Abstract
There is a considerable amount of evidence that supports the possibility of an increased risk of pneumonia associated with prolonged use of inhaled corticosteroids (ICS) in patients with chronic obstructive pulmonary disease (COPD). However, as yet, no statistically significant increase in pneumonia-related 30-day mortality in patients on ICS has been demonstrated. The lack of objective pneumonia definitions and radiological confirmations have been a major source of bias, because of the similarities in clinical presentation between pneumonia and acute exacerbations of COPD. One of the newer fluticasone furoate studies overcomes these limitations and also provides an assessment of a range of doses, suggesting that the therapeutic window is quite narrow and that conventional dosing has probably been too high, although the absolute risk may be different compared to other drugs. Newer studies were not able to rule out budesonide as responsible for pneumonia, as previous evidence suggested, and there is still need for evidence from head-to-head comparisons in order to better assess possible intra-class differences. Although the exact mechanisms by which ICS increase the risk of pneumonia are not fully understood, the immunosuppressive effects of ICS on the respiratory epithelium and the disruption of the lung microbiome are most likely to be implicated. Given that COPD represents such a complex and heterogeneous disease, attempts are being made to identify clinical phenotypes with clear therapeutic implications, in order to optimize the pharmacological treatment of COPD and avoid the indiscriminate use of ICS. If deemed necessary, gradual withdrawal of ICS appears to be well tolerated. Vaccination against pneumococcus and influenza should be emphasized in patients with COPD receiving ICS. Physicians should keep in mind that signs and symptoms of pneumonia in COPD patients may be initially indistinguishable from those of an exacerbation, and that patients with COPD appear to be at increased risk of developing pneumonia as a complication of ICS therapy.
Keywords: adverse effects, chronic obstructive pulmonary disease (COPD), inhaled corticosteroids, mortality, phenotypes, pneumonia
Introduction
Chronic obstructive pulmonary disease (COPD) has been shown to be a risk factor for the development of pneumonia [Farr et al. 2000; Almirall et al. 2008]. COPD has also been linked to hospitalization for and death due to pneumonia [Restrepo et al. 2006; Rello et al. 2006], although an increased mortality from pneumonia in patients with COPD has not been a universal finding [Fine et al. 1997; Snijders et al. 2010; Loke et al. 2013]. Prevention of acute exacerbation of COPD (AECOPD) is perhaps one of the most important aspects of the management of the disease since they constitute an important source of morbidity and mortality [Criner et al. 2015]. In 2011, the GOLD guidelines were reorganized so that forced expiratory volume in 1 second (FEV1) alone ceased to be the main determinant of disease severity, and acknowledged that the identification of patients at risk for exacerbations is a key factor in guiding maintenance therapy choices. The current four-quadrant classification system was then developed in an attempt to bring the personalized medicine paradigm to COPD management. While the low-risk A and B categories can be effectively treated with long-acting bronchodilators alone, in patients at high risk (categories C and D) ICS are indicated in combination with long acting β2-agonists (LABAs) or long-acting muscarinic antagonists (LAMAs) [GOLD, 2015]. However, since not all category C or D patients are placed in those categories because of frequent exacerbations, further subclassification has been proposed to better tailor maintenance therapy indications [Agusti and Fabbri, 2014].
In our previous review, we discussed a considerable amount of evidence from both randomized controlled trials (RCTs) and observational studies that signals an increase in the risk of pneumonia associated with prolonged use of ICS in patients with COPD [Marzoratti et al. 2013]. However, a major source of bias is that in virtually all those studies pneumonia was not an anticipated adverse event, so no objective pneumonia definition nor radiological confirmation were required. This could lead to misdiagnosis, mainly because of the similarities in clinical presentation between pneumonia and AECOPD. Nevertheless, since there is as yet no evidence of a statistically significant increase in pneumonia-related 30-day mortality in patients on ICS, at first glance it would seem that the risk–benefit equation remains significantly in favor of treating COPD patients with ICS. Although previous large RCTs reported improvements in symptoms, quality of life, FEV1 decline, frequency of exacerbations, and even a survival benefit with ICS/LABA combination versus LABA alone [Nannini et al. 2007], more recent publications have pointed out methodological flaws in the analysis of the data that challenge the conclusions in those trials [Nannini et al. 2012; Ernst et al. 2015]. Therefore, the appropriate role of ICS in the treatment of stable COPD remains yet to be fully elucidated.
Since our previous review, numerous studies of various designs addressing this issue have been published. Interestingly, at least some of the new prospective trials have been designed taking previous limitations into account, now including pneumonia events as a predefined outcome, and making radiologic confirmation of suspected pneumonia cases a requirement. In this updated review, we aim to summarize and analyze the newly available evidence on the risk of pneumonia associated with the long-term use of ICS, assess its potential impact in clinical practice, and establish the appropriate use of ICS in stable COPD, including those situations in which they could be safely discontinued or replaced by other therapies.
Randomized controlled trials
The most relevant RCTs published before 2011 were extensively reviewed elsewhere [Marzoratti et al. 2013] (Table 1). To summarize, the large 3-year TORCH study was the first one to report on unexpected pneumonia in patients assigned to any of the fluticasone propionate (FP)-containing arms [Calverley et al. 2007], and still continues to exert great influence on subsequent pooled analyses, due to its size and length (see the Meta-analyses section). From then on, many other trials have reported similar findings with FP [Kardos et al. 2007; Wedzicha et al. 2008; Ferguson et al. 2008; Anzueto et al. 2009]. Among the many limitations found in these trials, the most relevant are the lack of inclusion of pneumonia as a prespecified outcome together with objective pneumonia definitions, and the absence in many of them of radiological confirmation of suspected cases of pneumonia [Calverley et al. 2007; Kardos et al. 2007; Wedzicha et al. 2008].
Table 1.
Relevant RCTs that reported on the risk of pneumonia in patients treated with ICS for COPD.
| RCT | n | Follow up (months) | Treatment groups | Pneumonia (%)¶ | Mortality (%)Þ | Chest X-ray | Dose–effect relationship | Industry funding |
|---|---|---|---|---|---|---|---|---|
| Fluticasone propionate + salmeterol (FP/SAL) | ||||||||
|
Calverley
et al. [2007]
(TORCH) |
6112 | 36 | FP/SAL 500/50 µg FP 500 µg SAL 50 µg Placebo |
19.6 18.3 13.3 12.3 |
12.6 16.0 13.5 15.2 |
No | No | GlaxoSmithKline |
| Kardos et al. [2007] | 994 | 10 | FP/SAL 500/50 µg SAL 50 µg |
4.5 1.4 |
1.4 1.9 |
No | No | GlaxoSmithKline |
|
Wedzicha
et al. [2008]
(INSPIRE) |
1323 | 24 | FP/SAL 500/50 µg TIO 18 µg |
8.0 4.0 |
3.0 6.0 |
No | No | GlaxoSmithKline |
| Ferguson et al. [2008] | 782 | 12 | FP/SAL 250/50 µg SAL 50 µg |
7.0 4.0 |
1.5 0.8 |
Yes | No | GlaxoSmithKline |
| Anzueto et al. [2009] | 797 | 13 | FP/SAL 250/50 µg SAL 50 µg |
7.0 2.0 |
1.0 1.5 |
Yes | No | GlaxoSmithKline |
| Fluticasone furoate + vilanterol (FF/VIL) | ||||||||
| Dransfield et al. [2013] | 3255 | 12 | FF/VIL 50/25 µg FF/VIL 100/25 µg FF/VIL 200/25 µg VIL 25 µg |
5.9 6.3 6.8 3.3 |
0Ŧ
0.1Ŧ 0.9Ŧ 0Ŧ |
Yes | No | GlaxoSmithKline |
| Budesonide + formoterol (BUD/FOR) | ||||||||
|
Tashkin
et al. [2008]
(SHINE) |
1704 | 6 | BUD/FOR 320/9 µgƒ
BUD/FOR 160/9 µg BUD 320 µg FOR 9 µg Placebo |
1.1 2.5 1.8 1.8 1.3 |
1.1 1.4 0.7 0.3 0.3 |
No | No | AstraZeneca |
| Rennard et al. [2009] | 1964 | 12 | BUD/FOR 320/9 µg BUD/FOR 160/9 µg FOR 9 µg Placebo |
3.0 3.0 3.4 4.8 |
0.6 1.2 0.4 0.8 |
No | No | AstraZeneca |
| Sharafkhaneh et al. [2012] | 1219 | 12 | BUD/FOR 320/9 µg BUD/FOR 160/9 µg FOR 9 µg |
6.4 4.7 2.7 |
1.7 2.2 2.5 |
No | No | AstraZeneca |
RCT, randomized controlled trial; ICS, inhaled corticosteroids; COPD, chronic obstructive pulmonary disease; FP, fluticasone propionate; FF, fluticasone furoate: SAL, salmeterol; VIL, vilanterol; TIO, tiotropium; NR, not reported.
‘Any pneumonia’ events (regardless of severity).
All-cause mortality unless otherwise specified.
Only pneumonia-related mortality rates were reported.
Single-inhaler and separate inhalers arms pooled together for this dose.
More recently, Crim and colleagues published a predefined analysis of the previously reported 1-year replicate studies by Dransfield and colleagues that compared three doses of the new fluticasone furoate/vilanterol (FF/VIL) combination (50, 100 or 200 µg of FF, combined with 25 µg of VIL via dry powder inhaler) in COPD patients with at least one moderate or severe AECOPD in the previous year [Dransfield et al. 2013; Crim et al. 2015]. Chest X-rays were required by protocol within 48 hours of any suspected pneumonia or moderate/severe AECOPD, and were available for 91% of the nonhospitalized and for all of the hospitalized pneumonia cases. In the pooled analysis of the results from both studies, the investigators found an at least two-fold increase in the incidence of radiologically confirmed pneumonia with the ICS-containing formulations (2% for VIL alone; 4%, 4% and 5% for the combination with 50 µg, 100 µg, or 200 µg FF, respectively). However, no dose-related increase associated with FF/VIL could be demonstrated. There was a significant excess of pneumonia-related mortality with the FF/VIL combination, with seven out of the eight deaths registered in the FF/VIL 200/25 µg arm (see the ICS-induced pneumonia mortality section). All-cause mortality was similar in the treatment and control groups.
Compared with the 3-year TORCH study with FP and salmeterol (FP/SAL) [Calverley et al. 2007], this 1-year FF/VIL study is much less affected by high differential drop-out rates and has a much higher percentage of radiological confirmation of pneumonia events. The overall incidence of pneumonia in the ICS-containing arms is about one third of that reported in TORCH, and much closer to those reported in the 2-year INSPIRE study and in the 1-year studies by both Ferguson and colleagues and Anzueto and colleagues [Calverley et al. 2007; Ferguson et al. 2008; Anzueto et al. 2009] (Table 1). In their original publication, Dransfield and colleagues reported a number needed to treat (NNT) of 3.3 treated with FP/VIL 100/25 µg for 1 year to prevent one moderate or severe exacerbation, and a number needed to harm (NNH) of 23 patients treated with the same combination over the same time to cause an additional pneumonia [Dransfield et al. 2013]. However, this event-based approach to NTT/NNH calculation may not be appropriate for this design and the kind of outcomes being measured (see the Risk/benefit ratio and indications section).
Previous budesonide/formoterol (BUD/FOR) trials did not find significant differences in pneumonia incidence in the ICS-containing groups [Tashkin et al. 2008; Rennard et al. 2009]. However, in contrast with these findings, Sharafkhaneh and colleagues conducted a 1-year trial to assess the effect of two doses (320/9 and 160/9 µg) of BUD/FOR pMDI inhaler versus FOR alone (9 µg DPI) on COPD exacerbations [Sharafkhaneh et al. 2011]. Along with reduced exacerbations rates with both doses and prolonged time to first exacerbation with the 320/9 µg formulation, they reported a somewhat elevated incidence of pneumonia in the BUD/FOR groups (6.4% and 4.7% of patients in the BUD/FOR 320/9 µg and 160/9 µg, respectively, compared with 2.7% in the FOR group). The main limitations of these studies were that pneumonia diagnosis was based on clinical judgment with no radiological confirmation, and that the drop-out rate was higher in the FOR-only arm. Randomized trials cannot necessarily control adequately for differences in severity of disease, patient age, types and severity of co-morbidities, phenotypes and disease progression over time. These factors can affect events that occur relatively infrequently and give the impression of significantly increased risk of an adverse event for one arm versus another, even when very large numbers of participants are enrolled. Additionally, the diagnosis and differentiation of AECOPD versus pneumonia may not be optimal and can be affected by study design and by the accuracy of the reporting of events by participating clinical trial sites. The ability of these factors to influence trial outcomes data may account for some of the disparate findings of various studies concerning risk of developing pneumonia with ICS therapies.
Meta-analyses
Previous systematic reviews have found a consistent rise in the risk of pneumonia (mainly with the use of FP-containing formulations) but with no increase in mortality, regardless of the drug being considered [Drummond et al. 2008; Rodrigo et al. 2009; Singh et al. 2009; Singh and Loke, 2010]. The main limitations of these studies include the lack of either an objective definition or a radiological confirmation of pneumonia, the fact that most trials were insufficiently powered to detect significant differences in mortality, the absence of patient-level data to adjust for potentially confounding variables, and the scarcity of trials with BUD, which precludes the detection of any possible intra-class differences between the available ICS. One meta-analysis that included patient-level data from more than 7000 subjects enrolled in seven RCTs with BUD concluded that its use was not associated with an increased risk of pneumonia [Sin et al. 2009]. All of these studies have been discussed in depth elsewhere [Marzoratti et al. 2013] (Table 2).
Table 2.
Relevant meta-analyses that reported on the risk of pneumonia in patients treated with ICS for COPD.
| Meta-analysis | Number of studies | ICS (number of studies) | n | Heterogeneity (I2) | Follow up (months) | Pneumonia RR (95% CI)Ŧ | Mortality RR (95% CI)# | Dose–effect relationship |
|---|---|---|---|---|---|---|---|---|
| Drummond et al. [2008] ǂ | 11 | FP (6) TRI (1) BUD (4) |
14,426 | 72%¶ | 6–40 | 1.34 (1.03–1.75) | 0.86 (0.68–1.09) | Yes |
| Singh et al. [2009] ǂ | 18 | FP (16) BUD (2) |
16,996 | 16% | 6–36 | 1.60 (1.33–1.92) | 0.96 (0.86–1.08) | NR |
| Sin et al. [2009] | 7 | BUD | 7042 | NR | 6–11 | 1.05 (0.81–1.37) | NR | NA |
| Singh and Loke [2010] | 24 | FP (16) BUD (7) MOM (1) Total |
23,096 | 0% 23% NA 15% |
NR | 1.67 (1.47–1.89) 1.19 (0.92–1.53)ǁ 2.00 (0.83–4.81) 1.57 (1.41–1.75) |
NR | NR |
| Halpin et al. [2011] ¥ | 12 | BUD (4) versus
FP (8) |
5502 9586 |
23.3% 0% |
6–12 2–36 |
0.47 (0.28–0.80) | 0.18 (0.01–4.10)£ | NR |
| Kew and Seniukovich [2014] | 43 | FP (26) BUD (17) FP versus BUD¥ |
21,247 10,150 |
0% 0% NA |
3–36 3–36 |
1.78 (1.50–2.12)Þ 1.62 (1.00–2.62)Þ 1.86 (1.04–3.34) |
0.99 (0.87–1.13) 0.90 (0.65–1.24) 1.24 (0.74–2.07) |
No Yes˫ |
ICS, inhaled corticosteroids; COPD, chronic obstructive pulmonary disease; FP, fluticasone propionate; TRI, triamcinolone; BUD, budesonide; MOM, mometasone; RR, relative risk; CI, confidence interval; OR, odds ratio; NR, not reported; NA, not applicable.
‘Any pneumonia’ events (regardless of severity).
All-cause mortality unless otherwise specified.
No distinction was made between the different ICS for the pooled analysis.
A re-analysis of the data shows a consistently increased risk of pneumonia after removal of the substantial statistical heterogeneity [Loke and Singh, 2009].
The exclusion of one trial demonstrates a statistically significant increased risk of pneumonia with BUD (RR 1.34, 95% CI 1.01–1.79) and removes the statistical heterogeneity (I2 = 0%) in a sensitivity analysis.
Adjusted indirect comparison with placebo as a common comparator (Bucher method).
Pneumonia-related mortality. Data extracted from 3 studies (2 with FP, 1 with BUD). The authors concluded there were too few events to draw any firm conclusions on pneumonia-related mortality.
Nonfatal serious adverse pneumonia events (requiring hospital admission). Data for ‘any pneumonia’ events were not pooled because of heterogeneity.
The 640 µg dose increased nonfatal serious adverse pneumonia events (OR 2.02, 95% CI 1.15–3.57), and no significant difference was observed for the 320 µg dose (OR 0.68, 95% CI 0.27–1.71).
Until now, only one meta-analysis has compared two different ICS against each other using an adjusted indirect comparison with placebo as a common comparator [Halpin et al. 2011]. It included eight FP trials and four BUD trials. There was a significantly lower proportion of serious pneumonia events [odds ratio (OR) 0.41; 95% confidence interval (CI) 0.19–0.86] with BUD/FOR compared with FP/SAL, with not enough events to draw conclusions on pneumonia-related mortality, according to the authors.
A 2014 Cochrane meta-analysis included 43 RCTs of at least 12 week’s duration (FP 26 studies, n = 21,247; BUD 17 studies, n = 10,150) to assess the risk of pneumonia with ICS in COPD [Kew and Seniukovich, 2014]. The authors reported a 78% increase in nonfatal serious pneumonia events with FP (irrespective of whether it was delivered alone or in combination with SAL or VIL). There was no evidence that dosing, treatment duration or baseline severity affected this outcome, and no significant differences in mortality were found (pneumonia-related or all-cause). BUD also increased the risk of nonfatal serious pneumonia events by 62%, with a larger effect at higher doses (640 µg). In addition, an indirect comparison of BUD versus FP monotherapy against placebo was performed, with the only significant difference being an 86% higher risk of less serious, community-managed pneumonia events with FP compared with BUD, with no significant differences with respect to serious pneumonia events or mortality. Although a rigorous selection methodology was applied to exclude studies with a high risk of bias and high or uneven withdrawal rates, the effect of BUD was generally based on shorter trials of inferior quality. The selected primary endpoint was nonfatal serious pneumonia events, as a way to compensate for the lack of radiologic confirmation in less severe, nonhospitalized pneumonia events. It must be noted that, although they are distinct molecules with completely different potency ratios and duration of action, FP and FF were pooled together for the analysis. Also, the 200 µg dose of FF included in this analysis is higher than the licensed dose. Given that there seems to be no additional benefit with 200 µg dose over the 100 µg dose, and that there is a potential increased risk of systemic corticosteroid-related adverse reactions, the 200 µg formulation is not indicated for patients with COPD [Electronic Medicines Compendium (eMC), 2015a].
Observational studies
We commented on previously available observational data regarding the increased risk of pneumonia with ICS use in our previous review [Marzoratti et al. 2013] (Table 3). The large Canadian study by Ernst and colleagues, being the most remarkable study at the time [Ernst et al. 2007], was designed as a nested case–control study and included data from more than 175,000 COPD patients extracted from Quebec’s health insurance program databases. It revealed a 70% higher risk of hospitalization due to pneumonia in current ICS users compared with nonusers. The increment in risk was dose-dependent, and still present even 12 months after drug discontinuation. Although many ICS doses were used, all doses were converted to FP equivalents and no intra-class risk subanalysis was performed.
Table 3.
Relevant case–control studies that reported on the risk of pneumonia in patients treated with ICS for COPD.
| Study | n | Country | Period | ICS | Pneumonia OR/HR (95% CI)¶ | Mortality OR/HR (95% CI)Þ | Dose–effect relationship | Industry funding |
|---|---|---|---|---|---|---|---|---|
| Increased risk of pneumonia with ICS | ||||||||
| Ernst et al. [2007] | 175,906 | Canada | Jan 1988 Dec 2001 |
No distinction | 1.70 (1.63–1.77) | 1.53 (1.30–1.80) | Yes | No |
| Joo et al. [2009] | 145,586 | US | Oct 1999 Sep 2002 |
No distinction | 1.38 (1.31–1.45) | 0.77 (0.75–0.80) | No | No |
| Thornton Snider et al. [2012] | 83,455 | US | Jan 2009 Sep 2011 |
No distinction | 1.26 (1.16–1.36) | NR | Yes | Novartis |
| Yawn et al. [2013] | 135,445 | US | Jan 2006 Sep 2010 |
No distinction | 1.51 (1.42–1.61) | NR | Yes | Novartis |
|
Janson
et al. [2013]
(PATHOS) |
5468 | Sweden | Jan 1999 Dec 2009 |
FP versus BUD | 1.74 (1.56–1.94) | 1.76 (1.22–2.53) | No | AstraZeneca ƒ |
| Suissa [2013] | 163,514 | Canada | Jan 1990 Dec 2005 |
FP BUD Total |
2.01 (1.93–2.10) 1.17 (1.09–1.26) 1.69 (1.63–1.75) |
NR | Yes | No |
| DiSantostefano et al. [2014] | 18,047 | UK | Jan 2002 Dec 2010 |
No distinction | 1.49 (1.22–1.83) | NR | Yes | GlaxoSmithKline Ŧ |
| Kern et al. [2015] | 7394 | US | NR | BUD versus FP | 0.92 (0.81–1.04) | NR | NR | AstraZeneca ƒ |
| No increased risk of pneumonia with ICS | ||||||||
| Mapel et al. [2010] | 5245 | US | Sep 2001 Aug 2003 |
ICS alone ICS/LABA |
1.29 (0.96–1.73) 1.03 (0.74–1.42) |
NR | NA | GlaxoSmithKline |
| Festic et al. [2014] | 589¥ | US | Mar 2009 Aug 2009 |
No distinction | 1.40 (0.95–2.09) | NR | NA | No |
| Gershon et al. [2014] | 11,872 | Canada | Sep 2003 Mar 2011 |
ICS/LABA versus
LABA alone |
1.01 (0.93–1.08) | 0.92 (0.87–0.97) | NA | No |
RCT, randomized controlled trial; ICS, inhaled corticosteroids; COPD, chronic obstructive pulmonary disease; LABA, long acting β2-agonist; FP, fluticasone propionate; BUD, budesonide; OR, odds ratio; HR, hazard ratio; CI, confidence interval; NR, not reported; NA, not applicable.
Hospitalization for, or death from, pneumonia, unless otherwise specified.
Pneumonia-related mortality unless otherwise specified.
This study was funded by AstraZeneca, which also took part in the data collection and analysis, the interpretation of the data, and the drafting of the manuscript.
This study was funded by GlaxoSmithKline, but did not have any additional role in the study design, data collection and analysis or preparation of the manuscript.
Subset of patients with COPD.
The same group of investigators recently published another nested case-control analysis in a new-user cohort of patients with COPD, to estimate the risk of serious pneumonia (hospitalization for, or death from, pneumonia) associated with current ICS use [Suissa et al. 2013]. With data from more than 163,000 patients from the same health insurance databases as above, they reported that current use of ICS translated into a 69% increase in the rate of serious pneumonia. The risk was found to be higher and dose-related with FP compared with BUD [relative risk (RR) = 2.01 versus 1.17], and did not persist beyond 6 months after ICS discontinuation.
The large observational, industry-sponsored PATHOS study retrospectively examined the mandatory healthcare registries of ICS/LABA-treated patients in Sweden to compare the effectiveness and safety of two of the most commonly prescribed ICS/LABA combination treatments for COPD [Larsson et al. 2013]. Using propensity score matching, a cohort of 2734 patients treated with FP/SAL were individually matched 1:1 with an equal number of patients treated with BUD/FOR. An initial analysis of the data found an overall reduction in the annual rate of moderate-to-severe exacerbations by 26% with BUD/FOR compared with FP/SAL. A reduction of 29% in the rates of COPD-related hospitalization, along with fewer hospital days due to COPD exacerbations with BUD/FOR compared with FP/SAL, were also reported. In a secondary analysis [Janson et al. 2013], the FP/SAL treatment group was associated with a 73% higher pneumonia rate (RR 1.73; 95% CI 1.57–1.90; p < 0.001) compared with the BUD/FOR group. Similarly, the FP/SAL group was associated with 74% higher pneumonia-related hospital admissions than the BUD/FOR group (RR 1.74; 95% CI 1.56–1.94; p < 0.001). The FP/SAL group was also associated with a 76% higher risk of pneumonia-related death compared with the BUD/FOR group (HR 1.76; 95% CI 1.22–2.53; p = 0.0025). No dose-related difference in the risk of pneumonia was found in either treatment group.
In contrast with these findings, another database study of similar design by Kern and colleagues reported no differences between BUD/FOR and FP/SAL in the overall exacerbation rates (48% versus 47%, RR 1.02) or pneumonia events (17.3% versus 19.0%, OR 0.92) in two matched cohorts of 3697 new users of either ICS/LABA combination. The inclusion criteria differed from PATHOS in that patients who were not currently being treated with ICS were also included in the analysis, and in the follow-up period was shorter (1 versus 2 years) [Kern et al. 2015].
Several other recent case-control studies confirm previous reports of an increased, dose-related pneumonia risk with ICS use in patients with COPD [Thornton Snider et al. 2012; Yawn et al. 2013; DiSantostefano et al. 2014]. One study also reported a 90% relative increase in the risk of recurrent pneumonia among high-risk individuals who survived a first episode of pneumonia [Eurich et al. 2013]. Surprisingly, in a number of other recent case–control studies, treatment with ICS was not associated with a significantly increased risk of developing pneumonia [Mapel et al. 2010; Festic et al. 2014; Gershon et al. 2014] (Table 3).
All of these studies are inherently subject to bias given their retrospective design. This implies that even after adjustment for covariates associated with the risk of pneumonia such as age, comorbidities, COPD severity, and vaccination status, still unknown confounding factors could be present. This could be the reason why the incidence of ICS-related pneumonia appears to be higher in observational studies than in RCTs. Also, as in most RCTs, pneumonia diagnosis had no standardized definition and was based on clinical diagnosis rather than radiographic findings. Nevertheless, in those in which only hospitalized pneumonia cases were considered, it is safe to assume that radiologic confirmation was obtained, since it is a common in-hospital procedure. ICS use was based on dispensed medications or prescription claims, which does not necessarily reflect actual use. On the other hand, this ‘real-world’ design allows for a large number of patients to be followed over a long period of time, and thus for a high number of pneumonia events to be detected. This translates in a greater generalizability of the findings to clinical practice compared with RCTs, and without the inherent risk of differential dropout rates between treatment and control groups.
Discussion
Proposed mechanism of action
Several mechanisms have been proposed by which ICS could increase the risk of pneumonia, which are mostly related to their immunosuppressive effects. To compare, inhaled FP in doses of 1000 μg daily raise serum cortisol levels in the same way as 10 mg daily of oral prednisone [Lipworth, 1999], a dose proven high enough to produce a two-fold increase in the risk of pneumonia in patients with rheumatoid arthritis [Wolfe et al. 2006].
Although it is generally accepted that ICS have a protective effect against the development of AECOPD, a secondary analysis of a large RCT has brought attention to the fact that a considerable amount of the pneumonia events associated with the use of FP were preceded by an unresolved AECOPD [Calverley et al. 2011]. The presence of chronic bacterial colonization of the airways has been implicated is the frequency and severity of AECOPD [Patel et al. 2002], particularly in patients with bronchiectasis [Patel et al. 2004]. However, even though ICS are known to increase bacterial load in stable COPD [Garcha et al. 2012], the role of changes in bacterial load as determinants of an AECOPD is not yet conclusive [Sethi et al. 2007]. Although traditionally it was believed that bacterial colonization in COPD developed in a previously sterile lung, advances in molecular microbiology have revealed the existence of a normal pulmonary microbiota, and that its displacement by other organisms may be detrimental to host health [Dickson et al. 2014]. COPD patients have alterations in their lung microbiome that may result in chronic infection with potentially pathogenic micro-organisms [Miravitlles and Anzueto, 2015]. Furthermore, the use of ICS may itself alter the lung microbiome [Pragman et al. 2012; Huang et al. 2014], but whether this has implications for the protection against AECOPD or for the development of pneumonia is as yet unknown.
The paradoxical double effect of ICS consisting of increased episodes of pneumonia despite their protective effect on exacerbations is another area of debate. Furthermore, ICS-induced pneumonia events have consistently shown unmodified or even lower mortality rates (see the ICS-induced pneumonia mortality section). A plausible explanation could be that, in the same way that ICS can favor the development of pneumonia, ICS could also have a beneficial counterbalancing modulating effect on the local anti-inflammatory response after the onset of pneumonia. The complexity of the interactions between these mechanisms precludes a definite explanation (Figure 1). It has been demonstrated that patients with COPD have a distinct inflammatory pattern in response to CAP compared with patients without COPD [Crisafulli et al. 2013]. Also, in those with COPD, both AECOPD and CAP display characteristic inflammatory profiles [Gutierrez et al. 2010; Huerta et al. 2013]. The potential immunomodulatory effect of ICS in COPD is supported to some extent by several studies. The inhibition of nuclear factor kappa B (NF-ƙB) by ICS in COPD, one of the proposed mechanisms for the therapeutic effect of ICS, could lead to the suppression of normal host responses to bacterial infection [Singanayagam et al. 2010]. A systematic review and meta-analysis showed that ICS therapy in stable COPD translated as lower CD4 and CD8 cell counts in bronchial biopsies and reduced neutrophil and lymphocyte counts in BAL [Jen et al. 2012]. Also, in a 2014 observational study, previous use of ICS in patients hospitalized for CAP was associated with a reduced systemic inflammatory response, as evidenced by lower interleukin (IL)-6 and tumor necrosis factor (TNF)-α levels [Ferrer et al. 2014]. Reduction of the pro-inflammatory response in pneumonia could thus lead to less subsequent organ dysfunction and, therefore, better clinical outcomes [Martinez et al. 2011]. Against this hypothesis, however, a 2011 observational study on the impact of ICS on pneumonia outcomes in patients with COPD found no significant difference in the levels of markers of systemic inflammation such as C-reactive protein and white cell count [Singanayagam et al. 2011]. Similarly, another prospective observational study found that, although patients with COPD and CAP have higher levels of C-reactive protein, procalcitonin, TNF-α and IL-6 compared with those with AECOPD, prior administration of ICS had no modulating effect on these early inflammatory biomarkers [Huerta et al. 2013].
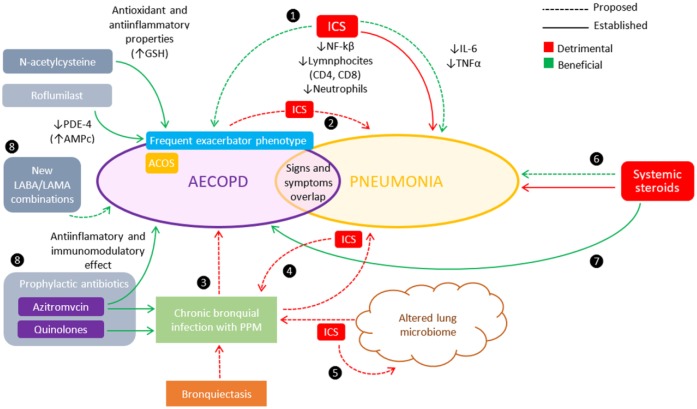
Figure 1.
Complexity and heterogeneity of the inflammatory response in acute exacerbation of chronic obstructive pulmonary disease (AECOPD) and pneumonia and possible impact of inhaled corticosteroids (ICS).
❶ There is a paradoxical double effect of ICS consisting of increased episodes of pneumonia despite their protective effect on exacerbations. ICS-induced pneumonia events have shown unmodified or even lower mortality rates, perhaps by modulating effect on the local anti-inflammatory response after pneumonia onset. The inhibition of nuclear factor kappa B (NF-ƙB) [Singanayagam et al. 2010], lower neutrophil and lymphocyte counts [Jen et al. 2012], and a reduced systemic inflammatory response (lower interleukin (IL)-6 and tumor necrosis factor (TNF)-α levels] [Ferrer et al. 2014] have been implicated. ❷ Many pneumonia events associated with the use of fluticasone propionate (FP) are preceded by an AECOPD [Calverley et al. 2011]. ❸ The presence of chronic bacterial colonization of the airways has been implicated is the frequency and severity of AECOPD [Patel et al. 2002], particularly in patients with bronchiectasis [Patel et al. 2004]. ❹ ICS are known to increase bacterial load in stable COPD [Garcha et al. 2012], but the role of these changes in the development of an AECOPD is uncertain [Sethi et al. 2007]. ❺ COPD patients have alterations in their lung microbiome that may result in chronic infection with potentially pathogenic microorganisms (PPM) [Miravitlles and Anzueto, 2015], and the use of ICS may further alter this microbiome [Pragman et al. 2012; Huang et al. 2014]. ❻ Chronic use of systemic steroids is a known risk factor for pneumonia [Wolfe et al. 2006]. Nevertheless, some studies have shown benefits when treating pneumonia with systemic corticosteroids to mitigate the immune response [Torres et al. 2015; Tagami et al. 2015; Blum, 2015]. ❼ The role of systemic steroids in the treatment of AECOPD is well established [GOLD, 2015]. ❽ Alternatives to ICS for the prevention of AECOPD include mycolitics, PDE-4 inhibitors such as roflumilast, and prophylactic antibiotics [GOLD, 2015; Criner et al. 2015]. There is also a potential role the new LABA/LAMA combinations (see the text).
In another observational study, previous use of ICS did not influence the inflammatory response, clinical presentation, severity, infectious etiology, hospital mortality, or readmission rates during an AECOPD [Crisafulli 2014].
Interestingly, clinical studies so far have failed to show a consistent benefit of systemic corticosteroid administration in CAP outcomes [Salluh et al. 2008; Nie et al. 2012], which also does not support the concept of a mitigating effect of ICS on local inflammatory response. Newer studies have recently reported that the use of systemic corticosteroids could be beneficial in severe CAP with a high inflammatory response [Torres et al. 2015], or with septic shock requiring vasopressors [Tagami et al. 2015]. Moreover, the Swedish multicenter STEP study showed that a 7-day treatment with prednisone in patients with CAP led to a reduction in time to clinical stability, to an overall reduction of length of hospital stay, and to a reduction in duration of intravenous antibiotic treatment, independent of severity [Blum, 2015]. Finally, a meta-analysis that included 13 RCTs and 2005 patients reported a reduction of 3% in mortality, 5% in the need for mechanical ventilation, and 1 day in the duration of hospitalization for patients with CAP treated with systemic corticosteroids. Despite these recent findings, it still remains a controversial subject that will need further clarification in future trials.
Finally, targeted inhibition of other anti-inflammatory pathways to prevent AECOPD, the mechanism of action of the oral PDE-4 inhibitor roflumilast, has not been associated with increased pneumonia rates in clinical trials to date [Chong et al. 2013; Yan et al. 2014]. Also, in the large REACT trial, in which roflumilast was administered to patients with severe COPD already under treatment with LABA/ICS combination, there were no differences in the rate of pneumonia between the LABA/ICS + roflumilast group versus the LABA/ICS-only group [Martinez et al. 2015].
All of the above findings reinforce the concept that, given the heterogeneity in the inflammatory response in CAP and AECOPD, the immunomodulatory effects that ICS have on the airways’ innate and adaptive defense mechanisms can lead to different patterns of cytokine activation, which can in turn impact differently on the occurrence of AECOPD and pneumonia. These mechanisms are quite complex and yet not fully elucidated.
ICS intra-class differences
Previous BUD RCTs and meta-analyses consistently failed to show an increase in pneumonia events [Tashkin et al. 2008; Rennard et al. 2009; Sin et al. 2009]. Surprisingly, newer studies were not entirely able to rule out BUD as responsible for increased pneumonia rates. The Canadian case–control study by Suissa and colleagues (see Observational studies section, above) found a moderate 17% increase in the rate of serious pneumonia, which did not increase with the dose of BUD [Suissa et al. 2013]. The trial with BUD/FOR pMDI by Sharafkhaneh and colleagues (see the Randomized controlled trials section) reported a 6.4% incidence of nonfatal pneumonia with the 320/9 µg formulation, compared with a 4.7% and 2.7% incidence with the 160/9 µg formulation and placebo, respectively [Sharafkhaneh et al. 2011]. In the Cochrane meta-analysis by Kew and Seniukovich (see Meta-analyses section), BUD also increased nonfatal serious adverse pneumonia events compared with placebo by 62%, with the largest effect also observed with the 640 µg dose [Kew and Seniukovich, 2014].
The absence of adequately powered, long-term, head-to-head trials precludes any definitive conclusions on intra-class differences in risk. Two previously mentioned meta-analyses (see the Meta-analyses section) compared BUD and FP against each other by means of an adjusted indirect comparison, using placebo as a common comparator [Halpin et al. 2011; Kew and Seniukovich, 2014]. Halpin and colleagues reported a 41% lower incidence of pneumonia-related serious adverse events with BUD/FOR compared with FP/SAL, but with not enough events to conclusively report on pneumonia-related mortality [Halpin et al. 2011]. Using the same statistical method, Kew and colleagues conducted an indirect comparison of BUD versus FP monotherapy against placebo. The only significant difference reported was a higher risk of nonserious (community-managed) pneumonia events with FP than with BUD [OR 1.86; 95% CI 1.04–3.34], with no significant differences with respect to serious pneumonia events or mortality [Kew and Seniukovich, 2014]. In the Swedish case–control study PATHOS, BUD was associated with a 73% fewer pneumonia events than with FP [Janson et al. 2013].
Differences in the molecular structures of ICS formulations are known to alter their relative potency ratios and duration of action. For this reason, intra-class differences between BUD and FP might be the result of BUD’s inferior bio-availability, duration of action and potency [Johnson, 1998]. However, evidence from the BUD studies has been invariably more inconsistent, as well as being derived from studies of shorter duration. Furthermore, FP has a much more widespread use in moderate-to-severe COPD than BUD, since only the former is commercially available in devices containing the high doses that are usually prescribed for those patients. Therefore, patients prescribed BUD might be those with less severe COPD, or asthma instead of COPD and, consequently, be at lower risk for pneumonia compared with subjects receiving FP. All of these factors make it difficult to draw conclusions on possible intra-class differences in the risk of causing pneumonia between FP and BUD. Only RCTs comparing both compounds head-to-head will be able to clarify this issue.
A pooled analysis of two large RCTs of identical design (total n = 2251) that compared the mometasone furoate/formoterol (MF/FOR) combination against each component alone and placebo reported that pneumonia was infrequent (<2%) across all treatment groups during the safety extension period [Tashkin et al. 2012].
The recently introduced long-acting FF, with structural similarities to FP and an allegedly superior pharmacological profile [Rossios et al. 2011], has also been shown to increase pneumonia rates in patients with COPD in a proportion similar to that reported for FP [Crim, 2015]. According to the manufacturer’s label information, 100 µg FF daily is approximately equivalent to twice daily 250 µg FP, and 200 µg FF daily is equivalent to twice daily 500 µg FP [Electronic Medicines Compendium (eMC), 2015a].
Dose–effect relationship
In many of the trials discussed so far, the unadjusted higher risk of pneumonia was associated with longer duration of use, more potent ICS formulations, and higher doses. This became especially clear in a large database study which showed that individuals receiving high daily doses of ICS (equivalent to >1000 µg of FP) had a 70% greater risk of pneumonia hospitalization [Ernst et al. 2007]. However, this dose–effect relationship could not be verified in subsequent observational studies [Joo et al. 2010; Janson et al. 2013] (see the Observational Studies section). Similarly, RCTs that used half that FP dose (500 µg daily) also showed an almost two-fold increase in the risk of pneumonia [Ferguson et al. 2008; Anzueto et al. 2009]. Many RCTs have also reported on this dose–effect relationship (Table 1). However, the FF/VIL combination trial on exacerbations assessed three different doses of that formulation (50, 100 and 200 µg FF) and showed no reduced pneumonia risk with the lower doses [Dransfield et al. 2013] (see the Randomized controlled trials section). A recent Cochrane meta-analysis of both BUD and FP found no dose-related effect for FP, but pneumonia rates appeared to be higher with the highest doses of BUD [Kew and Seniukovich, 2014] (see the Meta-analyses section).
Residual effect
Previously, based on the findings of two large retrospective studies [Ernst et al. 2007; Joo et al. 2010], we described the presence of a persistently increased risk of pneumonia even after 12 months of drug discontinuation [Marzoratti et al. 2013]. This residual effect was much shorter in a more recent observational study, which reported a 69% increase in the rate of pneumonia with a gradual decrease in risk after discontinuation, until it was no longer evident after six months [Suissa et al. 2013]. At the time of writing this review, the same group of investigators published online the results of a large case–control study, in which a 37% decrease in the rate of serious pneumonia was noted after ICS discontinuation. It took four months after ICS withdrawal for the risk of pneumonia to drop to 50%, after which it remained stable for the rest of the follow-up period [Suissa et al. 2015].
ICS-induced pneumonia mortality
As mentioned above, a remarkable finding in previous RCTs and meta-analyses was that, although there was a considerable amount of evidence in favor of ICS being responsible for an increased incidence of pneumonia, no parallel increase in mortality could be demonstrated [Drummond et al. 2008; Singh and Loke, 2010]. In the more recent FF/VIL study, concern was raised with regard to an excess of pneumonia-related mortality was reported. In fact, seven out of the eight deaths in the study were registered in the 200 µg arm [Dransfield et al. 2013]. The increased number of pneumonia-related deaths in the highest dose arm is statistically significant (not reported in the study paper). According to the full study report of the sponsoring pharmaceutical company, these seven deaths were reported in only one of the replicate studies (Study 1) and, even more importantly, four of them were recorded in the same center in the Philippines.
Two prospective observational studies that assessed the prevalence of ICS use and outcomes in patients hospitalized for pneumonia reported no significant increase in mortality after 6 and 12 months of ICS use, respectively [Singanayagam et al. 2011; Ferrer et al. 2014]. Some studies even found a decreased risk of short-term mortality. Two case–control studies assessed the impact of prior outpatient use of ICS on 30- and 90-day mortality in a cohort of hospitalized COPD patients with pneumonia, extracted from the database of the Department of Veterans Affairs [Malo de Molina et al. 2010 Chen et al. 2011]. Both studies reported a reduced 30- and 90-day mortality rate in the ICS users group. One of the studies also reported that there was less need for mechanical ventilation in the ICS group [Chen et al. 2011]. Less incidence of pulmonary complications, such as pleural effusion in current ICS users hospitalized for pneumonia, have also been reported [Sellares et al. 2013]. On the other hand, in a pooled analysis of observational data, Loke and colleagues concluded that prior ICS use was not consistently associated with lower mortality from CAP in COPD patients [Loke et al. 2013]. The authors emphasize the fact that reports of reduced mortality in current ICS users at the time of hospitalization came from three studies that enrolled patients from the same Veterans Affairs database [Joo et al. 2010; Malo de Molina et al. 2010; Chen et al. 2011], thus perhaps limiting the applicability of their findings to that particular population.
ICS and pneumonia in asthma
Given that ICS are the most effective treatment in asthma, findings from COPD trials of pneumonia as a consequence of ICS use implies that asthmatic patients treated with these drugs could also be at risk. Because patients with asthma do not generally have as many major confounding risk factors for developing pneumonia (i.e. advanced age and comorbid diseases) and the clinical manifestations of the disease do not overlap as much with the adverse effects of the medication used to treat it, patients with asthma may represent a more useful population in which to investigate the risk of pneumonia as a consequence of ICS use. On the other hand, for the reasons mentioned before, the number of pneumonia events in asthma studies is expected to be very low, so any adverse impact of ICS would be hard to measure.
O’Byrne and colleagues published the results of a retrospective, industry-sponsored analysis of a dataset of asthma patients who had been included in double-blind randomized trials of at least 3 months’ duration [O’Byrne et al. 2011]. With 86 trials included, and over 50,000 patients, they compared the effects of BUD versus controls and BUD versus FP. In their analysis, the authors found no differences in the risk of pneumonia, the dose - response relationship for the development of pneumonia with BUD, or any intra-class difference between BUD and FP. However, in another more recent case - control study of a UK primary care database, the authors reported a two-fold increase in the risk of pneumonia or lower respiratory tract infection with the highest dose of ICS, after adjusting for confounders [McKeever et al. 2013]. Lastly, another case-control cohort study in a Japanese population suggested that the risk of nontuberculous mycobacteriosis may be greater in asthmatic patients treated with ICS, with older individuals, who have more severe airflow limitation and receive higher doses of ICS therapy, being at greater risk [Hojo et al. 2012].
Despite these findings, the inflammatory profiles of asthma and COPD are quite different [Fabbri et al. 2003], so extrapolations between these two conditions regarding susceptibility to ICS-induced pneumonia are unlikely to be valid. COPD and asthma patients are almost invariably excluded from therapeutic clinical trials of the other condition, but the fact that clinical characteristics of both COPD and asthma can coexist in the same patient may have important therapeutic implications. Sputum eosinophilia has been demonstrated as predicting clinical response to systemic glucocorticoids [Bafadhel et al. 2012] and to ICS [Brightling et al. 2005; Kitaguchi et al. 2012]. The benefits of ICS treatment in the recently well-characterized asthma–COPD overlap syndrome (ACOS) is discussed later (see the Risk/benefit ratio and indications section).
Other ICS-related infections
The proposed immunosuppressive mechanism responsible an increase in bacterial pneumonia cases among long-term users of ICS suggests that the risk of infection by other micro-organisms could also be increased. In fact, several studies have raised safety concerns about the risk of tuberculosis (TB) and other pulmonary infections.
In a 2014 Japanese meta-analysis of RCTs of ICS therapy for COPD lasting at least six months, ICS treatment was associated with a significantly higher risk of TB (OR 2.29; 95% CI 1.04–5.03) but not influenza. The risk of TB was even higher for patients living in endemic areas [Dong et al. 2014]. Another 2014 meta-analysis reported that ICS use increases the risk of TB among patients with COPD and patients with history of past pulmonary TB [Songshi et al. 2014]. Two case–control studies also suggest that ICS may be a risk factor non-TB mycobacterial (NTM) pulmonary disease [Hojo et al. 2012; Andréjak et al. 2013].
Risk/benefit ratio and indications
While ICS have become a key element in the treatment of asthma, their role in patients with stable COPD remains unclear, and may be beneficial only for a specific subset of ICS responders, but not for everyone [Alcázar Navarrete et al. 2015]. Taking into consideration that an elevated risk of pneumonia with ICS use has been repeatedly found in studies of various designs, this safety concern should be balanced with the available evidence on efficacy, with particular emphasis on reduction of exacerbations and survival.
Trying to apply personalized medicine principles to such a complex and heterogeneous disease as COPD represents a real challenge. In order to achieve this goal, attempts are being made to identify clinical phenotypes with clear therapeutic implications, in order to optimize the pharmacological treatment of COPD [Han et al. 2010]. One of the best characterized phenotypes to date is the exacerbation-susceptibility phenotype which was defined in the ECLIPSE study [Hurst et al. 2010] as the presence of two or more AECOPD episodes in a year. This trait has been proven to be independent of disease severity, and stable over time and was found to be present in up to one third of COPD patients [Hurst et al. 2010]. Other clinically relevant subgroups or phenotypes such as chronic bronchitis, eosinophilic COPD, and asthma–COPD overlap syndrome (ACOS) have been described. Their definition and potential implications for targeted therapeutic strategies have been reviewed elsewhere [Miravitlles et al. 2013; Turner et al. 2015]. An elevated blood eosinophil count has been demonstrated to be a frequent trait in patients with COPD, with a prevalence of 37% in the ECLIPSE cohort [Singh et al. 2014], and peripheral blood eosinophilia could represent a potential biomarker of response to ICS in patients with COPD. In fact, in a post hoc analysis of data from the 1-year FF/VIL exacerbation study, the reduction in AECOPD across all doses of FF compared with VIL alone in patients with eosinophil is above the 2% cut-off point (29% versus 10%), progressively increased up to 42% for those with eosinophil counts ⩾6%. Patients treated with VIL alone, on the other hand, had higher exacerbation rates with increasing eosinophil count. However, there was no association observed between the blood eosinophil count and the risk of pneumonia [Pascoe et al. 2015].
In a recent consensus document from Spain, formal indications for ICS have been limited to the frequent exacerbator and the mixed asthma–COPD phenotypes. The participants also agreed on the feasibility of discontinuation of ICS outside these indications [Alcázar Navarrete et al. 2015]. It has already been demonstrated that in patients with COPD, at low risk for exacerbations, treatment with ICS can be withdrawn without serious detrimental effects on patients’ outcomes. In the INSTEAD trail, nonexacerbating patients with moderate COPD were successfully switched from FP/SAL to indacaterol (IND) with no impact on exacerbation rates over six months [Rossi et al. 2014b]. The OPTIMO study involving patients with FEV1 greater than 50% and less than two exacerbations per year reported similar results [Rossi et al. 2014a]. Evidence suggests that ICS withdrawal could also be well tolerated in more severe patients at a higher risk of exacerbations. The most recent and largest RCT on ICS withdrawal is the WISDOM study, in which the risk of moderate-to-severe AECOPD did not increase in patients with severe COPD and a history of exacerbations who gradually discontinued ICS therapy over 3 months. However, contrary to what it would have been expected, there was only a nonsignificant 5% reduction in the risk of pneumonia after 12 months in the withdrawal arm [Magnussen et al. 2014]. Sudden, instead of gradual, withdrawal of ICS seems to be well tolerated for COPD patients at low risk of exacerbation [Rossi et al. 2014a], but not for those with more severe disease and frequent exacerbations [Wouters et al. 2005]. At the time of writing this review, Suissa and colleagues published online the results of a case–control study from a cohort of more than 100,000 ICS users during a follow-up period of 4.9 years. They reported a 37% decrease in the rate of serious pneumonia with ICS discontinuation. The rate of risk reduction ranged from 20% in the first month to 50% by the fourth month after discontinuation, and was more evident for FP than for BUD (RR 0.58 versus 0.87) [Suissa et al. 2015].
Conversely, the widespread prescription of the ICS-containing inhalers for COPD regardless of severity stage or clinical phenotype carries the potential risk of overtreatment, with the associated increase in costs, and in serious adverse effects such as pneumonia. In a study that analyzed data extracted from records of London general practices, the investigators reported a 25% incidence of overtreatment with ICS when compared with the 2011 revised GOLD guidelines’ recommendations [White et al. 2013]. According to another observational study from Italy, ICS were over-prescribed in up to 62% of the subjects [Corrado and Rossi, 2012]. A post hoc analysis of data for 5162 patients from the phase III TONADO studies, which used a tiotropium/olodaterol (TIO/OLO) combination, reported that nearly 40% of patients who were classified as having GOLD A or B disease were on ICS maintenance therapy at study entry [Watz et al. 2015]. This lack of adherence to current GOLD recommendations may be favored for many reasons: (1) the fact that ICS and LABAs are frequently only available in a single combined inhaler that usually contains high doses of the ICS component; (2) uncertainty in the diagnosis of asthma versus COPD, or the inability to rule out an overlap between the two conditions; and (3) the concern of triggering an exacerbation by withdrawing the ICS component in a patient who maintains persistent clinical stability while on ICS/LABA maintenance therapy.
While ICS alone are not recommended in stable COPD, the ICS/LABA combination is commonly prescribed for COPD patients with severe disease or frequent exacerbations (or both), according to most current COPD management guidelines, [GOLD, 2015; Criner et al. 2015]. However, the methodological accuracy of previous trials that reported lower rates of exacerbations and improved survival with the ICS/LABA combination compared with monotherapy with long-acting bronchodilators, has been questioned extensively. The lack of a true intention-to-treat analysis for the outcome of exacerbations in the TORCH study is an example [Ernst et al. 2015]. Also worth mentioning is the inappropriate calculation of the number needed to treat (NNT) in most COPD exacerbations trials, as reported by Suissa [Suissa, 2013]. According to the investigators, the event-based approach to NNT or number needed to harm (NNH) calculation is not valid when patients have uneven follow-up time periods (which is true in most trials) and when, instead of binary outcomes that only occur once in each patient during the study period, there is the possibility of recurring events in the same patient over time (as is the case with AECOPD). In fact, they found that after the correct calculation of the NNT, in FP studies longer than 1 year such as TORCH and INSPIRE, considerably fewer patients needed to be treated with ICS to induce a pneumonia, compared with the number of patients needed to be treated in order to prevent an AECOPD [Suissa, 2013]. This undoubtedly has a major impact in clinical decisions and in cost–benefit analyses. Applying the same correction for the results of the newer FF/VIL trial by Dransfield and colleagues, we found that, similar to what Susissa and colleagues reported for two 1-year studies, the NNT for the 100 μg dose to prevent an exacerbation is 10, and the NNH to cause a pneumonia with the same dose is 35 (see the Randomized controlled trials section).
One 2012 updated meta-analysis challenges the superiority of ICS/LABA combination over LABA monotherapy in preventing exacerbations, and concludes that both treatments have similar effects on mortality [Nannini et al. 2012]. Also, in one of the two replicate COPD exacerbation studies with the new FF/VIL combination, no significant difference in exacerbation rate was found between the 200/25 μg FF/VIL and the VIL monotherapy arms [Dransfield et al. 2013]. LAMA monotherapy has also been investigated extensively. In the INSPIRE study, TIO was not inferior to FP/SAL for the prevention of AECOPD [Wedzicha et al. 2008]. A subsequent Cochrane meta-analysis reported similar findings [Welsh et al. 2013]. The new LABA/LAMA combinations have also shown some promising results. In the phase III LANTERN study, the subgroup of patients with a history of moderate to severe exacerbations experienced a 40% reduction in the rate of AECOPD with indacaterol/glycopyrronium (IND/GLY) compared with FP/SAL (RR 0.60; 95% CI 0.33–1.08) over a 26-week period. Moreover, a significant three-fold lower incidence of pneumonia was reported in the IND/GLY arm (0.8% versus 2.7%). Although the difference in the exacerbation rates did not reach statistical significance, these findings suggest that there is a potential role for LABA/LAMA combinations as replacements for ICS in preventing AECOPD [Zhong et al. 2015]. In this respect, an ongoing study will assess the rate of AECOPD over 52 weeks of treatment with IND/GLY or FP/SAL in approximately 3000 patients with a history of moderate to severe exacerbations in the previous year. Results are expected in 2016 [ClinicalTrials.gov identifier: NCT01782326]. Another ongoing study, with an estimated enrollment of 10,000 patients, will compare the annual rate of AECOPD in patients receiving umeclinidium/vilanterol (UME/VIL) against those receiving FF/VIL or the triple combination FF/UME/VIL. Results are expected in 2017 [ClinicalTrials.gov identifier: NCT02164513]. There is also an ongoing trial that will evaluate annual exacerbation rates with the TIO/OLO combination compared with TIO monotherapy, both delivered through the Respimat® (GlaxoSmithKline, UK) inhaler [ClinicalTrials.gov identifier: NCT02296138], but no studies comparing this novel LABA/LAMA combination against ICS in terms of exacerbations have been reported to date.
When exacerbations are not controlled with the usual doses of the ICS/LABA combination, increasing the dose of these drugs is not advisable because of the flat dose–response curve and the potentially harmful side effects. This was evidenced by the lack of additional benefit in terms of exacerbations, and the excess deaths from pneumonia, with the highest dose of FF/VIL in the trial by Dransfield and colleagues [Dransfield et al. 2013]. Alternatives to this approach include the use of roflumilast, mucolytics and macrolides in carefully selected patients who are more likely to benefit from these therapies [GOLD, 2015; Criner et al. 2015] (Figure 1).
Conclusion and recommendations
The evidence for an association between ICS use and the development of pneumonia in patients with COPD continues to persist according to the findings of newer trials, observational studies and meta-analyses. This has led to the inclusion of this adverse effect in the GOLD guidelines for the diagnosis, management and prevention of COPD [GOLD, 2015], the ACCP/CTS guidelines on prevention of AECOPD [Criner et al. 2015], and it being listed as a common adverse event in the safety section of the labels of most commonly prescribed FP- and FF -containing products [Electronic Medicines Compendium (eMC), 2015a; 2015b]. Despite the association of ICS with an increased risk of pneumonia, most studies found either no difference or even a reduction in pneumonia-related or overall mortality associated with the use of ICS or both. This makes the true relationship between COPD and pneumonia mortality even harder to establish. New studies were not able to rule out budesonide as responsible for pneumonia [Suissa et al. 2013; Kew and Seniukovich, 2014] as previous studies have suggested [Sin et al. 2009], and there is still need for evidence from head-to-head comparisons to assess the risks and benefits of the different ICS formulations. The immunomodulatory effects of ICS on the lung epithelium and microbiome are very complex, and proposed mechanisms that may lead to the development of pneumonia in ICS users are still speculative and warrant further research. Several flaws in the analysis of the data have been reported, challenging previous conclusions about reduced frequency of exacerbations and survival benefits with ICS/LABA combination, compared with monotherapy with long-acting bronchodilators [Ernst et al. 2015; Suissa, 2013]. New trials and meta-analysis were also unable to show a clear benefit in this respect [Nannini et al. 2012; Dransfield et al. 2013]. The role of long-acting bronchodilators combinations (LABA/LAMA) as candidates for ICS replacement in preventing exacerbations is another area of growing interest. Given that the appropriate role of ICS in the treatment of stable COPD remains controversial, efforts should be made to limit ICS use to those subgroups of patients in which a clear benefit has been demonstrated, mostly identified through the recent progress in the characterization of COPD phenotypes. Although vaccination against pneumococcus and influenza is recommended for all patients with COPD, regardless of treatment choice [Tomczyk et al. 2014; World Health Organization, 2012], given the increased risk of pneumonia with ICS, it seems advisable to emphasize vaccination among ICS users. We recommend that the lowest effective dose of ICS be used to prevent exacerbations in those at risk (frequent exacerbator or >2% blood eosinophils count/ACOS phenotypes), which would nominally be 250 μg twice daily of FP, 100 µg per day of FF, 200 µg twice daily of BUD. We also recommend considering a switch from an ICS/LABA to a LABA/LAMA in patients who have had pneumonia despite appropriate vaccination. Those with chronic carriage of bacteria in their sputum, especially those with bronchiectasis, could also benefit from this strategy, although more data is needed to make a strong recommendation in for this group. We suggest screening for atypical mycobacteria when considering ICS therapies, especially in patients with bronchiectasis and elderly individuals. Finally, physicians should keep in mind that signs and symptoms of pneumonia in COPD patients may be initially indistinguishable from those of an exacerbation, and that patients with COPD treated with ICS are at increased risk to develop pneumonia.
Acknowledgments
This review is dedicated to Dr Lucia Marzoratti, whose passion and invaluable contribution to the original review in 2013 inspired us to write this update. We will always remember you.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Waterer has spoken at GSK and AstraZeneca sponsored symposia in the past 5 years. Dr. Luna has acted as a member of the Advisory Board for AstraZeneca between 2013 and 2014. Dr. Iannella has no conflicts of interest to declare.
Contributor Information
Hernan Iannella, Hospital de Clínicas ‘José de San Martin’, Universidad de Buenos Aires, Av. Córdoba 2351, Ciudad de Buenos Aries, C1120AAR, Argentina.
Carlos Luna, Hospital de Clínicas ‘José de San Martin’, Universidad de Buenos Aires, Ciudad de Buenos Aires, Argentina.
Grant Waterer, Royal Perth Hospital, University of Western Australia, Western Australia, Australia.
References
- Agusti A., Fabbri L. (2014) Inhaled steroids in COPD: when should they be used? Lancet Respir Med 2: 869–871. [DOI] [PubMed] [Google Scholar]
- Alcázar Navarrete B., Casanova C., Miravitlles M., de Lucas P., Riesco J., Rodríguez González-Moro J., et al. (2015) “Correct use of inhaled corticosteroids in chronic obstructive pulmonary disease”: a consensus document. Arch Bronconeumol 51: 193–198. [DOI] [PubMed] [Google Scholar]
- Almirall J., Bolibar I., Serra-Prat M., Roig J., Hospital I., Carandell E., et al. (2008) New evidence of risk factors for community-acquired pneumonia: a population-based study. Eur Respir J 31: 1274–1284. [DOI] [PubMed] [Google Scholar]
- Andréjak C., Nielsen R., Thomsen V., Duhaut P., Sørensen H., Thomsen R. (2013) Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax 68: 256–262. [DOI] [PubMed] [Google Scholar]
- Anzueto A., Ferguson G., Feldman G., Chinsky K., Seibert A., Emmett A. (2009) Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD 6: 320–329. [DOI] [PubMed] [Google Scholar]
- Bafadhel M., McKenna S., Terry S., et al. (2012) Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med 186: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum C., Nigro N., Briel M., Schuetz P., Ullmer E., Suter-Widmer I., et al. (2015) Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 385: 1511–1518. [DOI] [PubMed] [Google Scholar]
- Brightling C., McKenna S., Hargadon B., Birring S., Green R., Siva R., et al. (2005) Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax 60: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley P., Anderson J., Celli B., Ferguson G., Jenkins C., Jones P., et al. (2007) Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 356: 775–789. [DOI] [PubMed] [Google Scholar]
- Calverley P., Stockley R., Seemungal T., Hagan G., Willits L., Riley J., et al. (2011) Reported Pneumonia in COPD: Findings From the INSPIRE Study. Chest 139: 505–512. [DOI] [PubMed] [Google Scholar]
- Chen D., Restrepo M., Fine M., Pugh M., Anzueto A., Metersky M., et al. (2011) Observational study of inhaled corticosteroids on outcomes for COPD patients with pneumonia. Am J Respir Crit Care Med 184: 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J., Leung B., Poole P. (2013) Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 11: CD002309. [DOI] [PubMed] [Google Scholar]
- Corrado A., Rossi A. (2012) How far is real life from COPD therapy guidelines? An Italian observational study. Respir Med 106: 989–997. [DOI] [PubMed] [Google Scholar]
- Crim C., Dransfield M., Bourbeau J., Jones P., Hanania N., Mahler D., et al. (2015) Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc 12: 27–34. [DOI] [PubMed] [Google Scholar]
- Criner G., Bourbeau J., Diekemper R., Ouellette D., Goodridge D., Hernandez P., et al. (2015) Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest 147: 894–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisafulli E., Menéndez R., Huerta A., Martinez R., Montull B., Clini E., et al. (2013) Systemic inflammatory pattern of patients with community-acquired pneumonia with and without COPD. Chest 143: 1009–1017. [DOI] [PubMed] [Google Scholar]
- Crisafulli E., Guerrero M., Menéndez R., Huerta A., Martinez R., Gimeno A., et al. (2014) Inhaled corticosteroids do not influence the early inflammatory response and clinical presentation of hospitalized subjects with COPD exacerbation. Respir Care 59: 1550–1559. [DOI] [PubMed] [Google Scholar]
- Dickson R., Erb-Downward J., Huffnagle G. (2014) Towards an ecology of the lung: new conceptual models of pulmonary microbiology and pneumonia pathogenesis. Lancet Respir Med 2: 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSantostefano R., Sampson T., Le H., Hinds D., Davis K., Bakerly N. (2014) Risk of pneumonia with inhaled corticosteroid versus long-acting bronchodilator regimens in chronic obstructive pulmonary disease: a new-user cohort study. PLoS One 9: e97149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Chang H., Lin Wu F., Shen L., Calverley P., Löfdahl C., et al. (2014) Use of inhaled corticosteroids in patients with COPD and the risk of TB and influenza. Chest 145: 1286–1297. [DOI] [PubMed] [Google Scholar]
- Dransfield M., Bourbeau J., Jones P., Hanania N., Mahler D., Vestbo J., et al. (2013) Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med 1: 210–223. [DOI] [PubMed] [Google Scholar]
- Drummond M., Dasenbrook E., Pitz M., Murphy D., Fan E. (2008) Inhaled corticosteroids in patients with stable COPD: a systematic review and meta-analysis. JAMA 300: 2407–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Electronic Medicines Compendium (eMC) (2015a) Relvar Ellipta 184 micrograms/22 micrograms inhalation powder, pre-dispensed. Updated 7 January 2015. www.medicines.org.uk/emc/medicine/28495 (accessed 2 June 2015).
- Electronic Medicines Compendium (eMC) (2015b) Seretide 100, 250, 500 Accuhaler. Updated 24 April 2015. www.medicines.org.uk/emc/medicine/2317 (accessed 17 June 2015).
- Ernst P., Gonzalez A., Brassard P., Suissa S., et al. (2007) Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med 176: 162–166. [DOI] [PubMed] [Google Scholar]
- Ernst P., Saad N., Suissa S. (2015) Inhaled corticosteroids in COPD: the clinical evidence. Eur Respir J 45: 525–537. [DOI] [PubMed] [Google Scholar]
- Eurich D., Lee C., Marrie T., Majumdar S. (2013) Inhaled corticosteroids and risk of recurrent pneumonia: a population-based, nested case-control study. Clin Infect Dis 57: 1138–1144. [DOI] [PubMed] [Google Scholar]
- Fabbri L., Romagnoli M., Corbetta L., Casoni G., Busljetic K., Turato G., et al. (2003) Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med 167: 418–424. [DOI] [PubMed] [Google Scholar]
- Farr B., Bartlett C., Wadsworth J., Miller D . (2000) Risk factors for community-acquired pneumonia diagnosed upon hospital admission. British Thoracic Society Pneumonia Study Group. Respir Med 94: 954–963. [DOI] [PubMed] [Google Scholar]
- Ferguson G., Anzueto A., Fei R., Emmett A., Knobil K., Kalberg C. (2008) Effect of fluticasone propionate/salmeterol (250/50 mg) or salmeterol (50 mg) on COPD exacerbations. Respir Med 102: 1099–1108. [DOI] [PubMed] [Google Scholar]
- Ferrer M., Torres A., Martínez R., Ramírez P., Polverino E., Montull B., et al. (2014) Inhaled corticosteroids and systemic inflammatory response in community-acquired pneumonia: A prospective clinical study. Respirology 19: 929–935. [DOI] [PubMed] [Google Scholar]
- Festic E., Bansal V., Gajic O., et al. (2014) Prehospital use of inhaled corticosteroids and point prevalence of pneumonia at the time of hospital admission: secondary analysis of a multicenter cohort study. Mayo Clin Proc 89: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine M., Auble T., Yealy D., Hanusa B., Weissfeld L., Singer D., et al. (1997) A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 336: 243–250. [DOI] [PubMed] [Google Scholar]
- Garcha D., Thurston S., Patel A., Mackay A., Goldring J., Donaldson G., et al. (2012) Changes in prevalence and load of airway bacteria using quantitative PCR in stable and exacerbated COPD. Thorax 67: 1075–1080. [DOI] [PubMed] [Google Scholar]
- Gershon A., Campitelli M., Croxford R., Stanbrook M., To T., Upshur R., et al. (2014) Combination long-acting β-agonists and inhaled corticosteroids compared with long-acting β-agonists alone in older adults with chronic obstructive pulmonary disease. JAMA 312: 1114–1121. [DOI] [PubMed] [Google Scholar]
- GOLD (2011) Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) (updated January 2015). http://www.goldcopd.org (accessed 26 February 2015).
- Gutierrez P., Closa D., Piñer R., Bulbena O., Menéndez R., Torres A. (2010) Macrophage activation in exacerbated chronic obstructive pulmonary disease with and without community acquired pneumonia. Eur Respir J 36: 285–291. [DOI] [PubMed] [Google Scholar]
- Halpin D., Gray J., Edwards S., Morais J., Singh D. (2011) Budesonide/formoterol vs. salmeterol/fluticasone in COPD: a systematic review and adjusted indirect comparison of pneumonia in randomized controlled trials. Int J Clin Pract 65: 764–774. [DOI] [PubMed] [Google Scholar]
- Han M., Agusti A., Calverley P., Celli B., Criner G., Curtis J., et al. (2010) Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 182: 598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo M., Iikura M., Hirano S., Sugiyama H., Kobayashi N., Kudo K. (2012) Increased risk of nontuberculous mycobacterial infection in asthmatic patients using long-term inhaled corticosteroid therapy. Respirology 17: 185–190. [DOI] [PubMed] [Google Scholar]
- Huang Y., Sethi S., Murphy T., Nariya S., Boushey H., Lynch S. (2014) Airway microbiome dynamics in exacerbations of chronic obstructive pulmonary disease. J Clin Microbiol 52: 2813–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta A., Crisafulli E., Menéndez R., Martínez R., Soler N., Guerrero M., et al. (2013) Pneumonic and nonpneumonic exacerbations of COPD: inflammatory response and clinical characteristics. Chest 144: 1134–1142. [DOI] [PubMed] [Google Scholar]
- Hurst J., Vestbo J., Anzueto A., Locantore N., Müllerova H., Tal-Singer R., et al. (2010) Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 363: 1128–1138. [DOI] [PubMed] [Google Scholar]
- Janson C., Larsson K., Lisspers K., Ställberg B., Stratelis G., Goike H. (2013) Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting β2 agonist: observational matched cohort study (PATHOS). BMJ 346: f3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen R., Rennard S., Sin D. (2012) Effects of inhaled corticosteroids on airway inflammation in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 7: 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. (1998) Development of fluticasone propionate and comparison with other inhaled corticosteroids. J Allergy Clin Immunol 101: S434–S439. [DOI] [PubMed] [Google Scholar]
- Joo M., Au D., Fitzgibbon M., Lee T. (2010) Inhaled corticosteroids and risk of pneumonia in newly diagnosed COPD. Respir Med 104: 246–252. [DOI] [PubMed] [Google Scholar]
- Kardos P., Wencker M., Glaab T., Vogelmeier C. (2007) Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 175: 144–149. [DOI] [PubMed] [Google Scholar]
- Kern D., Davis J., Williams S., Tunceli O., Wu B., Hollis S., et al. (2015) Comparative effectiveness of budesonide/formoterol combination and fluticasone/salmeterol combination among chronic obstructive pulmonary disease patients new to controller treatment: a US administrative claims database study. Respir Res 23 April 2015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew K., Seniukovich A. (2014) Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 10(3): CD010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaguchi Y., Komatsu Y., Fujimoto K., Hanaoka M., Kubo K. (2012) Sputum eosinophilia can predict responsiveness to inhaled corticosteroid treatment in patients with overlap syndrome of COPD and asthma. Int J Chron Obstruct Pulmon Dis 7: 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson K., Janson C., Lisspers K., Jørgensen L., Stratelis G., Telg G., et al. (2013) Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med 273: 584–594. [DOI] [PubMed] [Google Scholar]
- Lipworth B. (1999) Systemic adverse effects of inhaled corticosteroid therapy: A systematic review and meta-analysis. Arch Intern Med 159: 941–955. [DOI] [PubMed] [Google Scholar]
- Loke Y., Singh S. (2009) Inhaled corticosteroids in patients with COPD. JAMA 301: 1432–1434. [DOI] [PubMed] [Google Scholar]
- Loke, et al. (2011) [Google Scholar]
- Loke Y., Kwok C., Wong J., et al. (2013) Chronic obstructive pulmonary disease and mortality from pneumonia: meta-analysis. Int J Clin Pract 67: 477–487. [DOI] [PubMed] [Google Scholar]
- Magnussen H., Disse B., Rodriguez-Roisin R., et al. (2014) Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med 371: 1285–1294. [DOI] [PubMed] [Google Scholar]
- Malo de Molina R., Mortensen E., Restrepo M., Copeland L., Pugh M., Anzueto A. (2010) Inhaled corticosteroid use is associated with lower mortality for subjects with COPD and hospitalized with pneumonia. Eur Respir J 36: 751–757. [DOI] [PubMed] [Google Scholar]
- Mapel D., Schum M., Yood M., Brown J., Miller D., Davis K. (2010) Pneumonia among COPD patients using inhaled corticosteroids and long-acting bronchodilators. Prim Care Respir J 19: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R., Menendez R., Reyes S., Polverino E., Cillóniz C., Martínez A. (2011) Factors associated with inflammatory cytokine patterns in community-acquired pneumonia. Eur Respir J 37: 393–399. [DOI] [PubMed] [Google Scholar]
- Martinez F., Calverley P., Goehring U., Brose M., Fabbri L., Rabe K. (2015) Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 385: 857–866. [DOI] [PubMed] [Google Scholar]
- Miravitlles M., Soler-Cataluña J., Calle M., Soriano J. (2013) Treatment of COPD by clinical phenotypes: putting old evidence into clinical practice. Eur Respir J 41: 1252–1256. [DOI] [PubMed] [Google Scholar]
- Miravitlles M., Anzueto A. (2015) Role of infection in exacerbations of chronic obstructive pulmonary disease. Curr Opin Pulm Med 21:278–283. [DOI] [PubMed] [Google Scholar]
- Nannini L., Cates C., Lasserson T., Poole P. (2007) Combined corticosteroid and long-acting beta-agonist in one inhaler versus long-acting beta-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 4: CD006829. [DOI] [PubMed] [Google Scholar]
- Nannini L., Lasserson T., Poole P. (2012) Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 9: CD006829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie W., Zhang Y., Cheng J., Xiu Q., et al. (2012) Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One 7: e47926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni S., Fu Z., Zhao J., Liu H. (2014) Inhaled corticosteroids (ICS) and risk of mycobacterium in patients with chronic respiratory diseases: a meta-analysis. J Thorac Dis 6: 971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Byrne P., Pedersen S., Carlsson L., Radner F., Thorén A., Peterson S., et al. (2011) Risks of pneumonia in asthmatic patients taking inhaled corticosteroids. Am J Respir Crit Care Med 183: 589–595. [DOI] [PubMed] [Google Scholar]
- Pascoe S., Locantore N., Dransfield M., Barnes N., Pavord I., et al. (2015) Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med 3: 435–442. [DOI] [PubMed] [Google Scholar]
- Patel I., Seemungal T., Wilks M., Lloyd-Owen S., Donaldson G., Wedzicha J. (2002) Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 57: 759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel I., Vlahos I., Wilkinson T., Lloyd-Owen S., Donaldson G., Wilks M., et al. (2004) Bronchiectasis, exacerbations indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 170: 400–407. [DOI] [PubMed] [Google Scholar]
- Pragman A., Kim H., Reilly C., et al. (2012) The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS One 7: e47305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rello J., Rodriguez A., Torres A., Roig J., Sole-Violan J., Garnacho-Montero J., et al. (2006) Implications of COPD in patients admitted to the intensive care unit by community-acquired pneumonia. Eur Respir J 27: 1210–1216. [DOI] [PubMed] [Google Scholar]
- Rennard S., Tashkin D., McElhattan J., Goldman M., Ramachandran S., Martin U., et al. (2009) Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial. Drugs 69: 549–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo M., Mortensen E., Pugh J., Anzueto A. (2006) COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J 28: 346–351. [DOI] [PubMed] [Google Scholar]
- Rodrigo G., Castro-Rodriguez J., Plaza V. (2009) Safety and efficacy of combined long-acting beta-agonists and inhaled corticosteroids vs. long-acting betaagonists monotherapy for stable COPD: a systematic review. Chest 136: 1029–1038. [DOI] [PubMed] [Google Scholar]
- Rossi A., Guerriero M., Corrado A. (2014a) Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: A real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO). Respir Res 15: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A., van der Molen T., del Olmo R., Papi A., Wehbe L., Quinn M., et al. (2014) INSTEAD: a randomized switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J 44: 1548–1556. [DOI] [PubMed] [Google Scholar]
- Rossios C., To Y., To M., Ito M., Barnes P., Adcock I., et al. (2011) Long-acting fluticasone furoate has a superior pharmacological profile to fluticasone propionate in human respiratory cells. Eur J Pharmacol 670: 244–251. [DOI] [PubMed] [Google Scholar]
- Salluh J., Póvoa P., Soares M., Castro-Faria-Neto H., Bozza F., Bozza P. (2008) The role of corticosteroids in severe community-acquired pneumonia: a systematic review. Crit Care 12(3): R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellares J., López-Giraldo A., Lucena C., Cilloniz C., Amaro R., Polverino E., et al. (2013) Influence of previous use of inhaled corticoids on the development of pleural effusion in community-acquired pneumonia. Am J Respir Crit Care Med 187: 1241–1248. [DOI] [PubMed] [Google Scholar]
- Sethi S., Sethi R., Eschberger K., Lobbins P., Cai X., Grant B., et al. (2007) Airway bacterial concentrations and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 176: 356–361. [DOI] [PubMed] [Google Scholar]
- Sharafkhaneh A., Southard J., Goldman M., Uryniak T., Martin U. (2012) Effect of budesonide/formoterol pMDI on COPD exacerbations: a double-blind, randomized study. Respir Med 106: 257–268. [DOI] [PubMed] [Google Scholar]
- Siemieniuk R., Meade M., Alonso-Coello P., Briel M., Evaniew N., Prasad M., et al. (2015) Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med 163: 519–528. [DOI] [PubMed] [Google Scholar]
- Sin D., Tashkin D., Xuekui Zhang X., et al. (2009) Budesonide and the risk of pneumonia: a meta-analysis of individual patient data. Lancet 374: 712–719. [DOI] [PubMed] [Google Scholar]
- Singanayagam A., Chalmers J., Hill A. (2010) Inhaled corticosteroids and risk of pneumonia: evidence for and against the proposed association. QJM 103: 379–385. [DOI] [PubMed] [Google Scholar]
- Singanayagam A., Chalmers J., Akram A., Hill A. (2011) Impact of inhaled corticosteroid use on outcome in COPD patients admitted with pneumonia. Eur Respir J 38: 36–41. [DOI] [PubMed] [Google Scholar]
- Singh S., Amin A., Loke Y. (2009) Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta-analysis. Arch Intern Med 169: 219–229. [DOI] [PubMed] [Google Scholar]
- Singh S., Loke Y. (2010) Risk of pneumonia associated with long-term use of inhaled corticosteroids in COPD: A critical review and update. Curr Opin Pulm Med 16: 118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D., Kolsum U., Brightling C., Locantore N., Agusti A., Tal-Singer R. (2014) Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J 44: 1697–1700. [DOI] [PubMed] [Google Scholar]
- Snijders D., Van Der Eerden M., De Graaff C., Boersma W. (2010) The influence of COPD on mortality and severity scoring in community-acquired pneumonia. Respiration 79: 46–53. [DOI] [PubMed] [Google Scholar]
- Suissa S. (2013a) Number needed to treat in COPD: exacerbations versus pneumonias. Thorax 68: 540–543. [DOI] [PubMed] [Google Scholar]
- Suissa S., Patenaude V., Lapi F., Ernst P. (2013b) Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax 68: 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suissa S., Coulombe J., Ernst P. (2015) Discontinuation of inhaled corticosteroids in COPD and the risk reduction of pneumonia. Chest 148: 1177–1183. [DOI] [PubMed] [Google Scholar]
- Tagami T., Matsui H., Horiguchi H., Fushimi K., Yasunaga H. (2015) Low-dose corticosteroid use and mortality in severe community-acquired pneumonia patients. Eur Respir J 45: 463–472. [DOI] [PubMed] [Google Scholar]
- Tashkin D., Rennard S., Martin P., Ramachandran S., Martin U., Silkoff P., et al. (2008) Efficacy and safety of budesonide and formoterol in one pressurized metered-dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6-month randomized clinical trial. Drugs 68: 1975–2000. [DOI] [PubMed] [Google Scholar]
- Tashkin D., Doherty D., Kerwin E., Matiz-Bueno C., Knorr B., Shekar T., et al. (2012) Efficacy and safety characteristics of mometasone furoate/formoterol fumarate fixed-dose combination in subjects with moderate to very severe COPD: findings from pooled analysis of two randomized, 52-week placebo-controlled trials. Int J Chron Obstruct Pulmon Dis 7: 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton Snider J., Luna Y., Wong K., Zhang J., Chen S., Gless P., et al. (2012) Inhaled corticosteroids and the risk of pneumonia in Medicare patients with COPD. Curr Med Res Opin 28: 1959–1967. [DOI] [PubMed] [Google Scholar]
- Tomczyk S., Bennett N., Stoecker C., Gierke R., Moore M., Whitney C., et al. (2014) Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ⩾65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 63: 822–825. [PMC free article] [PubMed] [Google Scholar]
- Torres A., Sibila O., Ferrer M., Polverino E., Menendez R., Mensa J., et al. (2015) Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 313: 677–686. [DOI] [PubMed] [Google Scholar]
- Turner A., Tamasi L., Schleich F., Hoxha M., Horvath I., Louis R., et al. (2015) Clinically relevant subgroups in COPD and asthma. Eur Respir Rev 24: 283–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watz H., Ferguson G., Gronke L., Vob F., Abrahams R., Buhl R. (2015) Inhaled Corticosteroid Plus Long-Acting β2-Agonist Therapy is Overused in the Treatment of Patients with Chronic Obstructive Pulmonary Disease: Post Hoc Analyses of Two 1-Year Studies. ATS Conference, Denver Thematic Poster Session D37: A5784. [Google Scholar]
- Wedzicha J., Calverley P., Seemungal T., Hagan G., Ansari Z., Stockley R. (2008) The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med 177: 19–26. [DOI] [PubMed] [Google Scholar]
- Welsh E., Cates C., Poole P. (2013) Combination inhaled steroid and long-acting beta2-agonist versus tiotropium for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 5: CD007891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P., Thornton H., Pinnock H., Georgopoulou S., Booth H. (2013) Overtreatment of COPD with inhaled corticosteroids–implications for safety and costs: cross-sectional observational study. PLoS One 8: e75221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2012) Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec 87: 461–476. [PubMed] [Google Scholar]
- Wolfe F., Caplan L., Michaud K. (2006) Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease modifying anti-rheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum 54: 628–634. [DOI] [PubMed] [Google Scholar]
- Wouters E., Postma D., Fokkens B., Hop W., Prins J., Kuipers A., et al. (2005) Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomised controlled trial. Thorax 60: 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Gu W., Pan L. (2014) Efficacy and safety of roflumilast in patients with stable chronic obstructive pulmonary disease: A meta-analysis. Pulm Pharmacol Ther 27: 83–89. [DOI] [PubMed] [Google Scholar]
- Yawn B., Li Y., Tian H., Zhang J., Arcona S., Kahler K. (2013) Inhaled corticosteroid use in patients with chronic obstructive pulmonary disease and the risk of pneumonia: a retrospective claims data analysis. Int J Chron Obstruct Pulmon Dis 8: 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N., Wang C., Zhou X., Zhang N., Humphries M., Wang L., et al. (2015) LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J Chron Obstruct Pulmon Dis 10: 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]