of this pathway increases expression of MMP-13 and thereby lung cancer metastasis. An in vivo lung cancer metastasis test performed in a study by Yan and colleagues showed that ATM depletion reduced the number of metastatic nodules and cancer nests in lung tissues, thus verifying the critical role of ATM in metastasis.28 Therefore, inhibition of ATM might be a promising strategy for the treatment and prevention of inflammation-induced lung cancer metastasis.
ATM gene and lung cancer treatment
At present, treatment options for lung cancer in the clinic are still limited to conventional chemotherapy and radiotherapy. At the same time, radiation resistance and drug resistance of the tumor are the main causes of treatment failure. High ATM mRNA expression in tumors has been shown to be a negative prognostic marker in NSCLC patients.29 In order to improve treatment outcome, it is therefore crucial to find new targets and treatment strategies.
Hadian and Krappmann30 found that following DNA DSB, ATM protein moves out from the nucleus, and activates ubiquitin ligase to produce tumor necrosis factor receptor associated factor-6 (TRAF6) and NF-κB inhibitor ELKS. This process leads to tumor proliferation and increase in the resistance of tumor cells to radiotherapy and chemotherapy.
Studies have shown that the lower the ATM protein expression, the higher the sensitivity to radiotherapy.31,32 It is speculated that selective inhibition of ATM protein can breakdown DNA DSB repair barriers and cell cycle checkpoints, resulting in tumor cell apoptosis, and ultimately increased sensitivity to radiation (radiosensitivity).
Research on radiation-induced cell death in the lung cancer cell-line H1299 found that MAPK14 contributed to radiosensitization of H1299 cells and that ATM promoted radiation-induced autophagy via the MAPK14 pathway, mTOR pathway, and Beclin1–PI3KIII complexes.33 However, different tissues respond to ATM inhibition differently. Hammond and Muschel suggest that rapidly proliferating cancer tissues are more sensitive to the effects of ATM gene deletion than non-cancer tissues, thus making ATM inhibitors promising radiation sensitizers in cancer therapy.34
Many other drugs, including cisplatin and metformin, are also known to increase radiosensitivity, and it has been proposed that the ATM pathway is responsible for their radiosensitizing effects. A study using NSCLC cell-lines found that cisplatin radiosensitized these cells and that inhibition of ATM by its pharmacological inhibitors markedly potentiated this effect.35 Using a similar model,36 it has also been demonstrated that metformin at clinically achievable doses can inhibit NSCLC cell and tumor growth and sensitize them to radiation by a sustained activation of the ATM–AMPK–p53/p21 pathway.
The ATM gene and protein not only have the potential to increase the sensitivity to radiotherapy of lung cancer, but might also be involved in the complications of radiation therapy. Studies indicate that genetic polymorphisms in the ATM–p53 pathway influences susceptibility to radiation-induced pneumonitis. Thus genotyping p53 and ATM polymorphisms might help to identify patients at high risk of developing radiation-induced pneumonitis after receiving radiotherapy, and actively prevent this complication from occurring.37
Some lung cancers develop multidrug resistance (MDR), which is an important problem adversely affecting the response to therapy and the prognosis. The commonly used chemotherapy agents camptothecin and cisplatin can upregulate expression of the MDR-related genes ABCG2 and MRP2. This upregulation is impaired by ATM and NF-κB inhibitors, indicating a relationship between ATM, NF-κB activation, and MDR formation in lung cancer chemotherapy, and making ATM a potential molecular target for a solution of the MDR problem in lung cancer chemotherapy.38
The addition of an ATM inhibitor can increase response to conventional cancer treatment. One study has shown that ATM inhibition facilitates the gefitinib-dependent repression of the phosphorylation of EGFR and/or its downstream factors, leading to a synergistic effect inhibiting cell growth and inducing apoptosis in NSCLC cell-lines carrying the sensitive EGFR mutation when the ATM inhibitor KU55933 and the EGFR-tyrosine kinase inhibitor gefitinib are combined.39
In summary, ATM affects the prognosis of lung cancer and is highly involved in the response to conventional radiotherapy and chemotherapy. ATM may be not only a target for improving response to radiation therapy and chemotherapy for lung cancer, but may also predict radiation injury and poor prognoses. Further studies are needed to determine an optimal balance point whereby the expression level of ATM protein meets the needs of treatment while also only minimally affecting normal physiological function in the body.
Conclusions
Interaction between genes and the environment lies behind the pathogenesis of many diseases, including lung cancer. The challenge for modern medicine is to identify the subgroup in a population at greatest risk and then prevent them from developing diseases.
Variations in the ATM gene and its encoding product, the ATM protein, are found to affect the pathogenesis, development, response to treatment and prognosis of lung cancer. However, only a few of ATM’s many genetic polymorphisms have been explored, and other genetic loci and their interactions with environment and disease remain largely unknown. Further understanding of ATM gene polymorphisms and the multiple functions of the ATM protein can provide a more accurate and reliable theoretical basis for ATM’s role as a possible new target for the prediction and treatment of lung cancer.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Yanling Xu, Department of Geriatrics and General Medicine, the Second Affiliated Hospital of Jilin University, Changchun, Jilin 130041, China.
Peng Gao, Department of Respiratory Medicine, the Second Affiliated Hospital of Jilin University, Changchun, Jilin 130041, China.
Xuejiao Lv, Department of Respiratory Medicine, the Second Affiliated Hospital of Jilin University, Changchun, Jilin 130041, China.
Lin Zhang, Department of Respiratory Medicine, the Second Affiliated Hospital of Jilin University, Changchun, Jilin 130041, China.
Jie Zhang, Department of Respiratory Medicine, the Second Affiliated Hospital of Jilin University, No. 218 Ziqiang Street, Nanguan District, Changchun, Jilin 130041, China.
References
- 1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–132. [DOI] [PubMed] [Google Scholar]
- 2. Rami-Porta R. Reflections on the revisions in the international system for staging lung cancer. Chest 1998; 113: 1728–1729. [DOI] [PubMed] [Google Scholar]
- 3. Rothblum-Oviatt C, Wright J, Lefton-Greif MA, et al. Ataxia telangiectasia: a review. Orphanet J Rare Dis 2016; 11: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uziel T, Savitsky K, Platzer M, et al. Genomic organization of the ATM gene. Genomics 1996; 33: 317–320. [DOI] [PubMed] [Google Scholar]
- 5. Matsuoka S, Ballif BA, Smogorzewska A, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 2007; 316: 1160–1166. [DOI] [PubMed] [Google Scholar]
- 6. Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev 2001; 15: 2177–2196. [DOI] [PubMed] [Google Scholar]
- 7. Shiloh Y, Kastan MB. ATM: genome stability, neuronal development, and cancer cross paths. Adv Cancer Res 2001; 83: 209–254. [DOI] [PubMed] [Google Scholar]
- 8. Giunta S, Belotserkovskaya R, Jackson SP. DNA damage signaling in response to double-strand breaks during mitosis. J Cell Biol 2010; 190: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rothkamm K, Kruger I, Thompson LH, et al. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol 2003; 23: 5706–5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 2003; 421: 499–506. [DOI] [PubMed] [Google Scholar]
- 11. Alexander A, Walker CL. Differential localization of ATM is correlated with activation of distinct downstream signaling pathways. Cell Cycle 2010; 9: 3685–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maillet P, Chappuis PO, Vaudan G, et al. A polymorphism in the ATM gene modulates the penetrance of hereditary non-polyposis colorectal cancer. Int J Cancer 2000; 88: 928–931. [DOI] [PubMed] [Google Scholar]
- 13. Angele S, Falconer A, Edwards SM, et al. ATM polymorphisms as risk factors for prostate cancer development. Br J Cancer 2004; 91: 783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hall J. The ataxia-telangiectasia mutated gene and breast cancer: gene expression profiles and sequence variants. Cancer Lett 2005; 227: 105–114. [DOI] [PubMed] [Google Scholar]
- 15. Wu X, Zhao H, Suk R, et al. Genetic susceptibility to tobacco-related cancer. Oncogene 2004; 23: 6500–6523. [DOI] [PubMed] [Google Scholar]
- 16. Spitz MR, Wei Q, Dong Q, et al. Genetic susceptibility to lung cancer: the role of DNA damage and repair. Cancer Epidemiol Biomarkers Prev 2003; 12: 689–698. [PubMed] [Google Scholar]
- 17. Thorstenson YR, Shen P, Tusher VG, et al. Global analysis of ATM polymorphism reveals significant functional constraint. Am J Hum Genet 2001; 69: 396–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borresen AL, Andersen TI, Tretli S, et al. Breast cancer and other cancers in Norwegian families with ataxia-telangiectasia. Genes Chromosomes Cancer 1990; 2: 339–340. [DOI] [PubMed] [Google Scholar]
- 19. Kim JH, Kim H, Lee KY, et al. Genetic polymorphisms of ataxia telangiectasia mutated affect lung cancer risk. Hum Mol Genet 2006; 15: 1181–1186. [DOI] [PubMed] [Google Scholar]
- 20. Yang H, Spitz MR, Stewart DJ, et al. ATM sequence variants associate with susceptibility to non-small cell lung cancer. Int J Cancer 2007; 121: 2254–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lo YL, Hsiao CF, Jou YS, et al. ATM polymorphisms and risk of lung cancer among never smokers. Lung Cancer 2010; 69: 148–154. [DOI] [PubMed] [Google Scholar]
- 22. Hsia TC, Tsai CW, Liang SJ, et al. Effects of ataxia telangiectasia mutated (ATM) genotypes and smoking habits on lung cancer risk in Taiwan. Anticancer Res 2013; 33: 4067–4071. [PubMed] [Google Scholar]
- 23. Liu J, Wang X, Ren Y, et al. Effect of single nucleotide polymorphism Rs189037 in ATM gene on risk of lung cancer in Chinese: a case-control study. PLoS One 2014; 9: e115845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shen L, Yin Z, Wu W, et al. Single nucleotide polymorphism in ATM gene, cooking oil fumes and lung adenocarcinoma susceptibility in Chinese female non-smokers: a case-control study. PLoS One 2014; 9: e96911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bhowmik A, Nath S, Das S, et al. ATM rs189037 (G > A) polymorphism and risk of lung cancer and head and neck cancer: a meta-analysis. Meta Gene 2015; 6: 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schneider J, Illig T, Rosenberger A, et al. Detection of ATM gene mutations in young lung cancer patients: a population-based control study. Arch Med Res 2008; 39: 226–231. [DOI] [PubMed] [Google Scholar]
- 27. Jiang YN, Yan HQ, Huang XB, et al. Interleukin 6 trigged ataxia-telangiectasia mutated activation facilitates lung cancer metastasis via MMP-3/MMP-13 up-regulation. Oncotarget 2015; 6: 40719–40733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yan HQ, Zhang D, Shi YY, et al. Ataxia-telangiectasia mutated activation mediates tumor necrosis factor-alpha induced MMP-13 up-regulation and metastasis in lung cancer cells. Oncotarget 2016; 7: 62070–62083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xing J, Wu X, Vaporciyan AA, et al. Prognostic significance of ataxia-telangiectasia mutated, DNA-dependent protein kinase catalytic subunit, and Ku heterodimeric regulatory complex 86-kD subunit expression in patients with nonsmall cell lung cancer. Cancer 2008; 112: 2756–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hadian K, Krappmann D. Signals from the nucleus: activation of NF-kappaB by cytosolic ATM in the DNA damage response. Sci Signal 2011; 4: pe2. [DOI] [PubMed] [Google Scholar]
- 31. Liu S, Opiyo SO, Manthey K, et al. Distinct roles for DNA-PK, ATM and ATR in RPA phosphorylation and checkpoint activation in response to replication stress. Nucleic Acids Res 2012; 40: 10780–10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parsons JL, Khoronenkova SV, Dianova II, et al. Phosphorylation of PNKP by ATM prevents its proteasomal degradation and enhances resistance to oxidative stress. Nucleic Acids Res 2012; 40: 11404–11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liang N, Zhong R, Hou X, et al. Ataxia-telangiectasia mutated (ATM) participates in the regulation of ionizing radiation-induced cell death via MAPK14 in lung cancer H1299 cells. Cell Prolif 2015; 48: 561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hammond EM, Muschel RJ. Radiation and ATM inhibition: the heart of the matter. J Clin Invest 2014; 124: 3289–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Toulany M, Mihatsch J, Holler M, et al. Cisplatin-mediated radiosensitization of non-small cell lung cancer cells is stimulated by ATM inhibition. Radiother Oncol 2014; 111: 228–236. [DOI] [PubMed] [Google Scholar]
- 36. Storozhuk Y, Hopmans SN, Sanli T, et al. Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J Cancer 2013; 108: 2021–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang M, Zhang L, Bi N, et al. Association of P53 and ATM polymorphisms with risk of radiation-induced pneumonitis in lung cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys 2011; 79: 1402–1407. [DOI] [PubMed] [Google Scholar]
- 38. Ke SZ, Ni XY, Zhang YH, et al. Camptothecin and cisplatin upregulate ABCG2 and MRP2 expression by activating the ATM/NF-kappaB pathway in lung cancer cells. Int J Oncol 2013; 42: 1289–1296. [DOI] [PubMed] [Google Scholar]
- 39. Misumi K, Sun J, Kinomura A, et al. Enhanced gefitinib-induced repression of the epidermal growth factor receptor pathway by ataxia telangiectasia-mutated kinase inhibition in non-small-cell lung cancer cells. Cancer Sci 2016; 107: 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]


