Abstract
Background:
A meta-analysis of the risk of pneumonitis associated with the use of immune checkpoint inhibitors in cancer patients has been conducted.
Methods:
Eligible publications included randomized trials of cancer patients on immune checkpoint inhibitors, describing events of all-grade and high-grade pneumonitis.
Results:
After exclusion of noneligible citations, a total of 11 clinical trials were eligible for the meta-analysis. The odds ratio was 3.96 [95% confidence interval (CI): 2.02–7.79; p < 0.0001] for all-grade pneumonitis and 2.87 (95% CI: 0.90–9.20; p = 0.08) for high-grade pneumonitis. Moreover, the odds ratio of all-grade pneumonitis with a nivolumab/ipilimumab combination versus ipilimumab monotherapy was 3.68 (95% CI: 1.59–8.50; p = 0.002) and, for high-grade pneumonitis, it was 1.86(95% CI: 0.36–9.53; p = 0.46). Subgroup analysis did not reveal a difference between lung cancer patients and other cancer patients in the risk of pneumonitis.
Conclusions:
Our analysis provided evidence that the use of immune checkpoint inhibitors is associated with an increased risk of all-grade pneumonitis compared with chemotherapy or placebo controls.
Keywords: ipilimumab, nivolumab, NSCLC, pembrolizumab, pneumonitis
Introduction
Immunotherapy has been considered to be one of the most important breakthroughs in cancer management in the past decade [Rosenberg et al. 2004]. Immune checkpoint inhibitors have topped the list of successful cancer immunotherapies and they include two categories of agents; namely: cytotoxic T lymphocyte antigen-4 (CTLA-4) inhibitors and programmed death 1 (PD-1) inhibitors [Momtaz and Postow, 2014].
Ipilimumab, which is a monoclonal antibody against CTLA-4, has been evaluated in clinical practice with documented survival benefit in a number of phase III studies in patients with metastatic melanoma leading to US Food and Drug Administration (FDA) approval for this indication in 2011 [Hodi et al. 2010; Robert et al. 2011]. Nivolumab is a PD-1 targeting agent that has been approved for the treatment of advanced melanoma, advanced non-small cell lung cancer (NSCLC) and advanced renal cell carcinoma (RCC) [Hamid et al. 2013]. Pembrolizumab is another PD-1 targeted agent which outperforms ipilimumab for advanced melanoma management and thus has been FDA approved for this indication [Robert et al. 2015b]. Moreover, it is being extensively evaluated in many other solid tumor indications [Garon et al. 2015]. Other PD-1 targeting agents in the phase of development include atezolizumab and pidilizumab, which have shown activity against many solid and hematologic malignancies [Berger et al. 2008; Armand et al. 2013; Spira et al. 2015].
The very specific mechanism of action of this group of agents brings about a very peculiar set of adverse events [Brahmer et al. 2012]. This includes a reportedly higher risk of immune-related hepatitis, colitis, thyroiditis, pneumonitis and vitiligo [Abdel-Rahman et al. 2015a,b,c; Westin et al. 2014]. The reports of pneumonitis caused by these agents are relatively scarce compared with other immune-related adverse events and thus we felt the need to conduct this work [Corsello et al. 2013; Torino et al. 2013]. We conducted a meta-analysis of randomized clinical trials to determine the overall risk of developing pneumonitis in cancer patients treated with different immune checkpoint inhibitors.
Methods
Data source
We conducted a thorough review of the MEDLINE and Google Scholar databases from January 2000 to December 2015 using ‘ipilimumab’ OR ‘pembrolizumab’ OR ‘nivolumab’ as search terms. The search was limited to randomized clinical trials published in English. In case of duplicate publications, only the most complete clinical report was included. Trials were chosen and reviewed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [Moher et al. 2009].
Study selection
Inclusion criteria were as follows.
(1) Randomized phase II and III studies in patients with solid tumors.
(2) Participants received treatment with one of the immune checkpoint inhibitors.
(3) Sample size and event rate available for all-grade (grades 1–4) and high-grade (grades 3–4) pneumonitis.
Exclusion criteria were are as follows.
(1) Phase I trials were excluded.
We screened those reports that included the search terms by their titles and abstracts for relevance. The full texts of the relevant articles were then assessed for eligibility.
Data extraction and clinical endpoints
We conducted data extraction independently. The following information were recorded for each study: first author’s name, date of publication, phase of the trial, underlying malignancy, type of immune checkpoint inhibitor, treatment arms, number of patients available for analysis, and number of events for both all-grade and high-grade pneumonitis. The quality of the included studies was assessed through the use of the Jadad score (Table 3) [Jadad et al. 1996].
Table 3.
Jadad quality assessment of the included studies.
| Study [year] | Randomization | Blinding | An account of all patients | Overall score |
|---|---|---|---|---|
| Kwon et al. [2014] | 2 | 2 | 1 | 5 |
| Robert et al. [2015] | 2 | 2 | 1 | 5 |
| Weber et al. [2015] | 2 | 0 | 1 | 3 |
| Brahmer et al. [2015] | 2 | 0 | 1 | 3 |
| Borghaei et al. [2015] | 2 | 0 | 1 | 3 |
| Motzer et al. [2015] | 2 | 0 | 1 | 3 |
| Ribas et al. [2015] | 2 | 0 | 1 | 3 |
| Herbst et al. [2015] | 2 | 0 | 1 | 3 |
| Robert et al. [2015] | 2 | 0 | 1 | 3 |
| Larkin et al. [2015] | 2 | 2 | 1 | 5 |
| Postow et al. [2015] | 2 | 2 | 1 | 5 |
Any discrepancies between us were resolved by consensus. In the included clinical trials, the common terminology criteria of adverse events (CTCAE) version 4.0 were utilized for recording the toxicity in the included studies.
Analysis of the data
Odds ratio (OR) and corresponding 95% confidence intervals (CIs) of all-grade (grades 1–4) and high-grade (grades 3–4) pneumonitis were our principal measures. We compared the number of events of each adverse event in participants randomized to immune checkpoint inhibitors with those randomized to control treatment in each trial. The heterogeneity of outcomes between assessed studies in the analysis was evaluated through Cochrane’s Q statistic. A fixed effect model was used in all the subanalyses because of the homogeneity of the results. Publication bias was been assessed through the use of funnel plots. Data analyses were performed using Review Manager 5.3 (Nordic Cochrane Centre; Copenhagen, Denmark).
Results
Search results
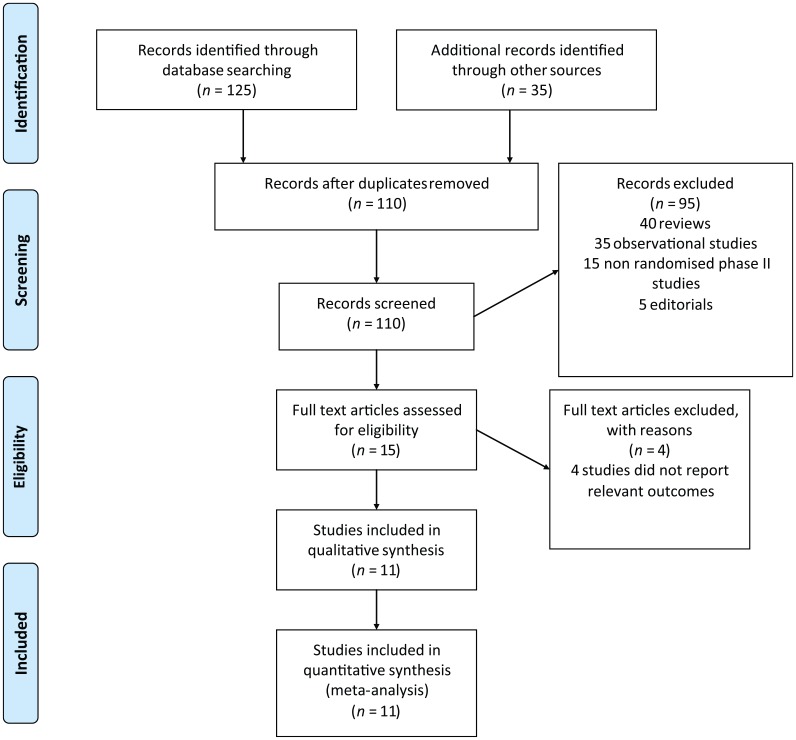
Our search strategy yielded 160 potentially relevant citations on immune checkpoint inhibitors from PubMed/MEDLINE and other databases. The reasons for study exclusion are shown in Figure 1.
Figure 1.
Flowchart of study selection procedure.
Thus, a total of 11 clinical trials were considered eligible for the meta-analysis, including 10 phase III trials and one randomized phase II trial (Tables 1 and 2). One study evaluated ipilimumab versus placebo [Kwon et al. 2014], four studies evaluated nivolumab versus chemotherapy control [Borghaei et al.2015; Brahmer et al. 2015; Robert et al. 2015a; Weber et al. 2015], one study evaluated nivolumab versus everolimus [Motzer et al. 2015], two studies evaluated pembrolizumab versus chemotherapy control [Herbst et al. 2015; Ribas et al. 2015], two studies evaluated a nivolumab/ipilimumab combination versus ipilimumab monotherapy [Larkin et al. 2015; Postow et al. 2015] and one study evaluated pembrolizumab versus ipilimumab [Robert et al. 2015b].
Table 1.
Baseline characteristics of included studies comparing immune checkpoint inhibitors to non immune checkpoint inhibitors.
| Study | Study type | Treatment arms | Indication | All-grade (grades 1–4) pneumonitis | High-grade (grades 3–4) pneumonitis |
|---|---|---|---|---|---|
| 1. Ipilimumab studies | |||||
| Kwon et al. [2014] | Phase III | Arm A: ipilimumab 10 mg/kg (393 pts) Arm B: placebo (396 pts) |
Metastatic castration-resistant prostate cancer | 5 (1.3%) versus 0 | 1 (0.3%) versus 0 |
| 2. Nivolumab studies | |||||
| Robert et al. [2015a] | Phase III | Arm A: nivolumab 3 mg/kg of body weight every 2 weeks (206
pts) Arm B: dacarbazine (205 pts) |
Stage III or IV unresectable melanoma without a BRAF mutation. | 3 (1.5%) versus 0 | 0 versus 0 |
| Weber et al. [2015] | Phase III | Arm A: nivolumab 3 mg/kg of body weight every 2 weeks (268
pts) Arm B: investigator choice chemotherapy (102 pts) |
Patients with advanced melanoma who progressed after anti-CTLA-4 treatment | 5 (1.9%) versus 0 | 0 versus 0 |
| Brahmer et al. [2015] | Phase III | Arm A: nivolumab 3 mg/kg of body weight every 2 weeks (131
pts) Arm B: docetaxel (129 pts) |
Advanced squamous cell NSCLC | 6 (5%) versus 0 | 0 versus 0 |
| Borghaei et al. [2015] | Phase III | Arm A: nivolumab 3 mg/kg of body weight every 2 weeks (292
pts) Arm B: docetaxel (290 pts) |
Advanced non squamous cell NSCLC | 8 (3%) versus 1 (<1%) | 3 (1%) versus 1 (<1%) |
| Motzer et al. [2015] | Phase III | 821 patients were randomly assigned (in a 1:1 ratio) to receive 3 mg of nivolumab per kilogram of body weight intravenously every 2 weeks or a 10 mg everolimus tablet orally once daily | Advanced clear-cell renal cell carcinoma for which they had received previous treatment with one or two regimens of antiangiogenic therapy | 16 (4%) versus 58 (15%) | 6 (1%) versus 11 (3%) |
| 3. Pembrolizumab studies | |||||
| Ribas et al. [2015] | Randomized phase II | Arm A: pembrolizumab (low dose 2 mg/kg): 179 pts Arm B: pembrolizumab (high dose 10 mg/kg):178 pts Arm C: chemotherapy control: 171 pts |
Ipilimumab-refractory advanced melanoma | 3 (2%) versus 3(2%) versus 0 | 0 versus 2(1%) versus 0 |
| Herbst et al. [2015] | Randomized phase II/III study | Arm A: pembrolizumab (low dose 2 mg/kg): 344 pts Arm B: pembrolizumab (high dose3 mg/kg ):346 pts Arm C: Docetaxel: 343 pts |
Previously treated NSCLC with PD-L1>1% | 16 (5%) versus 15 (4%) versus 6 (2%) | 7 (2%) versus 7 (2%) versus 2 (1%) |
CTLA-4, cytotoxic T lymphocyte antigen-4; NSCLC, non-small cell lung cancer; PD-L1, programmed death ligand 1; pts, patients.
Table 2.
Direct comparison among different immune checkpoint inhibitors.
| Study | Phase | Treatment arms | Indication | All-grade (grades 1–4) pneumonitis | High-grade (grades 3–4) pneumonitis |
|---|---|---|---|---|---|
| Robert et al. [2015b] | Phase III | 834 patients with advanced melanoma in a 1:1:1 ratio to
receive: • Arm A: pembrolizumab 10 mg/kg every 2 weeks (279 pts) • Arm B: pembrolizumab 10 mg/kg every 3 weeks (277 pts) • Arm C: ipilimumab (278 pts) |
Advanced melanoma | 1 (0.4%) versus 5 (1.8%) versus 1 (0.4%) | 0 versus 1(0.4%) versus 1(0.4%) |
| Larkin et al. [2015] | Phase III | 945 patients were randomized in a 1:1:1 fashion
into: • Nivolumab 3mg/kg combined with placebo (316 pts) • Ipilimumab (3 mg/kg) combined with nivolumab (1 mg/kg) (314 pts) • Ipilimumab (3 mg/kg) combined with placebo (315 pts) |
Advanced melanoma | 4 (1.3%) versus 20 (6.4%) versus 5 (1.6%) | 1 (0.3%) versus 3 (1%) versus 1 (0.3%) |
| Postow et al. [2015] | Phase III | 142 patients were randomized in a 2:1 fashion into
: • Ipilimumab (3 mg/kg) combined with nivolumab (1 mg/kg) (94 pts) • Ipilimumab (3 mg/kg) combined with placebo (46 pts) |
Advanced melanoma | 10 (11%) versus 2 (4%) | 2 (2%) versus 1(2%) NB One patient in the combination arm has died of drug-related pneumonitis. |
pts, patients.
Thus, the interventions evaluated in the analysis included ipilimumab monotherapy, nivolumab monotherapy, pembrolizumab monotherapy, nivolumab/ipilimumab combination, chemotherapy control (including docetaxel and/or dacarbazine), placebo control and everolimus control. Clinical indications for the evaluated studies included advanced melanoma (six studies), castrate-resistant prostate cancer (one study), advanced RCC (one study) and NSCLC (three studies).
Population characteristics
A total of 6671 patients were included in the analysis. According to the eligibility criteria of the majority of the trials, patients with impaired renal, hepatic or bone marrow function were excluded and most of patients have Eastern Cooperative Oncology Group (ECOG) performance status between 0 and 2. The baseline characteristics and the number of all-grade events in each trial are detailed in Table 1.
Quality of the included studies
Table 3 shows the different elements of the Jadad scale for each of the included studies, including randomization, blinding and an account of all patients in addition to the overall score.
Overall incidence of pneumonitis
For the incidence analysis, we considered only arms receiving one of the immune checkpoint inhibitors. All-grade pneumonitis was reported by all the included studies and ranged from 1.3% to 11%; high-grade (grades 3–4) pneumonitis was also reported by all the included studies and ranged from 0.3% to 2%.
Four studies reported detailed kinetics of immune-related pneumonitis (Table 4). In most of the cases, treatment with immune modulatory agents (IMM) resulted in complete resolution of the pneumonitis. Median time to resolution ranged from 3 to 6 weeks.
Table 4.
Kinetics of immune-related pneumonitis (as reported in some of the included studies).
| Study | Median time to onset | Treatment received | Median time to resolution |
|---|---|---|---|
| Brahmer et al. [2015] | Median time to onset of treatment-related pulmonary events was 15.1 weeks (range: 2.6–85.1) | All but one patient with pulmonary events received glucocorticoids | All cases resolved, with a median time to resolution of 5.0 weeks (range: 0.6–12.1). |
| Borghaei et al. [2015] | 31.1 weeks (range: 11.7–56.9) | 70% of patients received IMM | 80% of cases resolved with a median time to resolution of 5.7 (range: 2.7–28.3+) |
| Larkin et al. [2015] | Not reported | • Nivolumab monotherapy: 4/4 patients were treated with IMM
(including glucocorticoids) • Nivolumab/ipilimumab: 17/20 patients were treated with IMM • Ipilimumab monotherapy: 3/5 patients were treated with IMM. |
• Nivolumab monotherapy: all cases resolved with median time
to resolution of 3.3 weeks. • Nivolumab/ipilimumab: 94% of cases resolved with a median time to resolution of 6.1 weeks • Ipilimumab monotherapy: 66.7% of cases resolved with a median time to resolution of 6.1 weeks |
| Postow et al. [2015] | Not reported | • Nivolumab/ipilimumab: 80% were treated with
IMM • Ipilimumab: 100% were treated with IMM. |
• Nivolumab/ipilimumab: 70% of cases resolved with median
time to resolution of 6 weeks • Ipilimumab: 100% of cases resolved with median time to resolution of 3.2 weeks |
IMM, immune modulatory medication; N/R: not reported.
Treatment-related death secondary to high-grade pneumonitis was reported in one patient only in the combination arm of the study by Postow and colleagues [Postow et al. 2015].
OR of all-grade and high-grade pneumonitis
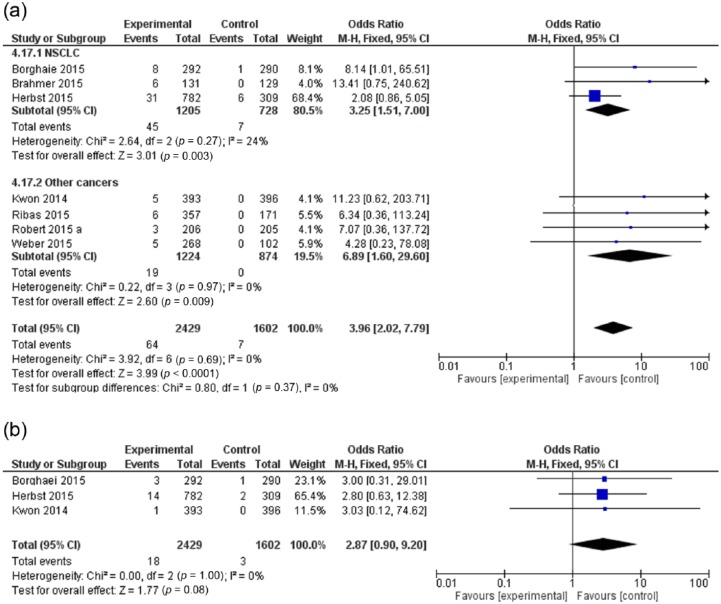
To evaluate the OR for all-grade and high grade pneumonitis, only studies evaluating immune checkpoint inhibitors compared with other agents were considered. Moreover, the study by Motzer and colleagues [Motzer et al. 2015] was excluded from the final analysis because everolimus (the control drug) is well known for a high risk of drug-related pneumonitis. The OR was 3.96 (95% CI: 2.02–7.79; p < 0.0001) for all-grade pneumonitis and 2.87 (95% CI: 0.90–9.20; p = 0.08) for high-grade pneumonitis (Figure 2). Thus, immune checkpoint inhibitors are associated with a higher risk of all-grade pneumonitis compared with control regimens.
Figure 2.
Forest plot for odds ratio of (a) all-grade and (b) high-grade pneumonitis for cancer patients receiving immune checkpoint inhibitors compared with control.
CI, confidence interval; df, degrees of freedom; M-H, Mantel–Haenszel; NSCLC, non-small cell lung cancer. (Robert 2015a)
Other relevant comparisons
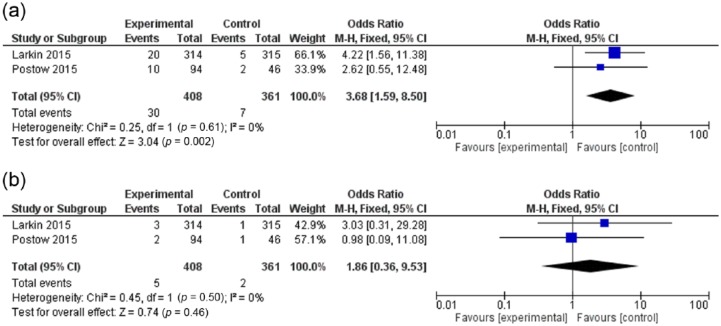
The OR of all-grade pneumonitis with a nivolumab/ipilimumab combination versus ipilimumab monotherapy (evaluated in two studies) was 3.68 (95% CI: 1.59–8.50; p = 0.002), and for high-grade pneumonitis, it was 1.86 (95% CI: 0.36–9.53; p = 0.46) (Figure 3a,b). Thus compared with ipilimumab, a nivolumab/ipilimumab combination is associated with all-grade rather than high-grade pneumonitis.
Figure 3.
Forest plot for odds ratio of (a) all-grade and (b) high-grade pneumonitis for cancer patients receiving a nivolumab/ipilimumab combination compared with ipilimumab monotherapy.
CI, confidence interval; df, degrees of freedom; M-H, Mantel–Haenszel.
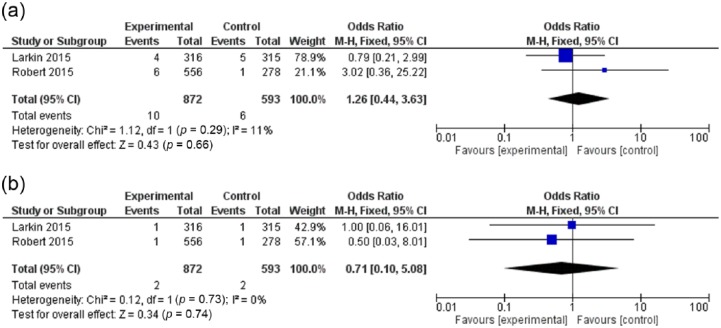
The OR of all-grade pneumonitis with PD-1 inhibitors (nivolumab and pembrolizumab) versus ipilimumab monotherapy (evaluated in 2 studies) was 1.26 (95% CI: 0.44–3.63; p = 0.66), and for high-grade pneumonitis, it was 0.71 (95% CI: 0.10–5.08; p = 0.74) (Figure 4a,b). Thus, no significant differences in the risk of all-grade or high-grade pneumonitis can be detected between PD-1 inhibitors and ipilimumab. Moreover, a funnel plot did not reveal evidence for publication bias for the primary analysis (Figure 5).
Figure 4.
Forest plot for OR of (a) all-grade; and (b) high-grade pneumonitis for cancer patients receiving PD-1 inhibitors compared to ipilimumab monotherapy.
CI, confidence interval; df, degrees of freedom; M-H, Mantel–Haenszel. (Robert 2015b)
Figure 5.
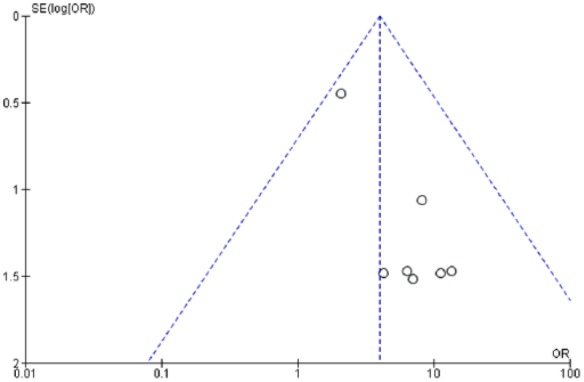
Forest plot for publication bias.
OR, odds ratio; SE, standard error.
Subgroup analysis
We conducted subgroup analysis according to the type of cancer treated (NSCLC versus other cancers) and we did not detect a significant difference among both subgroups (Figure 2a). We planned additionally to conduct a subgroup analysis (of the main comparison) between studies evaluating PD-1 inhibitors and those evaluating ipilimumab, but because ipilimumab is represented in this comparison by only one study, such subgroup analysis could not be made.
Discussion
To the extent of our knowledge, this is the most up-to-date analysis to provide an evaluation of the risk of pneumonitis in cancer patients receiving immune checkpoint inhibitors. Our analysis of data demonstrated a higher risk of all-grade pneumonitis with immune checkpoint inhibitors compared with control regimens. Moreover, the all-grade pneumonitis risk was higher with a nivolumab/ipilimumab combination than ipilimumab monotherapy.
The principal physiological role of the immune checkpoints is to restrict the power of T cells and to prevent them from overacting against normal self tissues [Intlekofer and Thompson, 2013]. This fact led to the hypothesis that inhibition of these checkpoints may lead to more powerful antitumor effects [Alegre et al. 1996; Topalian et al. 2012]. The two most important checkpoints upon which clinical development of targeted agents have been followed are CTLA-4 and PD-1 (and its ligand PD-L1) [Abdel-Rahman, 2016].
Ipilimumab, pembrolizumab and nivolumab were approved by the FDA for the treatment of advanced melanoma following a number of landmark phase III studies. Subsequently, further phase III data have encouraged the approval of nivolumab for NSCLC and a breakthrough designation for second-line treatment of advanced RCC. The results of a number of ongoing phase II and III studies are eagerly awaited to determine the role of these agents in the treatment of many other solid tumors.
Pathologically, drug-induced immune pneumonitis has been hypothesized to be similar to interstitial pneumonitis associated with collagen vascular disease. Clinical presentation of possible immune-related pneumonitis includes persistent cough, dyspnea, tachypnea and possibly hypoxia [Nishino et al. 2015]. Radiologically, evidence of interstitial pneumonitis may be apparent on body imaging, particularly high resolution computed tomography (CT) [Tirumani et al. 2015].
Despite being uncommon, drug-induced pneumonitis is considered a possible cause of extra morbidity and a potential reason for treatment interruption in immune checkpoint inhibitor clinical trials.
Drug-induced pneumonitis has been reported by other anticancer agents, notably erlotinib and gefitinib [Abdel-Rahman et al. 2015d]. Moreover, sporadic case reports have been reported with some other cytotoxic chemotherapies [Roychowdhury et al. 2002; Grande et al. 2007]. The usual policy in managing drug-induced pneumonitis reported with these agents has started with stopping the causative drug, then considering steroids in more severe conditions [Chow, 2013].
A recent review by Guibert and colleagues provided a useful algorithm for the management of suspected cases of pneumonitis with immune checkpoint inhibitors [Guibert et al. 2015]. This algorithm suggests that, upon clinical suspicion of pneumonitis (e.g. cough, dyspnea or interstitial infiltrates on chest X-ray), CT scanning should be requested. This should help confirm the diagnosis and radiology of pneumonitis or else suggest a different diagnosis. If after a CT scan, the diagnosis is not confirmed, bronchoscopy with additional bronchoalveolar lavage (BAL) should be considered.
The treatment of drug-induced pneumonitis secondary to immune checkpoint inhibitors follows the general management strategy of other immune-related adverse events (like hepatitis and colitis) [Weber et al. 2012]. For grade I pneumonitis, close observation has been suggested; while for higher grade pneumonitis, the causative agent has been held with initiation of IMM (most notably steroids). According to the data from the included studies in our analysis (Table 3), most of the cases resolved completely with the use of steroids. Rarely, a need for further use of other IMM such as azathioprine or cyclosporine may arise in some cases of drug-induced pneumonitis [Lai et al. 2011].
The impact of pneumonitis associated with these agents should be further stressed in view of the recent approvals of nivolumab in squamous and nonsquamous NSCLC as well as the encouraging data of pembrolizumab in the same indication [Garon et al. 2015]. Confusion may possibly arise in such cases, as it may be difficult to differentiate between the development of drug-induced pneumonitis versus progression of the disease. Careful multidisciplinary consultation in each suspected case of pneumonitis should be conducted to avoid improper management in such cases.
Another important aspect when approaching immune-related adverse events with immune checkpoint inhibitors has been the potential correlation between the appearance of some of these toxicities and the response to therapy. For example in a study by Bronstein and colleagues evaluating patients receiving anti-CTLA4 therapy, the disease control rate was 55% for the patients with, and 10% for the patients without radiologic manifestations of immune-related adverse events [Bronstein et al. 2011]. Although this was not directly linked to pneumonitis in any of the included studies of our analysis, this point needs further evaluation in future studies.
One point of caution in interpreting our study is that this is a meta-analysis at the study level rather than individual patient data level and therefore potential variables at the patient level were not imported in the analysis. Thus, we could not establish whether potential additional risk factors (e.g. background lung disease) may be potentially associated with the development of pneumonitis. Moreover, apparent heterogeneity of the drugs used as well as the cancers treated may weaken the results. We have tried to overcome this through appropriate subgroup analysis.
Conclusion
Our analysis of data demonstrated a higher risk of all-grade pneumonitis with immune checkpoint inhibitors compared with control regimens. Regarding high-grade pneumonitis, there is a trend in favor of a higher risk with immune checkpoint inhibitors compared with control regimens. Moreover, the all-grade pneumonitis risk was higher with a nivolumab/ipilimumab combination than with ipilimumab monotherapy. Management of suspected cases of pneumonitis requires vigilant early intervention and proper multidisciplinary consultation.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Omar Abdel-Rahman, Clinical Oncology Department, Faculty of Medicine, Ain Shams University, Lotfy Elsayed Street, Cairo 11665, Egypt.
Mona Fouad, Medical Microbiology and Immunology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
References
- Abdel-Rahman O. (2016) Immune checkpoints aberrations and gastric cancer; assessment of prognostic value and evaluation of therapeutic potentials. Crit Rev Oncol Hematol 97: 65–71. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman O., ElHalawani H. (2015d) Risk of fatal pulmonary events in patients with advanced non-small-cell lung cancer treated with EGF receptor tyrosine kinase inhibitors: a comparative meta-analysis. Future Oncol 11: 1109–1122. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman O., ElHalawani H., Fouad M. (2015a) Risk of gastrointestinal complications in cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Immunotherapy 7: 1213–1227. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman O., ElHalawani H., Fouad M. (2015b) Risk of cutaneous toxicities in patients with solid tumors treated with immune checkpoint inhibitors: a meta-analysis. Future Oncol 11: 2471–2484. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman O., ElHalawani H., Fouad M. (2015c) Risk of elevated transaminases in cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Expert Opin Drug Saf 14: 1507–1518. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman O., ElHalawani H., Fouad M. (2016) Risk of endocrine complications in cancer patients treated with immune check point inhibitors; a meta-analysis. Future Oncol 12: 413–425. [DOI] [PubMed] [Google Scholar]
- Alegre M., Noel P., Eisfelder B., Chuang E., Clark M., Reiner S., et al. (1996) Regulation of surface and intracellular expression of CTLA4 on mouse T cells. J Immunol 157: 4762–4770. [PubMed] [Google Scholar]
- Armand P., Nagler A., Weller E., Devine S., Avigan D., Chen Y., et al. (2013) Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol 31: 4199–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger R., Rotem-Yehudar R., Slama G., Landes S., Kneller A., Leiba M., et al. (2008) Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Research 14: 3044–3051. [DOI] [PubMed] [Google Scholar]
- Borghaei H., Paz-Ares L., Horn L., Spigel D., Steins M., Ready N., et al. (2015) Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. New Engl J Med 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J., Reckamp K.L., Baas P., Crinò L., Eberhardt W., Poddubskaya E., et al. (2015) Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. New Engl J Med 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J., Tykodi S., Chow L., Hwu W., Topalian S., Hwu P., et al. (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. New Engl J Med 366: 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein Y., Ng C., Hwu P., Hwu W. (2011) Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. Am J Roentgenol 197: W992–W1000. [DOI] [PubMed] [Google Scholar]
- Chow L. (2013) Exploring novel immune-related toxicities and endpoints with immune-checkpoint inhibitors in non-small cell lung cancer. In: Dizon D. (ed.), American Society of Clinical Oncology 2013 Educational Book. Alexandria, VA: American Society of Clinical Oncology, e280. [DOI] [PubMed] [Google Scholar]
- Corsello S., Barnabei A., Marchetti P., De Vecchis L., Salvatori R., Torino F. (2013) Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab 98: 1361–1375. [DOI] [PubMed] [Google Scholar]
- Garon E., Rizvi N., Hui R., Leighl N., Balmanoukian A., Eder J., et al. (2015) Pembrolizumab for the treatment of non–small-cell lung cancer. New Engl J Med 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- Grande C., Villanueva M., Huidobro G., Casal J. (2007) Docetaxel-induced interstitial pneumonitis following non-small-cell lung cancer treatment. Clin Transl Oncol 9: 578–581. [DOI] [PubMed] [Google Scholar]
- Guibert N., Delaunay M., Mazières J. (2015) Targeting the immune system to treat lung cancer: rationale and clinical experience. Ther Adv Respir Dis 9: 105–120. [DOI] [PubMed] [Google Scholar]
- Hamid O., Robert C., Daud A., Hodi F., Hwu W., Kefford R., et al. (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. New Engl J Med 369: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst R., Baas P., Kim D., Felip E., Pérez-Gracia J., Han J., et al. (2015) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. DOI: http://dx.doi.org/10.1016/S0140-6736(15)01281-7. [DOI] [PubMed]
- Hodi F., O’Day S.J., McDermott D., Weber R., Sosman J., Haanen J., et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. New Engl J Med 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer A., Thompson C. (2013) At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukocyte Biol 94: 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadad A., Moore R., Carroll D., Jenkinson C., Reynolds D., Gavaghan D., et al. (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clin Trials 17: 1–12. [DOI] [PubMed] [Google Scholar]
- Kwon E., Drake C., Scher H., Fizazi K., Bossi A., van den Eertwegh A., et al. (2014) Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 15: 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y., Lin P., Lai J., Hsu S., Kuo L., Chang S., et al. (2011) Successful treatment of erlotinib-induced acute hepatitis and acute interstitial pneumonitis with high-dose corticosteroid: a case report and literature review. Int J Clin Pharmacol Ther 49: 461–466. [DOI] [PubMed] [Google Scholar]
- Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J., Cowey C., Lao C., et al. (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. New Engl J Med 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. (2009) Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. Ann Intern Med 151: 264–269. [DOI] [PubMed] [Google Scholar]
- Momtaz P., Postow M. (2014) Immunologic checkpoints in cancer therapy: focus on the programmed death-1 (PD-1) receptor pathway. Pharmacogenomics Pers Med 7: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer R., Escudier B., McDermott D., George S., Hammers H., Srinivas S., et al. (2015) Nivolumab versus eerolimus in advanced renal-cell carcinoma. N Eng J Med 373: 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino M., Sholl L., Hatabu H., Ramaiya N., Hodi F. (2015) Anti-PD-1-related pneumonitis during cancer immunotherapy. New Engl J Med 373: 288–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow M., Chesney J., Pavlick A., Robert C., Grossmann K., McDermott D., et al. (2015) Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. New Engl J Med 372: 2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A., Puzanov I., Dummer R., Schadendorf D., Hamid O., Robert C., et al. (2015) Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 16: 908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C., Long G., Brady B., Dutriaux C., Maio M., Mortier L., et al. (2015a) Nivolumab in previously untreated melanoma without BRAF mutation. New Engl J Med 372: 320–330. [DOI] [PubMed] [Google Scholar]
- Robert C., Schachter J., Long G., Arance A., Grob J., Mortier L., et al. (2015b) Pembrolizumab versus ipilimumab in advanced melanoma. New Engl J Med 372: 2521–2532. [DOI] [PubMed] [Google Scholar]
- Robert C., Thomas L., Bondarenko I., O’Day S., Weber J., Garbe C., et al. (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. New Engl J Med 364: 2517–2526. [DOI] [PubMed] [Google Scholar]
- Rosenberg S., Yang J., Restifo N. (2004) Cancer immunotherapy: moving beyond current vaccines. Nat Med 10: 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychowdhury D., Cassidy C., Peterson P., Arning M. (2002) A report on serious pulmonary toxicity associated with gemcitabine-based therapy. Invest New Drugs 20: 311–315. [DOI] [PubMed] [Google Scholar]
- Spira A., Park K., Mazieres J., Vansteenkiste J., Rittmeyer A., Ballinger M. (2015) Efficacy, safety and predictive biomarker results from a randomized phase ii study comparing atezolizumab (MPDL3280a) vs docetaxel in 2l/3l NSCLC (Poplar). J Clin Oncol 33(suppl.): abstract 8010. [Google Scholar]
- Tirumani S., Ramaiya N., Keraliya A., Bailey N., Ott P., Hodi F., et al. (2015) Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res 3: 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S., Hodi F., Brahmer J., Gettinger S., Smith D., McDermott D., et al. (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Engl J Med 366: 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torino F., Barnabei A., Paragliola R., Marchetti P., Salvatori R., Corsello S. (2013) Endocrine side-effects of anti-cancer drugs: mabs and pituitary dysfunction: clinical evidence and pathogenic hypotheses. Eur J Endocrinol 169: R153–R164. [DOI] [PubMed] [Google Scholar]
- Weber J., D’angelo S., Minor D., Hodi F., Gutzmer R., Neyns B., et al. (2015) Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 TREATMENT (CHECKMATE 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16: 375–384. [DOI] [PubMed] [Google Scholar]
- Weber J., Kähler K., Hauschild A. (2012) Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 30: 2691–2697. [DOI] [PubMed] [Google Scholar]
- Westin J., Chu F., Zhang M., Fayad L., Kwak L., Fowler N., et al. (2014) Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol 15: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]