Abstract
With the advancement in understanding the biology of non-small cell lung cancer (NSCLC), therapies focused on novel molecular pathways have come to the forefront of NSCLC treatment. This review focuses on the preclinical and clinical aspects underlying the targeting of RAS aberrations in NSCLC with special focus on MEK inhibitors which work by inhibiting the principal downstream mediator of RAS aberrations with a view on how to optimize outcomes with these agents. Preclinical evidence of the activity of MEK inhibitors in KRAS-mutant NSCLC has pushed forward the clinical development of these agents (namely selumetinib and trametinib) in KRAS-mutant NSCLC particularly in combination with other agents. A number of randomized studies have been launched to confirm the activity of these agents and to establish their position in the treatment armamentarium of NSCLC.
Keywords: KRAS, lung cancer, MEK, selumetinib, trametinib
Introduction
Lung cancer represents a global health burden and a leading cause for mortality and morbidity and it tops the list of causes of cancer-related death [Thun et al. 2008]. Smoking has been considered the main etiologic factor for lung cancer and the incidence of lung cancer varies worldwide with the local smoking practices in different regions [Silvestri et al. 2013].
Until now, the prognosis of advanced non-small cell lung cancer (NSCLC) has looked bleak; thus, the search for more refined and effective approaches for this disease has been of prime importance for cancer researchers [Detterbeck et al. 2013]. RAS mutations have been among the most interesting molecular aberrations in NSCLC. This review focuses on the preclinical and clinical aspects underlying the targeting of RAS aberrations in NSCLC.
Basic biology of the RAS–ERK pathway and its role in lung carcinogenesis:
The mitogen-activated protein kinase (MAPK) pathway (also known as the RAS–Raf–MEK–ERK pathway) is an important pathway in both health and disease [Chang and Karin, 2001]. It involves a family of kinases that regulate many critical cellular processes such as proliferation, differentiation and survival [Thompson and Lyons, 2005]. This pathway works through transmitting extracellular signals from different external stimuli, e.g. growth factors, cytokines and hormones to intracellular pivotal molecules which consequently lead to the desired biological events [Roskoski, 2010].
RAS proteins are a family of proteins which represent the first relay point for this pathway. This family consists of essentially a group of small GTPases which function by transmitting signals from trans-membranous receptors into the principal pathways within the cell [Goodsell, 1999]. Because their function implies stimulation of growth and proliferation, they have been implicated in the carcinogenesis of many cancers [Downward, 2003]. The three most common oncogenic RAS subfamilies discovered in humans are KRAS, NRAS and HRAS [Malumbres and Barbacid, 2003].
KRAS mutation has been estimated to occur in a considerable proportion of NSCLC cases. Moreover, adenocarcinoma had a higher frequency of KRAS mutations than other subtypes of NSCLC (p < 0.001) [Boch et al. 2013]. Moreover, approximately 97% of KRAS mutations in NSCLC involve codons 12 or 13 [Forbes et al. 2006].
The incidence of KRAS mutations in NSCLC has been reported to correlate with some clinicopathological characteristics of the patients in some studies. For example, in a retrospective analysis of the distribution of KRAS mutations, Bauml and coworkers found that while KRAS mutation (occurring in this study at a frequency 28.1%) was not associated with race (p = 0.51 for African American versus White patients), it was more common among smokers (p < 0.001) and females (p = 0.01) [Bauml et al. 2013]. Moreover, similar incidence of KRAS mutations has been found in primary and in metastatic lesions. In a nice analysis of KRAS and BRAF genes mutation in the central nervous system (CNS) metastases of NSCLC conducted by Nicoś and coworkers, KRAS mutations were present in 21.4% of the metastatic lesions and in 23.3% of corresponding primary tumors. The majority of the mutations were found in codon 12 of KRAS. KRAS mutations were more frequent in smokers with adenocarcinoma [Nicoś et al. 2015].
Although KRAS mutations are the most prevalent and most frequently studied RAS mutations, other RAS mutations also indicate distinct clinicopathological and therapeutic characteristics. As an example, the frequency and clinical characteristics of lung cancer patients harboring NRAS mutations have been assessed in a recent publication by Ohashi and coworkers in which clinical data were assessed from patients with NRAS-mutant lung cancer reported in the COSMIC (Catalogue of Somatic Mutations in Cancer) study [Ohashi et al. 2013]. Moreover, six NRAS-mutant cell lines were screened for sensitivity against multikinase inhibitors (e.g. EGFR, ALK, MET, and MEK inhibitors). It was found that NRAS mutations define a distinct subset of lung cancer (∼1%) with potential sensitivity to MEK inhibitors (particularly trametinib and selumitinib). These mutations are more common in current/former smokers.
RAS mutations (particularly KRAS) are accompanied by an overactivation of the Raf/MEK/ERK signaling. First, direct targeting of mutant RAS has been proposed to overcome this overactivation. However, until the present, direct targeting was not successful because KRAS mutations impair GTPase binding so that RAS is predisposed to remain in a GTP-bound active state, which is difficult to target with small molecules [Riely et al. 2009]. As an alternative, alternative many groups are trying to target signaling downstream effectors of RAS; MEK inhibition is one example and the focus of this review [Yoon et al. 2010].Their role has been initially confirmed by the preclinical finding of increased susceptibility of 43 NSCLC cell lines to selumetinib (MEK 1–2 inhibitor) in the presence of RAS mutations [Garon et al. 2010]. Moreover, targeting of HRAS mutations through the use of farnesyl transferase inhibitors have been tried with initial preclinical encouraging results; however, this cannot be transferred widely to the clinic at present [Baines et al. 2011].
MEK inhibitors in preclinical and clinical phases of development
Currently, a number of MEK inhibitors are undergoing different phases of clinical assessment. These include trametinib, selumetinib, cobimetinib, binimetinib in addition to many other molecules under preclinical/early clinical development [Wang et al. 2007]. Trametinib has been the first agent to be approved by the US Food and Drug Administration (FDA) for BRAF-mutant advanced melanoma and it has been evaluated extensively in combination with dabrafenib in the same indication [Weber et al. 2012; Kim et al. 2013]. Selumetinib has been evaluated in NSCLC and BRAF-mutant advanced melanoma with very encouraging results [Robert et al. 2013]. Notably, a number of characteristic adverse events have been linked to different MEK inhibitors including diarrhea, hypertension, and acneiform skin eruption [Abdel-Rahman et al. 2015a, 2015b, 2015c].
Preclinical evidence of activities of MEK-targeting agents in NSCLC
A number of studies assessed preclinically the activity of MEK targeting agents both as a monotherapy in treatment-naïve cells and in combination with EGFR tyrosine kinase inhibitors (TKIs) in erlotinib- or gefitinib-resistant cells.
Monotherapy in treatment-naïve cells
In an interesting study (Table 1), human in vitro 3D lung adenocarcinoma models (OncoCilAir) mutated for KRAS were used to assess the antitumor efficacy of MEK inhibitors (selumetinib/trametinib). Remarkably, tumors showed a reduced growth in response to the MEK inhibitors, but stopping the selumetinib leads to tumor relapse [Mas et al. 2015]. Moreover, a murine lung cancer cotrial conducted concomitantly with a randomized phase II study of docetaxel with or without selumitinib showed that the addition of selumetinib provided substantial benefit for mice with lung cancer caused by KRAS [Chen et al. 2012].
Table 1.
Preclinical data with MEK inhibitors in non-small cell lung cancer.
| Study | Type | Treatments used | Endpoints | Outcome |
|---|---|---|---|---|
| Chen et al. [2012] | Preclinical: xenograft model (KRAS-mutant lung cancer) | Selumitinib plus docetaxel | Assessment of the synergistic effect of both agents | The addition of selumetinib provided substantial benefit for mice with lung cancer caused by KRAS and KRAS and p53 mutations, but mice with KRAS and Lkb1 mutations had primary resistance to this combination therapy. |
| Mas et al. [2015] | Preclinical: 3D microenvironment tumor cells (OncCilAir). | Selumetinib | Assessment of the antitumor effects of selumetinib. | Tumors showed a reduced growth in response to the MEK inhibitors, but halting the selumetinib dosing resulted in tumor relapse. |
These two studies provide initial encouraging data about the potential activity of MEK inhibitors in halting the progression of lung adenocarcinoma cell lines particularly those having KRAS mutations.
Combination MEK/EGFR inhibitors in cells resistant to EGFR-TKIs
A number of preclinical studies have shown an interesting synergistic effect of cotargeting MEK and EGFR in cells resistant to previous EGFR-TKIs therapy. Initially, Huang and coworkers have presented the results of a preclinical study in gefitinib-resistant PC-9 cells which harbor EGFR exon 19 deletion. They have shown that persistent activation of MAPK pathway boosts the acquired gefitinib resistance and that combined treatment with EGFR/ MEK inhibitors may be effective for some lung adenocarcinoma cells harboring EGFR mutations which confer resistance to EGFR-TKIs [Huang et al. 2013]. Moreover, another interesting study has shown that acquired resistance to EGFR inhibitor AZD9291 is associated with increased RAS signaling in preclinical models. This gives an additional clue to the possible role of combined EGFR/MEK inhibition in management of some NSCLC cases [Eberlein et al. 2015]. In a third study by Morgillo and coworkers, resistance of lung adenocarcinoma cell line CALU-3 to four TKIs (erlotinib, gefitinib, vandetanib and sorafenib) has been assessed. Interestingly enough, they found that treatment with selumetinib inhibited cell proliferation and migration both in vivo and in vitro for all four TKI-resistant CALU-3 cell lines. This gives an additional clue as to the potential role that may be played by MEK inhibitors in overcoming resistance of other targeted therapies in lung adenocarcinoma [Morgillo et al. 2011].
Thus, the above studies provide preclinical evidence for the activity of MEK inhibitors as an add-on therapy for overcoming the resistance to EGFR-TKIs. This idea has been investigated clinically in a randomized phase II study [ClinicalTrials.gov identifier: NCT01229150] evaluating selumetinib/erlotinib combination in NSCLC with either wild-type or mutant KRAS. The publication of the final results of this study is still awaited.
More recently, Qu and coworkers provided an additional innovative way by which MEK inhibitors overcome the potential resistance of EGFR-TKIs. In this study, selumetinib in combination with the PI3K/mTOR inhibitor BEZ235 were evaluated in gefitinib-resistant NSCLC xenograft models and actually such a combination has shown an enhanced antitumor and antiangiogenic effect. This gives an extra support for the clinical evaluation of this novel combination in the setting of EGFR-resistant NSCLC considering that PI3K/mTOR pathway is a potential escape pathway inducing resistance to therapies targeting the MAPK pathway [Qu et al. 2014].
Clinical evidence of activity of MEK-targeting agents in NSCLC
Initially, selumetinib has been evaluated in a previously treated unselected NSCLC patient population as compared with pemetrexed in a randomized phase II study (Table 2). Despite the clinical activity of selumetinib in this setting, it does not offer any advantage over standard treatment with pemetrexed. Thus, it has been suggested that it is better to evaluate these agents principally in patients with sensitive mutations (e.g. KRAS mutations) [Hainsworth et al. 2010].
Table 2.
Clinical data with MEK inhibitors in NSCLC.
| Study | Phase | Indication | Treatment regimen | OS | PFS | ORR | Toxicities |
|---|---|---|---|---|---|---|---|
| Hainsworth et al. [2010] | Randomized phase II | Previously treated NSCLC pts | Arm A: 100 mg oral selumetinib free-base suspension twice daily (42 pts). | N/R | 67 versus 90 days; p = 0.79 | 5% versus 5% | Dermatitis acneiform, diarrhea, nausea, and vomiting were the most frequently reported adverse events with selumetinib |
| Arm B: 500 mg/m2 intravenous pemetrexed once every 3 weeks | |||||||
| Blumenschein et al. [2015] | Randomized phase II | Second-line therapy for histologically-confirmed KRAS-mutant NSCLC | Arm A: trametinib (2 mg orally once daily) (87 pts) | 8 months in the trametinib arm and not reached in the docetaxel arm (p = 0.934) | 12 weeks in the trametinib arm and 11 weeks in the docetaxel arm (p = 0.5197) | 12% versus 12% (p = 1) | The most frequent adverse events (AEs) in ⩾20% of trametinib patients were rash, diarrhea, nausea, vomiting, and fatigue. |
| previously treated with one prior platinum-based chemotherapy | Arm B: docetaxel (75 mg/m2 IV every 3 weeks) (43 pts) | ||||||
| Jänne et al. [2013] | Randomized phase II | Second-line therapy for histologically-confirmed pretreated KRAS-mutant NSCLC | Arm A: oral selumetinib (75 mg twice daily in a 21 day cycle) plus docetaxel (75 mg/m2 on day 1 of a 21 day cycle) (44 pts) | 9.4 months in the selumetinib group and 5.2 months in the placebo group (p = 0.21) | 5.3 months in the selumetinib group and 2.1 months in the placebo group (p = 0.014) | 37% versus 0% (p = 0.0001) | The most common grade 3–4 adverse events were neutropenia [67%] in the selumetinib group versus [55%] in the placebo group), febrile neutropenia [18%] in the selumetinib group versus none in the placebo group. |
| Arm B: placebo plus docetaxel (43 pts) | |||||||
| Planchard et al. [2015] | Single arm phase II | BRAF V600E mutated metastatic NSCLC | Dabrafenib (dosed at 150 mg orally twice daily) plus Trametinib (at 2 mg once daily). | N/R | N/R | 63% | Most common (>20%) AEs were pyrexia, diarrhea, nausea, vomiting, decreased appetite, asthenia, cough, peripheral edema |
| Papadimitrakopoulou et al. [2014] | Four arm Phase II | Previously treated NSCLC (after excluding those with sensitive EGFR mutations and ALK fusion) | Erlotinib (E) (22 patients), E plus the AKT inhibitor MK-2206 (M) (42 patients) 135 mg q week, M plus the MEK inhibitor selumetinib (A) (73 patients) or sorafenib (61 patients) | N/R | N/R | N/R | N/R |
| Kelly et al. [2013] | Phase I/Ib | KRAS-mutant and WT advanced NSCLC | Trametinib plus pemetrexed | N/R | N/R | KRAS mutant: 15% | Nausea, fatigue, and peripheral edema were the three most-frequent toxicities. |
| KRAS wild type: 14% |
NSCLC, non-small cell lung cancer; OS, overall survival; PFS, progression-free survival; ORR, overall response rate; pts, patients; N/R, not reported; WT, wild-type.
Accordingly, all of the subsequent studies have implemented a strong accompanying biomarker program to help tailor MEK inhibitor therapy to a sensitive subset of NSCLC patients. This includes the MEK inhibitor monotherapy of KRAS-mutant NSCLC, combination of MEK/AKT inhibitors in KRAS-mutant NSCLC and combination MEK/RAF inhibitors in BRAF-mutant NSCLC. Moreover, the evaluation of a combination of a MEK inhibitor with systemic chemotherapy in KRAS wild-type and mutant NSCLC has been conducted.
In an interesting randomized phase II study reported by Blumenschein and coworkers, trametinib was compared with docetaxel in previously treated KRAS-mutant advanced NSCLC. Interestingly, trametinib has shown a similar progression-free survival [PFS; hazard ratio (HR) 1.14; 95% confidence interval (CI) 0.75–1.75; p = 0.5197] and response rate (p = 1.000) to docetaxel in this setting [Blumenschein et al. 2015].
Another randomized phase II study has evaluated selumetinib plus docetaxel versus placebo plus docetaxel in previously treated KRAS-mutant advanced NSCLC. This study has shown a promising activity for the combination arm versus docetaxel alone [PFS: 5.3 months in the selumetinib group and 2.1 months in the placebo group (p = 0.014)] with a higher rate of adverse events in the combination arm [Jänne et al. 2013]. Moreover, this study has been conducted concomitantly with a murine cotrial with the same design and it showed a similar preclinical activity for mice with KRAS-mutant lung cancer xenografted into them [Chen et al. 2012]. Based on the encouraging data of both studies, a randomized phase III study (SELECT-1) with the same design has been launched and is currently recruiting participants [ClinicalTrials.gov identifier: NCT01933932].
Moreover, in order to better evaluate the potential for MEK/AKT inhibitor combination in a biomarker-enriched program setting, the study of the Biomarker-integrated Approaches of Targeted Therapy for Lung Cancer Elimination (BATTLE-2) has been launched. As reported in the initial publication (in an abstract form in ASCO 2014), 198 patients were enrolled and randomized to either erlotinib (E) (22 patients), E plus the AKT inhibitor MK-2206 (M) (42 patients) 135 mg q week, M plus the MEK inhibitor selumetinib (A) (73 patients) or sorafenib (61 patients). Improved disease control rate was observed with EM and MA compared with E alone. Moreover, the MA combination is active in KRAS-mutant NSCLC and further study has been launched [Papadimitrakopoulou et al. 2014].
Another recently reported phase II trial by Planchard and coworkers has explored another aspect of the use of MEK inhibitors. This was a phase II study which evaluated combination trametinib plus dabrafenib in BRAF V 600 mutated NSCLC patients. ORR was released in ASCO 2015 as of 63% and further efficacy and toxicity data are expected to be released soon [Planchard et al. 2015].
In another study, Kelly and coworkers have evaluated in a phase I/Ib study the combination of trametinib and pemetrexed in both KRAS wild-type and mutant NSCLC. Interestingly, this combination shows evidence of activity not only in KRAS-mutant disease but also in KRAS wild type disease [Kelly et al. 2013]. Further research has been launched following the above paradigm of regimens incorporating pemetrexed and MEK inhibitors and the results are awaited.
Thus, the available clinical data indicate clearly the potential activity of selumetinib (and to a lesser extent trametinib) as a potential therapeutic option in KRAS-mutant NSCLC. This has fueled an enthusiastic research program with multiple phase III studies (as described in the following) further evaluating MEK inhibitors in NSCLC.
Resistance to MEK inhibitors and rationale for a combinational strategy
One of the emerging problems with MEK inhibitor therapy has been the development of resistance to these therapies. A number of mechanisms have been described to explain the development of this resistance and to help overcome it. For example, Troiani and coworkers have shown in a xenograft model of NSCLC that activation of cAMP-dependent protein kinase A is associated with selumetinib resistance. Moreover, combined targeting of both MEK and this kinase resulted in growth inhibition of selumetinib-resistant cancer cell lines in vitro and in vivo [Troiani et al. 2012]. The following are examples of potential combinational strategies that may be of value in boosting the antitumor effect of MEK inhibitors and potentially overcoming possible resistance out of these agents.
Tolcher and coworkers have recently published the results of a phase I study of combined AKT and MEK inhibition (using MK-2206 at 135 mg weekly and selumetinib at 100 mg once daily) in RAS-mutant solid tumors. They found generally durable responses in KRAS-mutant cancer which may provide an extra rationale for the use of this combination in KRAS-mutant lung adenocarcinoma [Tolcher et al. 2015] .
Ku and coworkers evaluated the effects of combining BYL719 which is a selective inhibitor of phosphoinositide 3-kinase (PI3K) with selumetinib on KRAS-mutant NSCLC cell lines. Such a combination has shown a reliable evidence of synergy and this provides an additional modality to overcome the possible resistance to MEK inhibitors [Ku et al. 2015].
Takahashi and coworkers reported the results of an interesting preclinical study evaluating the combination of selumetinib and cediranib in NCI-H441 or NCI-H460 KRAS-mutant human NSCLC cells orthotopically injected into mice lung. It was clear that combining selumetinib with cediranib markedly increased their antitumor effects with almost complete disappearance of the lung lesions [Takahashi et al. 2012].
Lamba and coworkers have recently shown that RAF1 inhibition was synthetically lethal in KRAS mutant lung cancer cells treated with MEK inhibitors. This gives a rationale for developing combinational strategies of RAF/MEK inhibition in KRAS mutant lung cancer in order to avoid resistance to either agent alone [Lamba et al. 2014].
Thus, the above studies provide a strong rationale for the development of combinational strategies targeting simultaneously many critical pathways for NSCLC. This may be introduced clinically first in the setting of MEK inhibitor resistance but may then be extended to the upfront setting.
Ongoing projects
A number of ongoing studies have been launched to further evaluate MEK inhibitors in locally advanced/metastatic NSCLC (Table 3). The results of such studies are expected within the coming 5 years and may give practice-changing evidence for MEK inhibitors in NSCLC. Preliminary results of the NCT01229150 study which evaluated selumetinib with erlotinib in KRAS wild-type and KRAS-mutant advanced NSCLC have been published in ASCO 2013 and, unfortunately, this study failed to show improvement of combination therapy over single agent in KRAS wild-type and KRAS-mutant patients. Moreover, toxicity was increased in the combination arms [Carter et al. 2013].
Table 3.
Ongoing studies of MEK inhibitors in NSCLC.
| ClinicalTrials.gov identifier | Official title | Status | Estimated primary completion date |
|---|---|---|---|
| NCT01809210 | A Phase I, Open-Label, Multicentre Study to Assess the Safety, Tolerability, Pharmacokinetics and Preliminary Efficacy of Selumetinib (AZD6244; ARRY-142886) in Combination with First Line Chemotherapy Regimens in Patients with Non-Small Cell Lung Cancer (NSCLC) | Recruiting | May 2017 |
| NCT02185690 | A Phase I/Ib Study of MEK162, a MEK Inhibitor, in Combination With Carboplatin and Pemetrexed in Patients With Non-squamous Carcinoma of the Lung | Recruiting | July 2015 |
| NCT02276027 | A Phase II, Open Label, Multiple Arm Study of AUY922, BYL719, INC280, LDK378 and MEK162 in Chinese Patients With Advanced Non-small Cell Lung Cancer | Recruiting | October 2017 |
| NCT01912625 | A Phase 1 Study of Trametinib in Combination With Chemoradiation for KRAS Mutant Non-small Cell Lung Cancer | Recruiting | December 2016 |
| NCT01336634 | A Phase II Study of the BRAF Inhibitor Dabrafenib as a Single Agent and in Combination With the MEK Inhibitor Trametinib in Subjects With BRAF V600E Mutation Positive Metastatic (Stage IV) Non-small Cell Lung Cancer | Recruiting | September 2019 |
| NCT01933932 | A Phase III, Double-Blind, Randomised, Placebo-Controlled Study to Assess the Efficacy and Safety of Selumetinib (AZD6244; ARRY-142886) (Hyd-Sulfate) in Combination With Docetaxel, in Patients Receiving Second Line Treatment for KRAS Mutation-Positive Locally Advanced or Metastatic Non Small Cell Lung Cancer (Stage IIIB - IV) (SELECT 1) | Recruiting | March 2017 |
| NCT01229150 | Randomized Phase II Study of AZD6244 MEK-Inhibitor With Erlotinib in KRAS Wild Type and KRAS Mutant Advanced Non-Small Cell Lung Cancer | Ongoing but not recruiting participants | September 2016 |
| NCT02079740 | An Open Label, Two-Part, Phase Ib/II Study to Investigate the Safety, Pharmacokinetics, Pharmacodynamics, and Clinical Activity of the MEK Inhibitor Trametinib and the BCL2-Family Inhibitor Navitoclax (ABT-263) in Combination in Subjects With KRAS Mutation-Positive Advanced Solid Tumors. | Recruiting | September 2017 |
| NCT02258607 | A Phase 1b With Expansion Study Evaluating the Efficacy and Safety of Momelotinib Combined With Trametinib in Subjects With Metastatic KRAS-mutated Non-Small Cell Lung Cancer (NSCLC) Who Have Failed Platinum-Based Chemotherapy Preceded by a Dose-finding Lead-in Phase. | Recruiting | September 2017 |
| NCT02022982 | Phase I/II Study of the CDK4/6 Inhibitor Palbociclib (PD-0332991) in Combination With the MEK Inhibitor PD-0325901 for Patients With KRAS Mutant Non-Small Cell Lung Cancer and Other Solid Tumors | Recruiting | December 2020 |
NSCLC, non-small cell lung cancer.
Conclusions
The search for newer, more effective and more intelligent therapies to improve the outcome of advanced NSCLC patients is a real challenge for the cancer research community. In the past decade, huge advancement in understanding the lung cancer biology has made it possible to develop smarter agents with a more tailored style of therapy.
About 15% of NSCLC patients have been estimated to be KRAS driven. Thus, development of focused therapeutic strategies against KRAS mutations has been considered a priority. Preclinical evidence of activity of MEK inhibitors in KRAS-mutant NSCLC has pushed forward the clinical development of these agents (namely selumetinib and trametinib) in KRAS-mutant NSCLC. Until now, the results of the clinical studies of these agents have been very encouraging and a number of randomized studies have been launched to confirm the activity of these agents and to establish their position in the treatment armamentarium of NSCLC. However, it has to be noted that although targeting MEK signaling is important, but therapeutic combinations will likely be necessary in order to achieve the desired therapeutic effect.
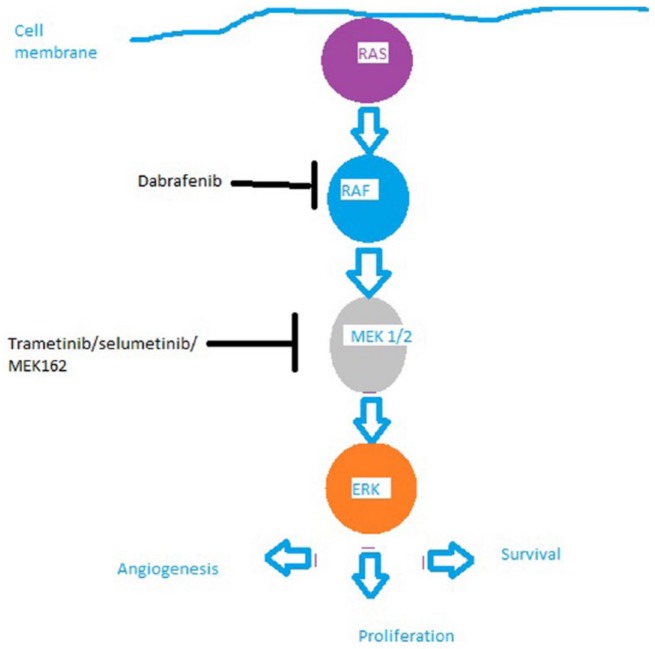
Figure 1.
The RAS–RAF–MEK–ERK pathway and the potential sites of action of RAF inhibitors and MEK inhibitors discussed in the text.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Abdel-Rahman O., Elhalawani H., Ahmed H. (2015a) Risk of selected cardiovascular toxicities in patients with cancer treated with MEK inhibitors: a comparative systematic review and meta-analysis.J Global Oncol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Rahman O., Elhalawani H., Ahmed H. (2015b) Risk of selected dermatological toxicities in cancer patients treated with MEK inhibitors: a comparative systematic review and meta-analysis. Future Oncol, in press. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman O., Elhalawani H., Ahmed H., Ellithy M. (2015c) Risk of selected gastrointestinal toxicities in cancer patients treated with MEK inhibitors: a comparative systematic review and meta-analysis. Expert Rev Gastroenterol Hepatol, in press. [DOI] [PubMed] [Google Scholar]
- Baines A., Xu D., Der C. (2011) Inhibition of RAS for cancer treatment: the search continues. Future Medicinal Chem 3: 1787–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauml J., Mick R., Zhang Y., Watt C., Vachani A., Aggarwal C., et al. (2013) Frequency of EGFR and KRAS mutations in patients with non small cell lung cancer by racial background: do disparities exist? Lung Cancer 81: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenschein G., Smit E., Planchard D., Kim D., Cadranel J., De Pas T., et al. (2015) A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC). Ann Oncol 26: 894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch C., Kollmeier J., Roth A., Stephan-Falkenau S., Misch D., Grüning W., et al. (2013) The frequency of EGFR and KRAS mutations in non-small cell lung cancer (NSCLC): routine screening data for Central Europe from a cohort study. BMJ Open 3: e002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C., Rajan A., Szabo E., Khozin S., Thomas A., Brzezniak C., et al. (2013) Two parallel randomized phase II studies of selumetinib (S) and erlotinib (E) in advanced non-small cell lung cancer selected by KRAS mutations. J Clin Oncol 31: abstr 8026. [Google Scholar]
- Chang L., Karin M. (2001) Mammalian MAP kinase signalling cascades. Nature 410: 37–40. [DOI] [PubMed] [Google Scholar]
- Chen Z., Cheng K., Walton Z., Wang Y., Ebi H., Shimamura T., et al. (2012) A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature 483: 613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detterbeck F., Lewis S., Diekemper R., Addrizzo-Harris D., Alberts W. (2013) Executive summary: diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. CHEST J 143: 7S–37S. [DOI] [PubMed] [Google Scholar]
- Downward J. (2003) Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 3: 11–22. [DOI] [PubMed] [Google Scholar]
- Eberlein C., Stetson D., Markovets A., Al-Kadhimi K., Lai Z., Fisher P., et al. (2015) Acquired resistance to mutant-selective EGFR inhibitor AZD9291 is associated with increased dependence on RAS signaling in preclinical models. Cancer Research: canres. 3167.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes S., Clements J., Dawson E., Bamford S., Webb T., Dogan A., et al. (2006) Cosmic 2005. Br J Cancer 94: 318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon E., Finn R., Hosmer W., Dering J., Ginther C., Adhami S., et al. (2010) Identification of common predictive markers of in vitro response to the MEK inhibitor selumetinib (AZD6244; ARRY-142886) in human breast cancer and non-small cell lung cancer cell lines. Mol Cancer Therapeut 9: 1985–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodsell D. (1999) The molecular perspective: the RAS oncogene. Oncologist 4: 263–264. [PubMed] [Google Scholar]
- Hainsworth J., Cebotaru C., Kanarev V., Ciuleanu T., Damyanov D., Stella P., et al. (2010) A phase II, open-label, randomized study to assess the efficacy and safety of AZD6244 (ARRY-142886) versus pemetrexed in patients with non-small cell lung cancer who have failed one or two prior chemotherapeutic regimens. J Thoracic Oncol 5: 1630–1636. [DOI] [PubMed] [Google Scholar]
- Huang M., Lee J., Chang Y., Tsai H., Lin Y., Lin A., et al. (2013) MEK inhibitors reverse resistance in epidermal growth factor receptor mutation lung cancer cells with acquired resistance to gefitinib. Molecular Oncol 7: 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänne P., Shaw A., Pereira J., Jeannin G., Vansteenkiste J., Barrios C., et al. (2013) Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol 14: 38–47. [DOI] [PubMed] [Google Scholar]
- Kelly K., Mazieres J., Leighl N., Barlesi F., Zalcman G., Gordon M., et al. (2013) Oral MEK1/MEK2 inhibitor trametinib (GSK1120212) in combination with pemetrexed for KRAS-mutant and wild-type (Wt) advanced non-small cell lung cancer (NSCLC): a phase I/Ib trial. J Clin Oncol 31: abstr 8027. [Google Scholar]
- Kim K., Kefford R., Pavlick A., Infante J., Ribas A., Sosman J., et al. (2013) Phase II study of the MEK1/MEK2 inhibitor trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor.J Clin Oncol 31: 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku B., Jho E., Bae Y., Sun J., Ahn J., Park K., et al. (2015) BYL719, a selective inhibitor of phosphoinositide 3-kinase Α, enhances the effect of selumetinib (AZD6244, ARRY-142886) in KRAS-mutant non-small cell lung cancer. Invest New Drugs 33: 12–21. [DOI] [PubMed] [Google Scholar]
- Lamba S., Russo M., Sun C., Lazzari L., Cancelliere C., Grernrum W., et al. (2014) RAF suppression synergizes with MEK inhibition in KRAS mutant cancer cells. Cell Reports 8: 1475–1483. [DOI] [PubMed] [Google Scholar]
- Malumbres M., Barbacid M. (2003) RAS oncogenes: the first 30 years. Nat Rev Cancer 3: 459–465. [DOI] [PubMed] [Google Scholar]
- Mas C., Boda B., Caulfuty M., Huang S., Wiszniewski L., Constant S. (2015) Antitumour efficacy of the selumetinib and trametinib MEK inhibitors in a combined human airway-tumour-stroma lung cancer model. J Biotechnol, in press. [DOI] [PubMed] [Google Scholar]
- Morgillo F., Cascone T., D’aiuto E., Martinelli E., Troiani T., Saintigny P., et al. (2011) Antitumour efficacy of MEK inhibitors in human lung cancer cells and their derivatives with acquired resistance to different tyrosine kinase inhibitors. Br J Cancer 105: 382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoś M., Krawczyk P., Jarosz B., Sawicki M., Szumiłło J., Trojanowski T., et al. (2015) Analysis of KRAS and BRAF genes mutation in the central nervous system metastases of non-small cell lung cancer. Clin Exp Med, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K., Sequist L., Arcila M., Lovly C., Chen X., Rudin C., et al. (2013) Characteristics of lung cancers harboring NRAS mutations. Clin Cancer Res 19: 2584–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitrakopoulou V., Lee J., Wistuba I. (2014) BATLLE-2: KRAS mutation and outcome in a biomarker-integrated study in previously treated patients (Pts) with advanced non-small cell lung cancer (NSCLC). In Program and abstracts of the American Society of Clinical Oncology Annual Meeting. [Google Scholar]
- Planchard D., Groen H., Kim T., Rigas J., Souquet P., Baik C., et al. (2015) Interim results of a phase II study of the BRAF inhibitor (BRAFI) dabrafenib (D) in combination with the MEK inhibitor trametinib (T) in patients (Pts) with BRAF V600e mutated (Mut) metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 33: abstr 8006. [Google Scholar]
- Qu Y., Wu X., Yin Y., Yang Y., Ma D., Li H. (2014) Antitumor activity of selective MEK1/2 inhibitor AZD6244 in combination with PI3K/MTOR inhibitor BEZ235 in gefitinib-resistant NSCLC xenograft models. J Exp Clin Cancer Res 33: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riely G., Marks J., Pao W. (2009) KRAS mutations in non-small cell lung cancer. Proc Am Thoracic Soc 6: 201–205. [DOI] [PubMed] [Google Scholar]
- Robert C., Dummer R., Gutzmer R., Lorigan P., Kim K., Nyakas M., et al. (2013) Selumetinib plus dacarbazine versus placebo plus dacarbazine as first-line treatment for BRAF-mutant metastatic melanoma: a phase 2 double-blind randomised study. Lancet Oncol 14: 733–740. [DOI] [PubMed] [Google Scholar]
- Roskoski R. (2010) RAF protein-serine/threonine kinases: structure and regulation. Biochem Biophys Res Commun 399: 313–317. [DOI] [PubMed] [Google Scholar]
- Silvestri G., Gonzalez A., Jantz M., Margolis M., Gould M., Tanoue L., et al. (2013) Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer. American College of Chest Physicians evidence-based clinical practice guidelines. CHEST J 143: e211S–e250S. [DOI] [PubMed] [Google Scholar]
- Takahashi O., Komaki R., Smith P., Jürgensmeier J., Ryan A., Bekele B., et al. (2012) Combined MEK and VEGFR inhibition in orthotopic human lung cancer models results in enhanced inhibition of tumor angiogenesis, growth, and metastasis. Clin Cancer Res 18: 1641–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N., Lyons J. (2005) Recent progress in targeting the RAF/MEK/ERK pathway with inhibitors in cancer drug discovery. Curr Opin Pharmacol 5: 350–356. [DOI] [PubMed] [Google Scholar]
- Thun M., Hannan L., Adams-Campbell L., Boffetta P., Buring J., Feskanich D., et al. (2008) Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med 5: e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolcher A., Khan K., Ong M., Banerji U., Papadimitrakopoulou V., Gandara D., et al. (2015) Antitumor activity in RAS-driven tumors by blocking AKT and MEK. Clin Cancer Res 21: 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiani T., Vecchione L., Martinelli E., Capasso A., Costantino S., Ciuffreda L., et al. (2012) Intrinsic resistance to selumetinib, a selective inhibitor of MEK1/2, by CAMP-dependent protein kinase a activation in human lung and colorectal cancer cells. Br J Cancer 106: 1648–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Boerner S., Winkler J., Lorusso P. (2007) Clinical experience of MEK inhibitors in cancer therapy. Biochim Biophys Acta Mol Cell Res 1773: 1248-1255. [DOI] [PubMed] [Google Scholar]
- Weber J., Flaherty K., Infante J., Falchook G., Kefford R., Daud A., et al. (2012) Updated safety and efficacy results from a phase I/II study of the oral BRAF inhibitor dabrafenib (GSK2118436) combined with the oral MEK 1/2 inhibitor trametinib (GSK1120212) in patients with BRAFI-naive metastatic melanoma. J Clin Oncol 30: abstr 8510. [Google Scholar]
- Yoon Y., Kim H., Han S., Oh D., Im S., Bang Y., et al. (2010) KRAS mutant lung cancer cells are differentially responsive to MEK inhibitor due to AKT or STAT3 activation: implication for combinatorial approach. Mol Carcinogen 49: 353–362. [DOI] [PubMed] [Google Scholar]