Abstract
Introduction:
Use of varenicline for as long as necessary to achieve abstinence has not been studied. The aim of this study was to test whether smokers with mild-to-moderate chronic obstructive pulmonary disease (COPD) are able to quit if they use varenicline for a sufficient length of time.
Methods:
A total of 30 heavy smokers with COPD took varenicline for sufficiently long enough for smoking cessation. Smokers were allowed to smoke without a fixed quit date. The main endpoints were the time of voluntary abstinence (VA) and the continuous abstinence rate (CAR) at 12 and 18 months.
Results:
Of 28 subjects, eight subjects continued to smoke and 20 subjects stopped smoking, demonstrating a CAR up to 18 months (71%). Median time of treatment was 6 (range 3–24) and 2 (range 1–8) months for abstainers and non-abstainers, respectively, and the median time of VA for abstainers was 4 (range 1–21) months.
Conclusions:
Use of varenicline for more than the traditional 12 recommended weeks may be a good strategy to increase the cessation rate in heavy smokers with mild COPD.
Keywords: chronic obstructive pulmonary disease, flexible quit date, non-motivated smokers, varenicline
Introduction
Tobacco smoking is the leading cause of chronic obstructive pulmonary disease (COPD), which is one of the most important causes of morbidity and mortality worldwide. COPD is expected to increase over the coming years [Ezzati and Lopez, 2003]. Therefore, smoking cessation is a cornerstone to both prevent and treat COPD [Vestbo et al. 2013]. However, an important number of subjects with COPD are unsuccessful in quitting smoking [Vestbo et al. 2013; Wagena et al. 2004], particularly those with mild-to-moderate COPD who cannot or do not want to quit. The large majority of these subjects continue smoking even though they have a strong desire to quit [Anthonisen et al. 1994; Fong et al. 2004] with the aid of pharmacologic agents for treating nicotine dependence [Tashkin et al. 2001]. Different pharmacological interventions have demonstrated variable rates of success in smokers [Ashare et al. 2012]. The best success is that observed with varenicline [Cahill et al. 2014] when used for 12 weeks [Gonzales et al. 2006; Jorenby et al. 2006]. Specifically for COPD, there is one clinical trial that compared varenicline with placebo in mild-to-moderate COPD when used for 12 weeks. The rate of success from 9–52 weeks was 18% [Tashkin et al. 2011]. Attempts to increase the rate of success have been made either by preloading [Hajek et al. 2011; Hawk et al. 2012] for several weeks before the target quit date (TQD) or by adding 12 extra weeks to the standard treatment [Tonstad et al. 2006]. A more recent strategy was to increase the dose to 3 mg [Jiménez-Ruiz et al. 2003]. However, the rate of successful cessation is still ~40% at best after 1 year of treatment.
Most smoking cessation guidelines [Fiore et al. 2008] recommend that smokers set a TQD. However, few specific randomized clinical trials [Lindson-Hawley et al. 2016] have been carried out to decide if this strategy is better than a flexible quit date (FQD) while smokers are under pharmacological support to quit smoking. Lindson-Hawley and colleagues found that quitting smoking abruptly is more likely to lead to lasting abstinence than cutting down first. However, the total follow-up time and the observed rate of success were still discouraging [Lindson-Hawley et al. 2016].
The vast majority of studies on smoking cessation are addressed at smokers who are motivated to quit. In this sense, the crude effect of the pharmacological intervention to eliminate the desire for smoking in subjects wanting to smoke either with or without COPD is unknown [Gonzales et al. 2006; Jorenby et al. 2006; Hawk et al. 2012].
These data together suggest at least three issues that have not yet been fully explored. One is that despite the fact that use of varenicline is efficacious [Brandon et al. 2011; Foll et al. 2012; Gonzales et al. 2006; Jorenby et al. 2006; Hawk et al. 2012] and safe even when used for up to 52 weeks [Williams et al. 2007], no studies have reported its use for >12 months or as long as necessary to quit smoking. The second issue is that there are no studies on smoking cessation in smokers with COPD who are not motivated to quit and the third issue is that there are no studies using a FQD during the time smokers are under pharmacological treatment.
Therefore, the aim of this study was to demonstrate that nonmotivated heavy smokers with mild or moderate COPD can quit if: (1) they receive proper treatment for a sufficient time to achieve success; and (2) as a part of the medical counseling, a FQD is used as a tool for smoking cessation. For this study, treatment was carried out with varenicline for as long as necessary to quit smoking along with psychological and medical assistance.
Methods
Design
This work was designed as an open pilot, observational clinical study in which the pharmacological intervention was varenicline and no placebo control group was included. Subjects had to take varenicline until they quit smoking and were willing to participate in a follow-up phase up to 18 months after quitting smoking. They also had to buy varenicline on their own. The study was reviewed and approved by the ethics review boards at CRM Comité de Ciencia y Bioética en Investigación.
Study population
Inclusion criteria for the participants were as follows: (1) males and/or females aged 30 years or older; (2) have COPD with mild or moderate airflow limitation according to the GOLD criteria [Vestbo et al. 2013]; (3) currently smoking 20 or more cigarettes/day; (4) having no abstinence periods over the past year; and (5) having declared initially little or no desire to quit. Participants were excluded if they had serious comorbidities such as: having a heart attack in the past 6 months; need for supplemental oxygen; cancer; drug or alcohol abuse in the past year; history of psychiatric and neurologic disorder; and use of a smoking cessation medication or intervention in the past month.
Induction
This study was performed in two private centers for smoking cessation addressed at heavy smokers who were apparently unmotivated to quit. Smokers with a pulmonary consultation for treatment of various diseases or, in some cases, relatives of patients who consulted for a specific pulmonary disease (COPD, pneumonia, asthma, etc.) were invited to participate.
All subjects were positively challenged to participate in a smoking cessation ‘experiment’. They were asked to participate without prejudice in relation to the possibility of adverse events or expectations in relation with the possibility of quitting smoking. In this experiment there was no date to quit and the only condition to participate was the commitment to attend the clinic according to a calendar of visits.
Subjects were instructed in great detail that the hypothesis of our study was that, with such an intervention, we were inducing them to quit by eliminating the desire for smoking. In other words, they were told that the aim of the medical intervention was to eliminate the desire to smoke. If such a phenomenon occurred, they would stop smoking only because they no longer had the desire to smoke. If not, they were free to continue smoking as usual during the trial. They were also encouraged to quit by using two additional strategies. One strategy was that they had brief advice during the medical visits. The second strategy was the certainty of not experiencing abstinence symptoms for two reasons: because they were allowed to smoke as much as they wanted and because the drug intervention was supposed to eliminate the desire for smoking, abstinence symptoms and reduction in smoking cue-elicited craving as long as subjects continued taking the treatment drug.
Interventions
Eligible smokers during the baseline visit had to take varenicline during the entire time course of the project at 0.5 mg once daily for 3 days, 0.5 mg twice daily (bid) for 4 days and then 1.0 mg bid during the entire time needed for voluntarily quitting. Participants received two baseline educational sessions. The first one was diseases related to smoking and benefits of smoking cessation. The second one included mechanisms of nicotine addiction, the role of dopamine and the potential benefits associated with actions of varenicline. All subjects received a planned 5 minute brief intervention at each onsite visit.
Provision of varenicline
Varenicline was purchased by the patients because medical insurance companies in Mexico do not cover the cost of drugs as auxiliary treatment for smoking cessation. Medical visits were compensated by the insurance companies with reimbursement in 70% of the subjects. The visits of the remaining subjects were not charged by the treating institution.
Onsite medical visits
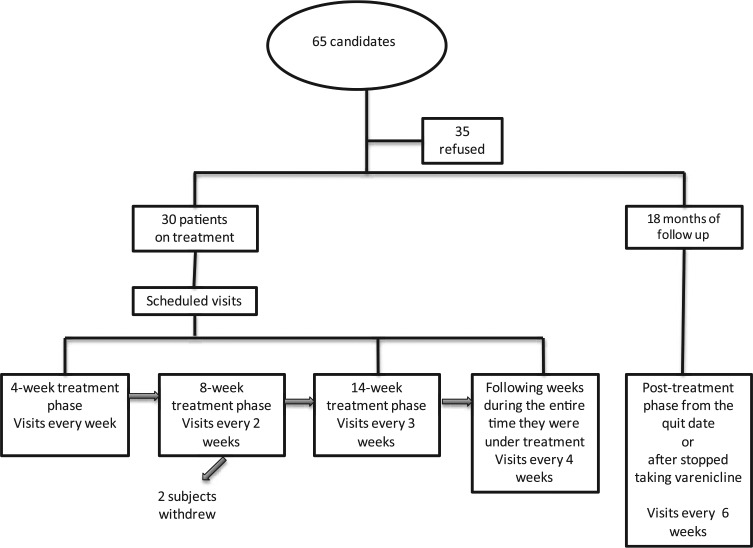
Smokers attended weekly sessions during the first 4-week treatment phase, every 2 weeks during the following 8-week treatment phase, every 3 weeks during the following 14-week phase treatment, every 4 weeks during the entire time they were under treatment and every 6 weeks during the post-treatment phase (Figure 1). Participants attended the clinic according to scheduled visits and received a telephone call 1 day prior to the visit to assure attendance at the clinic. During the 18-month nontreatment follow-up phase, participants attended the clinic at weeks 6, 12, 18, 24, 30, 36, 42, 48, 52 and 72. These visits were reinforced with telephone calls on the day prior to the visit. During each visit, all subjects received additional smoking cessation advice given by one of the psychologists.
Figure 1.
The graph shows the flow from the time of screening to finish the study.
TQD
No TQD was scheduled.
Baseline and follow-up assessment
The Fagerström Test for Cigarette Dependence (FTCD) was used for measuring physical dependence on nicotine. Nicotine use was determined at clinic visits to assess self-reported smoking or other use of nicotine or tobacco products by answering ‘yes’ or ‘no’ to the following question: have you smoked or used any nicotine products in the last 7 days? An exhaled carbon monoxide (eCO) measurement was taken at baseline and to assess smoking status when voluntary abstinence (VA) was declared and at 12 and 18 months of abstinence. An eCO value of <10 parts per million (ppm) was the criterion to confirm smoking abstinence. Abstinence symptoms were evaluated according to a short survey considering the most common symptoms in our population.
Efficacy
The primary endpoint was to determine the month in which VA started. VA was defined as the day during which cigarettes were no longer smoked and was reported as the proportion of participants who reported not smoking or not using any nicotine-containing products. The key secondary efficacy endpoint was the eCO confirmed continued abstinence rate (CAR) for weeks 52–72.
Safety evaluation
During the medical and psychological visits, patients were asked about neuropsychiatric adverse events such as depression, anxiety or suicide ideation.
Statistical analysis
The total number of analyzed subjects represented those who concluded the 72 weeks of follow up. Student’s t-test, the Welch approximation test and Mann–Whitney test were used to assess differences between abstainers and nonabstainers as appropriate. A multivariate logistic regression model was estimated to determine predicting variables of smoking cessation. No sample size was estimated. We included as many subjects who accepted to participate.
Results
Demographic data
From September 2009 to March 2012, 65 smokers with mild or moderate airflow limitations were invited to participate. A total of 35 subjects did not accept because were not able to afford the cost of varenicline. This was indeed the only limitation for these subjects to refuse to participate in the study. A total of 30 subjects accepted and signed informed consent; two patients discontinued treatment after 2 and 3 months of taking varenicline, respectively. A total of 28 smokers completed the entire project. Patients were classified as ‘abstainer group’ or ‘nonabstainer group’. Both groups were followed for 18 months regardless of whether they quit smoking or continued smoking. A total of 20 subjects quit smoking and eight subjects continued to smoke. Comparisons between abstainers and nonabstainers are given in Table 1. There was no difference between groups in age, lung function and tobacco consumption. All patients were heavy smokers with high nicotine dependence. The only difference was the higher baseline levels of eCO in the nonabstainers group (24 ± 8 ppm versus 18 ± 6 ppm, p < 0.029).
Table 1.
General characteristics and endpoints in abstinence and no abstinence groups.
| Abstinence group | No abstinence group | p | |
|---|---|---|---|
| Baseline characteristics | |||
| n (%) | 20 (71.4) | 8 (28.6) | |
| Age, years (SD) | 54 (11) | 56 (9) | 0.659* |
| FEV1%p (SD) | 76 (4) | 74(3) | 0.69* |
| FEV1/FVC (SD) | 0.62 (0.7) | 61(0.6) | 0.71* |
| Smoking age onset, years, median (range) | 17 (5–30) | 16 (14–23) | 0.252** |
| Cigarettes/day; median (range) | 24 (20–60) | 30 (20–35) | 0.436** |
| Pack/years; median (range) | 48 (19–119) | 54 (33–90) | 0.092** |
| Years of smoking; median (range) | 37 (20–58) | 36 (34–47) | 0.346** |
| eCO, ppm (SD) | 18 (6) | 24 (8) | 0.029* |
| Fagerström score; median (range) | 7 (3–9) | 7 (1–10) | 0.313** |
| Endpoints | |||
| Time of treatment, months; median (range) | 6 (3–24) | 2 (1–8) | 0.002** |
| Voluntary abstinence, months; median (range) | 4 (1–21) | 0 (0–11) | 0.005** |
| eCO ppm (12 months) (SD) | 4 (2) | 21 (4) | <0.0001*** |
| eCO ppm (18 months) (SD) | 4 (2) | 14 (13) | 0.02** |
Student’s t-test; **Mann–Whitney test; ***Welch’s approximation.
e-CO ppm, parts per million of exhaled carbon monoxide; FEV1%p, forced expiratory volume in 1 second percent predicted; FVC, forced vital capacity; SD, standard deviation.
Endpoints
Patients in the abstainer group had a higher number of months when they received varenicline (median 6; range 3–24 months) compared with the nonabstainer group (median 2; range 1–8 months) (p = 0.002)].
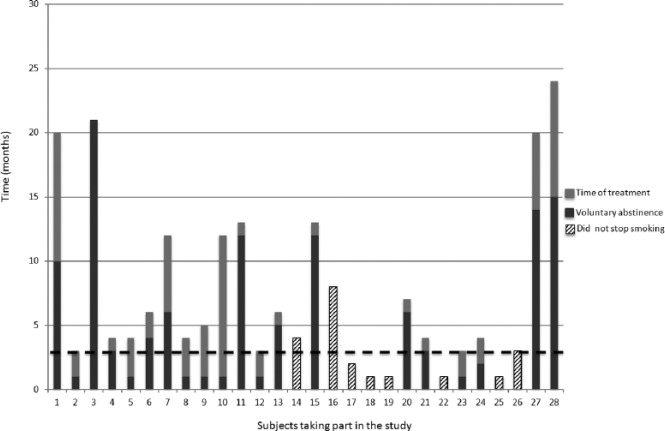
The median VA time for abstainers was 4 (range 1–21) months versus 0 months (range 0–11 months) for the nonabstainer group (p = 0.005); 71% of COPD patients who continued using varenicline quit smoking and remained as abstainers for at least 18 months after VA. After the VA time, the CAR validated with eCO was constant up to 18 months of follow up. Figure 2 shows the onset of voluntary abstinence and the total time of taking varenicline for each subject. Overall, subjects who took the treatment for less than 3 months treatment were less likely to maintain abstinence, while those who used varenicline for more than 3 months were more likely to succeed and maintain abstinence. A total of two subjects stopped smoking at months 14 and 15, but continued taking varenicline for 6 and 9 additional months, respectively; both subjects needed combined therapy either with nicotine replacement therapy as patches and bupropion during some point of the trial.
Figure 2.
The total time each subject took varenicline is shown as the sum of the grey bars. The two tone grey bars show the time when they started abstaining. Some subjects started voluntary abstinence from the first month of taking varenicline (subjects 2, 5, 8, 9, 10 and 12); however, they took varenicline for more than 3 months (4, 5, 5, 6, 12 and 4 months, respectively). However, the majority of subjects who kept smoking (striped bar) took varenicline for less than 3 months. The dotted line is a threshold showing that the minimum time needed to definitively quit is ⩾3 months (p < 0.001). Note that one subject (16) kept smoking despite the use of varenicline for up to 8 months.
A multivariate logistic regression analysis adjusted by pack/years (>20 versus ⩽20), Fagerström score (>6 versus ⩽6), time of treatment (>3 versus ⩽3 months), age at start (>17 versus ⩽17 years) and forced expiratory volume in 1 second percent predicted (FEV1%p) (<80 versus ⩾80) showed that time of treatment is the only predictor of abstinence [odds ratio (OR) 17; 95% confidence interval (CI): 2.3–128].
Abstinence and side effects
None of the patients who stopped smoking reported a desire to smoke (craving). Abstinence symptoms were mild. Three subjects reported headache, two subjects had difficulty in sleeping, three had anxiety, and two reported sweating. When subjects were questioned about side effects, all reported at some point of the study some of the following symptoms: nausea; headache; flatulence; and insomnia. Symptoms were transient and were not a cause for stopping treatment. No other side effect was observed.
Discussion
The main findings of this open study were as follows: (1) 71% (20 subjects) of COPD patients who continued using varenicline quit smoking and remained as abstainers at least 18 months after VA; (2) the desire for smoking was not observed once they quit; (3) abstinence symptoms were mild; (4) varenicline was well tolerated even if used for a period up to 24 months; and (5) not having a predetermined date to quit seems to be useful for smokers.
The high rate of failure in the traditional smoking cessation interventions in COPD patients probably depends on the belief that 3 months of treatment may be sufficient [González et al. 2006; Hawk et al. 2012]. However, it is unlikely that this duration works considering that the majority of heavy smokers with COPD have smoked for many years (>20 years). Therefore, new strategies for the population with COPD should be considered. What we hypothesized here is that if we eliminate the desire for smoking through the concept of pharmacological extinction framework [Rollema et al. 2007] without a predetermined date to quit and without a window for quitting, the likelihood to quit smoking increases (quit wish and quit win). The majority of these heavy smokers stopped smoking because they did not have the desire to continue smoking, despite the fact that they claimed not being motivated to quit but took varenicline on median time for 6 months.
In this work we present data exploring a previous hypothesis on the concept of preloading for smoking cessation [Rose et al. 1998]. In this sense, other studies [Hawk et al. 2012; Tashkin et al. 2001] found that abstinence rates were enhanced with 4 weeks of pre-quit varenicline compared with 1 week. Indeed, this paper goes beyond the hypothesis that extending the pre-quit period for varenicline (not just for 21 days as has been proposed [Tashkin et al. 2011] but for as long as necessary) would alter smoking behavior and subjective effects by reducing the positive reinforcement mechanism of smoking [Franklin et al. 2011; Patterson et al. 2012]. Another study that used a FQD to motivate subjects for quitting smoking using varenicline was that published by Rennard and colleagues [Rennard et al. 2011]. However, treatment was given only for 12 weeks and there was a window of time for quitting. Patients had an opportunity to quit smoking cigarettes between 8 and 35 days of starting medication; the vast majority of subjects stopped smoking within the first 9 weeks. In our study, patients continued smoking even for 4 months with a range from 1 to 21 months. Our results showed higher rates of success than the results by Rennard and colleagues.
Our work addressed two additional aspects of smoking cessation. One is that the subjects included in our study were not initially motivated to stop smoking. In one study in which varenicline was used in subjects who were not trying to quit, a decline in smoking was observed [Rennard et al. 2011; Tashkin et al. 2011], suggesting that varenicline gradually decreases the desire to smoke if taken over periods of days [Tashkin et al. 2011] or months [Rennard et al. 2011]. Furthermore, imaging studies showed that varenicline has actions in the reward-activated medial orbitofrontal cortex and in the reward-evaluating lateral orbitofrontal cortex, leading to a diminished smoking cue response in heavy smokers who were contemplating but who were not currently considering quitting [van der Meer et al. 2003]. This distinctive action of varenicline likely contributes to its clinical efficacy if taken for several months as observed in the current study. Other alternatives to the use of varenicline have been previously attempted in order to increase success rates. Jimenez-Ruiz and colleagues [Jorenby et al. 2006] used 3 mg of varenicline after 8 weeks of standard dosage. They achieved an eCO-validated continuous abstinence rate of ~40%.
Another issue addressed in this paper is the FQD, which is in parallel to the preloading concept in the direction that the longer the preloading time, the longer the quit date (FQD). In this work, the concept is integrative and we referred to it as the time of VA; it probably also explains the lack of abstinence symptoms. In addition, the low reported frequency of abstinence symptoms might also be associated with the personal decision to gradually reduce of the number of cigarettes smoked.
Study strengths
This was a group of subjects with mild/moderate airflow limitation receiving varenicline as long as necessary to quit (preloading time as needed). To the best of our knowledge, this is the first study in which smokers used varenicline for up to 24 months. Despite being an experiment, these results must be pivotal to establish a formal clinical trial. The issue is relevant once that the large clinical trials – TORCH, UPLIFT and others – showed that ~40% of smokers with COPD continued smoking.
Study limitations
The absence of a placebo control group and the nonblinding process are the main limitations of this study. Therefore, these results must be considered as a hypothesis-generating study and from this perspective provoke the need for carrying out a well-controlled placebo clinical trial. An additional limitation is the small number of subjects who accepted and completed the whole study. This may be explained by the costs. Another issue is that these smokers received very strong and continuous medical and brief psychological intervention. No control group received either intervention. These smokers were supposed to be nonmotivated to quit. However, during the time course they became very committed and motivated not only to continue with the project but also to purchase their medication. In this sense, this is a very peculiar group and we cannot apply these results to all smokers. The current cost of a pack containing 56 tablets is USD 101 (i.e. USD 3.3/day) whereas the cost of a cigarette/day depends on the number of cigarettes the smoker consumes. For example, if someone smokes, 20, 30 or 40 cigarettes/day the cost per day is USD 2.7, 4.0 and 5.0, respectively, whereas the daily cost of taking varenicline is always USD 3.3. Therefore, for those who were actually heavy smokers consuming more than 20 cigarettes a day, it could have been an economic incentive to purchase varenicline instead of smoking, whereas an economic limitation for those who smoke less than 20 cigarettes a day.
Conclusion
In summary, use of varenicline for more than the traditional 12 recommended weeks might be a good strategy to help heavy smokers with mild/moderate COPD to quit. Further properly designed research is certainly needed in this field.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Raúl H. Sansores, Departamento de Investigación en Tabaquismo y EPOC. Instituto Nacional de Enfermedades Respiratorias, Calzada de Tlalpan No. 4502, Delegación Tlalpan, Mexico City, 14080 Mexico, D.F., Mexico.
Alejandra Ramírez-Venegas, Departamento de Investigación en Tabaquismo y EPOC, Instituto Nacional de Enfermedades Respiratorias, Mexico City, Mexico; Centro Respiratorio de México, Mexico City, Mexico.
Rosario Arellano-Rocha, Centro Respiratorio de México, Mexico City, Mexico.
Valeri Noé-Díaz, Departamento de Investigación en Tabaquismo y EPOC, Instituto Nacional de Enfermedades Respiratorias, Mexico City, Mexico.
Leonor García-Gómez, Departamento de Investigación en Tabaquismo y EPOC, Instituto Nacional de Enfermedades Respiratorias, Mexico City, Mexico.
Oliver Pérez Bautista, Departamento de Investigación en Tabaquismo y EPOC, Instituto Nacional de Enfermedades Respiratorias, Mexico City, Mexico.
Mónica Velázquez Uncal, Departamento de Investigación en Tabaquismo y EPOC, Instituto Nacional de Enfermedades Respiratorias, Mexico City, Mexico.
References
- Anthonisen N., Connett J., Kiley J., Altose M., Bailey W., Buist A., et al. (1994) Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the Lung Health Study. JAMA 272:1497–1505. [PubMed] [Google Scholar]
- Ashare R., Tang K., Mesaros A., Blair I., Leone F., Strasser A. (2012) Effects of 21 days of varenicline versus placebo on smoking behaviors and urges among non-treatment seeking smokers. J Psychopharmacol 26: 1383–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon T., Drobes D., Unrod M., Heckman B., Oliver J., Roetzheim R., et al. (2011) Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology 218: 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill K., Stevens S., Lancaster T. (2014) Pharmacological treatments for smoking cessation. JAMA 311: 193–194. [DOI] [PubMed] [Google Scholar]
- Ezzati M., Lopez A. (2003) Estimates of global mortality attributable to smoking in 2000. Lancet 362: 847–852. [DOI] [PubMed] [Google Scholar]
- Fiore M., Jaén C., Baker T., Bailey W., Curry S., Wewers M. (2008) Treating tobacco use and dependence: 2008 update. Clinical practice guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service. [Google Scholar]
- Foll B., Chakraborty-Chatterjee M., Lev-Ran S., Barnes C., Pushparaj A., Gamaleddin I., et al. (2012) Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pretreatment time is used. Int J Neuropsychopharmacol 15: 1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong G., Hammond D., Laux F., Zanna M., Cummings M., Borland R., et al. (2004) The near-universal experience of regret among smokers in four countries: findings from the International Tobacco Control Policy Evaluation Survey. Nicotine Tob Res 3(Suppl.): S341–S351. [DOI] [PubMed] [Google Scholar]
- Franklin T., Wang Z., Suh J., Hazan R., Cruz J., Li Y., et al. (2011) Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry 68: 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D., Rennard S., Nides M., Oncken C., Azoulay S., Billing C., et al. (2006) Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist versus sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 296: 47–55. [DOI] [PubMed] [Google Scholar]
- Hajek P., McRobbie H., Myers K., Stapleton J., Dhanji A. (2011) Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Arch Intern Med 171: 770–777. [DOI] [PubMed] [Google Scholar]
- Hawk L., Ashare R., Lohnes S., Schlienz N., Rhodes J., Tiffany S., et al. (2012) The effects of extended pre-quit varenicline treatment on smoking behavior and short-term abstinence: a randomized clinical trial. Clin Pharmacol Ther 9: 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Ruiz C., Barrios M., Peña S., Cicero A., Mayayo M., Cristóbal M., et al. (2003) Increasing the dose of varenicline in patients who do not respond to the standard dose. Mayo Clin Proc 88: 1443–1445. [DOI] [PubMed] [Google Scholar]
- Jorenby D., Hays J., Rigotti N., Azoulay S., Watsky E., Williams K., et al. (2006) Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist versus placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 296: 56–63. [DOI] [PubMed] [Google Scholar]
- Lindson-Hawlely N., Banting M., West R., Michi S., Shinkins B., Aveyard P. (2016) Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Ann Intern Med 164: 582–592. [DOI] [PubMed] [Google Scholar]
- Patterson F., Jepson C., Strasser A., Loughead J., Perkins K., Frey J., et al. (2009) Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry 65: 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S., Hughes J., Cinciripini P., Kralikova E., Raupach T., Arteaga C., et al. (2011) A randomized placebo-controlled trial of varenicline for smoking cessation allowing flexible quit dates. Nicotine Tob Res 14: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H., Chambers L., Coe J., Glowa J., Hurst R., Lebel L., et al. (2007) Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52: 985–994. [DOI] [PubMed] [Google Scholar]
- Rose J., Behm F., Westman E. (1998) Nicotine-mecamylamine treatment for smoking cessation: the role of pre-cessation therapy. Exp Clin Psychopharmacol 6: 331–343. [DOI] [PubMed] [Google Scholar]
- Tashkin D., Kanner R., Bailey W., Buist S., Anderson P., Nides M., et al. (2001) Smoking cessation in patients with chronic obstructive pulmonary disease: a double blind, placebo-controlled, randomised trial. Lancet 357: 1571–1575. [DOI] [PubMed] [Google Scholar]
- Tashkin D., Rennard S., Hays J., Ma W., Lawrence D., Lee T. (2011) Effects of varenicline on smoking cessation in patients with mild to moderate COPD: a randomized controlled trial. Chest 139: 591–599. [DOI] [PubMed] [Google Scholar]
- Tonstad S., Tønnesen P., Hajek P., Williams K., Billing C., Reeves K. (2006) Varenicline. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 296: 64–71. [DOI] [PubMed] [Google Scholar]
- Van der Meer R., Wagena E., Ostelo R., Jacobs J., van Schayck C. (2003) Smoking cessation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2: CD002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestbo J., Hurd S., Agustí A., Jones P., Vogelmeier C., Anzueto A., et al. (2013) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 187: 347–365. [DOI] [PubMed] [Google Scholar]
- Wagena E., van der Meer R., Ostelo R., Jacobs J., van Schayck C. (2004) The efficacy of smoking cessation strategies in people with chronic obstructive pulmonary disease: results from a systematic review. Respir Med 98: 805–815. [DOI] [PubMed] [Google Scholar]
- Williams K., Reeves K., Billing C., Jr., Pennington A., Gong J. (2007) A double-blind study evaluating the long-term safety of varenicline for smoking cessation. Curr Med Res Opin 23: 793–801. [DOI] [PubMed] [Google Scholar]