Abstract
Progress in the treatment of patients with advanced stage squamous cell non-small cell lung cancer (NSCLC) has been limited. An improvement in the understanding of tumor immunosurveillance has resulted in the development of the immune checkpoint inhibitors such as nivolumab. Nivolumab (Opdivo®), a human immunoglobulin (Ig)G4 anti-programmed death (PD)-1 monoclonal antibody, was the first PD-1 inhibitor approved in the treatment of patients with advanced stage squamous cell NSCLC following platinum-based chemotherapy. CHECKMATE 017, a randomized phase III study of second-line nivolumab versus docetaxel, significantly improved overall survival (OS), progression-free survival (PFS), patient reported outcomes and the safety and tolerability favored patients treated with nivolumab. The ligand (PD-L1) expression did not predict for outcome. In this paper, we review the role of nivolumab in the treatment of NSCLC with particular attention on recent studies, ongoing combination studies, toxicity profile, current and potential predictive biomarkers.
Keywords: anti-programmed death-1, immunotherapy, nivolumab, non-small cell lung cancer
Introduction
Although the field of immune-oncology has been ongoing for more than a decade, only more recently has it generated great interest with the efficacy of immune checkpoint inhibitors (ICI). Ipilimumab, a cytotoxic T-cell Lymphocyte Antigen 4 (CTLA4) inhibitor, was amongst the first ICI to be approved by the United States (US) Food and Drug Administration (FDA), and this was subsequently followed by other ICIs such as antibodies against both programmed-cell death-protein 1 (PD-1) and its ligand (PD-L1). The ICIs have been shown to be effective in a range of hematological and solid tumors, including melanomas, non-small cell lung cancer (NSCLC), renal cell carcinoma and lymphomas [Sundar et al. 2015]. Nivolumab (BMS-936558), an anti-PD-1 antibody, was the first in its class to be approved by the US FDA in the second-line treatment of patients with advanced stage NSCLC (both squamous [Brahmer et al. 2015] and nonsquamous [Borghaei et al. 2015] histologies) after progression on platinum-based chemotherapy. Nivolumab is a human immunoglobulin (Ig)G4 anti-PD-1 monoclonal antibody. It acts by binding to PD-1, an inhibitory co-receptor expressed on antigen-activated T cells, thus preventing interaction with PD-L1, resulting in the loss of inhibitory signals in T cells, and tumor recognition by cytotoxic T cells and thus restoring T-cell function (Figure 1) [Wang et al. 2014]. In this article, we review the current standard of care for treatment of squamous NSCLC, the role of nivolumab in its treatment, toxicities and management of treatment-related adverse events.
Figure 1.
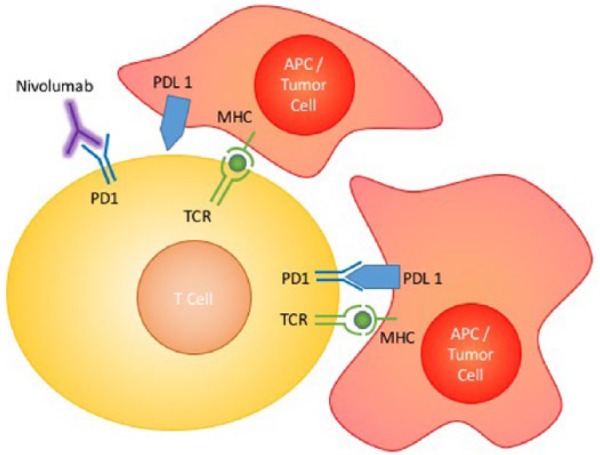
Mechanism of action of nivolumab.
The TCR signaling pathway is a major component of immune defense against tumors. Under normal circumstances, TCR recognition of the MHC on APCs will trigger a cascade of events causing activation of T cells and thus immune response against tumor cells. This signal is inhibited by tumor cells with activation of the PD-1/PD-L1 inhibitor co-signaling pathway, which ameliorates T-cell activation. Nivolumab acts by binding onto the PD-L1 on T cells, thus preventing inhibitor co-signaling and restoring T-cell activation.
APC, antigen presenting cell; MHC, major histocompatibility complex; PD-1, programmed death-1; PD-L1, programmed death-1 ligand; TCR, T-cell receptor.
Treatment of squamous non-small cell lung cancer
Adenocarcinoma and squamous cell carcinoma (SCC) histologic subtypes comprise 60–70% and 10–20% of NSCLC, respectively [De la Cruz et al. 2011]. The survival of patients with adenocarcinoma has improved. Patients with NSCLC adenocarcinoma treated with agents targeting tumor-specific oncogenes had a median survival of 3.5 years [Kris et al. 2014]. However, unlike adenocarcinoma, therapeutic progress in SCC of the lung has lagged, in part due to the paucity of relevant oncogenic drivers that can inform treatment. This evidence suggests that new drugs for metastatic squamous NSCLC are needed. The treatment of nonsquamous NSCLC has seen significant progress in the past decade with the identification of molecular subtypes [Kumarakulasinghe et al. 2015]. Data from the Cancer Genome Atlas Research Network (TCGA) showed significant differences in genetic profiles between the two most common histological subtypes of lung cancer, adenocarcinoma and SCC [Cancer Genome Atlas Research, 2012, 2014]. Overall, 62% of lung adenocarcinomas harbored known activating mutations in driver oncogenes, including mutations in the epidermal growth factor receptor (EGFR) gene and echinoderm microtubule-associated protein-like 4 – anaplastic lymphoma kinase (EML4-ALK) and ROS1 gene fusion and the use of tyrosine kinase inhibitors (TKIs) towards target proteins [Cancer Genome Atlas Research, 2014]. However similar driver mutations are uncommonly observed in SCCs [Cancer Genome Atlas Research, 2012]. As such, platinum-based doublet chemotherapy, such as carboplatin/ paclitaxel or gemcitabine/ cisplatin has remained the mainstay of first-line treatment of squamous NSCLC [Ang et al. 2015]. Recently, the addition of necitumumab, a second-generation EGFR monoclonal antibody, showed significant improvement in progression-free survival (PFS) (5.7 versus 5.5 months, 95% CI 0.74–0.98, p = 0.02) and overall survival (OS) (11.5 versus 9.9 months, 95% CI 0.74–0.96, p = 0.01) when added to the platinum doublet of cisplatin-gemcitabine in patients with metastatic SCC lung [Thatcher et al. 2015]. Further analysis of correlation between EGFR expression by IHC scoring and FISH analysis suggest possible predictive values towards response, although this will need to be further validated. Nonetheless, necitumumab is the first drug that has been approved in first-line treatment for SCC lung since the use of platinum-based doublet therapy in the past decade.
Traditionally the median OS in patients treated with second-line therapy for advanced NSCLC is about 8 months, with docetaxel the most frequent chemotherapy of choice [Shepherd et al. 2000; Kim et al. 2008]. Nonetheless, recent pooled analysis from various second-line docetaxel studies have suggested that patients with squamous histology tend to do worse than those with nonsquamous histologies, amidst already dismal survival improvements [Torri et al. 2015]. In a phase III study of second-line docetaxel and ramucirumab versus docetaxel (REVEL study), OS favored the combination arm with an OS of 10.5 versus 9.1 months [hazard ratio (HR) 0.86, 95% CI 0.75–0.98]. In the 26% of patients with SCC, the HR for OS was 0.761 (95% CI 0.606–0.957) [Garon et al. 2014]. In a phase III study of docetaxel and nintedanib versus docetaxel, among patients with SCC lung (42%), the addition of nintedanib was associated with a PFS of HR 0.77 (95% CI 0.62–0.96), and an OS of HR 1.01 (95%CI 0.85–1.21, p = 0.891) [Reck et al. 2014]. In a phase III study of second-line afatinib versus erlotinib, the median PFS was 2.6 versus 1.9 months (HR 0.81, 95% CI 0.69–0.96, p = 0.0103), and OS was 7.92 versus 6.77 months (HR 0.808, 95% CI 0.691–0.946, p = 0.0077) [Soria et al. 2015]. In these studies, the survival benefit, whilst statistically significant, is only modest and novel therapeutic approaches are needed. The success of ICIs in melanoma has led to its use in other solid tumors including NSCLC.
Nivolumab in lung cancer
Nivolumab (Opdivo®, Bristol Myers Squibb, USA) was the first ICI to be approved by the US FDA in the treatment of SCC lung in March 2015, with its approval extended to lung cancers of nonsquamous histologies in October 2015 [Sundar et al. 2015]. In addition, nivolumab has been approved by the US FDA for the following indications: unresectable or metastatic melanoma, classical Hodgkin’s lymphoma after failure of more than two treatment lines, second-line treatment of advanced renal cell carcinoma, and in combination with ipilimumab, a CTLA-4 inhibitor, in metastatic melanoma irrespective of BRAF status.
Pembrolizumab (Keytruda®, Merck, USA), another anti-PD-1 agent, was approved by the US FDA in October 2015 for use in a second-line setting post platinum-based chemotherapy for patients with metastatic NSCLC whose tumors express PD-L1.
The first in-human studies of nivolumab carried out were basket trials carried out in refractory solid tumors, of which 15.4% patients (six out of 39) had NSCLC [Topalian et al. 2012]. Patients on the study received a single dose of nivolumab at doses ranging from 0.3–10 mg/kg with provision for a repeat dose at 3-month intervals for patients who derived clinical benefit. Although drug efficacy was not the primary endpoint in this phase I trial, significant tumor regression was seen in one of the six patients with NSCLC, despite the fact that lung cancers were not known to be classically immunogenic compared with tumors like renal cell carcinomas and melanomas. The drug was well tolerated and maximum tolerated dose (MTD) was not reached, with only one patient developing the serious adverse event (SAE) of inflammatory colitis [Brahmer et al. 2010].
Based on these results, a dose-expansion cohort of patients with NSCLC were enrolled into trial. Of these 129 patients enrolled, 54 patients (41.2%) had SCC lung. The overall response rate (ORR) was 17% across all cohorts, and median OS was 9.9 months, with median survival in the 3 mg/kg cohort performing best at 14.9 months [Gettinger et al. 2015]. This was similar for both patients with squamous and nonsquamous histology. PD-L1 expression status did not appear to predict for tumor response, and subgroup analysis suggest that better responses were seen in former and current smokers (ORR 30% in patients with smoking history of more than 5 pack-years versus 0% in patients with smoking history of 5 pack-years or less) [Gettinger et al. 2015a]. Based on the promising results, subsequent phase II studies with nivolumab at a dose of 3 mg/kg every 2 weeks were conducted.
In a phase II single-arm study (CHECKMATE 063), 117 patients with advanced stage squamous cell NSCLC were treated with nivolumab in third-line and beyond. The ORR was 14.5%, 26% of patients had stable disease and the 6-month and 12-month PFS was 25.9% and 20.0% [Rizvi et al. 2015]. These results were noteworthy given 65% of patients were previously treated with three or more lines of systemic therapy, and in 61% of patients, disease progression was the best response to the most recent treatment. Updated survival results presented recently reported an OS of 8.2 months and 1-year OS rate of 41% [Horn et al. 2015]. In a Japanese phase II study (ONO-4538-05), of patients with advanced stage SCC lung (n = 35) and nonsquamous lung cancer (n = 76) treated with nivolumab, the ORR was 25.7% in the SCC group. In addition, the disease control rate (DCR) of 54.3% and the PFS of 4.2 months. The OS was not reached [Nakagawa et al. 2015] (Table 1). In another phase II study conducted at community research sites (CHECKMATE 153, CA209-153) patients with advanced stage NSCLC were treated with nivolumab till progression or unacceptable toxicities compared with being treated for a maximum of 1 year, then discontinuation with an intention to restart nivolumab at progression. Of the 824 patients enrolled, 227 patients (28%) had a SCC histologic subtype. The ORR and stable disease at first assessment was 13% and 50%, respectively, in patients with squamous cell NSCLC [Hussein et al. 2015]. While the second study involved patients with both squamous and nonsquamous subtypes, both phase II studies supported that nivolumab was effective in patients with SCC lung compared with historical outcomes of patients on conventional chemotherapy. As such, further phase III trial was undertaken for patients with SCC lung.
Table 1.
Trials of nivolumab in SCC lung.
| Phase | Author | Line of treatment | Sample size | ORR (%) | PFS (months) (95% CI) | OS (months) (95% CI) | Limitations |
|---|---|---|---|---|---|---|---|
| I | Topalian NEJM2012 [Topalian et al. 2012], Gettinger JCO2015 [Gettinger et al. 2015] | Second line and beyond | 54 | 16.7 | NA | 9.2 (7.3–12.5) | Small sample size Multiple treatment lines |
| I | Gettinger ASCO2015 [Gettinger et al. 2015] | First line | 13 | 15 | NA | 18.2 (CI not reported) | Small sample size First line only |
| II (CHECKMATE 063) | Rizvi Lancet 2015 [Rizvi et al. 2015] | Third line and beyond | 117 | 14.5 | 1.9 (1.8–3.2) | 8.2 (6.1–10.9) | Heavily pretreated population, with 20.5% of patients having 4 or more lines of previous treatment |
| II (ONO-4538-050) | Nakagawa JTO2015 [Nakagawa et al. 2015] | Second line and beyond | 35 | 25.7 | 4.2 (1.5–7.1) | Not reached (12.4–not reached) | Preliminary results Small sample size Asian population Multiple treatment lines |
| II (CHECKMATE 153) | Hussein WLCC2015 [Hussein et al. 2015] | Second line or more | 145 | 13 | NA | NA | Preliminary results Study compared 1 year versus continuous nivolumab Multiple treatment lines |
| III (CHECKMATE 017) | Brahmer NEJM2015 [Brahmer et al. 2015] | Second line | 272 | 20 | 3.5 (2.1–4.9) | 9.2 (7.3–13.3) | Retrospective analysis of PD-L1 expression Compared against docetaxel whereas newer regimens such as docetaxel + ramucirumab is superior to docetaxel |
CI, confidence interval; NA, not available; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; SCC, squamous cell carcinoma.
In a phase III study of nivolumab versus docetaxel in 272 patients with advanced SCC lung (CHECKMATE 017), the study was terminated early on the recommendation of the independent data and safety monitoring committee as pre-specified interim analysis succeeded in showing an improvement in their primary endpoint of OS. The OS with nivolumab and docetaxel was 9.2 and 6.0 months, respectively (HR 0.59, 95% CI 0.44–0.79, p < 0.001) [Brahmer et al. 2015]. Furthermore, the ORR and PFS favored nivolumab (20% versus 9%, p = 0.008) and HR 0.63 (95% CI 0.48–0.83, p < 0.01), respectively (Table 1). Again PD-L1 expression was neither prognostic nor predictive of outcomes. Lung Cancer Symptom Scale (LCSS) assessment of patients showed that patients who responded to nivolumab appeared to have a more sustained period of symptom improvement compared with patients on docetaxel. Time to first disease deterioration measured by LSCC Global Health Related Quality of Life was longer in patients treated with nivolumab compared with patients treated with docetaxel (HR 0.58, 95% CI 0.39–0.86) [Gralla et al. 2015]. With the positive outcome of this study, the US FDA approved nivolumab for the treatment of advanced SCC lung with progression on or after platinum-based chemotherapy. Nonetheless, certain limitations remain, including the role of PD-L1 expression as a predictive marker, that is still an area of active investigation. While nivolumab is superior to docetaxel, the efficacy of nivolumab compared with other recent active combinations such as docetaxel with ramucirumab or nindetanib remains to be seen.
Current trials are being undertaken to look at the efficacy of nivolumab compared with platinum doublet treatment in a first-line setting. CHECKMATE026 included patients with both squamous and nonsquamous NSCLC, has completed accrual and early results may be made available by 2017. In the squamous cohort, patients were randomized to either nivolumab or platinum doublet (cisplatin or carboplatin combined with either gemcitabine or paclitaxel) with the option of crossover at disease progression (Table 2).
Table 2.
Combination studies of nivolumab in non-small cell lung cancer.
| Phase | Author | Line of treatment | Sample size | ORR (%) (range) | PFS (months) (range) | OS (months) (range) |
|---|---|---|---|---|---|---|
| I | Antonia [Antonia et al. 2014a] | First line with chemotherapy | 56 | 33–57 | 4.1–6.1 | 50.5–NR |
| I | Rizvi [Rizvi et al. 2014] | EGFR+ but resistant nivolumab + erlotinib | 21 | 19 | NA | NA |
| I | Antonia [Antonia et al. 2014b] | Second line with ipilimumab | 148 | 13–39 | 4.9–10.6 | NR |
NA, not available; NR, not reported; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
Rationale for combination therapy
Preclinical data have suggested that combination of ICIs with chemotherapy or other targeted therapy like anti-angiogenic drugs may have synergistic effects. Akbay and colleagues showed that PD-L1 expression may be upregulated via the EGFR signaling pathway [Akbay et al. 2013], and D’Incecco and colleagues showed that there was a significant correlation between EGFR and KRAS mutations with PD-L1 and PD-1 expression respectively [D’Incecco et al. 2015]. The combination of nivolumab with EGFR-mutant NSCLC is being studied in patients who are treatment naïve or have progressed on first-line EGFR TKI therapy. Preliminary results reported an ORR of 19%, 6-month PFS of 51% and 1-year OS of 73% [Rizvi et al. 2014].
Conventional chemotherapy occurs largely through apoptosis, which is believed to be nonimmunogenic compared with necrosis, which is more inflammatory in nature. Preclinical data have shown that combination of chemotherapy with immunotherapy may lead to more pronounced cell death due to complementary pathways in cell killing [Apetoh et al. 2008]. The combination of nivolumab with platinum-based doublet in the first-line setting has shown encouraging outcomes. In a phase I trial of 56 patients with NSCLC, including both squamous and nonsquamous histology, patients were treated with four cycles of nivolumab plus platinum-based doublet in four different subgroups, with continuation of nivolumab alone till progression or unacceptable toxicities. The ORR ranged from 33–57%, PFS 21–31 weeks and median OS of 50.5 weeks to not reached [Antonia et al. 2014b].
In vitro studies have shown anti-CTLA4 and anti-PD-1 inhibition have synergistic effects in promoting cell death. Combined PD-1 and CTLA-4 blockade has been shown to increase effector T-cell infiltration in melanoma cells, that eventually leads to decreased anergy and increased activity that allows for the tumor microenvironment to shift from suppressive to inflammatory [Curran et al. 2010]. Encouraging preclinical data, combined with success of combination therapy in melanomas have fueled the extension of such studies in NSCLC. CHECKMATE012, a phase I trial, is currently investigating the combination of nivolumab with ipilimumab at various dose combinations. Initial results have been encouraging with ORR range from 13–39% and median PFS from 4.9–10.6 months in various treatment arms. Grade 3/4 SAEs were see in 28% of patients, including pneumonitis, diarrhea, colitis and raised liver function, with three patient deaths deemed attributable to drug-related toxicities, including respiratory failure following colitis, pulmonary hemorrhage and toxic epidermal necrolysis in a patient with history of ulcerative colitis. [Antonia et al. 2014].
Adverse events
Based on the mechanism of action of nivolumab, the anticipated side effects are immune-related adverse events (irAEs). In studies of patients with NSCLC treated with nivolumab, any grade adverse events were reported in 58–85% of patients, while rates of grade 3–4 toxicities ranged from 5.7–17% (Table 3). The common side effects included fatigue (16–33%), anorexia (11%), diarrhea (8–10%) and endocrinopathies [Rizvi et al. 2014; Brahmer et al. 2015; Nakagawa et al. 2015].
Table 3.
Ongoing trials of nivolumab in advanced NSCLC.
| Phase | Line of treatment | Control arm | Experimental arm | Clinical trial gov. number |
|---|---|---|---|---|
| I (CHECKMATE 012) | Second line and beyond | – | Nivolumab + ipilimumab, nivolumab + platinum doublet, nivolumab + erlotinib | NCT01454102 |
| III (CHECKMATE 026) | First line | Platinum doublet | Nivolumab | NCT02041533 |
| I | Second line and beyond | – | Nivolumab + EGF816 or INC280 | NCT02323126 |
| III (CHECKMATE 078) | Second line | Docetaxel | Nivolumab | NCT02613507 |
| III (CHECKMATE 227) | First line | Platinum doublet | Nivolumab, nivolumab + ipilimumab, nivolumab + platinum doublet | NCT02477826 |
| II (CHECKMATE 568) | First line | – | Nivolumab + ipilimumab | NCT02659059 |
| I | First line | – | Nivolumab + ceritinib | NCT02393625 |
The rate of treatment-related adverse events (TRAEs) leading to discontinuation and death is relatively low with nivolumab. In CHECKMATE 063 [Rizvi et al. 2015], 12% and 1.7% of patients experienced TRAE discontinuation and death, respectively, and in CHECKMATE 017 [Brahmer et al. 2015], it was 3% and 0%, respectively. In comparison, the rate of TRAE discontinuation and death in patients treated with docetaxel in CHECKMATE 017 was 10% and 2%, respectively. In addition, there was more neutropenia of any grade (33% versus 1%) and febrile neutropenia (11% versus 0%) in patients treated with docetaxel compared with nivolumab.
Pneumonitis was the most common irAE resulting in nivolumab discontinuation. Pneumonitis is generally mild and manageable with steroid treatment. In CHECKMATE 063, 5% of patients had pneumonitis of which 3% was grade 3 or higher. In this study, all patients responded to steroids and the median time to resolution was 3.4 weeks (1.6–13.4 weeks) [Rizvi et al. 2015]. In CHECKMATE 017, 5% of patients had pneumonitis (any grade) whereas no patients had pneumonitis is the docetaxel arm. In the phase I study, three patients had unresolved pneumonitis, resulting in mortality [Gettinger et al. 2015]. The onset of pneumonitis may be variable. In a recent report of patients with melanoma treated with nivolumab, the onset of pneumonitis was 7.4–24.3 months from treatment initiation [Nishino et al. 2015]. Onset can be insidious with symptoms like cough, while others developed dyspnea and hypoxia. Radiology may show ground glass changes with reticular opacities, although consolidative changes were also observed in one patient. The diagnosis of pneumonitis may be challenging in patients, especially in those with underlying airways disease or metastases confounding underlying baseline symptoms. It may also sometimes be difficult to discern development of pneumonitis from underlying disease progression from description of symptoms alone. It is important to have a high degree of suspicion and early access to imaging as this may be useful in differentiating the etiology of worsening respiratory symptoms.
The management of irAEs include the use of steroids and in more severe cases, immunomodulatory agents such as anti-metabolite (mycophenolate mofetil), anti-tumor necrosis factor (TNF)-α antibody (infliximab) and calcineurin inhibitors (tacrolimus and cyclosporine). Choice of steroids and immunomodulatory agents are largely dependent on grade of side effects, and are summarized in Table 4 [Naidoo et al. 2015; Spain et al. 2016; Bristol-Myers-Squibb, 2016]. While the Common Terminology Criteria for Adverse Events (CTCAE) is the most common platform used to grade toxicities, there have been concerns about whether it may underestimate certain toxicities like hypophysitis. With increased understanding of irAEs, better grading systems may eventually evolve.
Table 4.
Immune related adverse events (%) in patients treated with nivolumab.
| Study | CHECKMATE 063 [Horn et al. 2015] | ONO-4538-050 [Nakagawa et al. 2015] | CHECKMATE 017 [Brahmer et al. 2015] | |||
|---|---|---|---|---|---|---|
| Immune related adverse events | All grade | Grade 3/4 | Grade 3/4 | Grade 3/4 | All grade | Grade 3/4 |
| Skin | 15.5 | 0 | 0 | 0 | 28.6 | 0 |
| Gastrointestinal | 11.6 | 0.8 | 1 | 1 | 5.7 | 0 |
| Pulmonary | 7.0 | 2.3 | 1 | 1 | 5.7 | 0 |
| Endocrinopathies | 6.2 | 0 | 0 | 0 | 11.4 | 0 |
| Hepatitis | 4.7 | 0.8 | 0 | 0 | 5.7 | 0 |
| Renal injury | 3.1 | 0 | 1 | 1 | 2.9 | 0 |
Role of predictive biomarkers
In this era of precision medicine, companion diagnostics such as a validated predictive biomarkers that may be able to help select for patients who are most likely to respond to treatment, is paramount in optimizing patient outcomes. Studies involving nivolumab have collected immunohistochemical (IHC) data in terms of measurement of PD-1, PD-L1 and tumor infiltrating lymphocytes (TIL) expression levels in tumor samples for correlation with treatment outcomes. Thus far, none of the above IHC expression levels have conclusively proven to be either prognostic or predictive of response to treatment. In CHECKMATE 017, tumor expression of PD-L1 by immunohistochemistry does not seems not to be predictive for OS or PFS benefit to nivolumab [Brahmer et al. 2015]. However in CHECKMATE 057, PD-L1+ patients treated with nivolumab was associated with an improved OS, PFS and duration of response at the predefined 1%, 5%, and 10% cut-off points [Borghaei et al. 2015]. These findings suggest there may inherent differences in the tumor microenvironment between SCC versus nonsquamous cancer, consistent with the idea that these are two distinct diseases. Inherent challenges are present in the use of IHC expression levels, as differing antibodies and staining techniques may affect scoring. There are currently no standard criteria across various anti PD-1 and PD-L1 trials in terms of reporting of a positive expression, making it even more challenging to confirm the utility of IHC expression level with outcomes. To improve on patient selection for ICIs, other biomarkers have been explored. Tumor mutation and neo-antigen load are potentially promising predictive factors [Schumacher and Schreiber, 2015]. However, the clinical applicability of this biomarker approach remains to be defined.
Conclusion and future perspectives
The positive results of CHECKMATE 017 have established the role of nivolumab as a superior option as compared with docetaxel in the second-line therapy in lung SCC. Studies of nivolumab in first-line settings are ongoing and results are greatly anticipated. Currently, many further studies are underway to investigate nivolumab’s role both as a single agent and as combination therapy at various lines of treatment (Table 5). These studies will undoubtedly help to further define the role of nivolumab in the treatment algorithm of NSCLC. However, undefined areas remain and are actively being explored, including the role of ICIs in the adjuvant setting, in stage III following chemotherapy and radiotherapy and duration of treatment. Further studies to investigate predictive biomarkers and a better understanding of how we evaluate treatment response will further help to optimize the outcomes of patients on treatment.
Table 5.
Overview on the treatment of immune-related toxicities, adapted from Spain and colleagues [Spain et al. 2016], Naidoo and colleagues [Naidoo et al. 2015] and product insert [Bristol-Myers-Squibb, 2016].
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Skin | Topical steroid cream, antihistamines, can continue ICI | Initiate systemic steroids (prednisolone 0.5–1 mg/kg/day) and delay treatment until symptoms resolve to Grade 1 | Hold ICI. Commence steroids (prednisolone 1–2 mg/kg/day). Dermatology consult for skin biopsy | Permanently discontinue ICI. Management as per Grade 3. |
| Gastrointestinal | Supportive measures, can continue ICI | Hold ICI. Commence steroids (prednisolone 0.5–1 mg/kg/day) until symptoms improve to Grade 1. If symptoms do not improve after 5 days, consider increasing steroids to 1–2 mg/kg/day. | Hold ICI. Intravenous hydration, commence steroids (prednisolone 1–2 mg/kg/day). Add infliximab 5 mg/kg if no improvement | Permanently discontinue ICI. Acute treatment as per Grade 3, consider referral to gastroenterology and surgery for opinion in management. |
| Pulmonary | Consider commencement of steroids (prednisolone 0.5–1 mg/kg/day) | Hold ICI. Commence steroids (1–2 mg/kg/day) until improvement. consider empiric antibiotics. | Permanently discontinue ICI. High dose steroids (IV methylprednisolone 2–4 g/day) with empirical antibiotics. Add immunomodulatory agent if no improvement after 48 h. referral to pulmonologist for consideration of bronchoscopy. | Permanently discontinue ICI. Acute management as per Grade 3, consider intensive care support |
| Thyroid dysfunction | Continue ICI. Monitor thyroid function | Hold ICI. Referral to endocrinology for further management | Permanently discontinue ICI. Commence steroids (Prednisolone 1–2 mg/kg), referral to endocrinology for further management | Permanently discontinue ICI. Management as per Grade 3 |
| Hepatitis | Continue ICI. Rule out other causes of hepatitis including viral, metabolic and alcoholic causes | Hold ICI. Commence steroids (prednisolone 0.5–1 mg/kg/day) until symptoms improve to Grade 1. | Hold ICI, consider permanent discontinuation. Commence steroids as per Grade 2. | Permanent discontinue ICI. Acute treatment as per Grade 3, consider referral to hepatologist. |
| Renal | Can continue ICI. Supportive measures (encourage hydration, monitor renal function, medication reconciliation to avoid nephrotoxic drugs) | Hold ICI. Commence steroids (prednisolone 0.5–1 mg/kg/day) | Permanently discontinue ICI, commence steroids (prednisolone 1–2 mg/kg/day), consider high dose steroids. Referral to nephrologist for further management | Permanently discontinue ICI. Acute management as per Grade 3, consider acute hemodialysis |
ICI, immune checkpoint inhibitor; IV, intravenous.
In conclusion, ICI has proven benefit in the treatment of metastatic NSCLC, especially in the setting of SCC where targeted therapy with TKIs has not been particularly effective. It is currently a treatment of choice post-progression on platinum-based chemotherapy, and several studies are underway to examine its role in a first-line setting and also in combination with chemotherapy and other immunotherapeutic agents.
Acknowledgments
Ross Soo is supported by the National Research Foundation, Singapore and the Singapore Ministry of Education under its Research Centers of Excellence initiative. Joline Lim is supported by the National Medical Research Council, Singapore.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Ross Soo is supported by the National Research Foundation Singapore and the Singapore Ministry of Education under its Research Centres of Excellence initiative.
Conflict of interest statement: Joline Lim has no conflict of interest. Ross Soo has received honoraria from Astra-Zeneca, Boehringer Ingelheim, Lilly, Merck, Novartis, Pfizer, and Roche.
Contributor Information
Joline S. J. Lim, Cancer Science Institute of Singapore, National University of Singapore, Singapore
Ross A. Soo, Department of Haematology–Oncology, National University Cancer Institute, National University Health System, 1E Kent Ridge Road, NUHS Tower Block Level 7, Singapore 119228.
References
- Akbay E., Koyama S., Carretero J., Altabef A., Tchaicha J., Christensen C., et al. (2013) Activation of the Pd-1 pathway contributes to immune escape in Egfr-driven lung tumors. Cancer Discov 3: 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang Y., Tan H., Soo R. (2015) Best practice in the treatment of advanced squamous cell lung cancer. Ther Adv Respir Dis 9: 224–235. [DOI] [PubMed] [Google Scholar]
- Antonia S., Brahmer J., Gettinger S., Chow L., Juergens R., Shepherd F., et al. (2014a) Nivolumab (Anti-Pd-1; Bms-936558, Ono-4538) in combination with platinum-based doublet chemotherapy (Pt-Dc) in advanced non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 90: S2. [Google Scholar]
- Antonia S., Gettinger S., Chow L., Juergens R., Borghaei H., Shen Y., et al. (2014b) Nivolumab (Anti-Pd-1; Bms-936558, Ono-4538) and ipilimumab in first-line non-small cell lung cancer (NSCLC): Interim Phase 1 Results. J Clin Oncol 32(Suppl. 5): 8023. [Google Scholar]
- Apetoh L., Tesniere A., Ghiringhelli F., Kroemer G., Zitvogel L. (2008) Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res 68: 4026–4030. [DOI] [PubMed] [Google Scholar]
- Borghaei H., Paz-Ares L., Horn L., Spigel D., Steins M., Ready N., et al. (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small cell lung cancer. N Engl J Med 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J., Drake C., Wollner I., Powderly J., Picus J., Sharfman W., et al. (2010) Phase I study of single-agent anti-programmed death-1 (Mdx-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28: 3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J., Reckamp K., Baas P., Crino L., Eberhardt W., Poddubskaya E., et al. (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small cell lung cancer. N Engl J Med 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol-Myers-Squibb (2016) Opdivo Product Insert (version revised 01/2016). Available at: http://packageinserts.bms.com/pi/pi_opdivo.pdf (accessed 20 July 2016).
- Cancer Genome Atlas Research (2012) Comprehensive genomic characterization of squamous cell lung cancers. Nature 489: 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research (2014) Comprehensive molecular profiling of lung adenocarcinoma. Nature 511: 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran M., Montalvo W., Yagita H., Allison J. (2010) Pd-1 and Ctla-4 combination blockade expands infiltrating T-cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA 107: 4275–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Cruz C., Tanoue L., Matthay R. (2011) Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 32: 605–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Incecco A., Andreozzi M., Ludovini V., Rossi E., Capodanno A., Landi L., et al. (2015) Pd-1 and Pd-L1 expression in molecularly selected non-small cell lung cancer patients. Br J Cancer 112: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon E., Ciuleanu T., Arrieta O., Prabhash K., Syrigos K., Goksel T., et al. (2014) Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (revel): a multicentre, double-blind, randomised phase 3 trial. Lancet 384: 665–673. [DOI] [PubMed] [Google Scholar]
- Gettinger S., Hellmann M., Shepherd F., Antonia S., Brahmer J., Goldman J., et al. (2015a) First-line monotherapy with nivolumab in advanced non-small cell lung cancer: safety, efficacy and correlation of outcomes with Pd-1 ligand expression. J Clin Oncol 33(suppl): abstr 8025. [Google Scholar]
- Gettinger S., Horn L., Gandhi L., Spigel D., Antonia S., Rizvi N., et al. (2015b) Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, Bms-936558, Ono-4538) in patients with previously treated advanced non-small cell lung cancer. J Clin Oncol 33: 2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla R., Coon C., Taylor F., Penrod J., Derosa M., Dastani H., et al. (2015) Evaluation of disease-related symptoms in patients with advanced squamous non-small cell lung cancer treated with nivolumab or docetaxel. J Thorac Oncol 10(Suppl 2): S233. [Google Scholar]
- Horn L., Rizvi N., Mazieres J., Planchard D., Stinchcombe T., Dy G., et al. (2015) Longer-term follow-up of a phase 2 study (checkmate 063) of nivolumab in patients with advanced, refractory squamous non-small cell lung cancer. J Thorac Oncol 10(Suppl 2): S175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein M., McCleod M., Chandler J. (2015) Safety and efficacy of nivolumab in an ongoing trial of a Pd-L1+/- patient population with metastatic non-small cell lung cancer. J Thorac Oncol 10(Suppl 2): S175. [Google Scholar]
- Kim E., Hirsh V., Mok T., Socinski M., Gervais R., Wu Y., et al. (2008) Gefitinib versus docetaxel in previously treated non-small cell lung cancer (interest): a randomised phase III trial. Lancet 372: 1809–1818. [DOI] [PubMed] [Google Scholar]
- Kris M., Johnson B., Berry L., Kwiatkowski D., Iafrate A., Wistuba I., et al. (2014) Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 31: 1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarakulasinghe N., Van Zanwijk N., Soo R. (2015) Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC). Respirology 20: 370–378. [DOI] [PubMed] [Google Scholar]
- Naidoo J., Page D., Li B., Connell L., Schindler K., Lacouture M., et al. (2015) Toxicities of the anti-Pd-1 and anti-Pd-L1 immune checkpoint antibodies. Ann Oncol 26: 2375–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa K. M., Sakai H., Nogami N., Atagi S., Takahashi T., Nishio M., et al. (2015) Phase II studies of nivolumab in patients with advanced squamous (SQ) or nonsquamous (NSQ) non-small cell lung cancer (NSCLC). J Thorac Oncol 10(Suppl 2): S270. [Google Scholar]
- Nishino M., Sholl L., Hodi F., Hatabu H., Ramaiya N. (2015) Anti-Pd-1-related pneumonitis during cancer immunotherapy. N Engl J Med 373: 288–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M., Kaiser R., Mellemgaard A., Douillard J., Orlov S., Krzakowski M., et al. (2014) Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (Lume-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 15: 143–155. [DOI] [PubMed] [Google Scholar]
- Rizvi N., Mazieres J., Planchard D., Stinchcombe T., Dy G., Antonia S., et al. (2015) Activity and safety of nivolumab, an anti-pd-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (checkmate 063): a phase 2, single-arm trial. Lancet Oncol 16: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi N., Quan L., Borghaei H., Shen Y., Harbison C., Alparthy S., et al. (2014) Safety and response with nivolumab (anti-Pd-1; Bms-936558, Ono-4538) plus erlotinib in patients (Pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. J Clin Oncol 32(Suppl. 5): 8022. [Google Scholar]
- Schumacher T., Schreiber R. (2015) Neoantigens in cancer immunotherapy. Science 348: 69–74. [DOI] [PubMed] [Google Scholar]
- Shepherd F., Dancey J., Ramlau R., Mattson K., Gralla R., O’Rourke M., et al. (2000) Prospective randomized trial of docetaxel versus best supportive care in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18: 2095–2103. [DOI] [PubMed] [Google Scholar]
- Soria J., Felip E., Cobo M., Lu S., Syrigos K., Lee K., et al. (2015) Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (Lux-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 16: 897–907. [DOI] [PubMed] [Google Scholar]
- Spain L., Diem S., Larkin J. (2016) Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 44: 51–60. [DOI] [PubMed] [Google Scholar]
- Sundar R., Cho B., Brahmer J., Soo R. (2015) Nivolumab in NSCLC: latest evidence and clinical potential. Ther Adv Med Oncol 7: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher N., Hirsch F., Luft A., Szczesna A., Ciuleanu T., Dediu M., et al. (2015) Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small cell lung cancer (squire): an open-label, randomised, controlled phase 3 trial. Lancet Oncol 16: 763–774. [DOI] [PubMed] [Google Scholar]
- Topalian S., Hodi F., Brahmer J., Gettinger S., Smith D., Mcdermott D., et al. (2012) Safety, activity, and immune correlates of anti-Pd-1 antibody in cancer. N Engl J Med 366: 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torri V., Ando M., Rulli E., Floriani I., Kawaguchi T., Isa S., et al. (2015) Individual patients data analysis (IPD) of three randomized studies comparing erlotinib (E) with chemotherapy (CT) in patients with advanced wild-type epidermal growth factor receptor (WTEGFR) non-small cell lung cancer (NSCLC). J Clin Oncol 33: abstr 8048. [Google Scholar]
- Wang C., Thudium K., Han M., Wang X., Huang H., Feingersh D., et al. (2014) In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res 2: 846–856. [DOI] [PubMed] [Google Scholar]


