Abstract
Despite several therapeutic choices, 10–20% of patients with severe uncontrolled asthma do not respond to maximal best standard treatments, leading to a healthcare expenditure of up to 80% of overall costs for asthma. Today, there are new important therapeutic strategies, both pharmacological and interventional, that can result in improvement of severe asthma management, such as omalizumab, bronchial thermoplasty and other biological drugs, for example, mepolizumab, reslizumab and benralizumab. The availability of these new treatments and the increasing knowledge of the different asthmatic phenotypes and endotypes makes correct patient selection increasingly complex and important. In this article, we discuss the features of benralizumab compared with other anti-interleukin-5 biologics and omalizumab, the identification of appropriate patients, the safety profile and future developments.
Keywords: anti-interleukin-5 monoclonal antibodies, benralizumab, biomarkers, cytokines, eosinophils, interleukin 5, severe asthma
Introduction
Severe asthma is a chronic inflammatory disease affecting 5–10% of more than 300 million people with asthma worldwide [Masoli et al. 2012]. Despite the wide therapeutic armamentarium, 10–20% of patients [Global Asthma Report, 2014] with severe uncontrolled asthma do not respond to maximal best standard treatments due to the complexity of the different mechanisms underlying disease pathogenesis. Consequently, low symptom control, poor quality of life, unscheduled visits to the doctor’s office or emergency department, and hospitalizations continue to occur with major direct and indirect costs. In 2007 the incremental cost due to asthma was $56 billion in the United States, with even higher figures worldwide [Masoli et al. 2012; Barnett and Nurmagambetov, 2007]. After the advent of novel biological therapies that have recently been approved for severe asthma, health economists and society are looking closely to whether rearing direct costs due to these medications can be balanced by proportional benefits [Menzella et al. 2012].
Today, there are new important therapeutic strategies, both pharmacological and interventional, that have been shown to improve the management of severe asthma, such as omalizumab, bronchial thermoplasty (BT), and other biological drugs, for example mepolizumab. Different studies have shown that anti-interleukin 5 (anti-IL-5) therapies are effective only in a specific eosinophilic phenotype [Nair et al. 2009; Pavord et al. 2012] while trials on unselected patients have often missed their goal [Flood-Page et al. 2007].
The availability of these new treatment options and the increasing knowledge of the several asthmatic phenotypes and endotypes make correct patient selection increasingly complex but important. In this review we discuss the key and sometimes controversial aspects of benralizumab, a new and different approach to IL-5 antagonism, debating the current and potential fields of application of this innovative and promising molecule.
Eosinophils and IL-5 interaction
Eosinophils represent an important player in different acute and chronic inflammatory processes involved in airway narrowing and remodelling. Recruitment and survival of eosinophils in the airways and granule maturation are promoted by IL-3, granulocyte–macrophage colony-stimulating factor (GM-CSF) and in particular IL-5, a 134-amino acid protein forming a 52 kDa homodimer related to both GM-CSF and IL-3 [Klein et al. 2012; McKinnon et al. 1999; Menzies-Gow et al. 2003].
IL-5 stimulates the final steps in the differentiation of activated B cells to antibody-forming cells and enhances proliferation and differentiation of eosinophil precursors [Kouro and Takatsu, 2009]. There is evidence in experimental murine models of IL-5 involvement in airway remodelling [Tanaka et al. 2004].
Mature eosinophils circulate in the blood for 6–10 h [Berek, 2016; Becchetti et al. 2011] to end their life cycle 8–12 days after migration in connective tissues. The cell surface of eosinophils expresses immunoglobulin E (IgE) receptors involved in the activation of allergic reactions and the protection against parasites and worms through a mechanism of antigen–antibody complex internalization and release of inflammatory mediators (such as major basic protein) highly toxic for microbial agents. Eosinophils can release from cytoplasmic granules different mediators of allergic reactions, such as histaminase and arylsulfatase, and secrete leukotrienes, which have a significant role in asthma by sustaining inflammation, increasing mucus production and inducing bronchoconstriction [Baptista-dos-Reis et al. 2015; George and Brightling, 2016]. Eosinophilia is not only typical of some phenotypes of asthma, but can also be found in several other disorders originating from atopic conditions, helminthic infestations, hypersensitivity to drugs and cancer [Curtis and Ogbogu, 2015].
The life cycle of eosinophils is mainly regulated by IL-5, therefore this molecule and its receptor have been studied as a target in the treatment of eosinophilic disorders. IL-5 is secreted by T helper 2 (Th2) lymphocytes, type 2 cytotoxic T cells, eosinophils, mast cells, γδ-T cells and type 2 innate lymphoid cells. Small amounts of IL-5 can be released by epithelial cells, natural killer (NK) cells and NK T cells [Klein et al. 2012; McKinnon et al. 1999].
The IL-5 receptor complex is expressed in particular by eosinophils and basophils and is a heterodimer composed of an α chain (highly specific for IL-5) with a molecular weight of 60 Kd and a βc chain with a molecular weight of 130 Kd. The βc chain is also shared and recognized by IL-3 and GM-CSF [Kouro and Takatsu, 2009; Menzies et al. 2003] and belongs to the class I cytokine receptor family [Kaczmarski and Mufti, 1991]. The α chain is required for ligand-specific binding, whereas association with the βc chain leads to an increased binding affinity [Takagi et al. 1995]. The receptor gene is located on chromosome 5, close to the genes encoding IL-3, IL-4 and GM-CSF [Wells et al. 1994; Valent et al. 1994; Toba et al. 1999]. Several transcription factors (including GATA3) regulate the expression of IL-5.
Benralizumab: structure and mechanism of action
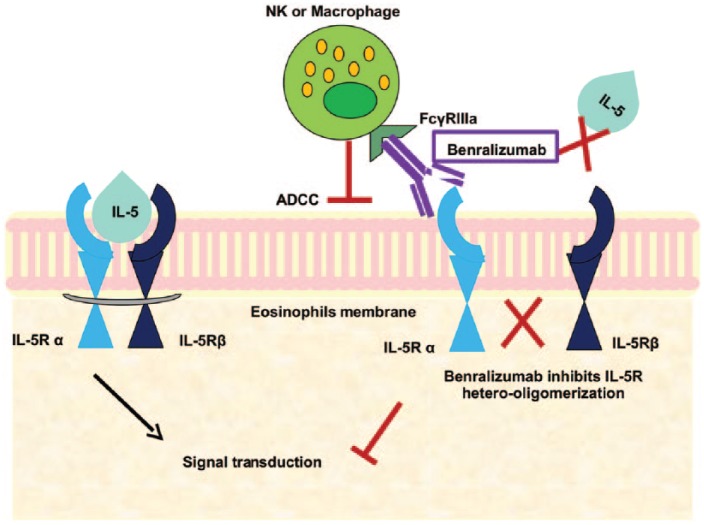
Benralizumab, formerly known as BIW-8405/MEDI-56 developed by MedImmune-AstraZeneca, Gaithersburg, Maryland (USA) is a humanized monoclonal antibody (mAb) (IgG1k) that binds with high affinity to the α chain of human IL-5R, resulting in inhibition of IL5-mediated receptor activation (Figure 1). The binding site of benralizumab is close to a conformationally distinct epitope within domain 1 of the IL-5R, further explaining its neutralizing activity. The molecular structure has been engineered without fucose sugar residue in the CH2 region: afucosylation enhances the interaction of benralizumab with its binding site and strongly induces antibody-dependent cell-mediated cytotoxicity (ADCC) of eosinophils and basophils [Rothenberg and Hogan, 2006; Brown, 2015; Castro et al. 2014].
Figure 1.
Benralizumab binds with the α chain of interleukin 5 receptor (IL-5R) resulting in inhibition of hetero-oligomerization of α and β subunits and thus no signal transduction occurs. Afucosylated site of benralizumab enhances its binding to FcγRIIIa leading to antibody-dependent cell-mediated cytotoxicity (ADCC).
The afucosylated IgGk mAb is characterized by a high-binding affinity for the Fcγ RIIIa region and it can overcome the inhibitory effects exhibited by serum IgG that limit the ADCC activity of their fucosylated counterparts [Walsh, 2013]. This is in contrast to the other anti-IL-5 biologics (i.e. mepolizumab and reslizumab) that block the activation of eosinophils by neutralizing circulating IL-5 (Table 1). Benralizumab targets the circulating effector cells and lung-tissue resident eosinophils, but it also induces the depletion of eosinophils in the circulation, bone marrow and target tissues, particularly the airways and lungs in patients with asthma [Iida et al. 2006]. Benralizumab decreases blood eosinophils and basophils close to the limit of detection and reduces eosinophil precursors in the bone marrow by 80% or more. Therefore, it may provide a marked depletion of airway eosinophils and basophils through enhanced ADCC, giving a possible explanation for the great reduction of exacerbations in asthma.
Table 1.
Benralizumab and the other anti-interleukin-5 monoclonal antibodies.
| Compound | Mechanism of action | Key points |
|---|---|---|
| Benralizumab | High-binding affinity to α-chain of IL-5R. Induces ADCC of both eosinophils and basophils | Decreases blood eosinophils and basophils close to the limit of detection and reduces eosinophil precursors in the bone marrow by 80%. FDA and EMA approval. Subcutaneous administration |
| Mepolizumab | N-glycosylated IgG1/κ humanized monoclonal antibody. Binds IL-5 with high specificity and affinity | First anti-IL-5, several studies available in literature. FDA and EMA approval. Subcutaneous administration |
| Reslizumab | IgG4/κ monoclonal antibody targeting circulating IL-5 with high affinity | Several efficacy data available. FDA approval. The intravenous administration could be a limiting factor |
ADCC, antibody-dependent cell-mediated cytotoxicity; EMA, European Medicines Agency; FDA, Food and Drug Administration; IL-5R, interleukin 5 receptor.
Preclinical studies
Kolbeck and colleagues used surface plasmon resonance to determine the binding affinity of MEDI-563 to FcgRIIIa [Kolbeck et al. 2010]. Human eosinophils and basophils were used to demonstrate antibody-dependent cell-mediated cytotoxicity. The binding epitope of MEDI-563 on IL-5Ra was determined by using site-directed mutagenesis and the drug effect on peripheral blood and bone marrow eosinophil depletion was studied on a monkey model (cynomolgus). The authors showed that benralizumab at various dose levels (0.1, 1, 10 and 30 mg/kg intravenously every 3 weeks × 12 weeks) binds to an epitope on IL-5Ra close to the IL-5 binding site, inhibits IL-5-mediated cell proliferation and causes antibody-dependent cell-mediated cytotoxicity on eosinophils and basophils in vitro. Blood eosinophils and eosinophil precursors in the bone marrow were markedly reduced at all dose levels and all eosinophils had been bound by benralizumab after incubation.
These encouraging results have promoted the clinical development of this biological agent, especially in asthma.
Clinical data on asthma
In November 2008 the first phase I randomized clinical trial (RCT) was completed on benralizumab for asthma [ClinicalTrials.gov identifier: NCT00512486]. This was a multicentre, open-label, single-administration, sequential dose escalation study in subjects with mild asthma with the primary outcome to evaluate the effects on the overall incidence of adverse events for each dose of the drug (BIW-8405/MEDI-56). Secondary outcome measures were in particular the assessment of eosinophil counts in peripheral blood, fractional exhaled nitric oxide (FeNO), and eosinophil cationic protein (ECP). Single escalating doses of MEDI-563 resulted in a marked reduction of peripheral blood eosinophil counts within 24 h after dosing, with an acceptable safety profile. The pharmacokinetic and pharmacodynamic relationships for benralizumab were also evaluated in the same study. The pharmacokinetic activity was dose proportional at doses of 0.03–3 mg/kg while the elimination half life was about 18 days. The most frequently reported adverse events were reduced white blood cell counts, nasopharyngitis and increased blood creatine phosphokinase [Busse et al. 2010].
Between April 2008 and April 2011, a phase I, double-blind, placebo-controlled trial was conducted [ClinicalTrials.gov identifier: NCT00659659] to evaluate the safety profile of MEDI-563-benralizumab and its effects on eosinophils in the airways, sputum, bone marrow and peripheral blood in adults with atopic asthma [Laviolette et al. 2013]. Twenty-seven patients were randomized in two cohorts to receive respectively an intravenous infusion of 1 mg/kg benralizumab or placebo, or 100 or 200 mg benralizumab or placebo divided into four subcutaneous injections. Induced sputum samples were collected for eosinophil evaluation at screening and at subsequent time points. Patients underwent a baseline bronchoscopy with airway mucosal biopsies at least 7 days after screening sputum induction and within 2 days before day 0. Some patients who provided informed consent had bone marrow aspiration at screening and day 28. Finally, blood samples were taken. The incidence of adverse events was similar among groups. In the two cohorts, intravenous or subcutaneous, benralizumab produced a median decrease from baseline of 61.9% and 95.8% in airway mucosal eosinophils, 18.7% and 89.9% in sputum and 100% in blood, respectively. Eosinophils were not detectable in bone marrow of subjects on active treatment and significant adverse events did not appear in both groups, demonstrating a good safety and efficacy profile for benralizumab (Table 2).
Table 2.
The recent history of benralizumab through its key studies.
| Author | Study population | Study design | Dosage | Results |
|---|---|---|---|---|
| Busse et al. [2010] | 44 with mild atopic asthma | Multicentre, open-label, single-administration, sequential dose escalation of BIW-8405/MEDI-563 | 0.03 mg/kg, 0.1 mg/kg, 0.3 mg/kg, 1.0 mg/kg, 3.0 mg/kg intravenous injection | Reduction of PB eosinophil counts within 24 h after dosing |
| Laviolette et al. [2013] | 27 adults with eosinophilic asthma | Multicentre, double-blind, placebo-controlled phase I study | Placebo or benralizumab 1 mg/kg intravenous injection or subcutaneous doses of placebo or benralizumab 100 or 200 mg | Single-dose intravenous and multiple-dose subcutaneous benralizumab reduced eosinophil counts in airway mucosa/submucosa and sputum, and suppressed eosinophils in bone marrow and PB |
| Gossage et al. [2010] | 25 with persistent adult asthma | Phase IIa, randomized, double-blind, placebo-controlled, dose-escalation study | Benralizumab subcutaneous dose 25 mg, 100 mg and 200 mg | Peripheral blood eosinophils depletion |
| Nowak et al. [2015] | 136 with severe asthma | Randomized, double-blind, placebo-controlled study | Intravenous infusion of placebo or benralizumab 0.3 mg/kg, n = 36 or 1.0 mg/kg | One dose of benralizumab, reduced rate and severity of exacerbations over 12 weeks in subjects who presented to the ED with acute asthma |
| Castro et al. [2014] | 324 with persistent eosinophilic and noneosinophilic asthma | Randomized, controlled, double-blind, dose-ranging phase IIb study | 2 mg benralizumab, 20 mg benralizumab, or 100 mg benralizumab | Reduced asthma exacerbations in adults with uncontrolled eosinophilic asthma and baseline blood eosinophils of at least 300 cells/μl |
| Park et al. [2016] | 106 adults with uncontrolled eosinophilic asthma | Multicentre, randomized, double-blind, placebo-controlled study | 20 mg and 100 mg benralizumab subcutaneously | Reduced asthma exacerbations, improved lung function and asthma control |
ED, emergency department; PB: Peripheral blood.
A phase IIa study [ClinicalTrials.gov identifier: NCT00783289] evaluated the safety and tolerability of multiple doses of benralizumab administered subcutaneously to adults with asthma [ClinicalTrials.gov identifier: NCT00783289]. In this trial, subjects received thrice monthly subcutaneous doses of benralizumab (25, 100 and 200 mg, respectively or placebo). A few days after dosing, an almost complete peripheral blood eosinophils depletion was observed, remaining stable for 160 days in all cohorts with an acceptable safety profile.
Sera of patients enrolled in the previous two trials [ClinicalTrials.gov identifier: NCT00659659 and NCT00783289] were collected (number of patients=14+24) and compared with sera of 20 healthy volunteers [Pham et al. 2016]. Blood eosinophils, IL-5, eosinophil derived neurotoxin (EDN), ECP, eotaxin/chemokine (CeC motif) 11 (CCL11), eotaxin-2/CCL24, tumour necrosis factor (TNF) and interferon ɤ (IFNɤ) were measured at baseline and post benralizumab administration. After treatment a significant reduction of blood eosinophils and sera EDN and ECP were found in comparison to baseline (p < 0.05). No changes in TNF or IFNɤ were observed, while serum IL-5, eotaxin/CCL11 and eotaxin-2/CCL24 increased after anti IL-5 mAb administration versus placebo (p < 0.05). These results suggest that cytotoxic granule proteins were not released after eosinophil reduction following treatment with benralizumab.
A subsequent phase II, multicentre, randomized, double-blind RCT was completed in 2011. The primary outcome was the evaluation of two intravenous dose regimens of MEDI-563 (0.3 and 1.0 mg/kg) in adult patients who required an urgent healthcare visit for an asthma exacerbation [ClinicalTrials.gov identifier: NCT00768079]. One hundred and ten subjects were stratified according to baseline blood eosinophil counts of at least 450 or greater than 450 cells/μl and randomized to benralizumab (0.3 mg/kg or 1.0 mg/kg) or placebo according to a 2:1 ratio. Patients were followed up for 168 days after drug administration. This study showed that one single dose of benralizumab added onto current standard maximal care with bronchodilators and systemic corticosteroids significantly reduced blood eosinophil counts, rate and severity of exacerbations (–49%), and hospitalizations (–60%) in subjects who presented to the emergency department with an asthma exacerbation [Nowak et al. 2015]. Another interesting finding was the persistent effect of the single dose of benralizumab on exacerbations beyond 12 weeks, independently of blood eosinophil levels, thereby reducing the use of healthcare resources; the effect on eosinophil reduction was independent of the compliance with usual oral and inhaled asthma treatments. Despite these positive outcomes, benralizumab had a poor impact on pulmonary function, asthma control and asthma quality of life [Nowak et al. 2015].
Further investigations have been planned to confirm the promising results that have emerged so far. A phase IIb dose-ranging study with the aim of assessing the efficacy and safety of benralizumab was completed in March 2012 [ClinicalTrials.gov identifier: NCT01238861]. Benralizumab 2 or 20 or 100 mg or placebo were randomly assigned to 324 ‘eosinophilic patients’ while 285 noneosinophilic subjects received 100 mg benralizumab or placebo [Castro et al. 2014]. In the eosinophilic pattern, benralizumab led to an 80% reduction of exacerbation rates compared with placebo in the 100 mg group; on the contrary, in the 2 and 20 mg groups the results were not significant (Table 2). In patients with a baseline blood eosinophil cutoff of 300 cells/μl, the efficacy of benralizumab was even more evident in reducing exacerbation rates, including the 20 mg group. In these patients, benralizumab also allowed improvements of mean forced expiratory volume in 1 second (FEV1) and the six-item Asthma Control Questionnaire (ACQ-6) score from baseline to week 52 compared with placebo; no adverse events were observed, apart from nasopharyngitis and injection site reactions.
A similar phase II RCT was conducted in a specific geographical setting in Korea and Japan [ClinicalTrials.gov identifier: NCT01412736]. One hundred and six patients were randomized into four groups: placebo, subcutaneous benralizumab at doses of 2, 20 and 100 mg. The primary endpoint, that is reduction in asthma exacerbation rate at week 52, was improved by 33%, 45% or 36% versus the placebo group when treated with 2, 20 or 100 mg benralizumab, respectively [Park et al. 2016], with a significant improvement even in lung function (Table 2).
These data confirm that patients with high levels of eosinophils are the real target of anti-IL-5 mAbs and in general the overall results of phase II RCTs underline that benralizumab is safe and effective in reducing eosinophils and improving asthma control compared with placebo.
AstraZeneca recently launched the phase III Windward program for benralizumab, including a series of RCTs, a few already completed with preliminary data available.
The first trial was CALIMA [ClinicalTrials.gov identifier: NCT01914757], with the primary outcome to evaluate the effect of benralizumab on asthma exacerbations in adult and adolescent patients with uncontrolled asthma despite best standard therapy. This study also assessed the safety and the effects on lung function, symptoms, emergency room access and hospitalization rates during a period of 56 weeks.
The Windward program includes two other phase III pivotal RCTs for benralizumab added to high-dose (SIROCCO) [ClinicalTrials.gov identifier: NCT01928771] or medium-dose (PAMPERO) [ClinicalTrials.gov identifier: NCT01947946] inhaled corticosteroids plus a long-acting β agonist. The preliminary results of these studies confirm the achievement of the primary endpoint, demonstrating significant reductions in the annual asthma exacerbation rate compared with placebo.
Another RCT is in progress with the primary outcome to evaluate the effect of two dosing regimens of benralizumab on oral corticosteroid use in terms of percentage reduction comparing final versus baseline oral corticosteroid dose (ZONDA) [ClinicalTrials.gov identifier: NCT02075255]. Finally, a long-term safety trial (BORA) is currently recruiting patients [ClinicalTrials.gov identifier: NCT02258542] with the main purpose of assessing safety and tolerability of two dosing regimens of benralizumab for adult patients, with a duration of 56 weeks. The end of this trial is expected in June 2018.
Other anti-IL-5 mAbs
The first and probably most well known anti-IL-5 agent is mepolizumab, a N-glycosylated IgG1/κ humanized mAb with a disulphide bridge binding two light chains and two heavy chains (molecular weight 149.2 kDa) [Zia-Amirhosseini et al. 1999]. This mAb has high specificity (IC50 < 1 nM) and affinity (Kd = 4.2 pM) for IL-5, inhibiting binding to the α chain of the IL-5 receptor complex expressed on the eosinophil cell surface [Hart et al. 2001; Clutterbuck and Sanderson, 1990]. In November 2015, mepolizumab received US Food and Drug Administration (FDA) approval for the treatment of severe eosinophilic asthma with the brand name of Nucala (GSK, 980 Great West Road Brentford Middlesex (UK)) at the dose of 100 mg administered subcutaneously once every 4 weeks (www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm471031.htm). In December 2015, the European Medicines Agency approved a marketing authorization as ‘medicine under additional monitoring’ valid throughout the European Union (http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003860/human_med_001933.jsp&mid=WC0b01ac058001d124).
The first studies with mepolizumab produced contradictory results in the clinical setting, raising questions about its efficacy in asthma treatment [Flood-Page et al. 2003; Leckie et al. 2000].
These problems were mainly related to an inaccurate selection of patients, who had not been carefully evaluated for airway eosinophilia. As a matter of fact, mepolizumab was associated with a significant reduction in blood and sputum eosinophils but a statistically significant change was not achieved in any of the clinical endpoints measured, in particular, decrease in exacerbation rates [Flood-Page et al. 2007].
Another limitation to reliably defining the effectiveness and safety of mepolizumab was represented by a relatively small number of patients and a short duration of studies.
Only when an ‘eosinophilic phenotype’ was identified as the potential subgroup responsive to mepolizumab was it realized that history had taken the right direction.
The MENSA trial enrolled 576 patients with eosinophilic inflammation and recurrent asthma exacerbations despite high doses of inhaled glucocorticoids [Ortega et al. 2014]. Patients were randomized to one of three study groups to receive intravenous mepolizumab 75 mg or a subcutaneous dose of 100 mg, or placebo, every 4 weeks for 32 weeks. The rate of exacerbations was the primary outcome, other endpoints were FEV1, St George’s Respiratory Questionnaire (SGRQ) and five-item ACQ (ACQ-5) scores. Exacerbation rate was reduced by 47% in the intravenous group and by 53% in the subcutaneous group. The mean increase from baseline in FEV1 at the end of the study was 100 ml greater than placebo for intravenous mepolizumab and 98 ml greater than placebo in patients receiving subcutaneous mepolizumab. Significant improvements in the SGRQ and ACQ-5 scores were found in comparison with placebo in both the intravenous and subcutaneous mepolizumab groups, with a safety profile of the active drug comparable to placebo. In contrast to previous studies, the MENSA trial confirmed the efficacy of mepolizumab in reducing asthma exacerbations, but improvements in markers of asthma control, including FEV1, were demonstrated too.
A randomized, double-blind trial (SIRIUS study) of 135 patients with severe eosinophilic asthma compared the glucocorticoid-sparing effect of subcutaneous mepolizumab 100 mg every 4 weeks for 20 weeks with placebo [Bel et al. 2014]. Mepolizumab was associated with a reduction in the glucocorticoid dose 2.39 times greater than in the placebo group (95% confidence interval, 1.25–4.56; p = 0.008); concomitant findings were a 32% relative reduction in the annualized rate of exacerbations (1.44 versus 2.12, p = 0.04) and a significant improvement in asthma symptoms (p = 0.004).
A milestone in the clinical positioning of the drug was the ‘Dose Ranging Efficacy and Safety with Mepolizumab’ (DREAM) study, a large multicentre, double-blind, placebo-controlled trial enrolling 621 subjects with a history of recurrent severe asthma exacerbations, and evidence of eosinophilic inflammation [Pavord et al. 2012]. Patients were randomly assigned to receive one of three doses of intravenous mepolizumab (75, 250 or 750 mg) or matched placebo (100 ml 0.9% NaCl). The active treatment significantly reduced blood and sputum eosinophil counts and the number of asthma exacerbations, despite a small effect on FEV1, Asthma Quality of Life Questionnaire (AQLQ) and ACQ scores compared with the placebo group. Tolerability at 12 months was very good.
Since patients affected by severe asthma with frequent exacerbations and persistent eosinophilia account for about 40% of patients with severe asthma, the above studies have offered evidence for new treatment options, provided that appropriate selection of patients can be carried out [Rothenberg et al. 2008].
Reslizumab (Cinqair; Teva; Corporate Headquarters. 5 Basel St., Petach Tikva Israel) is a second anti-IL-5 biological, recently approved by the FDA (http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491980.htm) as an add-on maintenance treatment for adults (18 years and older) with severe eosinophilic asthma. Reslizumab is a humanized monoclonal (IgG4/κ) antibody with high affinity for circulating IL-5, preventing its binding to specific receptor [Kips et al. 2003]. A preclinical study in allergic mice, monkeys and rabbits (Sch 55700) showed a long-term effect in reducing pulmonary eosinophilia and airway hyperresponsiveness [Egan et al. 1999].
Similarly to mepolizumab, early RCTs did not show clinically important results in patients with severe uncontrolled asthma [Kips et al. 2003] in terms of pulmonary function tests or symptoms, despite a significant reduction in circulating and sputum eosinophils. The selection of an hypereosinophilic asthmatic phenotype (sputum eosinophils > 3% or blood eosinophils 400/μl) led to a clear improvement of clinical outcomes. In a phase II trial of patients with asthma and nasal polyposis, asthma symptoms improved significantly (p = 0.012) along with asthma control as measured by the ACQ [Gevaert et al. 2006]. Significant improvements in FEV1 and ACQ score were found in subsequent phase III RCTs [Bjermer et al. 2014], particularly in the subgroup with nasal polyposis [Castro et al. 2011].
According to FDA indications, reslizumab should be administered once every 4 weeks via intravenous infusion by an experienced healthcare professional in an appropriate clinical setting to handle any possible adverse reactions or anaphylaxis.
Severe asthma phenotyping as a milestone
Large series with patients undergoing asthma phenotyping have allowed clinical and pathological features to be demonstrated that correlate either with eosinophilic or neutrophilic inflammation [Fahy, 2009]. Studies on induced sputum have identified two other inflammatory phenotypes of asthma, that is granulocytic and paucigranulocytic, most common in adults and children with stable asthma [Simpson et al. 2006]. Eosinophilic asthma is a distinct phenotype accounting for about 50% of patients with asthma, clinically characterized by severity of impairment correlating with level of eosinophilic inflammation and corticosteroid responsiveness. The main pathological peculiarity is represented by thickening of the basement membrane in the airway mucosa [Woodruff et al. 2009; Hasegawa et al. 2015], a feature atypical in noneosinophilic asthma, a relatively corticosteroid-resistant condition frequently associated with severe disease [Pepe et al. 2005]. Recurrent asthma exacerbations can predominate in a subgroup of patients with eosinophilic airway inflammation [Haldar et al. 2008]. At a biological level, eosinophilic infiltration is a key feature of Th2-driven inflammation, and eosinophils can be a useful biomarker in guiding treatment [Holgate, 2008]. As discussed so far, the greatest benefits in patients treated with anti-IL-5 mAbs and in particular benralizumab were seen in patients with blood eosinophil levels above 300 or 400 cells/μl [Park et al. 2016; Castro et al. 2014], who showed significant improvement in annual exacerbation rates, lung function and asthma control scores. Among asthma phenotypes, the literature shows that the potential responders to anti-IL-5 mAbs, in particular mepolizumab, are subjects with persistent systemic and airway eosinophilia (>0.3×109/liter in blood, >3% in sputum), possibly steroid responsive, with poor symptom control, treated with high-dose inhaled and systemic corticosteroids and a heavy impact on quality of life because of poor asthma control and high exacerbation rate [Ortega et al. 2014]. A better clinical response to mepolizumab has been reported in patients with eosinophilia greater than 500 cells/µl [Ortega et al. 2014], although to select patients correctly, a multimodal approach is necessary. An appropriate identification of the eligible population can indeed maximize the clinical benefits and reduce the costs of therapeutic failure.
Another interesting aspect is that the proportion of patients with severe asthma and IgE greater than 400 UI/ml is significantly greater than in series with moderate asthma [Dávila et al. 2015; European Network for Understanding Mechanisms of Severe Asthma, 2003]. This can lead to a possible overlap between omalizumab and anti-IL-5 biologics. At present, in severe allergic asthma, omalizumab is the gold standard treatment, with positive clinical outcomes represented by a reduction in exacerbations, a systemic corticosteroid sparing effect and an improvement in quality of life [Normansell et al. 2014]. In clinical trials, such as the INNOVATE and another six studies of patients with severe atopic asthma, baseline IgE was the only predictor of efficacy of omalizumab since statistical significance was reached in the upper IgE quartile only (p < 0.001) [Bousquet et al. 2007; Humbert et al. 2005]. According to the DREAM study, mepolizumab allowed a significant reduction in severe asthma exacerbations irrespective of the baseline IgE levels or atopic status [Pavord et al. 2012]. Since the populations eligible for mepolizumab or omalizumab partially overlap, a multicentre RCT has been planned in patients with severe eosinophilic asthma to evaluate the effect of mepolizumab when treatment with omalizumab has not allowed effective asthma control [ClinicalTrials.gov identifier: NCT02654145]. A recent study demonstrated that the anti-IL-5 agents can be effective even in patients who are nonresponders to omalizumab, with a steroid-sparing effect [Prazma et al. 2014]. The results will assist clinicians in understanding whether an anti-IgE or an anti-IL-5 mAb should be the first treatment of choice according to the phenotype of severe allergic asthma.
In the EXTRA trial, enrolling 850 patients, omalizumab was more effective in subjects with higher blood eosinophils (>260 cells/μl), high FeNO and periostin levels [Hanania et al. 2013]. In this phenotype anti-IL-5 agents should be studied as the first choice treatment in nonresponders to anti-IgE molecules.
Conversely, the DREAM study [Pavord et al. 2012] demonstrated that mepolizumab effectiveness was influenced by higher baseline peripheral blood eosinophils and frequent exacerbations. An appropriate patient selection according to these two variables would indicate mepolizumab as the first choice, also considering a steroid-sparing effect with a consistent reduction of related side effects in the long term.
To definitively clarify these doubts and uncertainties, there is a great need for RCTs comparing biological drugs head to head.
Biomarkers
The development of new biological therapies has stimulated much interest in biomarkers for the diagnosis and management of asthma to be used in combination with clinical and functional data that do not correlate with airways inflammation. The present gold standard to assess airway inflammation is represented by invasive procedures, that is, endoscopic biopsies and bronchoalveolar lavage, difficult to apply regularly in real-life settings [Djukanović, 1996; Lommatzsch et al. 2013]. An ideal biomarker should be cheap, readily available, reproducible, minimally invasive and clinically predictive. Unfortunately, none of the many molecules tested present those characteristics altogether.
The main biomarkers used at present in eosinophilic asthma are the level of eosinophils in sputum and blood. However, they have important limitations, since induced sputum is not practical to obtain, eosinophil levels in sputum are variable and not always reproducible, while circulating eosinophilia has a low value in predicting airway pathology [Al-Samri et al. 2010].
In the DREAM study, eosinophil counts in blood, but not in sputum, correlated with the response to mepolizumab [Pavord et al. 2012]. A post hoc analysis showed a peripheral blood eosinophil count at screening of at least 150 cells/ml was a good predictor of response in patients with unstable asthma and frequent exacerbations [Katz et al. 2014]. In contrast, a baseline eosinophil count of less than 150 cells/ml was associated with a limited reduction of asthma exacerbations. Although single analysis can be acceptable in RCTs, in clinical practice a single measurement might not be sufficient to evaluate patients accurately because blood eosinophil levels can show large spontaneous fluctuations over time. A common alternative is the expired FeNO, whose levels correlate with airway eosinophils [Jatakanon et al. 1998]. However, data are controversial because some authors reported that FeNO closely correlates with sputum eosinophils [Schleich et al. 2010], while others showed an association between FeNO and sputum eosinophilia only in 78% of cases [Wagener et al. 2015]. As a consequence, there is no agreement on the use of this biomarker for the prescription of IL-5 antagonizing agents [Westerhof et al. 2015].
Serum periostin (or osteoblast-specific factor 2) is a matrix protein originally identified in mesenchymal cells, for example osteoblasts, osteoblast-derived cells and periosteum, that can be secreted by bronchial epithelial cells following IL-13 stimulation. So far it has been studied to predict the response to lebrikizumab, an anti-IL-13 mAb [Westerhof et al. 2015]. The serum levels of this protein have been found to correlate with sputum eosinophilia and eosinophilic airway inflammation [Izuhara et al. 2016]. The present data for asthma are controversial, since in one study serum periostin levels were found to be an optimal predictor of airway eosinophilia [Jia et al. 2012], while in other studies periostin and total IgE did not discriminate between eosinophilic and noneosinophilic asthma [Westerhof et al. 2015; Wagener et al. 2015].
Other directions: chronic obstructive pulmonary disease
Eosinophilic airway inflammation is considered a feature of asthma, while a neutrophilic pattern is prevalent in chronic obstructive pulmonary disease (COPD). However, for many individual cases there is no clear distinction between asthma and COPD but a continuum in the variability of clinical and biological characteristics. The ‘rediscovery’ of overlap conditions, such as ACOS (asthma–COPD overlap syndrome) in recent studies has led to a scientific debate on acceptable definitions and optimal management strategies useful in clinical practice [Sin et al. 2016]. Eosinophilic inflammation is present in 20–40% of large and small airway tissue samples and induced sputum from patients with stable COPD [Saha and Brightling, 2006]. Eosinophils increase during exacerbations and might be a therapeutic target in COPD. Inhaled steroids are the key controller drugs in asthma but also have a role in severe and very severe COPD [Fabbri et al. 2003], while systemic steroids are indicated in the treatment of moderate and severe exacerbations both in asthma and in COPD [Fabbri et al. 2003; Bafadhel et al. 2014]. However, there is a subgroup of subjects with asthma and eosinophilia who are resistant to steroids and need targeted treatments; the same may occur in COPD [Grainge et al. 2016].
A phase II trial to evaluate the effectiveness of benralizumab in patients with moderate to severe COPD, aged 40–85 years, with sputum eosinophilia was completed in July 2013 [ClinicalTrials.gov identifier: NCT01227278]. Patients received placebo or three doses of 100 mg benralizumab subcutaneously every 4 weeks and then five doses every 8 weeks for a period of 48 weeks. The primary endpoint was the annualized rate of acute exacerbations of COPD at week 56 [Brightling et al. 2014]. Benralizumab showed a significant improvement in lung function and quality of life but was not able to reduce the annualized exacerbation rate, although the clinical effect on patients with airway eosinophilia (⩾200 cells/µl) was higher.
A few phase III RCTs that are now in progress, for example the GALATHEA study, have the primary outcome of evaluating the effect of benralizumab on COPD exacerbations in subjects with moderate to very severe COPD [ClinicalTrials.gov identifier: NCT02138916]. Another phase III study is TERRANOVA [ClinicalTrials.gov identifier: NCT02155660], a 56-week placebo-controlled, multicentre trial evaluating efficacy and safety of three subcutaneous doses of benralizumab in subjects with moderate to very severe COPD who are already receiving standard treatment with frequent exacerbations and hospitalizations. Outcomes include pulmonary function, health status, quality of life, respiratory symptoms, rescue medication use, severity, frequency, and duration of exacerbations.
Eosinophilia may be considered as a potential biomarker to identify and select patients with severe or very severe COPD who might benefit from inhaled corticosteroids [Watz et al. 2016] and anti-IL-5 biological treatments such as benralizumab.
Conclusion
The great increase in knowledge of pathophysiological and immunological mechanisms of asthma has led to the development of new and targeted therapeutic options for severe asthma. The available biological drugs have peculiarities that distinguish one from another and can meet the peculiar indications of specific subgroups. There are controversies about the appropriate criteria for patient selection and sometimes the partial overlap between the therapeutic potential of some biologicals, for example anti-IL-5 agents and omalizumab, makes it even more difficult to decide on the best individual treatment. The introduction of nonpharmacological approaches, such as BT, has brought further complexity. However, according to current evidence, the procedure should be considered in patients with severe asthma who are nonresponders to omalizumab or other biologics or who reject pharmacological therapies [Trivedi et al. 2016]. In any case, the complementary treatment of patients with either BT or biologics in addition to standard maximal therapies may help the clinician to meet the needs of a greater number of patients, but also can lead to a reduction in emergency room visits and hospitalizations, with considerable economic savings in the long term [Menzella et al. 2014].
As noted above, the ideal patients eligible for anti-IL-5 therapy are those with blood eosinophil levels above 300 or 400 cells/μl [Park et al. 2016; Castro et al. 2014], even if it seems that a better clinical response to mepolizumab has been reported in patients with eosinophilia greater than 500 cells/µl [Ortega et al. 2014]. This should be the patient subgroup to be considered for benralizumab and other anti-IL-5 mAbs; however, due to many similarities, the optimal anti-IL-5 agent to choose remains a matter of debate. Literature data show that all are safe and effective, but mepolizumab is the one with the largest scientific documentation and has already been approved for marketing. A limitation for reslizumab may be the intravenous route of administration, which is more complex and less well tolerated by patients in the long run. Conversely, benralizumab seems to have unique characteristics and advantages compared with its competitors due to its mechanism of action and pharmacodynamic properties. First, benralizumab binds the IL-5 receptor and not the IL-5 ligand, blocking in a more direct way the action of the cytokine and the effector cells. Benralizumab does not result in upregulation of IL-5 expression, as occurs for the other anti-IL-5 agents [Stein et al. 2008].
The effect of benralizumab is independent of the IL-5 circulating level, which tends to increase during asthma exacerbation [Sahid El-Radhi et al. 2000], and through ADCC and apoptosis drastically reduces levels of IL-5Rα-bearing cells, without evidence of cytolysis-mediated degranulation and release of toxic proteins [Busse et al. 2010]. The depletion of eosinophils through ADCC prevents the effects of a possible activation by other cytokines (IL-3 and GM-CSF), as may occur with mepolizumab and reslizumab [Tai et al. 1991]. As shown in preclinical studies, these characteristics also make it insensitive to the presence of receptors on the cell surface [Kolbeck et al. 2010], while the absence of the fucose sugar residue in its molecular structure gives benralizumab much higher affinity to the human Fcγ RIIIa receptor [Iida et al. 2006], overcoming the inhibitory effects determined by serum IgGs [Kolbeck et al. 2010]. Also the ability to reduce bone marrow precursors of eosinophils and basophils makes the anti-inflammatory effects of benralizumab more intense [Kolbeck et al. 2010]. Another peculiarity is a very rapid action in terms of eosinophil decrease compared with the other anti-IL-5 mAbs: in the study by Laviolette and coworkers a single intravenous administration of 1 mg/kg benralizumab produced a full depletion of eosinophils from the first day until 12 weeks after dosing [Laviolette et al. 2013]. Rapidity of action has also been confirmed in the clinical setting: one dose of benralizumab reduced the rate and severity of exacerbations in subjects on standard care presenting to the emergency department with an asthma exacerbation [Nowak et al. 2015]. The clinical benefits and improvements of lung function and AQLQ were confirmed in the long term, mostly in patients with blood eosinophil counts of at least 300 cells/μl [Castro et al. 2014], with a good safety profile.
Benralizumab seems to be a very promising and effective drug in a carefully selected population of patients with severe asthma. Extensive phase III trials, some already in progress, are needed to elucidate the real cost-effectiveness profile of the drug in clinical practice.
Footnotes
Funding: This work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Francesco Menzella and Luigi Zucchi participated in contracted research and clinical trials for Novartis, Sanofi, AstraZeneca and Glaxo Smith Kline.
The other authors report no conflicts of interest in this work.
Contributor Information
Francesco Menzella, Department of Cardio-Thoracic-Vascular and Intensive Care Medicine, Pneumology Unit, IRCCS – Arcispedale Santa Maria Nuova, Viale Risorgimento 56, 42123 Reggio Emilia, Italy.
Mirco Lusuardi, Unit of Respiratory Rehabilitation, AUSL Reggio Emilia, S. Sebastiano Hospital, Correggio, Italy.
Carla Galeone, Department of Cardio-Thoracic-Vascular and Intensive Care Medicine, Pneumology Unit, IRCCS – Arcispedale Santa Maria Nuova, Reggio Emilia, Italy.
Nicola Facciolongo, Department of Cardio-Thoracic-Vascular and Intensive Care Medicine, Pneumology Unit, IRCCS – Arcispedale Santa Maria Nuova, Reggio Emilia, Italy.
Luigi Zucchi, Department of Cardio-Thoracic-Vascular and Intensive Care Medicine, Pneumology Unit, IRCCS – Arcispedale Santa Maria Nuova, Reggio Emilia, Italy.
References
- Al-Samri M., Benedetti A., Préfontaine D., Olivenstein R., Lemière C., Nair P., et al. (2010) Variability of sputum inflammatory cells in asthmatic patients receiving corticosteroid therapy: a prospective study using multiple samples. J Allergy Clin Immunol 125: 1161– 1163. [DOI] [PubMed] [Google Scholar]
- Bafadhel M., Davies L., Calverley P., Aaron S., Brightling C., Pavord I. (2014) Blood eosinophil guided prednisolone therapy for exacerbations of COPD: a further analysis. Eur Respir J 44: 789– 791. [DOI] [PubMed] [Google Scholar]
- Baptista-dos-Reis R., Muniz V., Neves J. (2015) Multifaceted roles of cysteinyl leukotrienes in eliciting eosinophil granule protein secretion. Biomed Res Int 2015: 848762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett S., Nurmagambetov T. (2011) Costs of asthma in the United States: 2002–2007. J Allergy Clin Immunol 127: 145–52. [DOI] [PubMed] [Google Scholar]
- Becchetti E., Bani D., Baroni T. (2011) In: Idelson-Gnocchi (ed.), Istologia Umana. pp. 393–394. ISBN: 9788879475419. [Google Scholar]
- Bel E., Wenzel S., Thompson P., Prazma C., Keene O., et al. SIRIUS Investigators (2014) Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med 371: 1189–1197. [DOI] [PubMed] [Google Scholar]
- Berek C. (2016) Eosinophils: important players in humoral immunity. Clin Exp Immunol 183: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjermer C., Maspero J., Ciesielska M., O’Brien C., Zangrilli J. (2014) A randomized phase 3 study of the efficacy and safety of reslizumab in subjects with asthma with elevated eosinophils. Eur Resp J 44: 299. [Google Scholar]
- Bousquet J., Rabe K., Humbert M., Chung K., Berger W., Fox H., et al. (2007) Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respir Med 101: 1483–1492. [DOI] [PubMed] [Google Scholar]
- Brightling C., Bleecker E., Panettieri R., Jr, Bafadhel M., She D., Ward C., et al. (2014) Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir Med 2: 891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. (2015) FDA panel backs mepolizumab for severe eosinophilic asthma. Medscape Medical News 11 June. [Google Scholar]
- Busse W., Katial R., Gossage D., Sari S., Wang B., Kolbeck R., et al. (2010) Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol 125: 1237–1244. [DOI] [PubMed] [Google Scholar]
- Castro M., Mathur S., Hargreave F., Boulet L., Xie F., Young J., et al. (2011) Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med 184: 1125–1132. [DOI] [PubMed] [Google Scholar]
- Castro M., Wenzel S., Bleecker E., Pizzichini E., Kuna P., Busse W., et al. (2014) Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med 2: 879–890. [DOI] [PubMed] [Google Scholar]
- Clutterbuck E., Sanderson C. (1990) Regulation of human eosinophil precursor production by cytokines: a comparison of recombinant human interleukin-1 (rhIL-1), rhIL-3, rhIL-5, rhIL-6, and rh granulocyte-macrophage colony-stimulating factor. Blood 75: 1774–1779. [PubMed] [Google Scholar]
- Curtis C., Ogbogu P. (2015) Evaluation and differential diagnosis of persistent marked eosinophilia. Immunol Allergy Clin North Am 35: 387–402. [DOI] [PubMed] [Google Scholar]
- Davila I., Valero A., Entrenas L., Valveny N., Herráez L. (2015) Relationship between serum total IgE and disease severity in patients with allergic asthma in Spain. J Investig Allergol Clin Immunol 25(2): 120–127. [PubMed] [Google Scholar]
- Djukanović R. (1996) Bronchoscopy as a research tool for the study of asthma pathogenesis and effects of antiasthma drugs. J Allergy Clin Immunol 98(5 Pt 2): S41–5; discussion S64–S66. [DOI] [PubMed] [Google Scholar]
- Egan R., Athwal D., Bodmer M., Carter J., Chapman R., Chou C., et al. (1999) Effect of Sch 55700, a humanized monoclonal antibody to human interleukin-5, on eosinophilic responses and bronchial hyperreactivity. Arzneimittelforschung 49: 779–790. [DOI] [PubMed] [Google Scholar]
- European Network for Understanding Mechanisms of Severe Asthma (2003) The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. Eur Respir J 22: 470–477. [DOI] [PubMed] [Google Scholar]
- Fabbri L., Romagnoli M., Corbetta L., Casoni G., Busljetic K., Turato G., et al. (2003) Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med 167: 418–424. [DOI] [PubMed] [Google Scholar]
- Fahy J. (2009) Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc 6: 256–259. [DOI] [PubMed] [Google Scholar]
- Flood-Page P., Menzies-Gow A., Kay A., Robinson D. (2003) Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med 167: 199–204. [DOI] [PubMed] [Google Scholar]
- Flood-Page P., Swenson C., Faiferman I., Flood-Page P., Swenson C., Faiferman I., et al. (2007) A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med 176: 1062–1071. [DOI] [PubMed] [Google Scholar]
- George L., Brightling C. (2016) Eosinophilic airway inflammation: role in asthma and chronic obstructive pulmonary disease. Ther Adv Chronic Dis 7: 34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevaert P., Lang-Loidolt D., Lackner A., et al. (2006) Nasal IL-5 levels deterthe response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol 118(5): 1133–1141. [DOI] [PubMed] [Google Scholar]
- Global Asthma Report (2014) Global burden of disease due to asthma. Available at: http://www.globalasthmareport.org/burden/burden.php (accessed 4 July 2016).
- Gossage D., Geba G., Gillen A. (2010). A multiple ascending subcutaneous dose study of MEDI-563, A humanized anti-IL-5RA monoclonal antibody, in adult asthmatics (clinicaltrails.gov Identifier: NCT00783289). Annual Congress of the European Respiratory Society (ERS).
- Grainge C., Thomas P., Mak J., Benton M., Lim T., Ko F. (2016) Year in review 2015: asthma and chronic obstructive pulmonary disease. Respirology 21: 765–775. [DOI] [PubMed] [Google Scholar]
- Haldar P., Pavord I., Shaw D., Berry M., Thomas M., Brightling C., et al. (2008) Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 178: 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanania N., Wenzel S., Rosén K., Hsieh H., Mosesova S., Choy D., et al. (2013) Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med 187(8): 804–811. [DOI] [PubMed] [Google Scholar]
- Hart T., Cook R., Zia-Amirhosseini P., Minthorn E., Sellers T., Maleeff B., et al. (2001) Preclinical efficacy and safety of mepolizumab (SB-240563), a humanized monoclonal antibody to IL-5, in cynomolgus monkeys. J Allergy Clin Immunol 108: 250–257. [DOI] [PubMed] [Google Scholar]
- Hasegawa K., Stoll S., Ahn J., Bittner J., Camargo C., Jr (2015) Prevalence of eosinophilia in hospitalized patients with asthma exacerbation. Respir Med 109: 1230–1232. [DOI] [PubMed] [Google Scholar]
- Holgate S. (2008) Pathogenesis of asthma. Clin Exp Allergy 38: 872–897. [DOI] [PubMed] [Google Scholar]
- Humbert M., Beasley R., Ayres J., Slavin R., Hébert J., Bousquet J., et al. (2005) Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 60: 309–316. [DOI] [PubMed] [Google Scholar]
- Iida S., Misaka H., Inoue M., Shibata M., Nakano R., Yamane-Ohnuki N., et al. (2006) Nonfucosylated therapeutic IgG1 antibody can evade the inhibitory effect of serum immunoglobulin G on antibody-dependent cellular cytotoxicity through its high binding to FcgammaRIIIa. Clin Cancer Res 12:2879–2887. [DOI] [PubMed] [Google Scholar]
- Izuhara K., Conway S., Moore B., Matsumoto H., Holweg C., Matthews J., et al. (2016) Roles of periostin in respiratory disorders. Am J Respir Crit Care Med 193: 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatakanon A., Lim S., Kharitonov S., Chung K., Barnes P. (1998) Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax 53: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G., Erickson R., Choy D., Mosesova S., Wu L., Solberg O., et al. (2012) Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol 130: 647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarski R., Mufti G. (1991) The cytokine receptor superfamily. Blood Rev 5: 193–203. [DOI] [PubMed] [Google Scholar]
- Katz L., Gleich G., Hartley B., Yancey S., Ortega H. (2014) Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc 11: 531–536. [DOI] [PubMed] [Google Scholar]
- Kips J., O’Connor B., Langley S., et al. (2003) Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. Am J Respir Crit Care Med 167: 1655–1659. [DOI] [PubMed] [Google Scholar]
- Klein Wolterink R., Kleinjan A., van Nimwegen M., Bergen I., de Bruijn M., Levani Y., et al. (2012) Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol 42: 1106–1116. [DOI] [PubMed] [Google Scholar]
- Kolbeck R., Kozhich A., Koike M., Peng L., Andersson C., Damschroder M., et al. (2010) MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol 125: 1344–1353. [DOI] [PubMed] [Google Scholar]
- Kouro T., Takatsu K. (2009) IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol 21: 1303–1309. [DOI] [PubMed] [Google Scholar]
- Laviolette M., Gossage D., Gauvreau G., Leigh R., Olivenstein R., Katial R, et al. (2013) Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol 132: 1086–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckie M., ten Brinke A., Khan J., Diamant Z., O’Connor B., Walls C., et al. (2000) Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 356: 2144–2148. [DOI] [PubMed] [Google Scholar]
- Lommatzsch S., Martin R., Good J., Jr (2013) Importance of fiberoptic bronchoscopy in identifying asthma phenotypes to direct personalized therapy. Curr Opin Pulm Med 19: 42–48. [DOI] [PubMed] [Google Scholar]
- Masoli M., Fabian D., Holt S., Beasley R. (2012) The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 59: 469–478. [DOI] [PubMed] [Google Scholar]
- McKinnon M., Bank M., Solari R., Robinson G. (1999) Interleukin-5 and the interleukin receptor: targets for drug discovery in asthma. In: Sanderson C. (ed.), Interleukin-5: From Molecule to Drug Target for Asthma. New York: Marcel Dekker, Inc, pp. 299–320. [Google Scholar]
- Menzella F., Facciolongo N., Piro R., Formisano D., Roggeri A., Simonazzi A., et al. (2012) Clinical and pharmacoeconomic aspects of omalizumab: a 4-year follow up. Ther Adv Respir Dis 6: 87–95. [DOI] [PubMed] [Google Scholar]
- Menzella F., Zucchi L., Piro R., Galeone C., Castagnetti C., Facciolongo N. (2014) A budget impact analysis of bronchial thermoplasty for severe asthma in clinical practice. Adv Ther 31: 751–761. [DOI] [PubMed] [Google Scholar]
- Menzies-Gow A., Flood-Page P., Sehmi R., Burman J., Hamid Q., Robinson D., et al. (2003) Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol 111: 714–719. [DOI] [PubMed] [Google Scholar]
- Nair P., Pizzichini M., Kjarsgaard M., Inman M., Efthimiadis A., Pizzichini E., et al. (2009) Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med 360: 985–993. [DOI] [PubMed] [Google Scholar]
- Normansell R., Walker S., Milan S., Walters E., Nair P. (2014) Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 1: CD003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak R., Parker J., Silverman R., Rowe B., Smithline H., Khan F., et al. (2015) A randomized trial of benralizumab, an antiinterleukin 5 receptor α monoclonal antibody, after acute asthma. Am J Emerg Med 33: 14–20. [DOI] [PubMed] [Google Scholar]
- Ortega H., Liu M., Pavord I., Brusselle G., FitzGerald J., Chetta A., et al. (2014) Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 371: 1198–1207. [DOI] [PubMed] [Google Scholar]
- Park H., Kim M., Imai N., Nakanishi T., Adachi M., Ohta K., et al. (2016) A phase 2a study of benralizumab for patients with eosinophilic asthma in South Korea and Japan. Int Arch Allergy Immunol 169: 135–145. [DOI] [PubMed] [Google Scholar]
- Pavord I., Korn S., Howarth P., Bleecker E., Buhl R., Keene O. (2012) Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 380: 651–659. [DOI] [PubMed] [Google Scholar]
- Pepe C., Foley S., Shannon J., Lemiere C., Olivenstein R., Ernst P., et al. (2005) Differences in airway remodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol 116: 544–549. [DOI] [PubMed] [Google Scholar]
- Pham T., Damera G., Newbold P., Ranade K. (2016) Reductions in eosinophil biomarkers by benralizumab in patients with asthma. Respir Med 111: 21–29. [DOI] [PubMed] [Google Scholar]
- Prazma C., Wenzel S., Barnes N., Douglass J., Hartley B., Ortega H. (2014) Characterisation of an OCS-dependent severe asthma population treated with mepolizumab. Thorax 69(12): 1141–1142. [DOI] [PubMed] [Google Scholar]
- Rothenberg M., Hogan S. (2006) The eosinophil. Ann Rev Immunol 24: 147–174. [DOI] [PubMed] [Google Scholar]
- Rothenberg M., Klion A., Roufosse F., Kahn J., Weller P., Simon H., et al. (2008) Mepolizumab HES Study Group. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med 358: 1215–1228. [DOI] [PubMed] [Google Scholar]
- Saha S., Brightling C. (2006) Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis 1: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahid El-Radhi A., Hogg C., Bungre J., Bush A., Corrigan C. (2000) Effect of oral glucocorticoid treatment on serum inflammatory markers in acute asthma. Arch Dis Child 83: 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleich F., Seidel L., Sele J., Manise M., Quaedvlieg V., Michils A., et al. (2010) Exhaled nitric oxide thresholds associated with a sputum eosinophil count ⩾3% in a cohort of unselected patients with asthma. Thorax 65: 1039–1044. [DOI] [PubMed] [Google Scholar]
- Simpson J., Scott R., Boyle M., Gibson P. (2006) Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology 11: 54–61. [DOI] [PubMed] [Google Scholar]
- Sin D., Miravitlles M., Mannino D., Soriano J., Price D., Celli B., et al. (2016) What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J 23 June DOI: 10.1183/13993003.00436-2016. [DOI] [PubMed] [Google Scholar]
- Stein M., Villanueva J., Buckmeier B., Yamada Y., Filipovich A., Assa’ad A., et al. (2008) Anti-IL-5 (mepolizumab) therapy reduces eosinophil activation ex vivo and increases IL-5 and IL-5 receptor levels. J Allergy Clin Immunol 121: 1473–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P., Sun L., Spry C. (1991) Effects of IL-5, granulocyte/macrophage colony-stimulating factor (GM-CSF) and IL-3 on the survival of human blood eosinophils in vitro. Clin Exp Immunol 85: 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi M., Hara T., Ichihara M., Takatsu K., Miyajima A. (1995) Multi-colony stimulating activity of interleukin 5 (IL-5) on hematopoietic progenitors from transgenic mice that express IL-5 receptor alpha subunitconstitutively. J Exp Med 181: 889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Komai M., Nagao K., Ishizaki M., Kajiwara D., Takatsu K., et al. (2004) Role of interleukin-5 and eosinophils in allergen-induced airway remodeling in mice. Am J Respir Cell Mol Biol 31: 62–68. [DOI] [PubMed] [Google Scholar]
- Toba K., Koike T., Shibata A., Hashimoto S., Takahashi M., Masuko M., et al. (1999) Novel technique for the direct flow cytofluorometric analysis of human basophils in unseparated blood and bone marrow, and the characterization of phenotype and peroxidase of human basophils. Cytometry 35: 249–259. [PubMed] [Google Scholar]
- Trivedi A., Pavord I., Castro M. (2016) Bronchial thermoplasty and biological therapy as targeted treatments for severe uncontrolled asthma. Lancet Respir Med 4: 585–592. [DOI] [PubMed] [Google Scholar]
- Valent P. (1994) The phenotype of human eosinophils, basophils, and mast cells. J Allergy Clin Immunol 94: 1177–1183. [DOI] [PubMed] [Google Scholar]
- Wagener A., de Nijs S., Lutter R., Sousa A., Weersink E., Bel E., et al. (2015) External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax 70: 115–120. [DOI] [PubMed] [Google Scholar]
- Walsh G. (2013) Eosinophil apoptosis and clearance in asthma. J Cell Death 6: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watz H., Tetzlaff K., Wouters E., Kirsten A., Magnussen H., Rodriguez-Roisin R., et al. (2016) Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med 4: 390–398. [DOI] [PubMed] [Google Scholar]
- Wells T., Graber P., Proudfoot A., Arod C., Jordan S., Lambert M., et al. (1994) The three-dimensional structure of human interleukin-5 at 2.4-angstroms resolution: implication for the structures of other cytokines. Ann N Y Acad Sci 28(725): 118–127. [DOI] [PubMed] [Google Scholar]
- Westerhof G., Korevaar D., Amelink M., de Nijs S., de Groot J., Wang J., et al. (2015) Biomarkers to identify sputum eosinophilia in different adult asthma phenotypes. Eur Respir J 46: 688–696. [DOI] [PubMed] [Google Scholar]
- Woodruff P., Modrek B., Choy D., Jia G., Abbas A., Ellwanger A., et al. (2009) T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 180: 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zia-Amirhosseini P., Minthorn E., Benincosa L., Hart T., Hottenstein C., Tobia L., et al. (1999) Pharmacokinetics and pharmacodynamics of SB-240563, a humanized monoclonal antibody directed to human interleukin-5, in monkeys. J Pharmacol Exp Ther 291: 1060–1067. [PubMed] [Google Scholar]