Abstract
Tracheoesophageal fistulas (TEFs) often occur with esophageal or bronchial carcinoma. Currently, we rely on implantation of delicate devices, such as self-expanding and silicone stents, in the esophagus or trachea to cover the fistula and expand the stenosis in order to relieve patient pain. However, because each case is different, our approach may not be effective for every patient. Consequently, new devices and technology have emerged to address these situations, such as degradable stents, Amplatzer® devices, endobronchial one-way umbrella-shaped valves, and transplantation of mesenchymal stem cells. Although some studies have shown such alternatives can be reasonable solutions in special cases, further development of other new and effectual techniques is of utmost importance.
Keywords: covered self-expanding stents, silicone stents, tracheoesophageal fistula
Introduction
Tracheoesophageal fistula (TEF) is a pathological connection between the esophagus and trachea or bronchi, or in some cases, the lung, and can occur after surgery, radiotherapy, chemotherapy, or airway invasion.1,2 Approximately 5–15% of patients develop TEF due to esophageal malignancy, while 1% are caused by bronchogenic carcinoma.3,4,5 The most common clinical signs of TEF are coughing while swallowing or drinking, purulent bronchitis, pneumonia, and dysphagia. How serious the symptoms are and how long they persist depends on the size and location of the fistula. Patients diagnosed with TEF usually suffer from malnutrition and infection, in addition to other complications, and most TEFs are inoperable. Hence, TEF is associated with a soaring mortality rate, and development of more effective treatments is necessary to increase the quality of life for patients suffering from this pathological condition.
Cause
TEF can affect the esophagus, lung, or mediastinum. Most occur spontaneously due to tumoral invasion or as a complication of cancer therapies, such as radiotherapy, surgery, chemotherapy, laser treatment, instrumentation, or pressure necrosis caused by a previously implanted stent.2 TEF caused by other tumors, such as malignant mediastinal node disease and thyroid, and laryngeal carcinomas, represent only a small percentage of cases.6 One of the largest fistula studies to date reported TEF development in 4.94% of esophageal carcinoma patients studied (n = 1943 patients), 0.16% lung cancer patients (n = 5714 patients), and 14.75% of patients with tracheal cancer (n = 41 patients).1 In contrast, other studies have estimated the incidence of TEF to be much higher at up to 10% of patients with esophageal cancer.7,8 Balazs and colleagues4 showed that 243 out of 264 patients with TEF had esophageal cancer, 19 had pulmonary tumors, and two had mediastinal tumors. Moreover, some reports suggest that antiangiogenic therapies, such as bevacizumab, may play a role in TEF development when combined with radiation.9,10 Because TEFs are an opening between the esophagus and airways, saliva, food, and possibly acidic gastric contents could get into the lungs, promoting pneumonia, and in some situations, can lead to sepsis. Consequently, the normal route for obtaining nutrients becomes hazardous, making it necessary to bypass the esophagus via nasogastric tube or jejunum nutrient catheter.
Diagnosis
Patients suffering from frequent cough, aspiration, with or without fever with pneumonia could be TEF candidates, especially those previously diagnosed with esophageal malignancy. Additionally, those presenting with severe septic conditions with or without aspiration pneumonia most frequently have TEF.4 Years ago, a diagnosis of TEF was made if barium or Gastrografin swallow unexpectedly outlined the airways. In recent years, the planned diagnostic approach has been flexible bronchoscopy, sometimes followed by gastroscopy, under normal conditions. TEFs located in the posterior wall of the trachea can usually be identified by endoscope. However, the location, size, and appearance of TEFs can vary widely in different patients, and standard diagnostic guidelines may not be applicable to every case. Furthermore, very small fistulas can be overlooked if the mucosa is red and swollen.
At present, there are very few clinical guidelines for proper localization of TEFs. Previously, Wang and colleagues11 developed a more comprehensive TEF classification system modeled on one established for central airway stenosis. The proposed system includes eight zones (see figure 1) which classify various fistula locations; locations I–III were defined the same as that for central airway stenosis, while locations IV–VIII pertaining to the trachea carina and right main, right middle, proximal, and distal bronchi, respectively, were new (see figures 2 and 3).12
Figure 1.
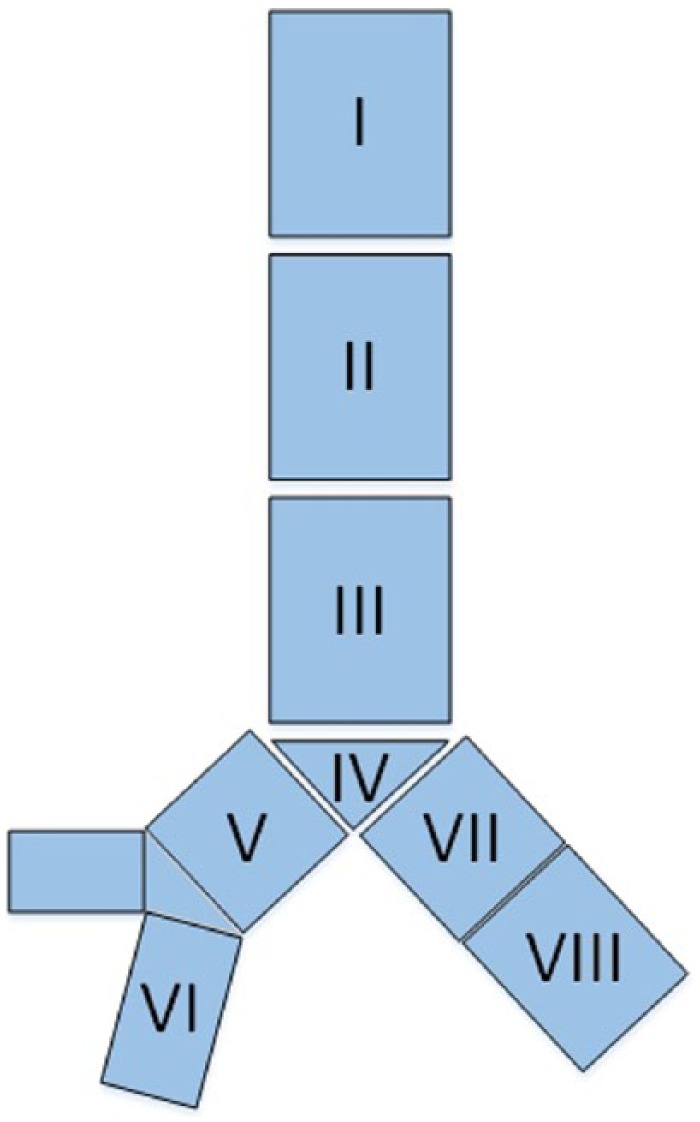
Eight locations of the central airway: (1) location I, upper third of the trachea; (2) location II, middle third of the trachea; (3) location III, lower third of the trachea; (4) location IV, trachea carina; (5) location V, right main bronchus; (6) location VI, right middle bronchus; (7) location VII, proximal of left main bronchus; (8) location VIII, distal of left main bronchus.11
Figure 2.
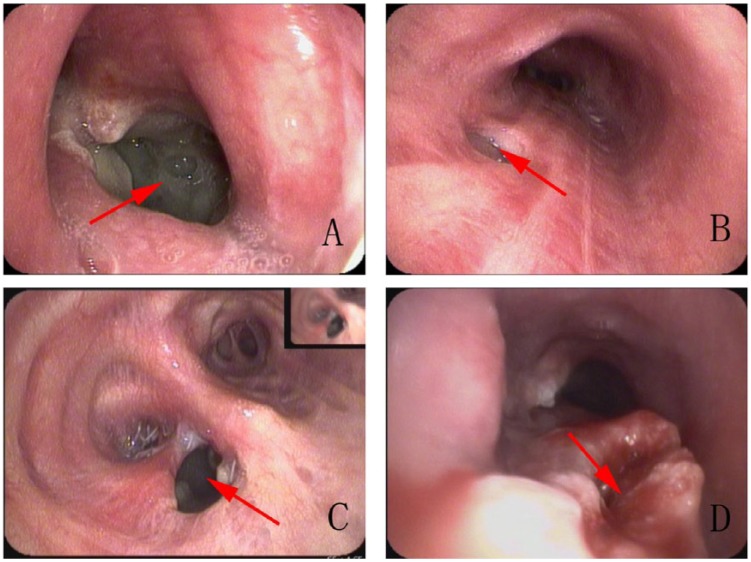
Picture A shows a right lung carcinoma patient who had been performed a partial lobectomy surgery 2 months ago, and presently suffering a fever and constantly secreting phlegm. Picture B is an esophagus cancer patient, in whom after resection surgery, a right main bronchus-located transesopogeal fistula (TEF) was formed. Picture C shows a patient just resembling patient A, with a different location of Part VII. Picture D shows a patient with tremendous high TEF, a great volume of gastric content draw back to trachea, then flow into the lungs via fistula attributed to the suction pressure of inspiration.
Figure 3.
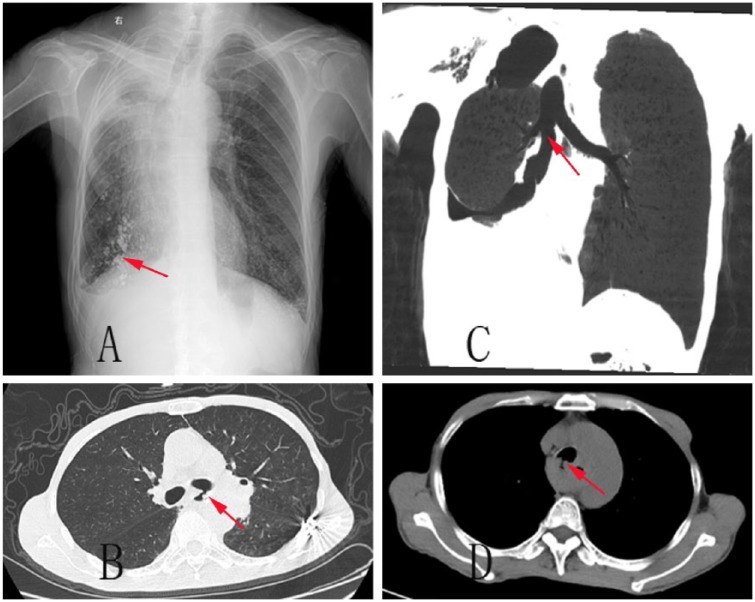
This picture ABCD shows the counterparts imaging of Figure 2. Picture A shows a lipodolo hysecrosalpingography of counterpart of Figure 2. Picture B shows the coronal view of the counterpart of Figure 2. Picture C and D show the common computerized tompography scan of counterpart of Figure 2.
While TEFs usually develop in the middle third of the esophagus, the lower-third usually corresponds to longer tumor life and local recurrence.4 Hopefully, this new classification system will enable more precise localization of TEFs and thereby, facilitate administration of more appropriate treatments in future.
Treatment
Because most patients diagnosed with TEF usually undergo chemotherapy or radiotherapy, their general condition is usually severely deteriorated and accompanied by recurrent aspiration pneumonia, which can lead to respiratory insufficiency. Although surgical intervention remains the first choice of treatment for these patients, major surgery for esophageal exclusion or extra-anatomical reconstruction of the gastrointestinal passage is not possible. Thus, approaches affording rapid and minimally invasive closure of the fistula to stop repeated aspiration are of utmost importance. Here, we describe a number of alternative treatments involving the use of stents for patients unsuitable for surgery.
Airway stents
The first insertion of a metallic stent into the trachea was in 1952.13 Since then, airway stents made of diverse materials has become important for the treatment of airway obstruction or fistula caused by malignant or benign diseases of the lung or esophagus. However, this technique is usually only selected for inoperable patients since surgical treatment is only justified in select cases and carries a very high morbidity and mortality.6 Recently, airway stent implantation has become overwhelmingly prevalent.
Covered self-expanding stents
There are two kinds of metallic stents used prevalently today, second- and third-generation (or current) stents. First-generation stents were largely discontinued due to their inflexibility (e.g. Gianturco stents) and are rarely used today.14 The second-generation metallic WallstentTM (Schneider Europe, Zurich, Switzerland) is a self-expanding, tubular mesh that is delivered in a constrained form and expands to a preset diameter once its released.15 Similarly, the Schneider prosthesis (Zurich, Switzerland) is a cobalt-based superalloy, tubular mesh that can be inserted into a variety of different bronchial or trachea diameters using a flexible fiber-optic bronchoscope. The UltraflexTM (Boston Scientific; Natick, MA, USA) stent is a third-generation WallstentTM made of nickel–titanium alloy with ‘shape memory’ capability, meaning that at low temperatures, the alloy deforms plastically into a martensitic state, and at higher temperatures, it returns to its original austenitic-state shape.16
When inserting a covered self-expanding stent via flexible bronchoscope, a guidewire must first be delivered through the bronchus to a point below the fistula, then the loaded delivery catheter can be advanced over the guidewire until the lower edges of the fistula can be observed by the bronchoscope.17 Metallic stents can expand firmly against the trachea wall with mere migration. At the same time, tumor or granulated tissue can grow through stent interstices, which make it impossible to remove a delivered stent. Moreover, metal stents will eventually fracture after a relatively long time (500–1000 days),18 which could be disastrous as metallic debris may impale the trachea and enter the mediastinum or even nearby arteries. Nonetheless, there are still significant advantages to this type of stent, especially its ability to accommodate different tracheal dimensions and facilitate better clearance of secretions.17,19
Silicone stents
Silicone stents came into use in the 1990s. Dumon and colleagues20 first introduced a dedicated tracheobronchial stent used to treat external compression of the main airway. Later, Weigert and colleagues21 reported short-term palliation of symptoms in eight patients suffering from esophagorespiratory fistulas caused by esophageal or bronchial carcinoma after implantation of a silicon-coated, modified Gianturco metal stent. Silicone was chosen as the main component of these stents due to its flexibility and compatibility with living tissue.20 While the outer shape of silicone stents is quite different from that of their metallic counterparts, they can be molded into a tubular shape, and placement of external studs at regular intervals on the outside prevent dislodgment. However, certain characteristics of the silicone material along with their shape make these stents incapable of fitting into a delivery device and expanding upon delivery. To circumvent this issue, a rigid bronchoscope can be used to provide a wider space for silicone stent delivery under general anesthesia.
Sometimes, a silicone stent may be the ultimate solution to a very complex case. For example, Jose and colleagues22 reported a case of poorly differentiated squamous cell carcinoma of the mid-esophagus that underwent surgery to remove foci, then covered the cavity with an expanding esophageal stent to palliate dysphagia. Unfortunately, a TEF developed 2 months later, and a DumonTM Y-stent (Novatech, La Ciotat, France) was applied via rigid bronchoscope, which elevated the patient’s health and quality of life. Nonetheless, while silicone is an excellent material, stent-associated respiratory tract infection is a serious problem to consider.23 When a silicone stent’s outer shape is completely covered and the inside space is fully expanded, it comes in close contact with and can adhere to the bronchi or trachea, which can hamper mucociliary clearance systems and promote infection. Although external studs prevent migration, dislodgement still ranks at the top of the silicone stent-related complications list.24 Sometimes a silicone stent can even be coughed out through the mouth. On the other hand, this disadvantage can also be advantageous if the stent should need removal after delivery.
Double stents
For anatomical reasons, an esophageal stent should not be implanted along because it may compress the trachea, leading to suffocation. Therefore, when use of double stents is proposed, tracheal stent implantation should be the initial procedure. In 2001, Yamamoto and colleagues25 reported on 11 patients (8 with combined esophago-airway stenosis; 3 with fistulas) who underwent successful double stent implantation and received significant relief from dyspnea and dysphagia. Moreover, fistulas were successfully closed in all except one ventilator-dependent patient. This palliation was also reported to last for a relatively long period of time (5 patients, >1 month; 3 patients, >2 months; 2 patients, >3 months). A prospective assessment of the influence of stent type and malignant airway esophageal fistulas on survival in 112 patients showed a mean survival of 219.1 days [95% confidence interval (CI): 197.3–240.9 days] for those with airway stents (n = 65), 262.8 days (95% CI: 244.4–281.3 days) for those with esophageal stents (n = 37), and 252.9 d (95% CI: 192.9–312.9 days) for patients who received double stents (n = 10).26 Though we cannot definitively conclude which stent type was best, based solely on survival time, we predict that those patients who received single airway stents might further benefit from implantation of a second, esophageal stent.
Over-the-scope clipping
OTSC (Ovesco Endoscopy GmbH, Tubingen, Germany) is a new technique first introduced by Kirschniak and colleagues27 that enables closure of gastrointestinal defects (fistula, perforations, leaks, etc.). Gastrointestinal OTSCs consist of a nitinol clip shaped like a bear claw which is attached to an applicator integrated onto the tip of an endoscope. After being adapted for TEF cases, both Traina and colleagues and Vinnamala and colleagues reported good outcomes.28,29 It is important to note that OTSCs need a soft and extensible tissue in order to launch; therefore, we usually apply it to the gastrointestinal side when TEFs develop. In this way, we significantly reduce the amount of secretions into the respiratory tract via gastrointestinal fistulas. Also, another kind of OTS-Clip named Padlock Clip (Aponos Medical, Kingston, NH, United States) has emerged in recent years. That owns a different out-shape as a nitinol ring, with six inner needles preassembled on an applicator cap, thumb press displaced by the Lock-It™ (Aponos Medical Corp., Kingston, New Hampshire, USA) delivery system. Because no requirement of the working channel, a more efficient suction to ensure tissue adhesion to the instrument tip can be achieved. Up to now, the Padlock Clip™ has been tested in survival porcine models for the closure of wall defects.30,31 And a few cases have been reported to apply this kind of device in recurrent or refractory GI bleeding or fistulas and get for a short time, good outcomes.32 There is no report of introducing it into tracheoesophageal treatment until now. OTSCs surpass conventional endoclips in terms of breadth and closure power, as well as launched shape, which does not block swallowing of food. As a result, this device should improve the number of therapeutic options available to more TEF cases.
Other treatments
Other airway fistula treatments include fibrin glue, degradable stents, Amplatzer® (AGA Medical; Golden Valley, Minnesota, USA) devices, endobronchial one-way umbrella-shaped valves, septal buttons, and transplantation of mesenchymal stem cells. Fibrin glue injections coagulate immediately, blocking bronchoplural fistulas and relieving symptoms. However, small fistulas (<5 mm) are more likely to be successfully treated endoscopically, whereas endoscopic closure alone is not suitable for large fistulas (>8 mm), and a combination of glue injection can fatally damage the bronchoscope if glue flows into the working channel.33 Degradable stents are inserted into patients that do not need permanent expansion of stenosis or covering of a fistula (e.g. infants and children). Attributable to its material, such stents usually have a lower radial force than other types, and only uncovered stents are available, making them inconvenient for malignant conditions or fistulas.34 Amplatzer® devices were originally designed for transcatheter closure of cardiac defects and may be suitable for both large and small BPFs that originate in the main and lobar bronchi.35 Endobronchial one-way umbrella-shaped valves were originally developed for endoscopic lung volume reduction surgery, but have also been shown to be a splendid tool for BPF treatment36 due to its ability to prevent air flow back through the disordered airway, which minimizes the air leak and allows the fistula to eventually close naturally. On the other hand, Schmitz and colleagues37 showed that simple insertion of a septal button through the TEF can significantly improve a patient’s quality of life after failure of standard approaches.
Lastly, Petrella and colleagues38 recently reported bronchoscopic transplantation of mesenchymal stem cells derived from bone marrow and successfully closed a bronchopleural fistula that developed after right extrapleural pneumonectomy for early-stage malignant mesothelioma. Although these results are very promising, further research on the effects of mesenchymal stem cell transplantation therapy on pulmonary defects is required. Nevertheless, we predict such therapy will be most useful for treatment of smaller bronchopleural and distal fistulas and cracks. Furthermore, it is possible that palliative, and even curative therapies for TEFs involving mesenchymal stem cells derived from bone marrow may sustain longer and greater improvements in symptoms, while successfully treating tumors.
Conclusion
TEF, together with malignant esophageal and pulmonary tumors, can be fatal apart from the carcinoma itself. This is largely due to the fact that most of these patients cannot undergo surgery owing to their poor overall condition. In these cases, stents offer an alternative to major surgery and can sometimes be a superior way to alleviate symptoms. In general, two main types of stents are used currently. Covered self-expanding stents are relatively simple to insert, can firmly expand and cover the damaged area, and have a low migration rate. However, a group of sophisticated physicians is necessary to ensure proper and precise insertion. Silicone stents are empty cylinders with a fully closed outer shape with external studs to prevent dislodgement. While this new type of stent is flexible, migration and adhesion are still major issues affecting airway tissues. Other nonsurgical, nonstent treatments include transplantation of mesenchymal stem cells derived from bone marrow which can successfully treat 3 mm bronchopleural fistulas, one-way umbrella-shaped endobronchial valves, and fibrin glue injection. However, we chose the very technology for the very patient to alleviate agony and improve health-related quality of life.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Changzhi Zhou, Department of Respiratory, Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Yi Hu, Department of Respiratory, Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Shengli Street No. 26, Wuhan 430014, China.
Yang Xiao, Department of Respiratory, Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Wen Yin, Department of Respiratory, Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
References
- 1. Martini N, Goodner JT, D’Angio GJ, et al. Tracheoesophageal fistula due to cancer. J Thorac Cardiovasc Surg 1970; 59: 319–324. [PubMed] [Google Scholar]
- 2. Morgan RA, Ellul JP, Denton ER, et al. Malignant esophageal fistulas and perforations: management with plastic-covered metallic endoprostheses. Radiology 1997; 204: 527–532. [DOI] [PubMed] [Google Scholar]
- 3. Shin JH, Song HY, Ko GY, et al. Esophagorespiratory fistula: long-term results of palliative treatment with covered expandable metallic stents in 61 patients. Radiology 2004; 232: 252–259. [DOI] [PubMed] [Google Scholar]
- 4. Balazs A, Kupcsulik PK, Galambos Z. Esophagorespiratory fistulas of tumorous origin: non-operative management of 264 cases in a 20-year period. Eur J Cardiothorac Surg 2008; 34: 1103–1107. [DOI] [PubMed] [Google Scholar]
- 5. Chen YH, Li SH, Chiu YC, et al. Comparative study of esophageal stent and feeding gastrostomy/jejunostomy for tracheoesophageal fistula caused by esophageal squamous cell carcinoma. PLoS One 2012; 7: e42766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reed MF, Mathisen DJ. Tracheoesophageal fistula. Chest Surg Clin N Am 2003; 13: 271–289. [DOI] [PubMed] [Google Scholar]
- 7. Smith JL, Michaletz PA, Tabibian N, et al. Improved palliation of a respiratory-esophageal fistula with a cuffed esophageal prosthesis. Am J Gastroenterol 1987; 82: 1175–1176. [PubMed] [Google Scholar]
- 8. Tomaselli F, Maier A, Sankin O, et al. Successful endoscopical sealing of malignant esophageotracheal fistulae by using a covered self-expandable stenting system. Eur J Cardiothorac Surg 2001; 20: 734–738. [DOI] [PubMed] [Google Scholar]
- 9. Goodgame B, Veeramachaneni N, Patterson A, et al. Tracheo-esophageal fistula with bevacizumab after mediastinal radiation. J Thorac Oncol 2008; 3: 1080–1081. [DOI] [PubMed] [Google Scholar]
- 10. Gore E, Currey A, Choong N. Tracheoesophageal fistula associated with bevacizumab 21 months after completion of radiation therapy. J Thorac Oncol 2009; 4: 1590–1591. [DOI] [PubMed] [Google Scholar]
- 11. Wang H, Tao M, Zhang N, et al. Airway covered metallic stent based on different fistula location and size in malignant tracheoesophageal fistula. Am J Med Sci 2015; 350: 364–368. [DOI] [PubMed] [Google Scholar]
- 12. Freitag L, Ernst A, Unger M, et al. A proposed classification system of central airway stenosis. Eur Respir J 2007; 30: 7–12. [DOI] [PubMed] [Google Scholar]
- 13. Harkins WB. An endotracheal metallic prosthesis in the treatment of stenosis of the upper trachea. Ann Otol Rhinol Laryngol 1952; 61: 663–676. [DOI] [PubMed] [Google Scholar]
- 14. Shaffer JP, Allen JN. The use of expandable metal stents to facilitate extubation in patients with large airway obstruction. Chest 1998; 114: 1378–1382. [DOI] [PubMed] [Google Scholar]
- 15. Carre P, Rousseau H, Lombart L, et al. Balloon dilatation and self-expanding metal Wallstent insertion: for management of bronchostenosis following lung transplantation. The Toulouse Lung Transplantation Group. Chest 1994; 105: 343–348. [DOI] [PubMed] [Google Scholar]
- 16. Vinograd I, Klin B, Brosh T, et al. A new intratracheal stent made from nitinol, an alloy with “shape memory effect”. J Thorac Cardiovasc Surg 1994; 107: 1255–1261. [PubMed] [Google Scholar]
- 17. Madden BP, Datta S, Charokopos N. Experience with ultraflex expandable metallic stents in the management of endobronchial pathology. Ann Thorac Surg 2002; 73: 938–944. [DOI] [PubMed] [Google Scholar]
- 18. Chung FT, Lin SM, Chen HC, et al. factors leading to tracheobronchial self-expandable metallic stent fracture. J Thorac Cardiovasc Surg 2008; 136: 1328–1335. [DOI] [PubMed] [Google Scholar]
- 19. Monnier P, Mudry A, Stanzel F, et al. The use of the covered wallstent for the palliative treatment of inoperable tracheobronchial cancers: a prospective, multicenter study. Chest 1996; 110: 1161–1168. [DOI] [PubMed] [Google Scholar]
- 20. Dumon JF. A dedicated tracheobronchial stent. Chest 1990; 97: 328–332. [DOI] [PubMed] [Google Scholar]
- 21. Weigert N, Neuhaus H, Rosch T, et al. Treatment of esophagorespiratory fistulas with silicone-coated self-expanding metal stents. Gastrointest Endosc 1995; 41: 490–496. [DOI] [PubMed] [Google Scholar]
- 22. Melendez J, Chu D, Bakaeen FG, et al. Tracheoesophageal fistula due to migration of a self-expanding esophageal stent successfully treated with a silicone “Y” tracheobronchial stent. J Thorac Cardiovasc Surg 2011; 141: e43–e44. [DOI] [PubMed] [Google Scholar]
- 23. Agrafiotis M, Siempos II, Falagas ME. Infections related to airway stenting: a systematic review. Respiration 2009; 78: 69–74. [DOI] [PubMed] [Google Scholar]
- 24. Mitsuoka M, Sakuragi T, Itoh T. Clinical benefits and complications of dumon stent insertion for the treatment of severe central airway stenosis or airway fistula. Gen Thorac Cardiovasc Surg 2007; 55: 275–280. [DOI] [PubMed] [Google Scholar]
- 25. Yamamoto R, Tada H, Kishi A, et al. Double stent for malignant combined esophago-airway lesions. Jpn J Thorac Cardiovasc Surg 2002; 50: 1–5. [DOI] [PubMed] [Google Scholar]
- 26. Herth FJ, Peter S, Baty F, et al. Combined airway and oesophageal stenting in malignant airway-oesophageal fistulas: a prospective study. Eur Respir J 2010; 36: 1370–1374. [DOI] [PubMed] [Google Scholar]
- 27. Kirschniak A, Kratt T, Stuker D, et al. A new endoscopic over-the-scope clip system for treatment of lesions and bleeding in the GI tract: first clinical experiences. Gastrointest Endosc 2007; 66: 162–167. [DOI] [PubMed] [Google Scholar]
- 28. Traina M, Curcio G, Tarantino I, et al. New endoscopic over-the-scope clip system for closure of a chronic tracheoesophageal fistula. Endoscopy 2010; 42(Suppl. 2): E54–E55. [DOI] [PubMed] [Google Scholar]
- 29. Vinnamala S, Murthy B, Parmar J, et al. Rendezvous technique using bronchoscopy and gastroscopy to close a tracheoesophageal fistula by placement of an over-the-scope clip. Endoscopy 2014; 46(Suppl. 1) UCTN: E301. [DOI] [PubMed] [Google Scholar]
- 30. Desilets DJ, Romanelli JR, Earle DB, et al. Gastrotomy closure with the lock-it system and the Padlock-G clip: a survival study in a porcine model. J Laparoendosc Adv Surg Tech A 2010; 20: 671–676. [DOI] [PubMed] [Google Scholar]
- 31. Guarner-Argente C, Cordova H, Martinez-Palli G, et al. Yes, we can: reliable colonic closure with the Padlock-G clip in a survival porcine study (with video). Gastrointest Endosc 2010; 72: 841–844. [DOI] [PubMed] [Google Scholar]
- 32. Armellini E, Crino SF, Orsello M, et al. Novel endoscopic over-the-scope clip system. World J Gastroenterol 2015; 21: 13587–13592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Glover W, Chavis TV, Daniel TM, et al. Fibrin glue application through the flexible fiberoptic bronchoscope: closure of bronchopleural fistulas. J Thorac Cardiovasc Surg 1987; 93: 470–472. [PubMed] [Google Scholar]
- 34. Dutau H, Musani AI, Laroumagne S, et al. Biodegradable airway stents – bench to bedside: a comprehensive review. Respiration 2015; 90: 512–521. [DOI] [PubMed] [Google Scholar]
- 35. Fruchter O, Kramer MR, Dagan T, et al. Endobronchial closure of bronchopleural fistulae using amplatzer devices: our experience and literature review. Chest 2011; 139: 682–687. [DOI] [PubMed] [Google Scholar]
- 36. Travaline JM, Mckenna RJ, Jr, De Giacomo T, et al. Treatment of persistent pulmonary air leaks using endobronchial valves. Chest 2009; 136: 355–360. [DOI] [PubMed] [Google Scholar]
- 37. Schmitz S, Van Damme JP, Hamoir M. A simple technique for closure of persistent tracheoesophageal fistula after total laryngectomy. Otolaryngol Head Neck Surg 2009; 140: 601–603. [DOI] [PubMed] [Google Scholar]
- 38. Petrella F, Spaggiari L, Acocella F, et al. Airway fistula closure after stem-cell infusion. N Engl J Med 2015; 372: 96–97. [DOI] [PubMed] [Google Scholar]