Abstract
Malignant pleural mesothelioma (MPM) represents a significant diagnostic and therapeutic challenge and is almost always a fatal disease. Imaging abnormalities are common, but have a limited role in distinguishing mesothelioma from metastatic pleural disease. Similarly, minimally invasive biomarkers have shown promise but also have limitations in the diagnosis of mesothelioma. In experienced centers, cytology and immunohistochemistry are now sufficient to diagnose the epithelioid subtype of mesothelioma, which can reduce the need for more invasive diagnostic investigations. Prognosis of MPM is modestly impacted by oncological treatments. Chemotherapy with cisplatin and pemetrexed is considered the standard of care, though the addition of bevacizumab to the platinum doublet may be the new standard of care. New targeted therapies have demonstrated some promise and are being addressed in clinical trials. This review focuses on the current data on the diagnostic and therapeutic issues of MPM.
Keywords: angiogenesis, chemotherapy, clinical trials, diagnosis, immunotherapy, mesothelioma, symptom management
Introduction
Malignant mesothelioma is a progressive, incurable malignancy of the pleura or peritoneum that is almost exclusively due to exposure to asbestos [Robinson and Lake, 2005]. Given the long latency period of mesothelioma, the incidence of mesothelioma is expected to plateau and then reduce over the coming decades in countries where asbestos control measures have been instituted. However, a rising incidence of mesothelioma remains an increasing problem in underdeveloped nations, where asbestos use continues unchecked [Leigh et al. 2002; Robinson and Lake, 2005; Le et al. 2011]. This review updates the reader on current diagnostic pathways in mesothelioma, followed by an overview of accepted management options and current avenues of research in mesothelioma treatment.
Diagnosis and imaging of mesothelioma
Malignant mesothelioma is one of the most challenging malignancies to diagnose. The existence of mesothelioma was initially doubted by prominent pathologists prior to the current epidemic from asbestos [Willis, 1960]. There are three main histological subtypes, epithelioid, sarcomatoid and biphasic, all with individual diagnostic challenges and prognoses [Brims and Maskell, 2013]. Symptoms from malignant pleural mesothelioma (MPM) include dyspnea, weight loss and chest wall pain, and have an insidious onset. Confirmation of the diagnosis is often prolonged as distinguishing MPM from benign pleural effusions related to asbestos or pleural effusions from other malignancies is challenging. Many factors cause this delay including identifying asbestos exposure and, when confirming the diagnosis, there are limitations of imaging, biomarkers and pathological sampling [Madsen et al. 2013].
Imaging investigations in suspected MPM can guide sampling of tissue and provide additional information on staging, in particular, invasion and extra pleural spread. Phenotypically, MPM can develop pleural based nodules, pleural effusions or diffuse pleural thickening [Finn et al. 2012]. Imaging of suspected MPM cases reflects these abnormalities. Computed tomography (CT) is more sensitive for pleural effusions and chest wall invasion than chest X-ray (CXR). CT features suggesting malignancy rather than benign pleural disease include circumferential pleural thickening, parietal thickening >1 cm, nodular pleural thickening and mediastinal pleural involvement. While these findings are specific, they are not sensitive and they do not differentiate MPM from metastatic pleural disease [Leung et al. 1990]. Loss of normal extrapleural fat planes on CT indicates the presence of chest wall invasion, which in conjunction with pathological findings can confirm the diagnosis. Magnetic resonance imaging (MRI), however, is more sensitive for identifying local invasion [Heelan et al. 1999]. Ultrasound is increasingly used in the assessment of pleural effusions and can identify suitable sites for biopsy [Havelock et al. 2010]. Likewise, fluorodeoxyglucose positron emission tomography (FDG PET) can identify sites of lymphadenopathy or metastasis, which are common, or sites suitable for direct biopsy [Kruse et al. 2013]. Dual-time point FDG PET, which includes a repeated delayed PET acquisition, may assist in differentiating malignant and benign pleural disease [Mavi et al. 2009]. Greater FDG uptake, measured as standardized uptake value (SUVmax), total glycolytic volume or total lesion glycolyis, is associated with poorer prognosis [Nowak et al. 2010; Terada et al. 2012]. While the role for multimodality treatment of MPM is controversial, in those who are considering surgery, comprehensive imaging is necessary for complete staging.
The role of biomarkers
Biomarkers are objectively measured characteristics that indicate biological processes, pathogenic processes or pharmacologic responses to therapeutic intervention. Biomarkers can act as potential diagnostic, prognostic and predictive factors [Strimbu and Tavel, 2010]. Evaluating the role of a biomarker for the diagnosis of MPM is challenging. An ideal MPM diagnostic biomarker needs to show excellent discrimination at an early stage of disease not only between MPM and normal controls, but also between malignant and benign pleural effusions and against otherwise healthy asbestos exposed patients. Additionally, any biomarker needs to be easily sampled with a minimally invasive technique and have an acceptable cost.
Three MPM specific biomarkers have been most extensively evaluated: osteopontin, soluble mesothelin and fibulin-3. Osteopontin is a mediator of cell–matrix interactions through binding with integrins and CD44 receptors. When compared with healthy asbestos exposed patients, serum osteopontin is elevated in MPM with the area under the curve (AUC) ranging from 0.72 to 0.89 [Pass et al. 2005]. Pleural or serum osteopontin cannot discriminate between MPM and other pleural effusions [Grigoriu et al. 2007]. Therefore, osteopontin has a limited role in the diagnosis of MPM in the context of a new pleural effusion.
Mesothelin is an antigen on normally differentiated mesothelial cells. The full length protein is cleaved into a C-terminal membrane bound fragment and an N-terminal fragment that is released into the blood and which is called megakaryocyte potentiating factor (MPF). Some of the membrane bound C-terminal fragment is released into the blood and is named soluble mesothelin. Both MPF and soluble mesothelin have been assessed as potential biomarkers. Serum and pleural soluble mesothelin is able to discriminate between MPM of the epitheliod subtype and healthy asbestos controls, benign pleural diseases and other malignant pleural effusions. It is unclear if MPF or soluble mesothelin is superior [Creaney et al. 2008; Iwahori et al. 2008].
Fibulin-3 initially showed promise as a diagnostic MPM biomarker. Early reports showed plasma fibulin-3 levels had an AUC of 0.87 when comparing patients with MPM and asbestos exposed controls [Pass et al. 2012]. Subsequent testing reported an AUC of 0.822 for mesothelin compared with 0.671 for fibulin-3, suggesting that soluble mesothelin remains the most useful diagnostic biomarker available [Creaney et al. 2014]. Changes in microRNA expression may also differentiate MPM from lung adenocarcinoma and MPM from benign asbestos related pleural effusions, but more work is required [Benjamin et al. 2010; Ak et al. 2015].
Histopathology and cytology diagnosis
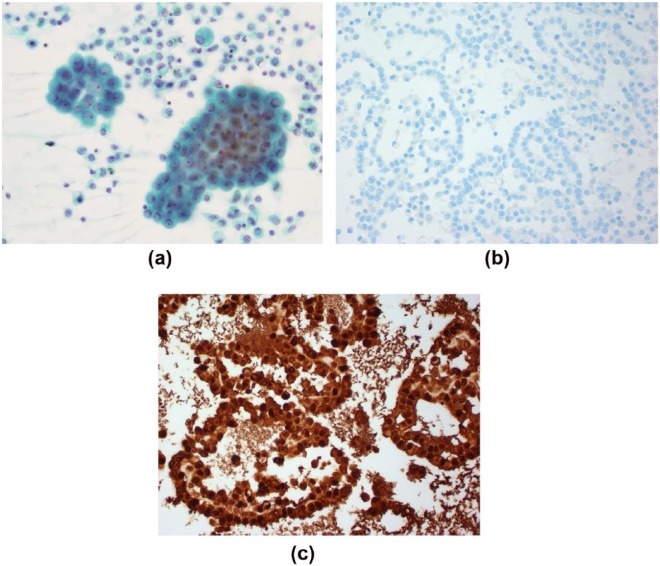
Cellular material for analysis is usually obtained via cytological examination of pleural fluid or biopsy of solid mass. Previous recommendations state that MPM should only be confirmed with histological material [Husain et al. 2009]. In experienced hands, however, MPM can be diagnosed on pleural fluid cytology alone [Segal et al. 2013]. This is advantageous as sampling of pleural fluid is minimally invasive and may lead to earlier confirmation of the diagnosis. The combination of cytomorphological features and immunohistochemistry (IHC) is sufficient (Figure 1). IHC antibodies for MPM include epithelial membrane antigen (EMA), calretinin, Ck5/6 and mesothelin. While these have individual varying specificity, a panel of such markers can differentiate MPM from major malignant differentials [Dejmek and Hjerpe, 2000]. Consequently, recent recommendations from the International Mesothelioma Interest Group now advise that cytological diagnosis of epitheliod mesothelioma is possible [Hjerpe et al. 2015]. Effusions in sarcomatoid MPM are often paucicellular, which limits the role of cytology analysis.
Figure 1.
Pleural fluid cytology examination from a patient with malignant mesothelioma. There are malignant cells seen on Papanicolaou stain (a) that are negative for the adenocarcinoma immunohistochemical stain MOC-31 (b) but positive for mesothelioma immunohistochemical stain Calretinin (c).
Systemic chemotherapy for mesothelioma
A majority of the unselected population with a new diagnosis of mesothelioma pursue palliative anticancer treatment rather than aggressive surgical management of this disease [Kao et al. 2013]. Hence we initially describe palliative systemic therapy for mesothelioma.
First-line chemotherapy
Despite extensive research into mesothelioma therapeutics, cytotoxic chemotherapy remains one of the only therapeutic options with a proven survival benefit in patients with MPM. The nature of cytotoxic chemotherapy in clinical practice has remained unchanged since 2003, but there is new research on the horizon in targeted therapies that may alter the current standard of care. The EMPHACIS trial in 2003 was the pivotal phase III trial which demonstrated that cisplatin (75 mg/m2) in combination with pemetrexed (500 mg/m2) every 21 days compared with cisplatin alone improved median survival from 9.3 to 12.1 months [hazard ratio (HR) 0.74, p = 0.003] [Vogelzang et al. 2003]. On the basis of these data, cisplatin and pemetrexed has become the standard of care (SOC) first-line therapy worldwide for patients with advanced unresectable MPM. Another anti-metabolite raltitrexed(3 mg/m2) in combination with cisplatin (80 mg/m2) compared with cisplatin alone did produce a 2.6 month survival benefit [van Meerbeeck et al. 2005]. However, this combination did produce a lower objective radiological response rate (23.6%) compared with cisplatin and pemetrexed. Raltitrexed is therefore an active alternative, but this drug is not approved for this indication in many jurisdictions.
Ongoing research over the past decade has investigated strategies to improve the response rate and outcomes of first-line chemotherapy. Research into tumor angiogenesis and targeted therapies paved the way for the recently published and well-designed MAPS trial, which evaluated bevacizumab (15 mg/kg for 6 cycles) plus SOC (cisplatin/pemetrexed for 6 cycles) followed by maintenance bevacizumab until progression versus SOC alone in 448 patients with advanced mesothelioma [Zalcman et al. 2015]. Median overall survival (OS) in the triplet arm was 18.8 months compared with 16.1 months in the SOC arm (HR 0.76, p = 0.012). This 2.7 month survival benefit was statistically and clinically significant, and may place this triplet therapy as the new SOC first-line agent [Zalcman et al. 2015]. There is unlikely to be a randomized trial testing the addition of bevacizumab to carboplatin and pemetrexed in mesothelioma as the US Food and Drug Administration (FDA) does not recognize carboplatin and pemetrexed as an approved first-line regimen for mesothelioma.
Whether this is adopted as a new standard will depend on the interplay between clinical effectiveness and health economics. As this stage, there are no clear clinical predictors of efficacy or biomarkers to select the patients most likely to benefit from this triplet therapy. On subgroup analysis, there was no indication that patients with sarcomatoid/biphasic histology or poor performance status did not derive benefit from this triplet combination. Nevertheless, a subgroup analysis from the only other randomized trial of chemotherapy with or without bevacizumab in mesothelioma demonstrated that patients with a high pretreatment plasma vascular endothelial growth factor (VEGF) concentration had worse OS and progression-free survival (PFS), and that a benefit to the addition of bevacizumab was confined to those with baseline VEGF levels below the median; this trial did not show any overall benefit for the addition of bevacizumab [Kindler et al. 2012]. Identification of biomarkers for appropriate patient selection would benefit the cost-effectiveness of using this monoclonal antibody.
Despite the use of the platinum/pemetrexed combination for more than a decade, there remain a number of outstanding questions around the optimal use of chemotherapy. Irrespective of the lack of randomized evidence, carboplatin is often substituted for cisplatin due to simpler administration and perceived lower toxicity. There have been no direct phase III comparisons between the two platinum agents, although phase I and II studies have shown similar activity and similar objective radiological response rates of either in combination with pemetrexed [Ceresoli et al. 2006, 2008]. Patients are often offered carboplatin in the setting of medical contraindications to cisplatin.
The optimum number of cycles of chemotherapy has not yet been defined in the management of patients with mesothelioma. For patients with a good performance status, adequate organ function and stable or responding disease, usual practice is a maximum of six cycles of platinum/pemetrexed. At this stage, it is unclear if four cycles would provide a similar survival benefit but lower toxicity than six cycles, as shown in patients with nonsmall cell lung cancer (NSCLC) [Socinski et al. 2002]. Also in NSCLC, pemetrexed maintenance therapy following four cycles of pemetrexed–cisplatin was shown to significantly increase PFS and OS [Paz-Ares et al. 2012]. The question is whether this strategy would also work in MPM. A small nonrandomized Dutch study of 13 patients showed that pemetrexed maintenance therapy is well tolerated [van den Bogaert et al. 2006]. A randomized phase II trial [ClinicalTrials.gov identifier: NCT01085630] is currently still recruiting participants to a study comparing maintenance pemetrexed with observation.
Another outstanding question is whether, in asymptomatic patients with MPM, it is appropriate to delay treatment until symptoms develop, or whether early treatment of asymptomatic patients provides a survival benefit. A phase II trial evaluated 21 patients in an early treatment group (ETG) and 22 patients in a delayed treatment group (DTG). Median survival was 14 months in the ETG compared with 10 months in the DTG [O’Brien et al. 2006]. This trial suggested a 4 month survival benefit in early treatment; however, of note, the chemotherapy used in this study was later demonstrated to be inactive against best supportive care (BSC) in the MS01 trial [Muers et al. 2008]. This strongly questions the interpretation of these results as supporting early treatment.
Second-line chemotherapy
Patients inevitably experience disease recurrence or progression after initial chemotherapy. If the individual had a good response to first-line platinum–pemetrexed and there has been a sufficient relapse-free interval, it is reasonable to consider retreatment with a platinum–pemetrexed combination and observational data supports this practice, although there is no randomized trial evaluating re-treatment of pemetrexed following first-line use [Ceresoli et al. 2011]. A phase III trial compared OS of second-line pemetrexed plus BSC versus BSC alone; however, this study recruited before first-line pemetrexed based treatment became standard care. In this trial, second-line pemetrexed did elicit a significant tumor response and delayed disease progression [Jassem et al. 2008]. Taken together, there is sufficient evidence to support re-treatment with the platinum–pemetrexed combination in patients with adequate performance status and reasonable control after first-line treatment.
There are a number of chemotherapeutic agents with second-line activity, although none of the agents have been tested by means of a randomized controlled trial. A phase II trial assessed the safety and efficacy of single agent vinorelbine in 62 patients [Stebbing et al. 2009]. The trial demonstrated an objective response rate of 16% and an OS of 9.6 months. Given the tolerability of vinorelbine and its response rate, it has been favored as the second-line agent in practice. Cisplatin and gemcitabine were incorporated as second-line chemotherapy agents on the basis of phase II trials demonstrating activity in the upfront setting [Nowak et al. 2002; Castagneto et al. 2005]. Based on these phase II trials, gemcitabine-based doublets seem to have moderate activity in MPM and may be considered in the second-line setting.
In summary, although a number of cytotoxic agents have shown modest second-line activity, this setting is awaiting an agent that has demonstrated response rates and OS gains by means of a randomized controlled trial.
New approaches: targeted therapy and immunotherapy
At present no actionable driver mutations have been identified for targeted agents in MPM. Nevertheless, over the last decade a number of targeted agents have been trialed empirically in this disease. Perhaps, the best way to structure a logical discussion around the diversity of targeted therapies is by classification based on Hanahan and Weinberg’s Hallmarks of Cancer [Hanahan and Weinberg, 2011] which divide the incredibly complex neoplastic process into simplified principles.
Sustaining proliferative signaling
Tumor cells have the capacity to release growth factors that facilitate and accelerate tumor cell growth, and drugs have been developed to impact these growth factors. A majority of the trials have been negative and are summarized in Table 1. There are two upcoming trials targeting growth factors, using cetuximab and the insulin-like growth factor (IGF) inhibitor cixutumumab (Table 1).
Table 1.
Clinical trials targeting sustained proliferative signaling through growth factors.
| Negative trials | ||||
|---|---|---|---|---|
| Mechanism | Rationale | Drug | Trial design | Reference |
| EGFR inhibition | Activating EGFR mutations are rare but EGFR is over-expressed between 50% and 95% of patients [43,44] | Erlotinib | Phase II | [Garland et al. 2007] |
| First line | ||||
| Gefitinib | Phase II | [Govindan et al. 2005] | ||
| First line | ||||
| PDGF inhibition | PDGF binds to PDGF receptor and induced mesothelial cell proliferation | Imatinib | Phase I | [Mathy et al. 2005] |
| First line | ||||
| +platinum/ pemetrexed | ||||
| Dasatinib | Phase II | [Dudek et al. 2012] | ||
| First-line | ||||
| + Gemcitabine | ||||
| Multi-receptor tyrosine kinase inhibitors | Sorafenib | Phase II | [Dubey et al. 2010] | |
| Monotherapy | ||||
| Pretreated patients | ||||
| Sunitinib | Phase II | [Nowak et al. 2012] | ||
| Monotherapy | ||||
| Pretreated patients | ||||
| Upcoming trials | ||||
| Mechanism | Rationale | Drug | Trial design | ClinicalTrials.gov identifier |
| IGF inhibition | IGF supports tumor cell growth and division | Cixutumumab | Phase II | NCT01160458 |
| Monotherapy | ||||
| Pretreated patients | ||||
| EGFR inhibition | Cetuximab | Phase II | NCT00996567 | |
| Monotherapy | ||||
| First-line + platinum/ pemetrexed | ||||
EGFR, epidermal growth factor receptor; IGF, insulin like growth factor; PDGF, platelet derived growth factor.
Avoiding immune destruction
Mesothelioma, like other tumors, can be immunogenic but has developed several mechanisms to evade immune destruction. Furthermore, the host immune response has powerful mechanisms to restrain potentially autoreactive T cells and prevent tissue damage in normal states, namely ‘checkpoint molecules’. The first checkpoint molecule to be successfully blocked for cancer treatment was cytotoxic T lymphocyte antigen-4 (CTLA-4). CTLA-4 is normally upregulated following T-cell activation and is a negative regulator, which competes with the costimulatory molecule CD28 to bind with B7 ligands, turning a positive costimulatory signal into a negative signal for activated T cells. Under normal circumstances, this mechanism functions to restrain autoimmunity; however, it also restrains antitumor immunity. By blocking CTLA-4, a strategy known as ‘checkpoint blockade’, the antitumor immune response can be restored. This has been established as a new drug class with therapeutic efficacy in other cancers, particularly melanoma [Hodi et al. 2010].
Examples of monoclonal antibodies against CTLA4 receptor include ipilimumab and tremelimumab. Tremelimumab was investigated in a phase II single arm study of 29 pretreated patients with MPM [Calabro et al. 2013]. The median PFS was 6.2 months and median OS was 10.7 months. This study did not meet its primary endpoint for overall response rate, with a partial response rate of 7% and a disease control rate of 31%. However, this trial dosed patients at 15 mg/kg every 90 days, a schedule that did not prove efficacious in melanoma either. Tremelimumab has subsequently been further tested in mesothelioma using 10 mg/kg every 28 days, reporting an immune-related partial response in 4 of 29 patients (14%) and a disease control rate of 52%, with a median OS of 11.3 months and median immune-related PFS of 6.2 months [Calabro et al. 2015]. There is an ongoing phase IIb trial randomizing pretreated patients to either monotherapy with tremelimumab or placebo [ClinicalTrials.gov identifier: NCT01843374], which has closed to recruitment at the time of writing.
Escape mechanisms to evade immune destruction include expression of programmed death 1 ligand (PD-L1). PD-L1 has recently been shown to be expressed in 40% of 106 samples of human mesothelioma, including almost all those with a sarcomatoid subtype, and was associated with significantly poorer outcomes, with a median survival of 5 months for those whose tumors expressed PD-L1 versus 14.5 months for those whose tumors did not (p < 0.0001)[Mansfield et al. 2014]. PD-L1 is the ligand for PD-1, which is a co-inhibitory receptor expressed by activated T cells. Binding of programmed death 1 (PD-1) to PD-L1 downregulates effector T-cell function and abrogates the immune response. These receptors can be targeted by monoclonal antibodies which block this negative regulation of T-cell function, hence ‘releasing the brakes’ at the effector site on the antitumor response. Examples of PD-1 receptor blocking monoclonal antibodies include pembrolizumab and nivolumab (Table 2).
Table 2.
Other upcoming and ongoing trials of agents in malignant pleural mesothelioma.
| Class | Agent | Phase | ClinicalTrials.gov identifier |
|---|---|---|---|
| Chemotherapy | Maintenance pemetrexed | II | NCT01085630 |
| Antibody against CTLA4 | Tremelimumab | IIb | NCT01843374 |
| Antibody against PD-L1 | Pembrolizumab | II | NCT02399371 |
| Antibody against PD-1 | Nivolumab | II | NCT02497508 |
| MET tyrosine kinase inhibitor | Tivantinib | I | NCT02049060 |
| MET tyrosine kinase inhibitor | Tivantinib | II | NCT01861301 |
| Monoclonal antibody against mesothelin | Amatuximab | II | NCT02357147 |
| Anti-mesothelin antibody-drug conjugate | BAY94-9343 | I | NCT01439152 |
| Pan-VEGF receptor tyrosine kinase inhibitor | Cediranib | I/II | NCT01064648 |
| FAK inhibitor | Defactinib | II | NCT02004028 |
| HSP90 inhibitor | Ganetespid | I/II | NCT01590160 |
CTLA4, cytotoxic T lymphocyte antigen-4; FAK, focal adhesion kinase; HSP90, heat shock protein 90; PD-1, programmed death 1; PD-L1, programmed death 1 ligand; VEGF, vascular endothelial growth factor.
The first report of the efficacy of PD-1/PD-L1 pathway blockade was presented at the American Association of Cancer Research meeting in April 2015. A subgroup of patients with pretreated mesothelioma was treated with the anti-PD1 antibody pembrolizumab in the KEYNOTE-028 trial, a multi-cohort phase Ib trial. Eligible patients had tumors with more than 1% positive membranous expression of PD-L1 on tumor or stromal cells. From 80 patients, 34 patients showed sufficient PD-L1 expression and 25 were enrolled in the cohort. Early reported results are encouraging, with a partial response in 28% and stable disease in 48% of participants, and prolonged responses observed albeit with a relatively short follow-up time [Alley et al. 2015]. Other monoclonal antibodies against PD-L1 and PD-1 are currently being tested in phase II trials for patients with MPM [ClinicalTrials.gov identifiers: NCT02399371, NCT02497508]. We await the results of these trials to further clarify the role of these drugs in the management of MPM.
Activating invasion and metastasis
MET is a tyrosine kinase receptor that is activated by ligand hepatocyte growth factor (HGF) [Feng et al. 2012]. Although mutations in the c-MET gene are rare in MPM, the c-MET receptor is overexpressed in this disease [Jagadeeswaran et al. 2006]. Tivantinib is a small molecule tyrosine kinase inhibitor (TKI) that targets MET kinase activity and is being tested in a first-line phase I study in combination with platinum–pemetrexed [ClinicalTrials.gov identifier: NCT02049060] and in pretreated patients in an ongoing phase II trial [ClinicalTrials.gov identifier: NCT01861301].
The differentiation antigen mesothelin is overexpressed in MPM [Hassan et al. 2008]. Its function in mesothelioma is not entirely clear, but it has been thought to promote tumor invasion. It is also a tumor antigen, which is an appropriate target for immunotherapy [Servais et al. 2012]. Amatuximab, a chimeric monoclonal antibody against mesothelin, was tested in phase II trial with chemotherapy in 89 patients with MPM [Hassan et al. 2014a]. Median PFS was 6.1 months and median OS was 14.8 months. The combination of amatuximab and chemotherapy was relatively well tolerated. A safety and efficacy study of amatuximab in combination with cisplatin–pemetrexed is currently underway [ClinicalTrials.gov identifier: NCT02357147]. Anetumab ravtansine (BAY 94-9343) is a fully human antimesothelin antibody–drug conjugate that showed promising results in preclinical studies [Golfier et al. 2014]. This drug is currently being studied in a phase I trial in patients with advanced solid tumors [ClinicalTrials.gov identifier: NCT01439152] and a randomized phase II study in mesothelioma in the second-line setting, with a comparator arm of single agent vinorelbine, is opening in early 2016 [EudraCT number: 2012-003650-88]. SS1P is a recombinant antimesothelin immunotoxin that was evaluated in combination with chemotherapy in a phase II study of 21 patients [Hassan et al. 2014b]. This study suggested the combination of SS1P with cisplatin–pemetrexed was well tolerated and associated with a significant response. Mesothelin-targeted CAR T-cell therapy is also under investigation [Adusumilli et al. 2014]. Together, these trials provide support for targeting this single antigen as a potentially effective therapy in this disease.
Inducing angiogenesis
Tumor cells can upregulate the expression of vascular endothelial growth factor receptor (VEGFR). When found in high levels, VEGF is associated with poor prognosis in MPM patients [Yasumitsu et al. 2010]. Thalidomide, an established drug with presumed anti-angiogenic properties, was investigated as a maintenance therapy after first-line platinum-pemetrexed for MPM in the phase III NVALT5/MATES study [Buikhuisen et al. 2013a]. This study was a negative study and thalidomide did not slow progression nor improve survival.
Two small molecule TKIs, vatalanib and axitinib, have not shown significant therapeutic activity in MPM in phase II studies [Jahan et al. 2012; Buikhuisen et al. 2013b]. Pazopanib, a multi-kinase angiogenesis inhibitor, was investigated as monotherapy in a phase II trial in chemotherapy- naïve and pretreated patients with MPM. Pazopanib was well tolerated and the primary endpoint PFS rate at 6 months was 47% [Garland et al. 2011] [ClinicalTrials.gov identifier: NCT00459862]. Sunitinib, a similar multitargeted TKI, demonstrated variable results in patients with pretreated disease and has also not moved forward into further trials [Laurie et al. 2011; Nowak et al. 2012].
Cediranib, an oral pan-VEGF receptor TKI, was investigated as monotherapy in 47 patients with pretreated MPM. Of the 47 patients, 4 had objective responses and 16 patients had stable disease [Garland et al. 2011]. A phase I/II trial is currently recruiting MPM patients and will offer cisplatin–pemetrexed with or without cediranib [ClinicalTrials.gov identifier: NCT01064648].
Nintedanib is an intracellular inhibitor that targets multiple tyrosine kinases including VEGFR, fibroblast growth factor receptor (FGFR) and platelet-derived growth factor receptor (PDGFR). Nintedanib has been evaluated in a completed but as yet unreported randomized phase II study in combination with cisplatin–pemetrexed followed by maintenance nintedanib monotherapy versus placebo [ClinicalTrials.gov identifier: NCT01907100].
Overall, despite the MAPS trial data, results with VEGF targeting small molecule TKIs have been disappointing to date. At this stage, pending reporting of the randomized nintedanib trial, it is unclear whether these agents will assume a significant role in the emerging therapeutic landscape.
Genetic instability and mutations
Multiple genetic abnormalities have been identified in MPM through molecular genetic analysis. These genetic alterations may drive the development and progression of MPM. Asbestos fibers can induce chromosome and DNA damage in normal mesothelial cells through several possible mechanisms [Sekido, 2013]. In general, mesothelioma is characterized by loss of tumor suppressor genes rather than gain of function mutations.
Examples of genetic abnormalities which may have implications for therapy include BAP1 and NF2. BAP1 expression is lost in about 20% of patients with MPM and its expression has been reported as required for vinorelbine activity [Zauderer et al. 2013]. A trial is currently recruiting patients in an effort to determine the prevalence of germline and somatic BAP1 mutations in melanoma and mesothelioma [ClinicalTrials.gov identifier: NCT01773655]. NF2 is another tumor suppressor gene that is frequently inactivated in MPM patients. Inactivation of NF2 occurs in about 40% of patients with MPM [Poulikakos et al. 2006]. Cells lacking expression of NF2 products are susceptible to focal adhesion kinase (FAK) inhibition. Defactinib is an oral FAK inhibitor that is being studied in two trials which are currently recruiting participants or recently closed [ClinicalTrials.gov identifier: NCT01773655, NCT02004028].
Heat shock protein 90 (HSP90) is a molecular chaperone that has a multitude of roles, including stabilizing a number of proteins that promote cancer cell growth and survival [Neckers, 2007]. Ganetespid, an oral HSP90 inhibitor, is being evaluated in a phase II trial of MPM patients. The patients will be randomized to cisplatin–pemetrexed with or without ganetespid, with maintenance ganetespid until progression [ClinicalTrials.gov identifier: NCT01590160].
Multi-modality therapy
Surgery
Surgery for MPM may vary from minor procedures for diagnostic, staging and palliative purposes to more complex cytoreductive procedures, and when used with ‘curative intent’ is a controversial topic. It is nearly impossible to resect the pleura with an adequate margin that is also microscopically negative in all directions. The two main surgical procedures are pleurectomy/decortication (P/D) and extrapleural pneumonectomy (EPP). Debulking pleurectomy with a palliative intent (for symptom control) is a more common procedure as most patients with MPM are not suitable for resection with curative intent. At this stage, it is unknown if cytoreductive procedures enhance the efficacy of postoperative chemotherapy or radiotherapy.
EPP is an aggressive surgical procedure that attempts to remove all macroscopic evidence of tumor from the hemithorax. It entails resection of pleura, lung, pericardium, diaphragm and regional lymph nodes. EPP allows for better macroscopic clearance and this procedure is the debulking procedure of choice in specialized centers in North America, Europe and Australia [Grondin and Sugarbaker, 1999; Yan et al. 2011]. The pilot MARS trial randomized 24 patients to EPP and 26 patients to no EPP [Treasure et al. 2009]. Median survival was 14·4 months for the EPP group and 19·5 months for the no EPP group. This limited data suggested that radical surgery in the form of EPP within trimodality therapy offers no benefit and possibly harms patients. However, the trial has been subjected to a number of criticisms and the results should be regarded with caution [Weder et al. 2011]. More recent analyses of large surgical datasets have favored results of P/D over EPP, both in terms of OS and surgical mortality and morbidity [Flores et al. 2008].
Given the lack of clear supporting evidence, EPP should be restricted to high volume institutions with significant experience. The MARS 2 trial [ClinicalTrials.gov identifier: NCT02040272] is a multicenter study that is currently recruiting patients to compare (extended) P/D versus no P/D. We await the results of this trial to further clarify the role of surgery in the management of MPM.
Radiotherapy
Radiotherapy is widely used for patients with MPM largely for palliation of symptoms [Jenkins et al. 2011]. Systematic reviews have not shown evidence that radiotherapy prolongs survival in patients with MPM [Ung et al. 2006]; however, this is not unexpected given the palliative context in which it is usually used.
Hemithoracic radiotherapy can be given after EPP, but it is challenging to target viable disease while avoiding the vital thoracic structures. First experiences with high dose hemithoracic radiotherapy were not favorable as there was significant toxicity [Maasilta, 1991; Maasilta et al. 1991]. Intensity modulated radiotherapy (IMRT) is a newer more accurate technique. A study that assessed 28 patients treated with EPP followed by IMRT showed local control of 100% at 9 month follow up [Ahamad et al. 2003]. In a larger study of 63 patients treated with IMRT after EPP, median OS and 3 year survival were 14.2 months and 20%, respectively [Rice et al. 2007].
Radiotherapy has previously been delivered prophylactically to thoracoscopy or thoracotomy scars to avoid tumor seeding. However, this practice is not supported by available evidence and is discouraged [van Zandwijk et al. 2013], although uptake varies geographically.
Trimodality therapy
Trimodality therapy is a treatment strategy that combines chemotherapy, surgery and radiotherapy [Sugarbaker and Garcia, 1997]. Typically chemotherapy is administered first followed by surgery and then hemithoracic radiotherapy. The treatment course extends over 6 months and the EORTC phase II trial suggested completion rates of 60% or more were achievable [van Schil et al. 2010]. The evidence that supports this practice is derived from retrospective and prospective observational studies, and no randomized controlled trials have been conducted. Patients with good performance status, epithelioid histology and low burden of disease were most likely to benefit from trimodality therapy [Sugarbaker and Norberto, 1998; Edwards et al. 2006].
Conclusion
MPM represents a significant diagnostic and therapeutic challenge, and is almost always a fatal disease. Prognosis can be modestly impacted by chemotherapeutics and VEGF targeting. The optimal role of surgery remains unclear and is a controversial field. The current landscape indicates an emerging potential role for immunotherapies in particular, with the most promising approaches being directed at mesothelin, or incorporating immune checkpoint blockade.
Acknowledgments
This work was supported by the Western Australian Cancer and Palliative Care Network fellowship funding scheme (S.K. and D.M.). The National Centre for Asbestos Related Diseases (NCARD) is supported by the National Health and Medical Research Council of Australia. The authors would like to acknowledge Dr Ming Chai for providing the pleural cytology figures.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Sanjana Kondola, Department of Medical Oncology, Fiona Stanley Hospital, Murdoch, Western Australia, Australia.
David Manners, Department of Respiratory Medicine, Sir Charles Gairdner Hospital, Nedlands, Western Australia, Australia.
Anna K. Nowak, School of Medicine and Pharmacology, University of Western Australia, M503 35 Stirling Hwy Crawley, WA 6009 Australia.
References
- Adusumilli P., Cherkassky L., Villena-Vargas J., Colovos C., Servais E., Plotkin J., et al. (2014) Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med 6: 261ra151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahamad A., Stevens C., Smythe W., Vaporciyan A., Komaki R., Kelly J., et al. (2003) Intensity-modulated radiation therapy: a novel approach to the management of malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 55: 768–775. [DOI] [PubMed] [Google Scholar]
- Ak G., Tomaszek S., Kosari F., Metintas M., Jett J., Metintas S., et al. (2015) MicroRNA and mRNA features of malignant pleural mesothelioma and benign asbestos-related pleural effusion. Biomed Res Int 2015: 635748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley E., Molife R., Satoro A., Beckey K., Yuan S., Cheng J., et al. (2015) Clinical safety and efficacy of pembrolizumab (MK-3475) in patients with malignant pleural mesothelioma: preliminary results from KEYNOTE-028. Cancer Res 75: abstract CT103. [Google Scholar]
- Benjamin H., Lebanony D., Rosenwald S., Cohen L., Gibori H., Barabash N., et al. (2010) A diagnostic assay based on microRNA expression accurately identifies malignant pleural mesothelioma. J Mol Diagn 12: 771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brims F., Maskell N. (2013) Prognostic factors for malignant pleural mesothelioma. Curr Respir Care Rep 2: 100–108. [Google Scholar]
- Buikhuisen W., Burgers J., Vincent A., Korse C., van Klaveren R., Schramel F., et al. (2013a) Thalidomide versus active supportive care for maintenance in patients with malignant mesothelioma after first-line chemotherapy (NVALT 5): an open-label, multicentre, randomised phase 3 study. Lancet Oncol 14: 543–551. [DOI] [PubMed] [Google Scholar]
- Buikhuisen W., Vincent A., Scharpfenecker M., Korse C., Griffioen A., van Pel R. (2013b) A randomized phase II study adding axitinib to pemetrexed–cisplatin in patients with malignant pleural mesothelioma (MPM): clinical results of a single-center trial. J Clin Oncol 31(Suppl.): abstract 7528. [DOI] [PubMed] [Google Scholar]
- Calabro L., Morra A., Fonsatti E., Cutaia O., Amato G., Giannarelli D., et al. (2013) Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol 14: 1104–1111. [DOI] [PubMed] [Google Scholar]
- Calabro L., Morra A., Fonsatti E., Cutaia O., Fazio C., Annesi D., et al. (2015) Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med 3: 301–309. [DOI] [PubMed] [Google Scholar]
- Castagneto B., Zai S., Dongiovanni D., Muzio A., Bretti S., Numico G., et al. (2005) Cisplatin and gemcitabine in malignant pleural mesothelioma: a phase II study. Am J Clin Oncol 28: 223–226. [DOI] [PubMed] [Google Scholar]
- Ceresoli G., Castagneto B., Zucali P., Favaretto A., Mencoboni M., Grossi F., et al. (2008) Pemetrexed plus carboplatin in elderly patients with malignant pleural mesothelioma: combined analysis of two phase II trials. Br J Cancer 99: 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceresoli G., Zucali P., De Vincenzo F., Gianoncelli L., Simonelli M., Lorenzi E., et al. (2011) Retreatment with pemetrexed-based chemotherapy in patients with malignant pleural mesothelioma. Lung Cancer 72: 73–77. [DOI] [PubMed] [Google Scholar]
- Ceresoli G., Zucali P., Favaretto A., Grossi F., Bidoli P., Del Conte G., et al. (2006) Phase II study of pemetrexed plus carboplatin in malignant pleural mesothelioma. J Clin Oncol 24: 1443–1448. [DOI] [PubMed] [Google Scholar]
- Creaney J., Dick I., Meniawy T., Leong S., Leon J., Demelker Y., et al. (2014) Comparison of fibulin-3 and mesothelin as markers in malignant mesothelioma. Thorax 69: 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creaney J., Yeoman D., Demelker Y., Segal A., Musk A., Skates S., et al. (2008) Comparison of osteopontin, megakaryocyte potentiating factor, and mesothelin proteins as markers in the serum of patients with malignant mesothelioma. J Thorac Oncol 3: 851–857. [DOI] [PubMed] [Google Scholar]
- Dejmek A., Hjerpe A. (2000) Reactivity of six antibodies in effusions of mesothelioma, adenocarcinoma and mesotheliosis: stepwise logistic regression analysis. Cytopathology 11: 8–17. [DOI] [PubMed] [Google Scholar]
- Dubey S., Janne P., Krug L., Pang H., Wang X., Heinze R., et al. (2010) A phase II study of sorafenib in malignant mesothelioma: results of Cancer and Leukemia Group B 30307. J Thorac Oncol 5: 1655–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek A., Pang H., Kratzke R., Oetterson G., Hodgson L., Vokes E., et al. (2012) Phase II Dasatinib in patients with previously treated malignant mesothelioma (Cancer and Leukemia Group B 30601): a brief report. J Thorac Oncol 7: 755–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J., Stewart D., Martin-Ucar A., Muller S., Richards C., Waller D. (2006) The pattern of lymph node involvement influences outcome after extrapleural pneumonectomy for malignant mesothelioma. J Thorac Cardiovasc Surg 131: 981–987. [DOI] [PubMed] [Google Scholar]
- Feng Y., Thiagarajan P., Ma P. (2012) MET signaling: novel targeted inhibition and its clinical development in lung cancer. J Thorac Oncol 7: 459–467. [DOI] [PubMed] [Google Scholar]
- Finn R., Brims F., Gandhi A., Olsen N., Musk A., Maskell N., et al. (2012) Postmortem findings of malignant pleural mesothelioma: a two-center study of 318 patients. Chest 142: 1267–1273. [DOI] [PubMed] [Google Scholar]
- Flores R., Pass H., Seshan V., Dycoco J., Zakowski M., Carbone M., et al. (2008) Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 135: 620–626. [DOI] [PubMed] [Google Scholar]
- Garland L., Chansky K., Wozniak A., Tsao A., Gadgeel S., Verschraegen C., et al. (2011) Phase II study of cediranib in patients with malignant pleural mesothelioma: SWOG S0509. J Thorac Oncol 6: 1938–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland L., Rankin C., Gandara D., Rivkin S., Scott K., Nagle R., et al. (2007) Phase II study of erlotinib in patients with malignant pleural mesothelioma: a Southwest Oncology Group Study. J Clin Oncol 25: 2406–2413. [DOI] [PubMed] [Google Scholar]
- Golfier S., Kopitz C., Kahnert A., Heisler I., Schatz C., Stelte-Ludwig B., et al. (2014) Anetumab ravtansine: a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol Cancer Ther 13: 1537–1548. [DOI] [PubMed] [Google Scholar]
- Govindan R., Kratzke R., Herndon J., Niehans G., Vollmer R., Watson D., et al. (2005) Gefitinib in patients with malignant pleural mesothelioma: a phase II study by the Cancer and Leukemia Group B. Clin Cancer Res 11: 2300–2304. [DOI] [PubMed] [Google Scholar]
- Grigoriu B., Scherpereel A., Devos P., Chahine B., Letourneux M., Lebailly P., et al. (2007) Utility of osteopontin and serum mesothelin in malignant pleural mesothelioma diagnosis and prognosis assessment. Clin Cancer Res 13: 2928–2935. [DOI] [PubMed] [Google Scholar]
- Grondin S., Sugarbaker D. (1999) Pleuropneumonectomy in the treatment of malignant pleural mesothelioma. Chest 116: 450S–454S. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674. [DOI] [PubMed] [Google Scholar]
- Hassan R., Ho M. (2008) Mesothelin targeted cancer immunotherapy. Eur J Cancer 44: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan R., Kindler H., Jahan T., Bazhenova L., Reck M., Thomas A., et al. (2014a) Phase II Clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin Cancer Res 20: 5927–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan R., Sharon E., Thomas A., Zhang J., Ling A., Miettinen M., et al. (2014b) Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer 120: 3311–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelock T., Teoh R., Laws D., Gleeson F. (2010) Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax 6(Suppl. 2): ii61–ii76. [DOI] [PubMed] [Google Scholar]
- Heelan R., Rusch V., Begg C., Panicek D., Caravelli J., Eisen C. (1999) Staging of malignant pleural mesothelioma: comparison of CT and MR imaging. AJR Am J Roentgenol 172: 1039–1047. [DOI] [PubMed] [Google Scholar]
- Hjerpe A., Ascoli V., Bedrossian C., Boon M., Creaney J., Davidson B., et al. (2015) Guidelines for the cytopathologic diagnosis of epithelioid and mixed-type malignant mesothelioma: complementary statement from the International Mesothelioma Interest Group, also endorsed by the International Academy of Cytology and the Papanicolaou Society of Cytopathology. Diagn Cytopathol 43: 563–576. [DOI] [PubMed] [Google Scholar]
- Hodi F., O’Day S., McDermott D., Weber R., Sosman J., Haanen J., et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain A., Colby T., Ordonez N., Krausz T., Borczuk A., Cagle P., et al. (2009) Guidelines for Pathologic diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 133: 1317–1331. [DOI] [PubMed] [Google Scholar]
- Iwahori K., Osaki T., Serada S., Fujimoto M., Suzuki H., Kishi Y., et al. (2008) Megakaryocyte potentiating factor as a tumor marker of malignant pleural mesothelioma: evaluation in comparison with mesothelin. Lung Cancer 62: 45–54. [DOI] [PubMed] [Google Scholar]
- Jagadeeswaran R., Ma P., Seiwert T., Jagadeeswaran S., Zumba O., Nallasura V., et al. (2006) Functional analysis of C-MET/hepatocyte growth factor pathway in malignant pleural mesothelioma. Cancer Res 66: 352–361. [DOI] [PubMed] [Google Scholar]
- Jahan T., Gu L., Kratzke R., Dudek A., Otterson G., Wang X., et al. (2012) Vatalanib in malignant mesothelioma: a phase II trial by the Cancer and Leukemia Group B (CALGB 30107). Lung Cancer 76: 393–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassem J., Ramlau R., Santoro A., Schuette W., Chemaissani A., Hong S., et al. (2008) Phase III trial of pemetrexed plus best supportive care compared with best supportive care in previously treated patients with advanced malignant pleural mesothelioma. J Clin Oncol 26: 1698–1704. [DOI] [PubMed] [Google Scholar]
- Jenkins P., Milliner R., Salmon C. (2011) Re-Evaluating the role of palliative radiotherapy in malignant pleural mesothelioma. Eur J Cancer 47: 2143–2149. [DOI] [PubMed] [Google Scholar]
- Kao S., Clarke S., Vardy J., Corte P., Clarke C., van Zandwijk N. (2013) Patterns of care for malignant pleural mesothelioma patients compensated by the dust diseases board in New South Wales, Australia. Intern Med J 43: 402–410. [DOI] [PubMed] [Google Scholar]
- Kindler H., Karrison T., Gandara D., Lu C., Krug L., Stevenson J., et al. (2012) Multicenter, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin plus bevacizumab or placebo in patients with malignant mesothelioma. J Clin Oncol 30: 2509–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse M., Sherry S., Paidpally V., Mercier G., Subramaniam R. (2013) FDG PET/CT in the management of primary pleural tumors and pleural metastases. AJR Am J Roentgenol 201: W215–W226. [DOI] [PubMed] [Google Scholar]
- Laurie S., Gupta A., Chu Q., Lee C., Morzycki W., Feld R., et al. (2011) Brief report: a phase II Study of sunitinib in malignant pleural mesothelioma. The NCIC Clinical Trials Group. J Thorac Oncol 6: 1950–1954. [DOI] [PubMed] [Google Scholar]
- Le G., Takahashi K., Park E., Delgermaa V., Oak C., Qureshi A., et al. (2011) Asbestos use and asbestos-related diseases in Asia: past, present and future. Respirology 16: 767–775. [DOI] [PubMed] [Google Scholar]
- Leigh J., Davidson P., Hendrie L., Berry D. (2002) Malignant mesothelioma in Australia, 1945-2000. Am J Ind Med 41: 188–201. [DOI] [PubMed] [Google Scholar]
- Leung A., Muller N., Miller R. (1990) CT in differential diagnosis of diffuse pleural disease. AJR Am J Roentgenol 154: 487–492. [DOI] [PubMed] [Google Scholar]
- Maasilta P. (1991) Deterioration in Lung function following hemithorax irradiation for pleural mesothelioma. Int J Radiat Oncol Biol Phys 20: 433–438. [DOI] [PubMed] [Google Scholar]
- Maasilta P., Kivisaari L., Holsti L., Tammilehto L., Mattson K. (1991) Radiographic chest assessment of lung injury following hemithorax irradiation for pleural mesothelioma. Eur Respir J 4: 76–83. [PubMed] [Google Scholar]
- Madsen P., Laursen C., Davidsen J. (2013) Diagnostic delay in malignant pleural mesothelioma due to physicians fixation on history with non-exposure to asbestos. BMJ Case Rep. DOI: 10.1136/bcr-2012-007491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield A., Roden A., Peikert T., Sheinin Y., Harrington S., Krco C., et al. (2014) B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol 9: 1036–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathy A., Baas P., Dalesio O., van Zandwijk N. (2005) Limited efficacy of imatinib mesylate in malignant mesothelioma: a phase II trial. Lung Cancer 50: 83–86. [DOI] [PubMed] [Google Scholar]
- Mavi A., Basu S., Cermik T., Urhan M., Bathaii M., Thiruvenkatasamy D., et al. (2009) Potential of dual time point FDG-PET imaging in differentiating malignant from benign pleural disease. Mol Imaging Biol 11: 369–378. [DOI] [PubMed] [Google Scholar]
- Muers M., Stephens R., Fisher P., Darlison L., Higgs C., Lowry E., et al. (2008) Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicentre randomised trial. Lancet 371: 1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L. (2007) Heat SHOCK PROtein 90: the cancer chaperone. J Biosci 32: 517-530. [DOI] [PubMed] [Google Scholar]
- Nowak A., Byrne M., Williamson R., Ryan G., Segal A., Fielding D., et al. (2002) A multicentre phase II study of cisplatin and gemcitabine for malignant mesothelioma. Br J Cancer 87: 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak A., Francis R., Phillips M., Millward M., van der Schaaf A., Boucek J., et al. (2010) A Novel prognostic model for malignant mesothelioma incorporating quantitative FDG-PET imaging with clinical parameters. Clin Cancer Res 16: 2409–2417. [DOI] [PubMed] [Google Scholar]
- Nowak A., Millward M., Creaney J., Francis R., Dick I., Hasani A., et al. (2012) A phase II study of intermittent sunitinib malate as second-line therapy in progressive malignant pleural mesothelioma. J Thorac Oncol 7: 1449–1456. [DOI] [PubMed] [Google Scholar]
- O’Brien M., Watkins D., Ryan C., Priest K., Corbishley C., Norton A., et al. (2006) A randomised trial in malignant mesothelioma (M) of early (E) versus delayed (D) chemotherapy in symptomatically stable patients: the MED trial. Ann Oncol 17: 270–275. [DOI] [PubMed] [Google Scholar]
- Pass H., Levin S., Harbut M., Melamed J., Chiriboga L., Donington J., et al. (2012) Fibulin-3 as a Blood and effusion biomarker for pleural mesothelioma. N Engl J Med 367: 1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pass H., Lott D., Lonardo F., Harbut M., Liu Z., Tang N., et al. (2005) Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N Engl J Med 353: 1564–1573. [DOI] [PubMed] [Google Scholar]
- Paz-Ares L., De Marinis F., Dediu M., Thomas M., Pujol J., Bidoli P., et al. (2012) Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 13: 247–255. [DOI] [PubMed] [Google Scholar]
- Poulikakos P., Xiao G., Gallagher R., Jablonski S., Jhanwar S., Testa J. (2006) Re-Expression of the tumor suppressor NF2/Merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene 25: 5960–5968. [DOI] [PubMed] [Google Scholar]
- Rice D., Stevens C., Correa A., Vaporciyan A., Tsao A., Forster K., et al. (2007) Outcomes after extrapleural pneumonectomy and intensity-modulated radiation therapy for malignant pleural mesothelioma. Ann Thorac Surg 84: 1685–1692; discussion 1692–1683. [DOI] [PubMed] [Google Scholar]
- Robinson B., Lake R. (2005) Advances in malignant mesothelioma. N Engl J Med 353: 1591–1603. [DOI] [PubMed] [Google Scholar]
- Segal A., Sterrett G., Frost F., Shilkin K., Olsen N., Musk A., et al. (2013) A diagnosis of malignant pleural mesothelioma can be made by effusion cytology: results of a 20 year audit. Pathology 45: 44–48. [DOI] [PubMed] [Google Scholar]
- Sekido Y. (2013) Molecular pathogenesis of malignant mesothelioma. Carcinogenesis 34: 1413–1419. [DOI] [PubMed] [Google Scholar]
- Servais E., Colovos C., Rodriguez L., Bograd A., Nitadori J., Sima C., et al. (2012) Mesothelin overexpression promotes mesothelioma cell invasion and mmp-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clin Cancer Res 18: 2478–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socinski M., Schell M., Peterman A., Bakri K., Yates S., Gitten R., et al. (2002) Phase III trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage IIIb/IV non-small-cell lung cancer.J Clin Oncol 20: 1335–1343. [DOI] [PubMed] [Google Scholar]
- Stebbing J., Powles T., McPherson K., Shamash J., Wells P., Sheaff M., et al. (2009) The efficacy and safety of weekly vinorelbine in relapsed malignant pleural mesothelioma. Lung Cancer 63: 94–97. [DOI] [PubMed] [Google Scholar]
- Strimbu K., Tavel J. (2010) What are biomarkers? Curr Opin HIV AIDS 5: 463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarbaker D., Garcia J. (1997) Multimodality therapy for malignant pleural mesothelioma. Chest 112: 272S–275S. [DOI] [PubMed] [Google Scholar]
- Sugarbaker D., Norberto J. (1998) Multimodality management of malignant pleural mesothelioma. Chest 113: 61S–65S. [DOI] [PubMed] [Google Scholar]
- Terada T., Tabata C., Tabata R., Okuwa H., Kanemura S., Shibata E., et al. (2012) Clinical utility of 18-fluorodeoxyglucose positron emission tomography/computed tomography in malignant pleural mesothelioma. Exp Ther Med 4: 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treasure T., Waller D., Tan C., Entwisle J., O’Brien M., O’Byrne K., et al. (2009) The mesothelioma and radical surgery randomized controlled trial: the MARS feasibility study. J Thorac Oncol 4: 1254–1258. [DOI] [PubMed] [Google Scholar]
- Ung Y., Yu E., Falkson C., Haynes A., Stys-Norman D., Evans W., et al. (2006) The Role of Radiation therapy in malignant pleural mesothelioma: a systematic review. Radiother Oncol 80: 13–18. [DOI] [PubMed] [Google Scholar]
- Van den Bogaert D., Pouw E., Van Wijhe G., Vernhout R., Surmont V., Hoogsteden H., et al. (2006) Pemetrexed maintenance therapy in patients with malignant pleural mesothelioma. J Thorac Oncol 1: 25–30. [PubMed] [Google Scholar]
- Van Meerbeeck J., Gaafar R., Manegold C., van Klaveren R., van Marck E., Vincent M., et al. (2005) Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol 23: 6881–6889. [DOI] [PubMed] [Google Scholar]
- Van Schil P., Baas P., Gaafar R., Maat A., van de Pol M., Hasan B., et al. (2010) Trimodality therapy for malignant pleural mesothelioma: results from an EORTC phase II multicentre trial. Eur Respir J 36: 1362–1369. [DOI] [PubMed] [Google Scholar]
- Van Zandwijk N., Clarke C., Henderson D., Musk A., Fong K., Nowak A., et al. (2013) Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J Thorac Dis 5: E254–E307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzang N., Rusthoven J., Symanowski J., Denham C., Kaukel E., Ruffie P., et al. (2003) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21: 2636–2644. [DOI] [PubMed] [Google Scholar]
- Weder W., Stahel R., Baas P., Dafni U., De Perrot M., McCaughan B., et al. (2011) The MARS feasibility trial: conclusions not supported by data. Lancet Oncol 12: 1093–1094; author reply 1094–1095. [DOI] [PubMed] [Google Scholar]
- Willis R. (1960) Pathology of Tumours, 3rd edn London: Butterworths. [Google Scholar]
- Yan T., Cao C., Boyer M., Tin M., Kennedy C., Mclean J., et al. (2011) Improving survival results after surgical management of malignant pleural mesothelioma: an Australian institution experience. Ann Thorac Cardiovasc Surg 17: 243–249. [DOI] [PubMed] [Google Scholar]
- Yasumitsu A., Tabata C., Tabata R., Hirayama N., Murakami A., Yamada S., et al. (2010) Clinical significance of serum vascular endothelial growth factor in malignant pleural mesothelioma. J Thorac Oncol 5: 479–483. [DOI] [PubMed] [Google Scholar]
- Zalcman G., Mazieres J., Margery J., Greillier L., Audigier-Valette C., Moro-Siblot D., et al. (2015) Bevacizumab 15mg/kg Plus cisplatin-pemetrexed (CP) triplet versus CP doublet in malignant pleural mesothelioma (MPM): results of the IFCT-GFPC-0701 MAPS randomized phase 3 trial. J Clin Oncol 33(Suppl.): abstract 7500. [Google Scholar]
- Zauderer M., Bott M., McMillan R., Sima C., Rusch V., Krug L., et al. (2013) Clinical characteristics of patients with malignant pleural mesothelioma harboring somatic BAP1 mutations. J Thorac Oncol 8: 1430–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]