Abstract
First-line afatinib significantly improved progression-free survival, patient-reported outcomes, and quality of life compared with chemotherapy regimens in patients with advanced epidermal-growth-factor-receptor (EGFR) mutation-positive non-small cell lung cancer, based on results of the LUX-Lung 3 and LUX-Lung 6 trials. When the analysis of these trials was restricted to patients with common EGFR mutations only (exon 19 deletions and L858R), the advantage over chemotherapy was even more pronounced. A significant overall survival advantage was firstly demonstrated versus chemotherapy in patients with non-small cell lung cancer-harboring EGFR exon 19 deletion (del19) mutations. First-line afatinib was also effective in patients with certain uncommon EGFR mutation and patients with central nervous system metastasis. So far, these data are not sufficient to conclude that afatinib is better than first-generation EGFR inhibitors. In addition, the toxicity profile of afatinib was somewhat worse than that observed with either erlotinib or gefitinib. In the absence of direct comparisons, for each patient the choice among the available EGFR inhibitors should take into account all the clinically relevant endpoints, including disease control, survival prolongation, tolerability, and quality of life.
Keywords: afatinib, EGFR mutation, first-generation EGFR-TKI, non-small cell lung cancer
Introduction
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKI) are recognized as standard first-line therapies for non-small cell lung cancer (NSCLC) patients with activating epidermal growth factor receptor mutations (EGFR mutations) [Azzoli et al. 2011]. Findings from six pivotal randomized phase III studies done in this genetically selected subset of patients with lung cancer have shown better progression-free survival (PFS) and responses with gefitinib or erlotinib than with platinum-based chemotherapy [Mok et al. 2009; Maemondo et al. 2010; Mitsudomi et al. 2010; Zhou et al. 2011; Han et al. 2012; Rosell et al. 2012]. However, there were no differences in overall survival (OS) between EGFR-TKIs and chemotherapy in these studies, most likely because of the high proportion of crossover from chemotherapy to EGFR-TKIs observed after study completion and the strong response to EGFR-TKIs in the salvage setting. Moreover, all patients inevitably develop acquired resistance to these agents, primarily due to secondary EGFR-T790M mutations, molecular aberrations affecting other signaling pathways, or transformation to small-cell histology [Sequist et al. 2011; Yu et al. 2013]. Next-generation tyrosine kinase inhibitors (TKIs) (including afatinib as second-generation inhibitor and T790M-mutant-selective third-generation inhibitors) have been developed in order to improve survival benefits and possibly overcome acquired resistance to EGFR-TKIs.
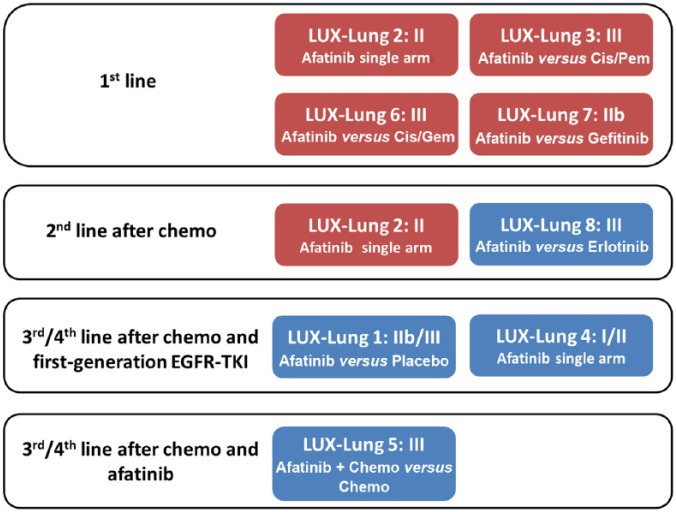
Afatinib, a second-generation irreversible TKI that inhibits signaling from all homodimers and heterodimers formed by ErbB receptor-family members (including EGFR, ErbB2, ErbB3, and ErbB4), has shown potent preclinical antitumor activity in both EGFR-TKI-naïve and -resistant cultured cells and xenograft models, providing biological rationale for the evaluation of afatinib in clinical trials [Li et al. 2008; Solca et al. 2012]. The implication was that this agent might work better in the long run and actually provide therapeutic salvage for patients whose tumors had progressed during treatment with first-line EGFR-TKIs. An intense program of clinical research (the LUX-Lung program, Figure 1) was developed in several categories of NSCLC patients (EGFR-mutated and wild-type tumors, reversible EGFR-TKIs-naïve or -resistant patients, and adenocarcinoma and squamous cell carcinoma histology). There was great hope that afatinib would be highly effective for patients with acquired resistance when it was developed. Nevertheless, it turned out to be rather disappointing in these patients, probably as a result of dose limitations from toxicity caused by inhibiting wild-type EGFR simultaneously [Miller et al. 2012]. Thus, afatinib cannot inhibit T790M mutation (the most common mechanisms of EGFR-TKI-acquired resistance) at tolerable doses in humans. The first global approval of afatinib was granted by the US FDA on 12 July 2013 for the first-line treatment of EGFR-mutation-positive metastatic NSCLC, supported by the results of LUX-Lung 3 (LL3) [Sequist et al. 2013]. After that, a lot of countries including Europe, Japan and Taiwan, have approved the use of afatinib in treatment-naïve or EGFR-TKI-naïve NSCLC. This article mainly focuses on data of afatinib in first-line treatment of EGFR-mutation-positive NSCLC. The use of afatinib in other indications is beyond the scope of this review.
Figure 1.
Summary box of LUX-Lung trials with afatinib in non-small cell lung cancers.
Red box: clinical trials in EGFR mutation-positive patients; Blue box: clinical trials in unselected patients
Cis, cisplatin; Pem, pemetrexed; Gem, gemcitabine; Chemo: chemotherapy.
Afatinib versus chemotherapy in the first-line treatment of epidermal-growth-factor receptor common mutation-positive non-small cell lung cancer
Progression-free survival benefit
The LL3 (345 patients recruited globally) and LUX-Lung 6 (LL6) (364 patients recruited in Asia) trials were the largest randomized, phase III trials ever to be undertaken in treatment-naïve patients with EGFR-mutation-positive advanced NSCLC [Sequist et al. 2013; Wu et al. 2014]. Patients were randomly assigned, with a 2:1 ratio, to receive afatinib 40 mg daily or up to six cycles of standard-of-care platinum-based chemotherapy every 21 days (cisplatin/pemetrexed in LL3 and cisplatin/gemcitabine in LL6). Mutation-positive patients were stratified by mutation type [exon 19 deletion (del19), L858R, or other] and race (Asian or non-Asian). Both trials met their primary endpoints of PFS by independent blinded review. Afatinib significantly prolonged median PFS versus chemotherapy in both LL3 [11.1 versus 6.9 months; hazard ratio (HR) = 0.58; 95% CI, 0.43 to 0.78; p < 0.001] and LL6 (11.0 versus 5.6 months; HR = 0.28; 95% CI, 0.20 to 0.39; p < 0.0001). Significantly higher response rates were observed with afatinib compared with chemotherapy, 56% versus 23% and 67% versus 23% in LL3 and LL6, respectively, according to independent assessments. When the analysis was restricted to patients with common EGFR mutations only (del19s and L858R), the advantage over chemotherapy was even more pronounced (Table 1). Median PFS in LL3 patients with EGFR common mutations was 13.6 months for afatinib and 6.9 months for chemotherapy (HR = 0.47; 95% CI, 0.34 to 0.65; p = 0.001). Overall, these results had confirmed the efficacy of afatinib in selected patients for EGFR mutations, and overlapped the previous trials with reversible EGFR-TKIs, as erlotinib and gefitinib in the first-line setting [Mok et al. 2009; Maemondo et al. 2010; Mitsudomi et al. 2010; Zhou et al. 2011; Han et al. 2012; Rosell et al. 2012; Wu et al. 2015].
Table 1.
Progression-free survival and overall survival benefit from LUX-Lung 3 and LUX-Lung 6 trials.
| n | Median PFS | HR for PFS | Median OS | HR for OS | |
|---|---|---|---|---|---|
| (months) | (95% CI) | (months) | (95% CI) | ||
| LUX-Lung 3 | |||||
| Del19 | 170 | 13.7 | 0.28 (0.18–0.44) | 33.3 versus 21.1 | 0.54 (0.36–0.79) |
| L858R | 138 | 11.0 | 0.73 (0.46–1.16) | 27.6 versus 40.3 | 1.30 (0.80–2.11) |
| Del19+L858R | 308 | 13.6 versus 6.9 | 0.47 (0.34–0.65) | 31.6 versus 28.2 | 0.78 (0.58–1.06) |
| LUX–Lung 6 | |||||
| Del19 | 186 | 13.7 | 0.20 (0.13–0.32) | 31.4 versus 18.4 | 0.64 (0.44–0.94) |
| L858R | 138 | 9.6 | 0.32 (0.19–0.54) | 19.6 versus 24.3 | 1.22 (0.81–1.83) |
| Del19+L858R | 324 | 11.0 versus 5.6 | 0.25 (0.18–0.35) | 23.6 versus 23.5 | 0.83 (0.62–1.09) |
EGFR-TKI versus chemotherapy.
PFS, progression-free survival; HR, hazard ratio; OS, overall survival; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor; CI, confidence interval.
Overall survival benefit
Moreover, a trend towards OS benefit was observed in a prespecified analysis of median OS in patients with common mutations (LL3, 31.6 versus 28.2 months; HR = 0.78, 95% CI, 0.58 to 1.06; p = 0.11; LL6, 23.6 versus 23.5 months; HR = 0.83, 95% CI, 0.62 to 1.09; p = 0.18), whereas in the overall dataset (all EGFR mutations), no significant difference was observed between the two arms (LL3, 28.2 versus 28.2 months; HR = 0.88, 95% CI, 0.66 to 1.17; p = 0.39; LL6, 23.1 versus 23.5 months; HR = 0.93; 95% CI, 0.72 to 1.22; p = 0.61) [Yang et al. 2015c]. As a subgroup analysis of a secondary endpoint (both LL3 and LL6 had PFS as the primary endpoint), patients who had the EGFR-del19 mutation and received afatinib had a median OS duration that was prolonged by 1 year compared with patients who received chemotherapy (LL3: HR = 0.54; 95% CI, 0.6 to 0.79; p = 0.0015; LL6: HR = 0.64; 95% CI, 0.44 to 0.94; p = 0.023). By contrast, no significant differences in OS were found by treatment group for patients with EGFR-L858R-positive tumors in either LL3 (HR = 1.30; 95% CI, 0.80 to 2.11; p = 0.29) or LL6 (HR = 1.22; 95% CI, 0.81 to 1.83; p = 0.34).
In the pooled analysis of these two randomized trials, afatinib had significantly improved OS compared with chemotherapy among patients with tumors harboring common EGFR mutations (HR = 0.81; 95% CI, 0.66 to 0.99; p = 0.037) [Yang et al. 2015c]. Consistent with individual study findings, subgroup analyses suggested that the OS benefit of afatinib was driven mainly by patients with lung adenocarcinoma harboring the EGFR-del19 mutation (HR = 0.59; 95% CI, 0.45 to 0.77; p = 0.0001), whereas in patients with L858R-positive tumors there was no difference between treatment arms (HR = 1.25; 95% CI, 0.92 to 1.71; p = 0.16). As emphasized by the investigators, the impressive advantage in OS reported in patients with lung adenocarcinoma harboring del19 mutations strongly suggested that the 19 deletions and L858R mutation represent two distinct subclasses of NSCLC, and should be studied separately in future trials.
Furthermore, although most patients in LL3 and the entire population of LL6 were Asian, a significant OS improvement with afatinib in the del19 subgroup was also noted in the smaller subpopulation of non-Asian patients in LL3 (33.6 versus 20.0 months; HR = 0.45; 95% CI, 0.21 to 0.95; p = 0.03). In the Chinese subgroup of LL6, median OS was 31.6 versus 16.3 months (HR = 0.61; 95% CI, 0.41 to 0.91; p = 0.015) in 19 deletions [Wu et al. 2014]. There was argument that there was less crossover to TKI in the chemotherapy arms therefore these arms were underperforming. However, in the LL3 subgroup analysis of Japanese patients, where there was 100% crossover, results showed afatinib was still associated with significantly improved OS in those with del19 mutations (46.9 versus 31.5 months; HR = 0.34; 95% CI, 0.13 to 0.87; p = 0.018) [Kato et al. 2015]. These subgroup data supported the concept that the OS benefit with afatinib over chemotherapy in patients with del19 mutation was a real phenomenon, independent of ethnicity.
Symptom and quality-of-life improvement
Patient-reported outcomes (PROs) are clinically meaningful treatment outcomes that are directly assessed by patients and reflect their disease-related symptoms, functional activity and health-related quality of life (QoL). In clinical trials for patients with advanced cancer such as NSCLC, the validity of PFS as a relevant primary endpoint requires not only rigorous and objective assessment of tumor progression but also a parallel benefit in PROs [Fallowfield and Fleissig, 2012; Damm et al. 2013]. Both LL3 and LL6 fully integrated comprehensive PRO evaluation into outcome analyses, demonstrating improvements in lung cancer-related symptoms and QoL, and a longer time to deterioration of these PROs [Yang et al. 2013; Geater et al. 2015]. Compared with chemotherapy, afatinib led to a significant delay in the time-to-deterioration for cough and dyspnea. The adverse-event (AE) profiles of both treatments were also reflected in the PRO symptom analysis, with worsening nausea, vomiting, and fatigue on the chemotherapy arm, and worsening diarrhea, dysphagia, and sore mouth on afatinib. Finally, and perhaps most importantly, afatinib was associated with significantly better mean scores in the longitudinal analysis of health status and QoL that captured patients’ perception of treatment that likely accounted for changes in both disease symptoms and treatment-related AEs during the study period.
As the latest two front-line studies comparing EGFR blockade with standard chemotherapy in patients with the EGFR mutation, LL3 and LL6 are distinguished by a number of factors. First of all, this is the first time that an OS benefit has been demonstrated in patients with tumors that contain the EGFR-del19 mutations but no such benefit was observed in patients with L858R-positive tumors. Besides, the PFS exceeding 13 months achieved with afatinib in those with common mutations appears superior in the context of previous studies with erlotinib and gefitinib. Secondly, both studies enrolled well over 300 patients to meet the regulatory requirements of different regions, making it far more robust and thereby tightening the CIs around the benefits already noted in similar studies. Of note, pemetrexed and cisplatin, the control arm in LL3, was considered a state-of-the-art chemotherapy regimen according to data from Scagliotti and colleagues [Scagliotti et al. 2008].
Afatinib versus first-generation epidermal-growth-factor-receptor-tyrosine-kinase inhibitor in the first-line treatment of epidermal-growth-factor-receptor-mutation-positive non-small cell lung cancer
Efficacy
A total of nine phase III randomized controlled trials (RCTs) of advanced NSCLC patients with either 19 or 21 exon alteration receiving first-line EGFR-TKIs were published [Mok et al. 2009; Maemondo et al. 2010; Mitsudomi et al. 2010; Zhou et al. 2011; Han et al. 2012; Rosell et al. 2012; Sequist et al. 2013; Wu et al. 2014; Wu et al. 2015]. In each of these studies, significant improvements in PFS and response were reported with EGFR TKI therapy versus chemotherapy. None of the drugs demonstrated an OS benefit versus chemotherapy in the overall population or in EGFR-del19 or L858R-mutation subgroups, with the notable exception of afatinib [Lee et al. 2013; Yang et al. 2015c]. Possible explanation for the impressive advantage in OS might lie in mechanistic differences between the irreversible ERBB-family blocker afatinib and first-generation reversible EGFR-TKIs. Data derived from indirect meta-analyses showed no statistically significant differences between afatinib and erlotinib or gefitinib in terms of PFS, but some numerical differences were observed, particularly in patients with common EGFR mutations [Popat et al. 2014]. The estimated HR (95% CI) for afatinib compared with gefitinib was 0.70 (0.40 to 1.16) and compared with erlotinib was 0.86 (0.50 to 1.50) in the total population, along with 0.60 (0.34 to 0.99) and 0.73 (0.42 to 1.24), respectively, in common mutants. The estimated probability of afatinib being the best treatment with regard to PFS in the total population was 70% versus 27% for erlotinib, 3% for gefitinib and 0% for chemotherapy. OS findings were not significantly different between treatments. Particularly, OS data for both afatinib trials were immature at the point of data cutoff for this analysis. According to another recently published meta-analysis, the pool HR for PFS was 0.24 in the del19 subgroup and 0.48 in the exon 21 L858R substitution subgroup. Compared with chemotherapy, treatment with EGFR-TKIs demonstrated 50% greater benefit in del19s than in exon 21 L858R mutations [Lee et al. 2015]. Increasing evidence demonstrates that they have different prognostic and predictive roles and are hence considerable as a stratification factor in clinical trials [Zhang et al. 2014; Lee et al. 2015; Yang et al. 2015c]. In the subgroup with del19s, the pooled HR for PFS was 0.24 (0.17 to 0.33) with afatinib and 0.25 (0.20 to 0.31) with first-generation EGFR-TKIs [Lee et al. 2015], prompting a similar effect among various EGFR inhibitors. The CTONG0901 study compared erlotinib versus gefitinib in patients with EGFR exon 19 or 21 mutations. There was no significant difference in either PFS (13.0 versus 10.4 months, p = 0.100) or OS (22.9 versus 20.1 months, p = 0.210) [Yang et al. 2015d].
The indirect retrospective comparison across completed studies of afatinib versus gefitinib/erlotinib did not seem to be convincing enough because of the differences in the chemotherapy comparator arms used, the populations evaluated, the ratio of del19s versus L858R mutations versus other mutations and nonsmokers versus smokers. LUX-Lung 7 (LL7) was the first prospective global randomized trial evaluating two EGFR-directed therapies in patients with EGFR-mutant NSCLC [Park et al. 2015]. The primary endpoint of PFS was met by 11.0 months versus 10.9 months (HR = 0.73; 95% CI, 0.57 to 0.95; p = 0.017). Afatinib treatment was associated with a significant improvement in response rate (70% versus 56%, p = 0.008) and time to treatment failure (13.7 months versus 11.5 months; HR = 0.73; 95% CI, 0.58 to 0.92; p = 0.007). The improvement in efficacy was observed in both del19 and L858R populations. OS data were immature.
Toxicity profile
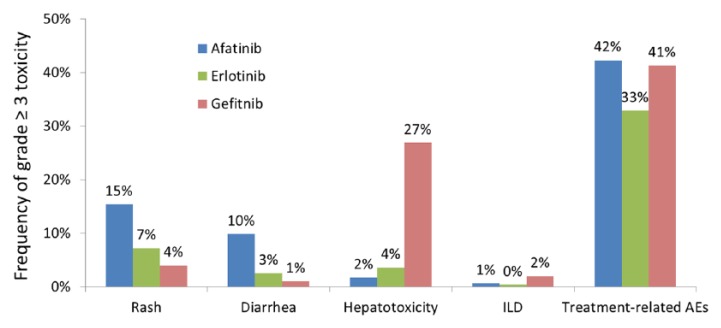
Given the unequal affinity for the kinase domain of EGFR, the toxicological properties of these EGFR-TKIs may differ from each other when observed in EGFR-mutant tumors. Thus, the toxicity data of afatinib in LL3 and LL6 [Sequist et al. 2013; Wu et al. 2014], erlotinib in OPTIMAL, EURTAC and ENSURE [Zhou et al. 2011; Rosell et al. 2012; Wu et al. 2015], and gefitinib in NEJ002 and WJTOG3405 [Maemondo et al. 2010; Mitsudomi et al. 2010] is summarized in Figure 1. The most common treatment-related adverse events, rash and diarrhea, were more frequent with afatinib therapy than with erlotinib or gefitinib therapy. Dose reduction due to afatinib occurred in more than 40% of patients. However, there was no reduction of efficacy for those who were dose reduced versus ones who had no dose reduction (11.3 months versus 11.0 months, respectively) [Yang et al. 2015a]. Treatment with gefitinib was associated with a higher frequency of severe (grade ⩾ 3) hepatotoxicity compared with erlotinib or afatinib. Frequency of interstitial lung disease (ILD) with a minimum of grade 3 was low for both first-generation and second-generation EGFR-TKIs. Treatment-related AEs with a minimum of grade 3 occurred in 42% of patients receiving afatinib, 33% of patients receiving erlotinib and 41% of patients receiving gefitinib. The direct comparison between afatinib and gefitinib was made in LL7, and the results were consistent with previous experience [Park et al. 2015].
Efficacy of afatinib in patients harboring uncommon epidermal-growth-factor-receptor mutations or with brain metastases
Uncommon EGFR mutations are defined as any mutation other than del19 or Leu858Arg, and account for approximately 10% of all mutation-positive NSCLC. The clinical data available regarding the activity of first-generation EGFR-TKIs in these tumors are inconclusive, anecdotal, and mostly retrospective [Asahina et al. 2006; De Pas et al. 2011; Wu et al. 2011; Watanabe et al. 2014]. Since preclinical data suggested that afatinib could irreversibly inhibit all ERBB family receptor tyrosine kinases, it was thought that this agent could be effective for patients with uncommon mutations, especially for patients with tumors that had the T790M mutation [Solca et al. 2012].
Figure 2.
The frequency of grade ⩾ 3 adverse events including rash, diarrhea, hepatotoxicity and interstitial lung disease.
ILD, interstitial lung disease, AEs, adverse events.
Therefore, a post-hoc analysis was conducted to assess the activity of afatinib in patients with uncommon EGFR mutations in the LUX-Lung clinical trials programme, with data from the nonrandomized phase II LUX-Lung 2 (LL2) study and the phase III randomized LL3 and LL6 trials [Yang et al. 2015b]. Of the total 600 patients given afatinib across the three trials, 75 (12%) patients had uncommon EGFR mutations. The investigators divided these patients into three cohorts: point mutations and duplications in exons 18–21 (group 1), de novo T790M mutation in exon 20 (group 2), or exon 20 insertions (group 3). The best response to afatinib (Objective Response Rate (ORR) = 71.1%; 95% CI, 54.1 to 84.6) was noted in group 1, especially in patients with G719X, L861G, and S768I that were the three most frequently reported types of uncommon EGFR mutations, suggesting that this group of uncommon mutations can be categorized as sensitive EGFR mutations and supporting the use of afatinib in these patients ( Table 2 ). However, patients had an objective response of less than 15% in groups 2 and 3, with a median PFS of 2.9 months and 2.7 months, respectively. Comparison between the 75 patients who received afatinib and the 25 patients who received chemotherapy was restricted due to the small size of the cohort and molecular heterogeneity within the genetic subgroups. The combination modality with afatinib plus cetuximab was explored in patients with the T790M mutation, although in the resistance setting [Janjigian, et al. 2014]. The efficacy was mild but the toxicity was quite serious. T790M mutant-selective inhibitors such as ADZ9291 are being tested in the first-line setting and may be the preferred treatment option for patients with de novo T790M mutation. It was imperative to assess uncommon EGFR mutations independently or appropriately grouped, but not as a whole group.
Table 2.
Response to afatinib or chemotherapy in patients with epidermal-growth-factor-receptor (EGFR) uncommon mutations.
| Mutations | n | Objective response | Median PFS | Median OS | |
|---|---|---|---|---|---|
| (95% CI) | (95% CI, months) | (95% CI, months) | |||
| Group 1 | L861G, G719X, S768I, etc. | 38 | 71.1% (54.1–84.6) | 10.7 (5.6–14.7) | 19.4 (16.4–26.9) |
| Group 2 | De novo T790M | 14 | 14.3% (1.8–42.8) | 2.9 (1.2–8.3) | 14.9 (8.1–24.9) |
| Group 3 | Exon 20 insertions | 23 | 8.7% (1.1–28.0) | 2.7 (1.8–4.2) | 9.2 (4.1–14.2) |
| Chemotherapy | All uncommon EGFR mutations | 25 | 24.0% (9.4–45.1) | 8.2 (5.2–10.8) | 30.2 (13.0–42.3) |
CI, confidence interval; EGFR, epidermal growth factor receptor.
In preclinical studies, afatinib demonstrated high potency of kinase inhibition with the median inhibitory concentration lower than that of first-generation EGFR-TKIs gefitinib or erlotinib [Solca et al. 2012]. This suggested that afatinib would penetrate into the central nervous system (CNS) with concentrations high enough to treat CNS metastases effectively. In LL3, 35 patients with stable brain metastases (asymptomatic, stable > 4 weeks with no treatment required) at baseline were included [Schuler et al. 2013]. Within the brain metastases group (afatinib: n = 20, pemetrexed/cisplatin: n = 15), median PFS by independent review was 11.1 months in the afatinib arm and 5.4 months in chemotherapy (HR = 0.52; 95% CI, 0.22 to 1.23; p = 0.13). Objective response in patients with brain metastases was 70% (afatinib) versus 20% (pemetrexed/cisplatin), odds ratio = 11.0, p = 0.007. By investigator review, progressive disease in the brain was observed for 4.2% (7/167) and 3.7% (3/82) of patients without brain metastases at baseline for afatinib and pemetrexed/cisplatin, respectively. The median (range) time to intracranial progression in this small group was 11.6 (1.3–20.2) months with afatinib and 5.5 (2.6–8.2) months with pemetrexed/cisplatin.
Conclusion
Afatinib is the first agent to demonstrate improvement in both PFS and OS versus standard-of-care platinum-doublet chemotherapy in a molecularly defined population of patients with NSCLC when used in the first-line setting. Maximal survival benefit is seen in patients with advanced NSCLC and the del19 mutant. Particularly in light of the OS advantage afatinib could take the place of gefitinib or erlotinib and be considered the preferred first-line therapy for patients with EGFR-del19 mutations. Further strategy development of EGFR-TKIs to enhance antitumor activity, particularly for tumors with exon 21 L858R mutations remains important. LL7 has confirmed the efficacy benefit of irreversible ERBB blockade with afatinib over reversible EGFR inhibition with gefitinib in treatment of EGFR-mutant NSCLCs, although the toxicity profile of afatinib is somewhat worse than that observed with first-generation TKIs.
To date, four molecules have been approved for the first-line treatment of EGFR-mutated lung cancer. Gefitinib and erlotinib are available in almost all countries. Afatinib has been approved by the US FDA and by the European Medicines Agency, and icotinib has been approved only in China. The concern of how to choose from a group of agents that share a similar mechanism may be quite crucial. For each patient, the choice among the available EGFR inhibitors should take into account all the clinically relevant endpoints, including disease control, survival prolongation, tolerability, and QoL.
The therapeutic landscape is still evolving. Other, more active, third-generation EGFR-TKIs with specific activity at T790M mutation, such as AZD9291 and CO-1686, seem to have a better efficacy and toxicity profile in early clinical trials as monotherapy, and the results are very encouraging in patients with advanced NSCLC who develop resistance to EGFR-TKI with secondary T790M mutation. Preclinical data suggest that AZD9291 could be highly effective as well in the front-line setting, and a clinical trial testing this agent in TKI-naïve patients is underway. Whether the use of AZD9291 in the first-line setting will extend the survival benefit for patients compared with erlotinib, gefitinib, or afatinib remains to be determined clinically. Besides, potential combined modality therapies are being developed to maximize the duration of disease control and further improve long-term outcomes.
Footnotes
Funding: This work was supported by the Key Technologies Research and Development Program of Guangzhou [grant number 2011Y2-00014]; the Key Laboratory Program of Guangdong [grant number 2012A061400006]; and the Special Fund for Research in the Public Interest from the National Health and Family Planning Commission of the People’s Republic of China [grant number 201402031].
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
E-E Ke, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China. Guangdong General Hospital and Guangdong Academy of Medical Sciences, Guangdong Lung Cancer Institute, Guangzhou, Guangdong, People’s Republic of China.
Yi-Long Wu, Guangdong General Hospital and Guangdong Academy of Medical Sciences, Guangdong Lung Cancer Institute, Guangzhou, Guangdong 510080, People’s Republic of China.
References
- Asahina H., Yamazaki K., Kinoshita I., Yokouchi H., Dosaka-Akita H., Nishimura M. (2006) Non-responsiveness to gefitinib in a patient with lung adenocarcinoma having rare EGFR mutations S768i and V769l. Lung Cancer 54: 419–422. [DOI] [PubMed] [Google Scholar]
- Azzoli C., Temin S., Aliff T., Baker S., Jr., Brahmer J., Johnson D., et al. (2011) 2011. Focused update of 2009 American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 29: 3825–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm K., Roeske N., Jacob C. (2013) Health-related quality of life questionnaires in lung cancer trials: a systematic literature review. Health Econ Rev 3: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pas T., Toffalorio F., Manzotti M., Fumagalli C., Spitaleri G., Catania C., et al. (2011) Activity of epidermal growth factor receptor-tyrosine kinase inhibitors in patients with non-small cell lung cancer harboring rare epidermal growth factor receptor mutations. Journal of Thoracic Oncology 6: 1895–1901. [DOI] [PubMed] [Google Scholar]
- Fallowfield L., Fleissig A. (2012) The value of progression-free survival to patients with advanced-stage cancer. Nat Rev Clin Oncol 9: 41–47. [DOI] [PubMed] [Google Scholar]
- Geater S., Xu C., Zhou C., Hu C., Feng J., Lu S., et al. (2015) Symptom and quality of life improvement in LUX-Lung 6: an open-label phase III study of afatinib versus cisplatin/gemcitabine in Asian patients with EGFR mutation-positive advanced non-small-cell lung cancer. J Thorac Oncol 10: 883–889. [DOI] [PubMed] [Google Scholar]
- Han J., Park K., Kim S., Lee D., Kim H., Kim H., et al. (2012) First-signal: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 30: 1122–1128. [DOI] [PubMed] [Google Scholar]
- Janjigian Y., Smit E., Groen H., Horn L., Gettinger S., Camidge D., et al. (2014) Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov 4: 1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Yoshioka H., Okamoto I., Yokoyama A., Hida T., Seto T., et al. (2015) Afatinib versus cisplatin plus pemetrexed in Japanese patients with advanced non-small cell lung cancer harboring activating EGFR mutations: subgroup analysis of Lux-Lung 3. Cancer Sci 106: 1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Brown C., Gralla R., Hirsh V., Thongprasert S., Tsai C., et al. (2013) Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 105: 595–605. [DOI] [PubMed] [Google Scholar]
- Lee C., Wu Y., Ding P., Lord S., Inoue A., Zhou C., et al. (2015) Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-mutant lung cancer: a meta-analysis. J Clin Oncol 33: 1958–1965. [DOI] [PubMed] [Google Scholar]
- Li D., Ambrogio L., Shimamura T., Kubo S., Takahashi M., Chirieac L., et al. (2008) BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 27: 4702–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maemondo M., Inoue A., Kobayashi K., Sugawara S., Oizumi S., Isobe H., et al. (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362: 2380–2388. [DOI] [PubMed] [Google Scholar]
- Miller V., Hirsh V., Cadranel J., Chen Y., Park K., Kim S., et al. (2012) Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phaseIIb/III randomised trial. The Lancet Oncology 13: 528–538. [DOI] [PubMed] [Google Scholar]
- Mitsudomi T., Morita S., Yatabe Y., Negoro S., Okamoto I., Tsurutani J., et al. (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase III trial. Lancet Oncology 11: 121–128. [DOI] [PubMed] [Google Scholar]
- Mok T., Wu Y., Thongprasert S., Yang C., Chu D., Saijo N., et al. (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. New England Journal of Medicine 361: 947–957. [DOI] [PubMed] [Google Scholar]
- Park K., Tan E., Zhang L., Hirsh V., O’Byrne K., Boyer M., et al. (2015) Afatinib versus gefitinib as first-line treatment for patients with advanced non-small cell lung cancer harboring activating EGFR mutations: LUX-Lung 7. In: 2015 ESMO Asia Congress, 18–21 December 2015, Singapore: abstr LBA2. [Google Scholar]
- Popat S., Mok T., Yang J., Wu Y., Lungershausen J., Stammberger U., et al. (2014) Afatinib in the treatment of EGFR mutation-positive NSCLC—a network meta-analysis. Lung Cancer 85: 230–238. [DOI] [PubMed] [Google Scholar]
- Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., et al. (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase III trial. The Lancet Oncology 13: 239–246. [DOI] [PubMed] [Google Scholar]
- Scagliotti G., Parikh P., Von Pawel J., Biesma B., Vansteenkiste J., Manegold C., et al. (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26: 3543–3551. [DOI] [PubMed] [Google Scholar]
- Sequist L., Waltman B., Dias-Santagata D., Digumarthy S., Turke A., Fidias P., et al. (2011) Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 3: 75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequist L., Yang J., Yamamoto N., O’Byrne K., Hirsh V., Mok T., et al. (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31: 3327–3334. [DOI] [PubMed] [Google Scholar]
- Solca F., Dahl G., Zoephel A., Bader G., Sanderson M., Klein C., et al. (2012) Target binding properties and cellular activity of afatinib (BIBW2992), an irreversible ERBB family blocker.J Pharmacol Exp Ther 343: 342–350. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Minegishi Y., Yoshizawa H., Maemondo M., Inoue A., Sugawara S., et al. (2014) Effectiveness of gefitinib against non–small-cell lung cancer with the uncommon EGFR mutations G719X and L861Q. Journal of Thoracic Oncology 9: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Yu C., Chang Y., Yang C., Shih J., Yang P. (2011) Effectiveness of tyrosine kinase inhibitors on ‘uncommon’ epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res 17: 3812–3821. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhou C., Hu C., Feng J., Lu S., Huang Y., et al. (2014) Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase III trial. The Lancet Oncology 15: 213–222. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhou C., Liam C., Wu G., Liu X., Zhong Z., et al. (2015) First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 26: 1883–1889. [DOI] [PubMed] [Google Scholar]
- Yang J., Ahn M., Dickgreber N., Halmos B., Hirsh V., Hochmair M., et al. (2015a) Influence of dose adjustment on afatinib safey and efficacy in patients (pts) with advanced EGFR mutation-positive (EGFRm+) non-samll cell lung cancer (NSCLC). J Clin Oncol 33: abstr 8073. [Google Scholar]
- Yang J., Hirsh V., Schuler M., Yamamoto N., O’Byrne K., Mok T., et al. (2013) Symptom control and quality of life in Lux-Lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol 31: 3342–3350. [DOI] [PubMed] [Google Scholar]
- Yang J., Sequist L., Geater S., Tsai C., Mok T., Schuler M., et al. (2015b) Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post hoc analysis of Lux-Lung 2, Lux-Lung 3, and Lux-Lung 6. The Lancet Oncology 16: 830–838. [DOI] [PubMed] [Google Scholar]
- Yang J., Wu Y., Schuler M., Sebastian M., Popat S., Yamamoto N., et al. (2015c) Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase III trials. The Lancet Oncology 16: 141–151. [DOI] [PubMed] [Google Scholar]
- Yang J., Zhou Q., Yan H., Zhang X., Chen H., Tu H., et al. (2015d) A randomized controlled trial of erlotinib versus gefitinib in advanced non-small-cell lung cancer harboring EGFR exon 19 or 21 mutations (CTONG0901). In: The 16th World Conference on Lung Cancer, 6–9 September 2015, Denver, Colorado: MINI16.13. [Google Scholar]
- Yu H., Arcila M., Rekhtman N., Sima C., Zakowski M., Pao W., et al. (2013) Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 19: 2240–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Sheng J., Kang S., Fang W., Yan Y., Hu Z., et al. (2014) Patients with exon 19 deletion were associated with longer progression-free survival compared to those with L858R mutation after first-line EGFR-TKIs for advanced non-small cell lung cancer: a meta-analysis. PLoS ONE 9: e107161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Wu Y., Chen G., Feng J., Liu X., Wang C., et al. (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (optimal, CTONG-0802): a multicentre, open-label, randomised, phase III study. The Lancet Oncology 12: 735–742. [DOI] [PubMed] [Google Scholar]