Abstract
Background:
Although lobectomy is still the preferred treatment for patients with stage I non-small cell lung cancer (NSCLC), segmentectomy or wedge resection is frequently performed on patients who cannot withstand the physiological rigors of lobectomy. The objective of this study was to compare the overall survival (OS), cancer-specific survival (CSS), and disease-free survival outcomes among patients with stage I NSCLC who have undergone these procedures.
Methods:
A systematic electronic search in three online databases was conducted from their earliest publication dates to June 2015. The studies were evaluated according to rigorous, predefined inclusion criteria. The hazard ratio (HR) was used as the outcome measure for data combining.
Results:
There were nine eligible studies. These studies included 1181 patients who underwent segmentectomy and 2003 patients who underwent wedge resection. Stage I NSCLC patients who underwent segmentectomy had significantly better OS (HR 0.80; 95% confidence interval [CI], 0.68–0.93; p = 0.004) and CSS (HR 0.42; 95% CI, 0.20–0.88; p = 0.02) rates than those who underwent wedge resection. However, there were no significant differences in OS (HR 0.39; 95% CI, 0.15–1.02; p = 0.06) and CSS (HR 1.87; 95% CI, 0.29–12.06; p = 0.51) rates between segmentectomy and wedge resection in patients with stage Ia NSCLC with tumor size ⩽ 2 cm.
Conclusions:
For patients with stage I NSCLC, segmentectomy results in higher survival rates than wedge resection, whereas the outcomes of wedge resection are comparable to those of segmentectomy for patients with stage Ia NSCLC with tumor size ⩽ 2 cm. Considering the limitations and heterogeneity of the included studies, this conclusion should be further confirmed by rigorous randomized clinical trials.
Keywords: non-small cell lung cancer, segmentectomy, stage I, wedge resection
Introduction
Lung cancer is usually diagnosed in older adults. In the USA, more than 50% of lung cancer cases are diagnosed in patients who are over 65 years of age, with a peak incidence between 65 years and 74 years [Jemal et al. 2008]. Currently, lobectomy combined with mediastinal lymph node sampling or dissection is the preferred treatment for patients with stage I non-small cell lung cancer (NSCLC) [Spira and Ettinger, 2004]. However, many patients are not candidates for complete lobectomy because of severely compromised pulmonary function, advanced age, or other comorbidities. For these patients, the surgical technique of limited resection is frequently used [Wisnivesky et al. 2010]. Moreover, results from several previously published studies suggest that limited resection may provide an adequate alternative for patients with stage I NSCLC with a tumor size of 2 cm or less (T1a), especially for elderly patients [Okada et al. 2001, 2006; Koike et al. 2003; Watanabe et al. 2005; Kates et al. 2011].
The limited resection operative approaches include wedge resection and segmentectomy. Wedge resection is a nonanatomical resection that involves the removal of cancerous lung tissue surrounded by a margin of normal lung parenchyma. Although segmentectomy is more technically challenging, it is an anatomic resection and can usually be used to dissect the lymph nodes more extensively. The wedge resection is generally considered less effective than anatomic segmentectomy for the following two reasons: (a) in wedge resection, the regional lymph nodes of the tumor are usually not removed immediately; (b) compared with segmentectomy, the margin of the staple line in wedge resection is closer to the tumor. However, several recently published articles comparing the main outcomes of patients with stage I NSCLC who were treated with one of these procedures reported conflicting results, which makes it difficult to determine which procedure is the best limited resection approach for patients with stage I NSCLC [Okada et al. 2005; Nakamura et al. 2011; Hamatake et al. 2012; Tsutani et al. 2014]. The outcomes of wedge resection and segmentectomy for patients with stage I NSCLC may be clarified by two ongoing randomized controlled trials: JCOG0802/WJOG4607L and CALGB 140503 [Bao et al. 2014; Cao et al., 2014]. However, neither of these studies has reported the final results. In this study, we use the meta-analysis method to combine the currently published data and further provide powerful evidence to promote common consensus on the topic.
The aim of this meta-analysis of published studies was to compare the outcomes of overall survival (OS), cancer-specific survival (CSS), and disease-free survival (DFS) for patients with stage I NSCLC who underwent either wedge resection or segmentectomy. In the subgroup analysis, we compared the OS and CSS results of segmentectomy and wedge resection in patients with stage Ia NSCLC and a tumor size of 2 cm or less. This is an important meta-analysis focused on comparing the outcomes of segmentectomy and wedge resection for patients with NSCLC in stage I, including stage Ia and T1a (tumor size ⩽ 2 cm). Through this pooled analysis, we hope to promote consensus about the surgical options for patients with stage I NSCLC undergoing limited resection.
Methods
Search strategy for published studies
According to the recommendations of the Cochrane Collaboration, we established a rigorous study protocol at the beginning of the search. To ensure that the highest quality of literature was included in this meta-analysis, we prespecified the objective, inclusion and exclusion criteria, primary outcome, and methods of synthesis [Honda et al. 2013].
A systematic and rigorous electronic search was independently performed by two investigators using MEDLINE, EMBASE, and the Cochrane Library database CENTRAL from their earliest publication dates to June 2015. All articles involving patients with stage I NSCLC who underwent segmentectomy or wedge resection were included in the analysis to allow our search strategy to reach the maximum sensitivity, and to ensure that all potentially relevant studies were identified. The search terms were ‘lung cancer’, ‘NSCLC’, ‘stage I’, ‘segmentectomy’, ‘segmental resection’, ‘sublobar’, ‘sublobectomy’, and ‘wedge’. Medical subject headings (MeSH) ‘NSCLC’ (MeSH), ‘sublobar’ (MeSH), ‘wedge’ (MeSH), and ‘segmentectomy’ (MeSH) were used in combination with the Boolean operators AND or OR. In this study, ethical approval was waived because it was a meta-analysis and did not involve patients.
Selection criteria
Eligible studies were identified as those articles in which outcomes, including OS, CSS, or DFS, were presented for patients with stage I NSCLC who underwent wedge resection or segmentectomy. Most of the patients included in the analysis were high-risk lobectomy patients. The definition of high risk was not consistent across studies, but most definitions were similar to the definitions used in the published criteria [Fernando et al. 2011]. According to the included studies, high-risk patients were defined as NSCLC patients with a predicted forced expiratory volume in 1 s ⩽ 60%, severe emphysema, poor left ventricular function (defined as an ejection fraction of 40% or less), or severe coronary heart disease. The search results were evaluated according to the prespecified inclusion and exclusion criteria. The following inclusion criteria were established before conducting the search: (a) the surgical procedure could include both wedge resection and segmentectomy; (b) one of the outcomes included OS, CSS, or DFS; (c) articles were original and published andwhose study subjects were limited to patients with clinical stage I NSCLC; (d) the median follow-up time was to exceed 2 years. The exclusion criteria were as follows: (a) letters to the editor, articles published in books, reviews, and papers not published in English; (b) duplicate trials published by centers with accumulating patient numbers or with extended follow-up times; in such cases, only the latest and most informative article was included in the meta-analysis for rigorous qualitative appraisal.
Quality assessment
We assessed the quality of the included studies based on the Newcastle–Ottawa scale (NOS) for evaluating the quality of case-control and cohort studies. A star system for the NOS (range, 0–9 stars) was developed for the evaluation. The values for the included studies are shown in Table 1.
Table 1.
Characteristics of included trials.
| Author | Year | Surgical procedure |
Tumor stage | NOS score | |
|---|---|---|---|---|---|
| Segmentectomy | Wedge resection | ||||
| Okada et al. | 2005 | 123 | 35 | Ia (⩽ 20 mm) | 6 |
| Okada et al. | 2005 | 64 | 14 | Ia (20–30 mm) | |
| Okada et al. | 2005 | 34 | 6 | Ib (> 30 mm) | |
| Okada et al. | 2006 | 214 | 30 | Ia | 7 |
| Sienel et al. | 2008 | 56 | 31 | Ia | 7 |
| Sienel et al. | 2008 | 35 | 25 | Ia (⩽ 20 mm) | |
| Yamato et al. | 2008 | 153 | 93 | Ia (⩽ 20 mm) | 8 |
| Sugi et al. | 2010 | 33 | 15 | Ia | 8 |
| Nakamura et al. | 2011 | 38 | 84 | I | 9 |
| Hamatake et al. | 2012 | 32 | 34 | Ia (⩽ 10 mm) | 8 |
| Smith et al. | 2013 | 378 | 1568 | I | 7 |
| Tsutani et al. | 2014 | 56 | 93 | Ia | 8 |
NOS, Newcastle–Ottawa scale for quality of case-control and cohort studies.
Statistical analysis
The meta-analysis was completed by combining the OS/CSS/DFS data in the published articles. The logarithm of the hazard ratio (HR) and its standard error (SE) were used as the outcome measures for data combination [Cao et al. 2014]. Using the techniques described by Tierney and colleagues, we obtained or calculated the HR and the associated variance for each selected study [Tierney et al. 2007; Cao et al. 2014]. The SE was calculated as (upper 95% confidence interval [CI]-lower 95% CI)/3.92 [Bao et al. 2014]. As the HR of the OS/CSS/DFS data could not be obtained directly from some studies, data were extracted from the Kaplan–Meier survival curves of these studies to calculate the HR and SE of the OS/CSS/DFS data [Bao et al. 2014]. Kaplan–Meier curves were read using the Engauge Digitizer version 4.1 software, and calculations were performed independently by two researchers. The two researchers discussed any discrepancies to reach consensus. We used the Review Manager version 5.1.2 software to summarize the statistical analysis. Statistical heterogeneity among the included clinical trials was evaluated using Higgins I2 statistic, which represents the total variation percentage across studies. If the I2 statistic was more than 50%, the random-effects model was used to pool studies; otherwise, the fixed-effect model was used.
Results
Characteristics of included trials
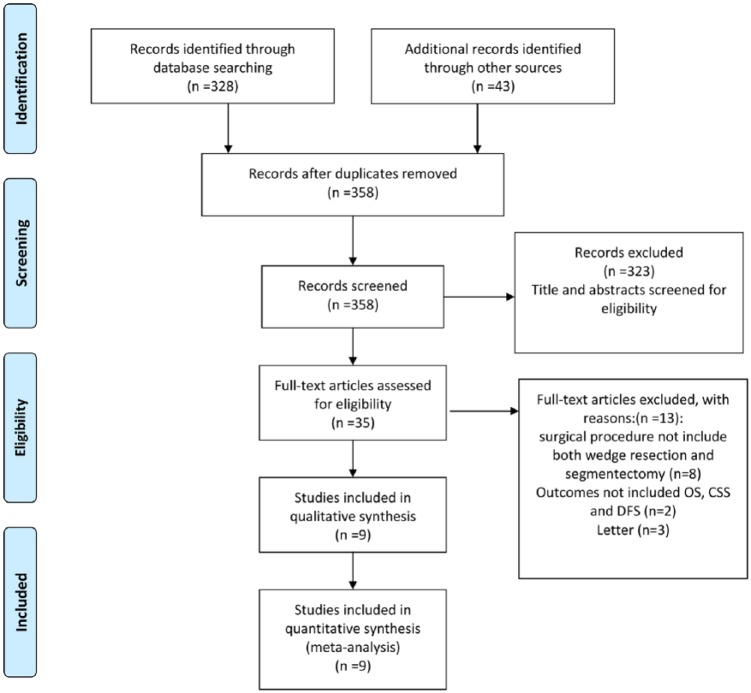
Nine studies (1181 patients who underwent segmentectomy and 2003 patients who underwent wedge resection) that met the inclusion criteria were identified; all were performed between 2005 and 2015. Figure 1 shows a flow chart of the literature search for the meta-analysis based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [Moher et al. 2010]. Our search strategy yielded a total of 339 articles. We reviewed the full text of 22 articles and finalized a list of 9 comparable studies with a total of 3184 patients for inclusion in the analysis. Table 1 shows the details of each trial, including baseline characteristics, publication year of the study, surgical procedure, and tumor stage for each trial [Okada et al. 2005, 2006; Sienel et al. 2008; Yamato et al. 2008; Sugi et al. 2010; Nakamura et al. 2011; Hamatake et al. 2012; Smith et al. 2013; Tsutani et al. 2014].
Figure 1.
The literature search based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. CSS, cancer-specific survival; DFS, disease-free survival; OS, overall survival.
Primary outcome measures
Pooled estimates were calculated for the primary outcomes of OS, CSS, or DFS following the surgical procedure (segmentectomy or wedge resection). Subgroup analyses were conducted according to the tumor stage and tumor size.
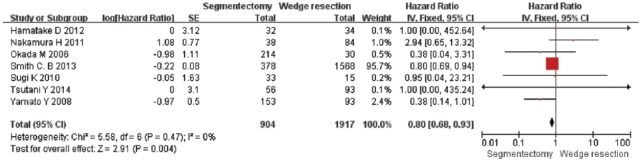
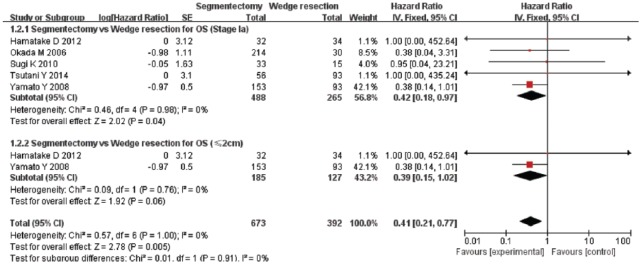
A total of seven studies were included in the analysis of the OS rate. These studies included 904 patients who underwent segmentectomy and 1917 patients who underwent wedge resection. The combined HR of the OS for segmentectomy and wedge resection for patients with stage I NSCLC was 0.80 [95% CI, 0.68–0.93; p = 0.004] (Figure 2). However, in the subgroup analysis of segmentectomy and wedge resection in patients with stage Ia NSCLC with T1a, the combined HR for OS was 0.42 (95% CI, 0.18–0.97; p = 0.04), and 0.39 (95% CI, 0.15–1.02; p = 0.06), respectively (Figure 3). This result indicated that the OS for segmentectomy was superior to that of wedge resection in patients with stage I and stage Ia NSCLC. However, based on our subgroup analysis, the OS for segmentectomy was comparable to that of wedge resection in patients with T1a NSCLC.
Figure 2.
Forest plot of comparison: the overall survival (OS) of segmentectomy versus wedge resection in patients with stage I non-small cell lung cancer. Seven studies were included. CI, confidence interval; df, degrees of freedom; SE, standard error.
Figure 3.
Forest plot of comparison: segmentectomy overall survival (OS) versus wedge resection OS in patients with stage Ia and tumor size ⩽ 2 cm (T1a) non-small cell lung cancer . Seven studies were included. CI, confidence interval; df, degrees of freedom; SE, standard error.
The CSS data were reported in five studies that included 655 patients who underwent segmentectomy and 1654 patients who underwent wedge resection. As shown in Figure 4, the comparative data showed a significant difference because the combined HR for CSS was 0.42 (95% CI, 0.20–0.88; p = 0.02). Another subgroup analysis of segmentectomy and wedge resection was also performed according to tumor stage. In this subgroup analysis, the combined HR for CSS was 0.40 (95% CI, 0.18–0.86; p = 0.02) for patients with stage Ia NSCLC and 1.87 (95% CI, 0.29–12.06; p = 0.51) for patients with T1a NSCLC (Figure 5). Based on the above analysis, the CSS of patients with stage I and stage Ia NSCLC treated with segmentectomy was superior to that of patients treated with wedge resection. However, for patients with stage I NSCLC with a tumor size of 2 cm or less, the CSS for segmentectomy was comparable to that of wedge resection.
Figure 4.
Forest plot of comparison: the cancer-specific survival (CSS) of segmentectomy versus wedge resection in patients with stage I non-small cell lung cancer. Five studies were included. CI, confidence interval; df, degrees of freedom; SE, standard error.
Figure 5.
Forest plot of comparison: the cancer-specific survival (CSS) of segmentectomy versus wedge resection in patients with stage Ia and tumor size ⩽ 2 cm (T1a) non-small cell lung cancer. Three studies were included. CI, confidence interval; df, degrees of freedom; SE, standard error.
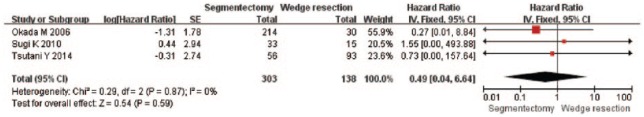
Only three studies reported the DFS. These studies included 303 patients who underwent segmentectomy and 138 patients who underwent wedge resection. All patients included in the two studies were in stage Ia NSCLC. As shown in Figure 6, no statistically significant difference was found between the two surgical procedure groups for DFS, and the combined HR was 0.49 (95% CI, 0.04–6.64; p = 0.59).
Figure 6.
Forest plot of comparison: segmentectomy disease-free survival (DFS) versus wedge resection DFS in patients with stage Ia non-small cell lung cancer. Three studies were included. CI, confidence interval; df, degrees of freedom; SE, standard error.
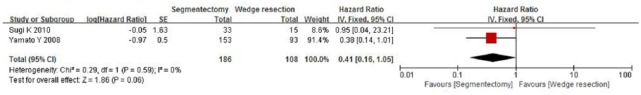
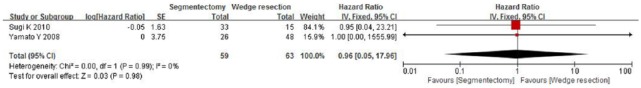
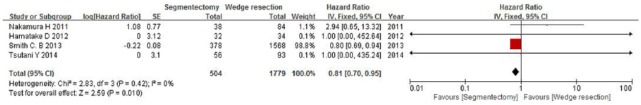
The included studies span 2005–2015, which includes the periods before and after the introduction and routine use of positron emission tomography scanners. We performed a further subgroup analysis to compare the OS rates of the publication year ranges 2005–2010 and 2010–2015. When comparing the OS rates for segmentectomy and wedge resection for patients with stages I and Ia NSCLC by pooling the studies published in 2005–2010, only two studies were included, and the combined HR was 0.41 (95% CI, 0.16–1.05; p = 0.06) and 0.96 (95% CI, 0.05–17.96; p = 0.98), respectively (Figures 7 and 8). However, when comparing the segmentectomy and wedge resection OS rates for patients with stage I NSCLC by pooling the studies published in 2011–2015, four studies were included, and the combined HR was 0.81 (95% CI, 0.70–0.95; p = 0.01) (Figure 9).
Figure 7.
Forest plot of comparison: segmentectomy overall survival (OS) versus wedge resection OS in patients with stage I non-small cell lung cancer in the period 2005–2010. Two studies were included. CI, confidence interval; df, degrees of freedom; SE, standard error.
Figure 8.
Forest plot of comparison: segmentectomy overall survival (OS) versus wedge resection OS in patients with stage Ia non-small cell lung cancer in the period 2005–2010. Two studies were included. CI, confidence interval; df, degrees of freedom; SE, standard error.
Figure 9.
Forest plot of comparison: segmentectomy overall survival (OS) versus wedge resection OS in patients with stage I non-small cell lung cancer in the period 2010–2015. Four studies were included. CI, confidence interval; df, degrees of freedom; SE, standard error.
Sensitivity analysis and publication bias
The findings were similar whether fixed- or random-effects models were used. A funnel plot estimating the precision of the trials (plots of the logarithm of the HR for efficacy against sample size) was examined for asymmetry to determine publication bias.
Discussion
Anatomic lobectomy is considered to be the standard surgical procedure for patients with stage I NSCLC. However, sublobar resection is an alternative operation for patients with stage I NSCLC who are considered to be at high risk for complications or mortality with lobectomy [Okada et al. 2006; Smith et al. 2013]. Although wedge resection and segmentectomy are the two sublobar resections that can be used to treat these patients, the type of sublobar resection that produces the better outcomes remains controversial [Okada et al. 2006; Sienel et al. 2008]. Therefore, we combined relevant studies and performed a meta-analysis to reach a conclusion on the topic. This analytical approach to studying patients with stage I NSCLC who have undergone segmentectomy or wedge resection has not been performed in the previously published literature.
In this meta-analysis, we found that segmentectomy is associated with significantly better CSS and OS in patients with stage I and stage Ia NSCLC than wedge resection, which suggests the use of segmentectomy decreases stage I NSCLC deaths at a greater rate than wedge resection. However, based on subgroup analyses in patients with T1a NSCLC, we found that the wedge resection OS and CSS rates were equivalent to those of segmentectomy. Furthermore, we noticed that the DFS outcome of wedge resection was comparable to that of segmentectomy in patients with stage Ia NSCLC. For patients with stage I NSCLC, including T1a,bN0M0, T2aN0M0, and T2bN0M0 patients, the above analysis indicates that the relative survival results of segmentectomy and wedge resection in T2aN0M0 NSCLC patients are still unknown. We could not collect the original T2aN0M0 data from all the included studies, and this is a weakness of our study. However, through this meta-analysis, we found a trend and tested a hypothesis that for patients with stage I NSCLC, the larger the tumor size, then the more obvious the advantage of segmentectomy over wedge resection would be. In addition, the results of the subgroup analysis based on the publication year periods 2005–2010 and 2011–2015 did not contradict this conclusion. In this subgroup analysis we also found that during the period 2005–2010 the OS of segmentectomy and wedge resection in patients with stage I NSCLC was comparable (Figure 7). However during the period 2010–2015 the OS of segmentectomy was superior to wedge resection in patients with stage I NSCLC (Figure 9).
There are several limitations and strengths of this study that should be noted. (a) The level of evidence was relatively low because most of the included articles were retrospective studies. (b) The radiotherapy and chemotherapy data for the cohorts could not be collected and analyzed, which might affect the survival rates of patients with stage I NSCLC in some way. (c) The most important factor affecting the results in each included study was the choice of indications for limited resection. In some included studies, patients who underwent wedge resection were at higher risk of death due to their underlying physiology and comorbidity. In addition, we assumed in this study that anyone who received sublobar resection was at high risk for negative outcomes, but not all included studies provided such detailed information. (d) Details of demographic data (e.g. size, age, histology, and margin status), which may influence the results, were not available for analysis in this study. Despite the existence of the above limitations, the results of this meta-analysis provide important evidence of the relative outcomes of segmentectomy and wedge resection.
The role of segmentectomy and wedge resection may be clarified by two ongoing randomized controlled clinical trials: a phase III randomized controlled trial (WJOG4607L/JCOG0802) launched by the West Japan Oncology Group and Japan Clinical Oncology Group in 2009 and a phase III randomized trial (CALGB 140503) launched by the National Cancer Institute in 2008. In CALGB 140503, more than 1200 patients in a multi-center design will be recruited for a randomized study comparing lobectomy and sublobar resection (segmentectomy and wedge resection) for stage Ia NSCLC with peripheral tumors that are no larger than 2 cm in diameter. Although the main purpose of this research is to compare lobectomy and sublobar resection, it is believe that a subgroup analysis comparing segmentectomy and wedge resection will also be performed. The follow-up period is 3 years, and the accrual period is 5 years. There is no doubt that the final outcomes of these two clinical trials will have an important impact on the surgical management (segmentectomy or wedge resection) of patients with stage I NSCLC who are undergoing limited resection.
In conclusion, this meta-analysis disclosed two important findings: (a) OS and CSS rates for patients with stage I NSCLC after segmentectomy are superior to those obtained after wedge resection; (b) wedge resection and segmentectomy produce similar CSS and OS rates for patients with NSCLC in stage Ia with a tumor size of 2 cm or less. Considering the limitations and heterogeneity of the included studies, the results and conclusions of the meta-analysis should be further confirmed by rigorous randomized clinical trials.
Acknowledgments
The authors thank the study participants for their contribution to the research, as well as current and past researchers and staff.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by funding from the General Program of the National Natural Science Foundation of China (81472188). The work was supported by the Third Military Medical University.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Bing Hou, Department of Thoracic Surgery, Xinqiao Hospital, Third Military Medical University, Chongqing China.
Xu-Feng Deng, Department of Thoracic Surgery, Xinqiao Hospital, Third Military Medical University, Chongqing China.
Dong Zhou, Department of Thoracic Surgery, Xinqiao Hospital, Third Military Medical University, Chongqing China.
Quan-Xing Liu, Xinqiao Hospital, Third Military Medical University, Chongqing 400037, China.
Ji-Gang Dai, Xinqiao Hospital, Third Military Medical University, Chongqing 400037, China.
References
- Bao F., Ye P., Yang Y., Wang L., Zhang C., Lv X., et al. (2014) Segmentectomy or lobectomy for early stage lung cancer: a meta-analysis. Eur J Cardiothorac Surg 46: 1–7. [DOI] [PubMed] [Google Scholar]
- Cao C., Gupta S., Chandrakumar D., Tian D., Black D., Yan T. (2014) Meta-analysis of intentional sublobar resections versus lobectomy for early stage non-small cell lung cancer. Ann Cardiothorac Surg 3: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando H., Landreneau R., Mandrekar S., Hillman S., Nichols F., Meyers B., et al. (2011) Thirty- and ninety-day outcomes after sublobar resection with and without brachytherapy for non-small cell lung cancer: results from a multicenter phase III study. J Thorac Cardiovasc Surg 142: 1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamatake D., Yoshida Y., Miyahara S., Yamashita S., Shiraishi T., Iwasaki A. (2012) Surgical outcomes of lung cancer measuring less than 1 cm in diameter. Interact Cardiovasc Thorac Surg 15: 854–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda M., Kuriyama A., Noma H., Nunobe S., Furukawa T. (2013) Hand-sewn versus mechanical esophagogastric anastomosis after esophagectomy: a systematic review and meta-analysis. Ann Surg 257: 238–248. [DOI] [PubMed] [Google Scholar]
- Jemal A., Thun M., Ries L., Howe H., Weir H., Center M., et al. (2008) Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 100: 1672–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates M., Swanson S., Wisnivesky J. (2011) Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer <=1 cm in size: a review of SEER data. Chest 139: 491–496. [DOI] [PubMed] [Google Scholar]
- Koike T., Yamato Y., Yoshiya K., Shimoyama T., Suzuki R. (2003) Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. J Thorac Cardiovasc Surg 125: 924–928. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D., Group P. (2010) Preferred reporting items for systematic reviews and meta-analyses: the Prisma statement. Int J Surg 8: 336–341. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Taniguchi Y., Miwa K., Adachi Y., Fujioka S., Haruki T., et al. (2011) Comparison of the surgical outcomes of thoracoscopic lobectomy, segmentectomy, and wedge resection for clinical stage I non-small cell lung cancer. Thorac Cardiovasc Surg 59: 137–141. [DOI] [PubMed] [Google Scholar]
- Okada M., Koike T., Higashiyama M., Yamato Y., Kodama K., Tsubota N. (2006) Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 132: 769–775. [DOI] [PubMed] [Google Scholar]
- Okada M., Nishio W., Sakamoto T., Uchino K., Yuki T., Nakagawa A., et al. (2005) Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg 129: 87–93. [DOI] [PubMed] [Google Scholar]
- Okada M., Yoshikawa K., Hatta T., Tsubota N. (2001) Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg 71: 956–961. [DOI] [PubMed] [Google Scholar]
- Sienel W., Dango S., Kirschbaum A., Cucuruz B., Horth W., Stremmel C., et al. (2008) Sublobar resections in stage Ia non-small cell lung cancer: segmentectomies result in significantly better cancer-related survival than wedge resections. Eur J Cardiothorac Surg 33: 728–734. [DOI] [PubMed] [Google Scholar]
- Smith C., Swanson S., Mhango G., Wisnivesky J. (2013) Survival after segmentectomy and wedge resection in stage I non-small-cell lung cancer. J Thorac Oncol 8: 73–78. [DOI] [PubMed] [Google Scholar]
- Spira A., Ettinger D. (2004) Multidisciplinary management of lung cancer. N Engl J Med 350: 379–392. [DOI] [PubMed] [Google Scholar]
- Sugi K., Kobayashi S., Sudou M., Sakano H., Matsuda E., Okabe K. (2010) Long-term prognosis of video-assisted limited surgery for early lung cancer. Eur J Cardiothorac Surg 37: 456–460. [DOI] [PubMed] [Google Scholar]
- Tierney J., Stewart L., Ghersi D., Burdett S., Sydes M. (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutani Y., Miyata Y., Nakayama H., Okumura S., Adachi S., Yoshimura M., et al. (2014) Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage Ia lung adenocarcinoma: wedge resection or segmentectomy. Chest 145: 66–71. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Okada A., Imakiire T., Koike T., Hirono T. (2005) Intentional limited resection for small peripheral lung cancer based on intraoperative pathologic exploration. Jpn J Thorac Cardiovasc Surg 53: 29–35. [DOI] [PubMed] [Google Scholar]
- Wisnivesky J., Henschke C., Swanson S., Yankelevitz D., Zulueta J., Marcus S., et al. (2010) Limited resection for the treatment of patients with stage Ia lung cancer. Ann Surg 251: 550–554. [DOI] [PubMed] [Google Scholar]
- Yamato Y., Koike T., Yoshiya K., Shinohara H., Toyabe S. (2008) Results of surgical treatment for small (2 cm or under) adenocarcinomas of the lung. Surg Today 38: 109–114. [DOI] [PubMed] [Google Scholar]