Abstract
Background:
The objective of this study was to compare the cost-effectiveness of the fixed-dose combination (FDC) of tiotropium + olodaterol Respimat® FDC with tiotropium alone for patients with chronic obstructive pulmonary disease (COPD) in the Italian health care setting using a newly developed patient-level Markov model that reflects the current understanding of the disease.
Methods:
While previously published models have largely been based around a cohort approach using a Markov structure and GOLD stage stratification, an individual-level Markov approach was selected for the new model. Using patient-level data from the twin TOnado trials assessing Tiotropium + olodaterol Respimat® FDC versus tiotropium, outcomes were modelled based on the trough forced expiratory volume (tFEV1) of over 1000 patients in each treatment arm, tracked individually at trial visits through the 52-week trial period, and after the trial period it was assumed to decline at a constant rate based on disease stage. Exacerbation risk was estimated based on a random-effects logistic regression analysis of exacerbations in UPLIFT. Mortality by age and disease stage was estimated from an analysis of TIOSPIR trial data. Cost of bronchodilators and other medications, routine management, and costs of treatment for moderate and severe exacerbations for the Italian setting were included. A cost-effectiveness analysis was conducted over a 15-year time horizon from the perspective of the Italian National Health Service.
Results:
Aggregating total costs and quality-adjusted life years (QALYs) for each treatment cohort over 15 years and comparing tiotropium + olodaterol Respimat® FDC with tiotropium alone, resulted in mean incremental costs per patient of €1167 and an incremental cost-effectiveness ratio (ICER) of €7518 per additional QALY with tiotropium + olodaterol Respimat® FDC. The lung function outcomes observed for tiotropium + olodaterol Respimat® FDC in TOnado drove the results in terms of slightly higher mean life-years (12.24 versus 12.07) exacerbation-free months (11.36 versus 11.32) per patient and slightly fewer moderate and severe exacerbations per patient-year (0.411 versus 0.415; 0.21 versus 0.24) versus tiotropium. Probabilistic sensitivity analyses showed tiotropium + olodaterol Respimat® FDC to be the more cost-effective treatment in 95.2% and 98.4% of 500 simulations at thresholds of €20,000 and €30,000 per QALY respectively.
Conclusion:
Tiotropium + olodaterol Respimat® FDC is a cost-effective bronchodilator in the maintenance treatment of COPD for the Italian health care system.
Keywords: bronchodilators, COPD, cost-effectiveness, economic evaluation, fixed-dose combination
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation that is not fully reversible, resulting in declining respiratory function and quality of life (QoL) and an increased risk of mortality. According to World Health Organization (WHO) estimates, 210 million people worldwide had COPD in 2011 and it is projected to become the fifth leading cause of disability-adjusted life years (DALYs) lost and the third leading cause of death worldwide in 2030 [World Health Organization, 2011]. The decline in lung function of COPD patients tends to be accompanied by intermittent acute exacerbations. Exacerbations often result in hospitalization, increasing the economic burden of COPD and have a considerable negative impact on QoL and daily activities [Burge and Wedzicha, 2003].
Effective pharmacological interventions for COPD are available, and international guidelines recommend that all patients who are symptomatic merit a trial of drug treatment. The mainstay of COPD maintenance treatment are long-acting bronchodilators, such as long-acting muscarinic antagonists (LAMAs) and long-acting beta adrenergics (LABAs). Combinations of LAMAs and LABAs increase forced expiratory volume in one second (FEV1) among other spirometric variables, reduce dyspnea and improve QoL versus one bronchodilator given alone [Tashkin and Ferguson, 2013]. Tiotropium + olodaterol Respimat®, a fixed-dose combination (FDC) of tiotropium (LAMA) and olodaterol (LABA), has recently been approved and marketed in the US [US Food and Drug Administration, 2015] and Europe [European Medicines Agency, 2016] to relieve symptoms in adult patients with COPD. The 1-year effectiveness of tiotropium + olodaterol Respimat® FDC versus its mono components was assessed in the TOnado trials, two twin 52-week clinical trials, where tiotropium + olodaterol Respimat® FDC demonstrated superiority in lung function, QoL assessed by Saint George’s Respiratory Questionnaire, and breathlessness assessed by the Transition Dyspnea Index.
A cost-effectiveness model was developed to assess the long-term benefits and costs of tiotropium + olodaterol Respimat® FDC as compared with tiotropium, given tiotropium’s place as the most prescribed long-acting bronchodilator for COPD. Understanding of disease progression has evolved over time, thus raising the need for new model structures to more accurately predict outcomes in COPD patients compared with previous published models. For instance, when analysing long-term patient data [Tantucci and Modina, 2012; Tashkin and Ferguson, 2013] it has been shown that lung function decline is faster in the less severe patients while it slows as severity worsens. When it comes to the modelling of exacerbations, ECLIPSE data also showed that the most important predictor of an exacerbation are previous ones, followed by lung function [Hurst et al. 2010] rather than lung function alone.
This article describes a newly developed cost-effectiveness model using an individual patient-level approach to assess tiotropium + olodaterol Respimat® FDC compared with tiotropium in the Italian health care setting. The model reflects the current understanding of COPD and was developed to inform healthcare decision-makers on the long-term effectiveness and value for money of tiotropium + olodaterol Respimat® FDC.
Methods
Previous COPD models
Given the chronic nature of COPD and recurrent exacerbations, previous COPD models have been based around a Markov model structure [Hoogendoorn et al. 2014]. In a Markov model, the current status of a patient is described using a discrete health state for a fixed cycle of time, during which patients accrue the costs and benefits associated within this health state. Most previous models have utilized a cohort approach whereby patients were distributed to health states following Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations which classify COPD severity as GOLD stages I, II, III and IV. In these models, disease severity based on lung function is assessed after the initial improvement following treatment initiation. Commonly, a fixed rate of lung function decline is used to estimate a transition probability matrix that is then applied to obtain the posterior distribution to severity stages. The assumptions on long-term lung function are supported by clinical trial results assessing bronchodilator treatments, which have shown that after initial lung function improvement, annual decline in lung function does not differ significantly between treatments [Tashkin and Ferguson, 2013; US Food and Drug Administration, 2015; European Medicines Agency, 2016]. Exacerbations risk, utility values, mortality and costs are then estimated by GOLD stage [Oostenbrink et al. 2005; Rutten-Van Molken et al. 2007; Price et al. 2011, 2013; Ariza et al. 2012].
Markov model structures are most appropriate when each health state can sufficiently represent the potential patient heterogeneity within these states, and the ECLIPSE study has highlighted the substantial heterogeneity of COPD patients [Vestbo et al. 2008]. For example, two COPD patients classified to a GOLD stage at baseline may initiate treatment with lung function values at extreme ends of the range defining that GOLD stage. When assuming a constant annual decline in lung function following the initial improvement, the time at which these two patients progress to the next severe disease stage may differ significantly, whereas Markov models would overlook these differences in progression. Furthermore, the memoryless feature of Markov models is only justified when the risk of experiencing an event (e.g. exacerbation) in future cycles is dependent on the current health state, and independent of disease history (e.g. the number or severity of events in past cycles) [Drummond et al. 1999].
Based on the review of previous COPD modelling methods, the new model, assessing the cost-effectiveness of tiotropium + olodaterol Respimat® FDC, integrated several aspects of the previous models while also modifying elements common to the model structures of these models. Model development adhered to ISPOR Modelling Good Research Practices on model conceptualization [Roberts et al. 2012] and the development of state-transition models [Siebert et al. 2012]. In particular, the conceptualization of the model structure was determined by the biological understanding of COPD and was further informed by expert consultation to convert the conceptualization into an appropriate model structure, as recommended by the guidelines.
Lung function improvement and decline
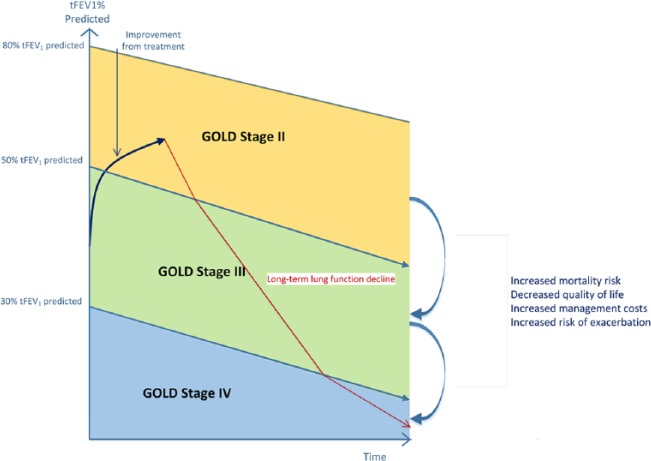
Rather than classifying a cohort of patients into discrete health states using a distribution of GOLD stages, outcomes were modelled based on the trough FEV1 (tFEV1) of all patients included in the tiotropium + olodaterol Respimat® FDC and tiotropium cohorts, based on patient-level data from the TOnado trials (see Table 1). Individual patient tFEV1 was tracked at baseline and at clinical visits through the 52-week trial duration, during which tFEV1 could improve from baseline (Figure 1). Beyond 52 weeks, tFEV1 was modelled assuming a constant linear decline by disease stage reported for the tiotropium arm in the UPLIFT trial: GOLD II: 37.1 ml/year; GOLD III: 30.7 ml/year; GOLD IV: 24.2 ml/year (Table 2) [Tantucci and Modina, 2012].
Table 1.
Baseline characteristics.
| Tiotropium 5 µg | Tiotropium + olodaterol Respimat®
FDC 5/5 μg |
|
|---|---|---|
| Participants | 1033 | 1029 |
| Male | 755 (73.1) | 733 (71.2) |
| Pre-bronchodilator FEV1 ml | 1200 ± 504 | 1180 ± 493 |
| Post-bronchodilator FEV1 ml | 1370 ± 521 | 1344 ± 505 |
| GOLD II | 517 (50.0) | 508 (48.8) |
| GOLD III | 347 (37.5) | 408 (39.7) |
| GOLD IV | 128 (12.4) | 119 (11.6) |
FDC, fixed-dose combination; FEV1, forced expiratory volume in one second; GOLD, Global Initiative for Chronic Obstructive Lung Disease
Figure 1.
Model structure.
GOLD, Global Initiative for Chronic Obstructive Lung Disease; tFEV1, trough forced expiratory volume in one second.
Table 2.
Clinical inputs assessed by GOLD stage.
| Annual tFEV1 decline | Utility value | |
|---|---|---|
| GOLD II | 37.1 ml | 0.787 (0.771–0.802) |
| GOLD III | 30.7 ml | 0.750 (0.731–0.768) |
| GOLD IV | 24.2 ml | 0.647 (0.590–0.0695) |
GOLD, Global Initiative for Chronic Obstructive Lung Disease; tFEV1, trough forced expiratory volume in one second.
Classification of disease severity
In each cycle, tFEV1 was converted to a post-bronchodilator value to calculate percentage FEV1 predicted to classify patients to different disease stages. A conversion factor of 1.16 was derived by comparing pre- to post-bronchodilator FEV1 at baseline of all patients in the TOnado trials. The GOLD 2007 criteria was then used for severity assessment defining GOLD II as 50% ⩽ FEV1 predicted < 80%; GOLD III as 30% ⩽ FEV1 predicted < 50%; and GOLD IV as FEV1 predicted < 30%, consistent with the criteria used in TOnado. The classification of patient-level FEV1 predicted values into GOLD stages enabled the modelling of clinical outcomes (lung function decline, mortality, QoL) as well as costs in each cycle for individual patients.
Exacerbation risk
While some previously published models in COPD have estimated the risk of both severe and moderate exacerbations by both treatment and GOLD stage, [Ariza et al. 2012; Price et al. 2013] others have estimated overall exacerbation risk by treatment only [Oostenbrink et al. 2005; Price et al. 2011] or estimated the proportion of severe to nonsevere exacerbations by GOLD stage [Hoogendoorn et al. 2014]. These approaches come with limitations, in that the estimated probability of exacerbation is not affected by past experience of exacerbation. A recent history of exacerbations has been shown to be the most reliable predictor for future exacerbations [Donaldson and Wedzicha, 2006; Tashkin and Ferguson, 2013].
In the current model, monthly exacerbation risk was estimated based on a random-effects logistic regression analysis of patient-level data of exacerbation history in the 4-year UPLIFT clinical trial which compared treatment with tiotropium to placebo. Using data from the tiotropium treatment group (given it was common to both the UPLIFT and TOnado trials), the dichotomous outcomes of moderate exacerbation and hospitalization due to severe exacerbation within a month were estimated. A COPD exacerbation was defined as a complex of lower respiratory events/symptoms (an increase or new onset of symptoms) relating to the underlying COPD, with a duration of 3 days or more. Moderate exacerbations were those that required antibiotics or systemic steroids without hospitalization and severe ones were those requiring hospitalization The explanatory variables in the regression analyses included number of moderate exacerbations in the previous 12 months (moving annual mean), number of hospitalizations due to severe exacerbation in the previous 12 months (moving annual mean), FEV1% predicted (measurement closest to the beginning of a month), and age. Results of the logistic regression analysis for both moderate and severe exacerbation risk are reported in Table 3, which also includes the random intercept distributions for each analysis used to make predictions of moderate and severe exacerbations.
Table 3.
Exacerbation risk logistic regression analysis.
| Moderate exacerbation risk logistic regression analysis | ||||||
|---|---|---|---|---|---|---|
| Coeff | SEM | Z | p >|z| | 95% CI | ||
| Moderate exacerbation in previous year | 0.2962704 | 0.0328924 | 9.01 | 0.000 | 0.2318024 | 0.3607383 |
| Hospitalization in previous year | 0.0421766 | 0.040818 | 1.03 | 0.301 | −0.0378252 | 0.1221784 |
| FEV1% predicted | −0.0120495 | 0.0016381 | −7.36 | 0.000 | −0.0152602 | −0.0088388 |
| Age in months | 0.000375 | 0.0002307 | 1.63 | 0.104 | −0.0000772 | 0.0008272 |
| Constant | −3.2075 | 0.1962957 | −16.34 | 0.000 | −3.592233 | −2.822768 |
| Random intercept | 0.6296274 | 0.0589371 | 0.5240898 | 0.7564174 | ||
| Severe exacerbation risk logistic regression analysis | ||||||
| Moderate exacerbation in previous year | 0.1723609 | 0.0345583 | 4.99 | 0.000 | 0.1046279 | 0.2400939 |
| Hospitalization in previous year | 0.3673385 | 0.0594212 | 6.18 | 0.000 | 0.2508752 | 0.4838019 |
| FEV1% predicted | −0.0506315 | 0.0035473 | −14.27 | 0.000 | −0.0575841 | −0.043679 |
| Age in months | 0.002943 | 0.0004814 | 6.11 | 0.000 | 0.0019993 | 0.0038866 |
| Constant | −5.272451 | 0.4052518 | −13.01 | 0.000 | −6.06673 | −4.478172 |
| Random intercept | 1.115954 | 0.0860875 | 0.959362 | 1.298106 | ||
CI, confidence interval; GOLD, Global Initiative for Chronic Obstructive Lung Disease; FEV1, forced expiratory volume in one second; SEM, standard error of the mean.
Moderate exacerbations in the previous year was not only a significant predictor, but a strongly significant predictor of future moderate exacerbation risk (p > 0.000). The tFEV1% predicted was also a statistically significant explanatory variable of future moderate exacerbation risk. In the severe exacerbation regression analysis, all explanatory variables included were statistically significant. Patients in the model were assumed to experience only one exacerbation (either moderate or severe) per 1-month cycle.
Mortality
Mortality was estimated based on two sources: a large trial of COPD patients, in which mortality was the primary outcome [Wise et al. 2013] and national statistics reporting mortality risk in the general population [Istat.It, 2015]. All-cause mortality was estimated from an analysis of TIOSPIR trial data, an international randomized controlled trial including 17,135 patients with COPD, which compared the safety and efficacy of tiotropium at three different doses using two types of inhalers [Wise et al. 2013]. The risk of death at 1 year in TIOSPIR was estimated for nine subgroups: GOLD stages II–IV combined with the age groups of (a) <60 years, (b) 60 ⩽ age < 70 years, and (c) ⩾70 years. The probabilities of death were compared with the mortality in the Italian population for the same age groups. To ensure an equivalent comparison, age group mortality rates in the Italian general population were based on the weighted average mortality using the male–female split (males = 71.1% across all age groups) and age distribution within age groups (mean age of 65.0; SD 9.1, assuming a normal distribution) from the TIOSPIR trial [Wise et al. 2013]. The risks of death in TIOSPIR and Italian population age groups were compared and standard mortality ratios (SMRs) were estimated for all nine subgroups (Table 4). Mortality was not adjusted by the incidence of exacerbations to prevent double counting.
Table 4.
COPD-related mortality risk (pooled) by age and severity stage.
| Patient subgroup | 365-day mortality in TIOSPIR | SMR compared with general population in Italy | |
|---|---|---|---|
| Age < 60 | GOLD Stage I–II | 0.437% | 1.15 |
| GOLD Stage III | 1.44% | 3.80 | |
| GOLD Stage IV | 3.93% | 10.37 | |
| 60 ⩽ age < 70 | GOLD Stage I–II | 1.37% | 1.37 |
| GOLD Stage III | 2.13% | 2.13 | |
| GOLD Stage IV | 4.56% | 4.56 | |
| Age ⩾70 | GOLD Stage I–II | 3.06% | 0.88 |
| GOLD Stage III | 4.00% | 1.15 | |
| GOLD Stage IV | 8.43% | 2.41 |
COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SMR, standard mortality ratio.
Costs
The base case analysis took the perspective of the Italian National Health Service including direct medical costs, where costs were expressed in Euros in 2015. Cost of bronchodilators and other medications, routine management, and costs of treatment of exacerbations were included in the analysis (Table 5). Tiotropium + olodaterol Respimat® FDC was not marketed in Italy at the time of the analysis, thus we assumed parity price to other LAMA/LABA FDCs; both this price and Tiotropium’s price were obtained from the AIFA database [Agenxia Italiana Del Farmaco, 2015]. Costs of routine management included prescription costs (excluding bronchodilators) [Koleva et al. 2007] and nonprescription drug costs [Zaniolo et al. 2012] which were derived from Italian observational studies. Costs of exacerbation treatment were obtained from an Italian observational study conducted in 25 hospitals in Italy in order to estimate costs associated with COPD exacerbations in Italy [Lucioni et al. 2005]. Costs of moderate and severe exacerbations were applied on occurrence.
Table 5.
Cost inputs.
| Costs (2015 €) | |
|---|---|
| Medication costs per month | |
| Tiotropium + olodaterol Respimat® FDC 5/5 µg | 43.95 |
| Tiotropium 5 µg | 31.23 |
| Routine management costs per month | |
| GOLD Stage II | 123.87 |
| GOLD Stage III | 135.65 |
| GOLD Stage IV | 199.03 |
| Exacerbation treatment costs per episode | |
| Severe exacerbation | 1730.78 |
| Moderate exacerbation | 400.78 |
FDC, fixed-dose combination; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Quality of life
QoL was also assessed based on GOLD stage (Table 2) [Rutten-Van Molken et al. 2006] and the incidence of exacerbations, with utility multipliers (moderate: 0.85; severe: 0.50) [Paterson et al. 2000] applied on occurrence for the duration of one cycle [Spencer et al. 2003].
Base case analysis
Each individual patient progressed through the model over time and their clinical and economic outcomes were aggregated by treatment cohort. Mean outcomes were calculated for each patient subgroup and stratified in terms of 10-year age band, GOLD stage (evolving over time) and sex. These average values were then weighted by the baseline distribution across these subgroups. Subgroup distribution was developed as a modifiable input to enable validation of the model results with those of other long-term clinical trials.
The base case analysis was run over a time horizon of 15 years, with direct medical costs and COPD health outcomes discounted at a rate of 3.0% according to the recommendations for health economic evaluations in Italy [Fattore, 2009].
Validation
As a validation exercise, the model was run over a 1-year time horizon to compare the model’s estimate of exacerbation incidence and all-cause mortality with what was reported in the TOnado trial. A scenario analysis was also conducted in which the model was run using a 30 ml constant annual rate of lung decline across GOLD stages as in previous COPD models, rather than the GOLD-stage specific rates of decline to assess the impact on results.
Parameter uncertainty
Parameter uncertainty was modelled in several ways. For lung function improvement, patient-level data from the TOnado trials through 52 weeks were incorporated directly at a patient level and so no uncertainty around this parameter was modelled in sensitivity analysis. All other inputs, relating to lung function decline, exacerbation and mortality risk, as well as costs and QoL, were varied in probabilistic sensitivity analyses (PSAs) to explore the impact of parameter uncertainty on cost-effectiveness results. Costs were varied in the PSAs based on gamma distributions, lung decline and utility values based on beta distributions, SMRs based on lognormal distributions, and logistic regression parameters for exacerbation risk based on normal distributions.
Results
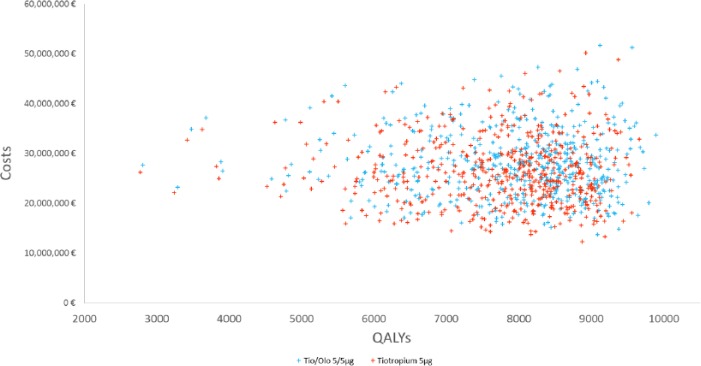
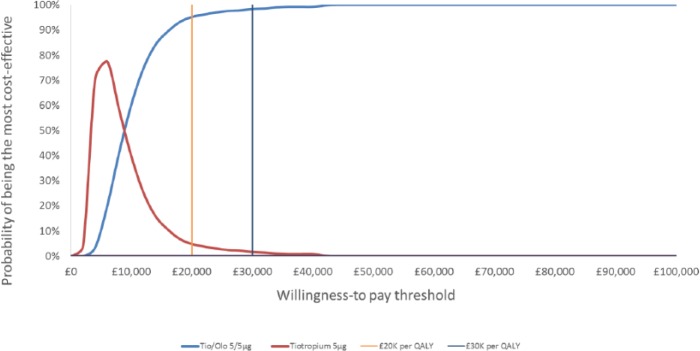
The model was run to assess the cost-effectiveness of tiotropium + olodaterol Respimat® FDC versus tiotropium over a time horizon of 15 years with results presented in Table 6 in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) for economic evaluations [Husereau et al. 2013]. Comparing total costs and quality-adjusted life years (QALYs) per patient in the tiotropium + olodaterol Respimat® FDC treatment cohort over 15 years with the tiotropium cohort showed incremental costs of €1,166.95 per patient, incremental QALYs of 0.16 per patient, and an incremental cost-effectiveness ratio (ICER) of €7,518.49 per additional QALY over 15 years. PSAs showed tiotropium + olodaterol Respimat® FDC to be the more cost-effective treatment in 95.2% and 98.4% of 500 simulations at thresholds of €20,000 and €30,000 per QALY respectively [See PSA scatterplot results in Figure 2 and cost-effectiveness acceptability curves (CEAC) in Figure 3]. In deterministic sensitivity analysis (DSA), the ICER ranged from £2,905 to £9,621, and the severe exacerbation risk equation was the most sensitive parameter. The base case ICER was most impacted at shorter time horizons, with an ICER of £18,180 at 10 years.
Table 6.
Cost-effectiveness results at 15 years.
| Discounted costs per patient |
Discounted QALYs per patient |
|
|---|---|---|
| Tiotropium + olodaterol Respimat® FDC 5/5 µg | €27,597.77 | 7.43 |
| Tiotropium 5 µg | € 26,430.82 | 7.27 |
| ∆ Costs | € 1,166.95 | |
| ∆ QALYs | 0.16 | |
| ICER | € 7,518.46 per QALY gained | |
FDC, fixed-dose combination; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.
Figure 2.
PSA scatterplot.
Olo, olodaterol; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life years; Tio, tiotropium.
Figure 3.
Cost-effectiveness acceptability curve.
Olo, olodaterol; QALY, quality-adjusted life years; Tio, tiotropium.
The more favourable distribution over time of patients to the less severe GOLD stages based on TOnado data was reflected in the higher mean life-years per patient with tiotropium + olodaterol Respimat® FDC versus tiotropium (12.24 versus 12.07 respectively), as shown in Table 7. Similarly, the mean number of exacerbation-free months and QALYs per patient (11.36 versus 11.32; 7.44 versus 7.23) were higher and the number of moderate and severe exacerbations per patient-year was lower with tiotropium + olodaterol Respimat® FDC compared with tiotropium (0.411 versus 0.415; 0.21 versus 0.24 respectively).
Table 7.
Clinical outcomes at 15 years: base case.
| Life-years per patient | Exacerbation-free months per patient per year | Nonsevere exacerbations per patient-year | Severe exacerbations per patient-year | |
|---|---|---|---|---|
| Tiotropium + olodaterol Respimat® FDC 5/5 µg | 12.24 | 11.36 | 0.411 | 0.21 |
| Tiotropium 5 µg | 12.07 | 11.32 | 0.415 | 0.24 |
FDC, fixed-dose combination.
Table 8.
Clinical outcomes versus TOnado trial.
| Total exacerbations per patient-year | All-cause mortality | |
|---|---|---|
| TOnado, 1 year | 0.509 | 1.6% |
| Model, 1 year | 0.462 | 3.2% |
To validate the model’s results, the model was run for a period of 1 year to compare with results from the TOnado trial. Estimating the number of total exacerbations per patient-year using the logistic regression equations derived from UPLIFT trial data, the mean number of total exacerbations in the model fell within the confidence intervals of the results reported in TOnado [0.462 versus 0.509 per patient (95% CI 0.432, 0.599)] for the tiotropium arm. All-cause mortality estimated in the model for the tiotropium arm was slightly higher than rates reported in TOnado at 1 year (3.2% versus 1.7%).
As a scenario analysis, the model was run using a 30 ml constant annual rate of lung decline across GOLD stages, consistent with a previous COPD model deriving lung function decline from UPLIFT [Tantucci and Modina, 2012]. The mean number of moderate exacerbations per patient-year in the tiotropium arm was only slightly changed (0.402 versus 0.405) while mean severe exacerbations were unchanged. All-cause mortality rates at 15 years were only marginally impacted (tiotropium + olodaterol Respimat® FDC: 45.3%; tiotropium: 46.8%) compared with the base case (tiotropium + olodaterol Respimat® FDC: 45.6%; tiotropium: 47.1%).
Discussion
The cost-effectiveness model incorporated patient-level data from over 2000 participants of the TOnado trials. This more granular approach enabled a reflection of the heterogeneity in the level of short-term lung function improvement following bronchodilator treatment initiation. Treatment benefit of tiotropium + olodaterol Respimat® FDC was modelled based on the lung function improvement observed in the treatment cohort of the TOnado trial, and the benefit in terms of exacerbation risk was modelled indirectly. Instead of estimating exacerbation incidence merely by GOLD stage, the current approach accounted for patient data on age and lung function over time as well as history of recent moderate and severe exacerbations. Rather than being mere indicators of COPD severity, the incidence of past exacerbations was used to predict future exacerbations, and by preventing an exacerbation now, future exacerbations are thus prevented.
The lung function treatment benefit reported for patients treated with tiotropium + olodaterol Respimat® FDC in the 52-week TOnado trials translated into lower risk of exacerbations and lower mortality. The ICER of €7,518/QALY obtained in the comparison of tiotropium + olodaterol Respimat® FDC versus tiotropium is well below commonly used cost-effectiveness thresholds. These results changed only marginally when parameter values were varied in the DSA for inputs related to costs, utilities, mortality and moderate exacerbations (1–6% change versus base case ICER). The results were most sensitive to variations to the parameters of the severe exacerbation risk equation (28–61% change versus base case ICER).
The modelling methods improved upon the approaches taken in previous models, which were based on a strict, cohort-based Markov structure and relied on a memoryless estimation of transition probabilities. Furthering the objective of more appropriately modelling disease progression by reflecting patient heterogeneity at baseline and post-improvement, the long-term lung function decline was applied based on COPD severity rather than using a single rate of decline for all patients. Excess mortality rates took into account data on age and GOLD stage for individual patients to more accurately estimate risk of death during the time horizon.
The consistency of the model results was validated using the ISPOR-SMDM good research practices on model transparency and validation [Eddy et al. 2012]. This validation involved tests of face validity evaluating model structure, data sources, assumptions and results. In addition, validation was expanded to include external validity, comparing model results with those observed in other clinical trials. When comparing the model results to what was observed in the TOnado trial, overall the model predicted similar rates of all-cause mortality to what has been observed in the trial, despite only using Italian life tables to estimate mortality rather than reflecting the multiple nationalities of TIOSPIR patients. Rate of exacerbations per patient-year were also closely aligned between the model and what observed in the TOnado trial.
Modelling over a time horizon of 15 years introduces additional uncertainty into the analysis given the assumption of indefinite use of bronchodilators. This assumption did not allow the model to account for new treatments entering the market, which might impact outcomes over the long term, although this is inherent in most cost-effectiveness analysis modelling over the long term. However, a time horizon extending to 15 years is reasonable given that COPD is a chronic condition for which patients receive life-long treatment. The mean age of patients in the TOnado trials was 64 years old and so the 15-year time horizon could be expected to cover a relevant duration even for the most severe patients at baseline (GOLD IV) of which approximately 40% were still alive at 15 years.
Furthermore, treatment switching due to specified stopping rules (e.g. following a 4-point drop on the St George’s Respiratory Questionnaire (SGRQ)) were not included in the analysis, which may have affected the outcomes predicted in the model. The model focussed exclusively on lung function following treatment initiation without taking into account other treatment effects, which can be both beneficial and harmful and could impact some clinically-relevant outcomes. Future models would benefit from including available data on treatment adherence and its impact on clinical outcomes as well as costs.
Given the focus on lung function improvement as the primary outcome in previous economic evaluations, the patient-level approach taken in the current model based on two large, well-matched patient cohorts demonstrated a feasible method of reflecting the heterogeneity of this improvement.
Conclusion
The cost-effectiveness model incorporated patient-level lung function improvement data observed in a recent clinical trial of tiotropium + olodaterol Respimat® FDC, a newly developed combination bronchodilator treatment in COPD. Lung function improvement assessed at clinical trial visits was used in combination with individual patient characteristics and history of exacerbations to estimate both moderate and severe exacerbations and mortality based on statistical analyses of large international clinical trial data in COPD. The analysis demonstrated that in the long term when comparing tiotropium + olodaterol Respimat® FDC with tiotropium alone, the number of moderate and severe exacerbations, and the number of patients in the most severe GOLD stages was reduced, thus increasing the number of life-years and QALYs. These benefits were achieved at a cost below cost-effectiveness thresholds used in Italian health care decision-making [Fattore, 2009].
In the analysis, the benefits observed in a short-term clinical trial impacted long-term outcomes, a finding supported by the current understanding of COPD. Future cost-effectiveness models would benefit from using the current approach, which robustly reflects this current understanding of COPD.
Footnotes
Funding: This study was funded by Boehringer Ingelheim GmbH.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Carl Selya-Hammer, Amaris Consulting, 188 York Way, 2nd Floor, London, N7 9AS, UK.
Nuria Gonzalez-Rojas Guix, Boehringer Ingelheim GmbH., Ingelheim, Germany.
Michael Baldwin, Boehringer Ingelheim GmbH., Ingelheim, Germany.
Andrew Ternouth, Boehringer Ingelheim Ltd., Ellesfield Avenue, Bracknell, Berkshire, UK.
Marc Miravitlles, Pneumology Department, University Hospital Vall d’Hebron, Ciber of Respiratory Diseases (CIBERES), Barcelona, Spain.
Maureen Rutten-van Mölken, University of Rotterdam, iMTA, Rotterdam, The Netherlands.
Lucas M.A. Goosens, University of Rotterdam, iMTA, Rotterdam, The Netherlands
Nasuh Buyukkaramikli, University of Rotterdam, iMTA, Rotterdam, The Netherlands.
Valentina Acciai, Boehringer Ingelheim SpA., Milan, Italy.
References
- Agenxia Italiana Del Farmaco (2015) Tabelle farmaci di classe A e H al 15/09/2015, Vol. 2016 http://www.agenziafarmaco.gov.it/it/content/tabelle-farmaci-di-classe-e-h-al-15092015 [Google Scholar]
- Ariza J., Thuresson P., Machnicki G., Mungapen L., Kraemer M., Asukai Y., et al. (2012) The cost-effectiveness and budget impact of introducing indacaterol into the Colombian health system. Value in Health Regional Issues 1: 165–171. [DOI] [PubMed] [Google Scholar]
- Burge S., Wedzicha J. (2003) COPD exacerbations: definitions and classifications. Eur Respir J Suppl 41: 46s–53s. [DOI] [PubMed] [Google Scholar]
- Drummond M.F., Sculpher M.J., Torrance G.W., O’Brien B.J., Stoddart G.L. (1997) Methods For The Economic Evaluation Of Health Care Programmes. 2nd edn Oxford: Oxford University Press. [Google Scholar]
- Donaldson G., Wedzicha J. (2006) COPD exacerbations. 1: epidemiology. Thorax 61:164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy D., Hollingworth W., Caro J., Tsevat J., McDonald K., Wong J., et al. (2012) Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Value Health 15: 843–850. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency (2016) Spiolto Respimat 2.5 microgram/2.5 microgram, inhalation solution. Vol. 2016 https://www.medicines.org.uk/emc/medicine/30495 [Google Scholar]
- Fattore G. (2009) Proposta di linee guida per la valutazione economica degli interventi sanitari in Italia. Pharmaco Economics Italian Research Articles 11: 83–93. http://link.springer.com/article/10.1007%2FBF03320660#page-2 [Google Scholar]
- Hoogendoorn M., Feenstra T., Asukai Y., Borg S., Hansen R., Jansson S., et al. (2014) Cost-effectiveness models for chronic obstructive pulmonary disease: cross-model comparison of hypothetical treatment scenarios. Value Health 17: 525–536. [DOI] [PubMed] [Google Scholar]
- Hurst J., Vestbo J., Anzueto A., Locantore N., Mullerova H., Tal-Singer R., et al. (2010) Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 363: 1128–1138. [DOI] [PubMed] [Google Scholar]
- Husereau D., Drummond M., Petrou S., Carswell C., Moher D., Greenberg D., et al. (2013) Consolidated Health Economic Evaluation Reporting Standards (CHEERS) - explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 16: 231–250. [DOI] [PubMed] [Google Scholar]
- Istat.It. (2015) Popolazione e Famiglie, Mortalita Vol. 2016. http://www.istat.it/en/population-and-households (accessed 24 June 2016).
- Koleva D., Motterlini N., Banfi P., Garattini L. Study Group B.I.C. (2007) Healthcare costs of COPD in Italian referral centres: a prospective study. Respir Med 101: 2312–2320. [DOI] [PubMed] [Google Scholar]
- Lucioni D., Donner C., De Benedetto F., Lusuardi M., Mazzi S., Paggiaro P., et al. (2005) I costi della broncopneumopatia cronica ostruttiva: la fase prospettica dello studio ice (Italian costs for exacerbations in COPD). Pharmacoeconomics Italian research articles 7: 119–134. [Google Scholar]
- Oostenbrink J., Rutten-Van Molken M., Monz B., Fitzgerald J. (2005) Probabilistic Markov model to assess the cost-effectiveness of bronchodilator therapy in COPD patients in different countries. Value Health 8: 32–46. [DOI] [PubMed] [Google Scholar]
- Paterson C., Langan C., Mckaig G., Anderson P., Maclaine G., Rose L., et al. (2000) Assessing patient outcomes in acute exacerbations of chronic bronchitis: the measure your medical outcome profile (MYMOP), Medical Outcomes Study 6-Item General Health Survey (MOS-6A) and EUROQOL (EQ-5D). Qual Life Res 9: 521–527. [DOI] [PubMed] [Google Scholar]
- Price D., Asukai Y., Ananthapavan J., Malcolm B., Radwan A., Keyzor I. (2013) A UK-based cost-utility analysis of indacaterol, a once-daily maintenance bronchodilator for patients with COPD, using real world evidence on resource use. Appl Health Econ Health Policy 11: 259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D., Gray A., Gale R., Asukai Y., Mungapen L., Lloyd A., et al. (2011) Cost-utility analysis of indacaterol in Germany: a once-daily maintenance bronchodilator for patients with COPD. Respir Med 105: 1635–1647. [DOI] [PubMed] [Google Scholar]
- Roberts M., Russell L., Paltiel A., Chambers M., McEwan P., Krahn M., et al. (2012) Conceptualizing a model: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-2. Value Health 15: 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten-Van Molken M., Oostenbrink J., Miravitlles M., Monz B. (2007) Modelling the 5-year cost effectiveness of tiotropium, salmeterol and ipratropium for the treatment of chronic obstructive pulmonary disease in Spain. Eur J Health Econ 8: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten-Van Molken M., Oostenbrink J., Tashkin D., Burkhart D., Monz B.U. (2006) Does quality of life of COPD patients as measured by the generic EUROQOL five-dimension questionnaire differentiate between COPD severity stages? Chest 130: 1117–1128. [DOI] [PubMed] [Google Scholar]
- Siebert U., Alagoz O., Bayoumi A., Jahn B., Owens D., Cohen D., et al. (2012) State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-3. Value Health 15: 812–820. [DOI] [PubMed] [Google Scholar]
- Spencer S., Jones P., Group G. (2003) Time course of recovery of health status following an infective exacerbation of chronic bronchitis. Thorax 58: 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantucci C., Modina D. (2012) Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis 7: 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashkin D., Ferguson G. (2013) Combination bronchodilator therapy in the management of chronic obstructive pulmonary disease. Respir Res 14: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashkin D., Li N., Halpin D., Kleerup E., Decramer M., Celli B., et al. (2013) Annual rates of change in pre- versus post-bronchodilator FEV1 and FVC over 4 years in moderate to very severe COPD. Respir Med 107: 1904–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STIOLTO™ RESPIMAT® (tiotropium bromide and olodaterol) inhalation spray, for oral inhalation use Initial U.S. Approval: 2015. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206756s001lbl.pdf
- Vestbo J., Anderson W., Coxson H., Crim C., Dawber F., Edwards L., et al. (2008) Evaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE). Eur Respir J 31: 869–873. [DOI] [PubMed] [Google Scholar]
- Wise R., Anzueto A., Cotton D., Dahl R., Devins T., Disse B., et al. (2013) Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med 369: 1491–1501. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2011) Chronic obstructive pulmonary disease (COPD). Fact sheet No. 315. Available from: http://www.who.int/mediacentre/factsheets/fs315/en
- Zaniolo O., Iannazzo S., Pradelli L., Miravitlles M. (2012) Pharmacoeconomic evaluation of tiotropium bromide in the long-term treatment of chronic obstructive pulmonary disease (COPD) in Italy. Eur J Health Econ 13: 71–80. [DOI] [PubMed] [Google Scholar]