Abstract
Background:
Direct hemoperfusion using polymyxin B-immobilized fiber column (PMX-DHP) therapy has been approved for sepsis-associated acute respiratory distress syndrome, but its efficacy for other rapidly progressive interstitial pneumonias (RPIPs) is unclear. The purpose of this study was to examine the efficacy of PMX-DHP therapy for acute respiratory failure in patients with RPIPs, when compared with a historical control receiving conventional treatment without PMX-DHP.
Methods:
This study comprised 77 patients with RPIPs in our institute between January 2002 and December 2015. The initial 36 patients between January 2002 and March 2007 were treated without PMX-DHP (historical control group), and the following 41 patients between April 2007 and December 2015 were treated with PMX-DHP (PMX-DHP group) once daily for two successive days concurrently with corticosteroids and/or immunosuppressive agents. The 90-day mortality and clinical factors were compared between the groups. Cox proportional hazards models were constructed to analyze 90-day mortality and identify predictors.
Results:
The 90-day mortality rate was significantly lower in the PMX-DHP group than in the controls (41.5% versus 66.7%, p = 0.019). PMX-DHP therapy was significantly associated with mortality (hazard ratio 0.505; 95% confidence interval, 0.270–0.904; p = 0.032). There were significant differences in the serial changes in the PaO2/FiO2 ratio, SOFA score, and blood neutrophil counts from days 0–5 after PMX-DHP between the survivor and non-survivor groups (p = 0.015, p < 0.001, p = 0.035, respectively). The improved PaO2/FiO2 ratio on day 3 significantly correlated with the change in blood neutrophil counts (rs = −0.431, p = 0.006).
Conclusions:
PMX-DHP therapy may be effective in RPIPs patients accompanied by acute respiratory failure and is expected to reduce mortality rates.
Keywords: connective tissue disease-associated interstitial pneumonia, direct hemoperfusion using polymyxin B-immobilized fiber column, idiopathic interstitial pneumonias, PMX-DHP, rapidly progressive interstitial pneumonias
Introduction
Rapidly progressive interstitial pneumonias (RPIPs) such as acute interstitial pneumonia (AIP), acute exacerbation of idiopathic pulmonary fibrosis (IPF), and acute exacerbation of other interstitial pneumonias are life-threatening conditions characterized by progressive dyspnea and severe acute hypoxemia.1–6 Aggressive supportive therapies for RPIPs, including high-dose corticosteroids (CS) alone or in combination with immunosuppressive agents such as cyclophosphamide and cyclosporine, have been performed. However, these patients are occasionally refractory to conventional therapies, and the mortality remains high at 50–80%.1–3,6
Direct hemoperfusion using polymyxin B-immobilized fiber column (PMX-DHP) therapy as an extracorporeal blood filter has been developed to remove blood endotoxin and is used for the treatment of patients with endotoxemia and septic shock.7–9 PMX-DHP therapy has been reported to improve not only hemodynamic status, but also respiratory dysfunction in patients with septic acute respiratory distress syndrome (ARDS).10–12 These studies also demonstrated that PMX-DHP may adsorb and eliminate increased levels of inflammatory cytokines and mediators and activated leukocytes, which are thought to have an essential role in the pathogenesis of ARDS.
There have been several reports of case series in which a combination of hemoperfusion of PMX and conventional therapies was used for the treatment of RPIPs, including IPF, connective tissue disease-associated interstitial pneumonia (CTD-IP), and drug-induced pneumonia.13–15 Recently, a multicenter retrospective study, including acute exacerbation of IPF patients, revealed that the PMX-DHP treatment of RPIPs with acute respiratory failure might have a beneficial effect on oxygenation and survival.16 We also reported that a combination of PMX-DHP and conventional therapies was effective against fatal drug-induced interstitial pneumonia and RPIP due to clinically amyopathic dermatomyositis (DM).15,17 However, the effects of PMX-DHP treatment on acute respiratory failure during RPIPs have not been fully established. The aim of this study was to evaluate the efficacy of PMX-DHP as an add-on therapy for acute respiratory failure in patients with RPIPs by retrospectively comparing the outcomes of patients with RPIPs who were treated either with conventional therapies plus PMX-DHP (PMX-DHP group) or with conventional therapies alone (historical control group).
Methods
Study population
We performed a historical control study in a single institute, Kumamoto University Hospital. The study population comprised 77 patients with RPIPs, including idiopathic interstitial pneumonias (IIPs) and CTD-IP, who were treated with a combination of conventional therapies and PMX-DHP or conventional therapies alone from 2002 to 2015. Patients with IIPs, including IPF, nonspecific interstitial pneumonia (NSIP), and AIP were diagnosed according to the international consensus classification of the American Thoracic Society/European Respiratory Society.18,19 CTD-IP, including rheumatoid arthritis, DM, polymyositis (PM), systemic lupus erythematosus (SLE), systemic sclerosis (SSc), Sjögren syndrome (SjS), mixed connective tissue disease (MCTD), and microscopic polyangiitis (MPA) were diagnosed based on each established criteria.20 RPIPs including acute exacerbation of IPF were defined using the criteria proposed by Collard and colleagues,2 with slight modifications for adaptation to interstitial pneumonias other than IPF. Briefly, the criteria for diagnosis of RPIPs were as follows: (1) previous or concurrent diagnosis of interstitial pneumonias; (2) unexplained worsening of dyspnea within 1 month; (3) evidence of hypoxemia as defined by the partial pressure of arterial O2 (PaO2)/fraction of inspired O2 (FiO2) (P/F) ratio <300 mmHg; (4) high-resolution computed tomography (HRCT) findings with newly developed ground-glass opacities and/or consolidations; (5) no evidence of pulmonary infection on bronchoalveolar lavage and sputum culture and negative results on blood tests for other potentially infectious pathogens; and (6) exclusion of left heart failure, pulmonary embolism, pneumothorax, and other possible causes of acute respiratory failure.14 For inclusion in this study, written informed consent was obtained from each patient. When an unconscious patient under sedation for mechanical ventilation was not able to give consent, the consent was obtained from a family member. Blood endotoxin levels were determined by the Endospecy test.
From April 2007 to December 2015, 41 patients who met the inclusion criteria were eligible for treatment with a combination of PMX-DHP and conventional therapies (PMX-DHP group). From January 2002 to March 2007, 36 patients who met the inclusion criteria and who were treated with conventional therapies alone were nominated as the historical comparison controls (historical control group). All patient records were reviewed to obtain demographic data and details of initial presentation at the start of PMX-DHP and/or conventional therapies. In the historical control, three of seven IPF and one of eight idiopathic NSIP were pathologically confirmed by surgical lung biopsy. In the PMX-DHP group, two of five IPF and two of nine idiopathic NSIP were pathologically confirmed. This study followed the principles of the Declaration of Helsinki; the study protocol was approved by the Human Ethics Review Committee of Kumamoto University Hospital (No. 2034), and written informed consent in the PMX-DHP group was obtained from all patients or their family members for the use of PMX-DHP.
PMX-DHP and conventional therapies
We performed PMX-DHP therapy (Toraymyxin; Toray Medical Co., Tokyo, Japan) in RPIPs patients with acute respiratory failure who were resistant to conventional therapies, including high-dose CS and/or immunosuppressive agents. There were no pre-specified criteria for defining resistance to conventional therapy; however, in most cases resistance to the therapy was judged by two or more of the following: (1) worsening of dyspnea; (2) increase of parenchymal abnormalities on chest radiograph or HRCT; and (3) deterioration of P/F ratio. Inclusion criteria of PMX-DHP therapy for RPIPs patients were as follows: (1) current diagnosis of RPIPs; (2) resistance to conventional therapy judged by an attending physician. Exclusion criteria of PMX-DHP were as follows: (1) >85 years old; (2) needed continuous hemodiafiltration; (3) history of cerebrovascular disorder within 1 year; (4) pregnancy or breastfeeding; (5) liver cirrhosis or other serious liver disorders; (6) severe hemorrhagic diseases; (7) advanced cancer; and (8) considered ineligible for PMX-DHP by an attending physician. Direct hemoperfusion with PMX was performed using conventional equipment for hemoperfusion and a circuit for hemodialysis. For venous access, a double-lumen catheter was inserted into the femoral vein using Seldinger’s method. Direct hemoperfusion was performed at a flow rate of 80–100 ml/min for 4 h twice, with a time interval of approximately 24 h. Nafamostat mesylate (Torii Pharma Co. Ltd., Tokyo, Japan) was used as an anticoagulant. For the conventional therapies, CS alone or in combination with immunosuppressive agents, including cyclophosphamide, cyclosporine, or tacrolimus, were administered to all patients with RPIPs in this study. All patients received 1–3 courses of high-dose CS pulse therapy (methylprednisolone 1000 mg/day for 3 consecutive days) followed by tapering doses of prednisolone with or without cyclophosphamide (500 mg/m2 every 3–4 weeks) or cyclosporine (2–3 mg/kg/day, followed by adjustment to trough levels of 100–150 ng/ml) or both. Tacrolimus (1–3 mg/day, followed by adjustment to trough levels of 5–10 ng/ml), instead of cyclosporine, was also added to the regimen in some patients. Respiratory supports such as oxygen inhalation therapy, noninvasive positive pressure ventilation, and invasive positive pressure ventilation were provided to the patients with respiratory failure at the attending physician’s discretion in each case.
Data collection
We defined the day when PMX-DHP and/or conventional therapies were initiated as day 0. The patients were followed for 90 days after the therapies. All of the patients’ backgrounds, including age, sex, smoking status, clinical diagnosis, contents of previous treatments, peripheral blood counts, blood biochemistry, P/F ratio, Acute Physiology and Chronic Health Evaluation (APACHE) II score,21 and Sequential Organ Failure Assessment (SOFA) score,22 at the initial treatments were compared between the two groups. Blood samples were collected at diagnosis, just before PMX-DHP therapy, and at 3 and 5 days after the initial PMX-DHP. Survival time was defined as days from initial treatment for RPIPs to death. We also evaluated 90-day mortality rates. The mortality rates were calculated based on all causes of death, and the cumulative mortality rate was estimated using the Kaplan–Meier method; intergroup differences were tested using the log-rank test. In the PMX-DHP group, the enrolled patients were divided into the groups of survivors and non-survivors on day 90 to investigate the effects of PMX-DHP therapy by identifying clinical and laboratory differences by recording the clinical and physiological data just before and at 3 and 5 days after the PMX-DHP therapy.
Statistical analysis
Clinical and laboratory data were collected from the patients’ medical records. Continuous variables were expressed as the mean ± standard deviation unless otherwise stated and were compared using the Mann–Whitney U-test. Categorized variables were analyzed using the chi-square test or Fisher’s exact test as appropriate for the sample size. Spearman’s rank correlation (rs) test was used to assess the relationship between improved P/F ratio after PMX-DHP therapy and changes of neutrophil counts in blood. The Cox proportional hazards regression model was used for univariate and multivariate analyses to determine the significant predictors of survival. A p-value <0.05 was considered to indicate statistical significance. Comparisons of the P/F ratio, SOFA score, serum levels of C-reactive protein (CRP), lactate dehydrogenase (LDH), Krebs von den Lungen-6 (KL-6), and neutrophil counts in blood between the groups of survivors and non-survivors over time after PMX-DHP were analyzed by repeated measures analysis of variance (ANOVA) adjusted for the baseline values as a covariate and by post-hoc Bonferroni test. The last observation carried forward method for missing data was used for the analysis. Missing samples occurred because of death or samples not drawn. On the basis of the mortality in our retrospective study of acute exacerbation of IPF,23 if a 50% reduction in the hazard ratio (HR) could be achieved in the PMX-DHP group, then, assuming an α of 0.05 and 80% power, a sample size of at least 72 subjects was needed. All statistical analyses were performed using the Statistical Package for the Social Sciences version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics
During the study period, 77 patients fulfilled the inclusion criteria described above. Forty-one patients were eligible for the treatments with PMX-DHP (PMX-DHP group), and 36 patients were eligible for the treatments without PMX-DHP (historical control group). The baseline characteristics of the study population are shown in Table 1. There were no significant differences in age, sex, smoking status, clinical diagnosis, and previous therapy between the two groups. In the PMX-DHP group, four patients with DM were positive for anti-ARS antibody and the remaining eight patients were positive for anti-MDA-5 antibody. In the historical control, all five patients with DM were negative for anti-Jo-1 antibody. In the PMX-DHP group, 17 patients received CS therapy before the onset of RPIPs; eight of these patients underwent immunosuppressive therapy with cyclosporine (n = 7) or tacrolimus (n = 1), and one patient received pirfenidone as an antifibrotic agent. In the control group, 16 patients received CS before onset; seven patients underwent immunosuppressive therapy with cyclosporine (n = 6) or cyclophosphamide (n = 1). The mean white blood cell counts, neutrophils, CRP, LDH, KL-6, and surfactant protein-D were elevated in both groups, but were not significantly different. There was no significant difference in the respiratory failure indices between the groups on admission (mean P/F ratio; PMX-DHP group, 160.1 ± 60.9 mmHg versus control group, 172.1 ± 74.3 mmHg; p = 0.540). The APACHE II and SOFA scores were not significantly different between the two groups. Endotoxin levels before initial treatment and PMX-DHP therapy were within the normal range in all patients.
Table 1.
Baseline characteristics of RPIPs patients on initial therapy.
| Historical control group | PMX-DHP group | p-value | |
|---|---|---|---|
| Case no. | 36 | 41 | |
| Sex (male/female) | 15/21 | 20/21 | 0.692 |
| Smoking status (current/ex-/never) | 2/12/22 | 2/18/21 | 0.696 |
| Clinical diagnosis | |||
| IPF, AE | 7 | 5 | 0.531 |
| Idiopathic NSIP, AE | 8 | 9 | >0.99 |
| AIP | 4 | 6 | 0.743 |
| CTD-IP, AE | 17 | 21 | 0.903 |
| DM | 5 | 12 | 0.168 |
| PM | 1 | 4 | 0.363 |
| RA | 5 | 1 | 0.092 |
| SSc | 0 | 1 | >0.99 |
| MCTD | 0 | 1 | >0.99 |
| SLE | 1 | 0 | 0.468 |
| SjS | 1 | 0 | 0.468 |
| MPA | 4 | 2 | 0.410 |
| Previous therapy | |||
| No therapy | 20 | 23 | >0.99 |
| CS alone | 9 | 9 | 0.964 |
| CS + ISAs | 7 | 8 | >0.99 |
| Pirfenidone | 0 | 1 | >0.99 |
| Clinical parameters | |||
| White blood count (/μL) | 10,403 ± 4730 | 10,683 ± 4092 | 0.803 |
| Neutrophil (/μL) | 8857 ± 4670 | 9026 ± 4093 | 0.850 |
| CRP (mg/dL) | 7.5 ± 7.3 | 9.0 ± 6.7 | 0.198 |
| LDH (U/L) | 450 ± 127 | 432 ± 155 | 0.340 |
| KL-6 (U/mL) | 2027 ± 1404 | 1564 ± 1227 | 0.130 |
| SP-D (ng/mL) | 516 ± 501 | 350 ± 403 | 0.102 |
| PaO2/FiO2 ratio | 172.1 ± 74.3 | 160.1 ± 60.9 | 0.540 |
| APACHE II score | 13.3 ± 4.8 | 12.1 ± 3.6 | 0.286 |
| SOFA score | 3.8 ± 1.7 | 3.6 ± 1.5 | 0.576 |
Data are expressed as group means ± standard deviations or number of patients. The p-values refer to comparisons between the historical control group and PMX-DHP group.
AE, acute exacerbation; AIP, acute interstitial pneumonia; APACHE, Acute Physiology and Chronic Health Evaluation; CRP, C-reactive protein; CS, corticosteroids; CTD, connective tissue disease; DM, dermatomyositis; IP, interstitial pneumonia; IPF, idiopathic pulmonary fibrosis; ISAs, immunosuppressive agents; KL-6, Krebs von den Lungen-6; LDH, lactate dehydrogenase; MCTD, mixed connective tissue disease; MPA, microscopic polyangiitis; NSIP, nonspecific interstitial pneumonia; PaO2/FiO2, ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen; PM, polymyositis; PMX-DHP, direct hemoperfusion using polymyxin B-immobilized fiber column; RA, rheumatoid arthritis; SjS, Sjögren syndrome; SLE, systemic lupus erythematosus; SOFA, Sequential Organ Failure Assessment; SP-D, surfactant protein-D; SSc, systemic sclerosis.
Treatment for RPIPs
Therapeutic interventions performed during the study are shown in Table 2. After the diagnosis of RPIPs, all patients were treated with high-dose CS pulse therapy alone or combination therapy with high-dose CS pulse therapy and immunosuppressive agents such as cyclophosphamide, cyclosporine, or tacrolimus, followed by maintenance treatment with a tapered dose of CS. The combination therapy was applied to 33 of the 41 patients (80%) in the PMX-DHP group and 25 of the 36 patients (69%) in the control group. The number of patients receiving combination therapy with CS, cyclophosphamide, and cyclosporine or tacrolimus in the PMX-DHP group tended to be larger than that in the historical control group; however, there was no significant difference between the two groups (p = 0.056). Sivelestat sodium hydrate was administered to 26 patients (63%) in the PMX-DHP group and 20 patients (56%) in the control group. Mechanical ventilation was applied to 23 patients (56%) in the PMX-DHP group and 16 patients (45%) in the control group. There were no significant differences in the treatments, excluding PMX-DHP therapy, between the control and PMX-DHP groups. The mean interval between the diagnosis of RPIPs and PMX-DHP therapy was 6.3 ± 11.0 (range 0–48) days. Eight patients were treated without PMX-DHP and excluded after April 2007. Five of eight patients showed good response to the conventional therapy and the remaining three patients died before commencement of PMX-DHP therapy.
Table 2.
Therapeutic interventions in the study populations.
| Historical control group | PMX-DHP group | p-value | |
|---|---|---|---|
| PMX-DHP therapy | 0 | 41 | |
| Time to PMX-DHP (days) | – | 6.3 ± 11.0 | |
| CS therapy | 36 (100) | 41 (100) | >0.99 |
| CS alone | 11 (31) | 8 (20) | 0.392 |
| CS + ISAs | 25 (69) | 33 (80) | 0.392 |
| CS + cyclophosphamide | 9 (25) | 10 (24) | >0.99 |
| CS + cyclosporine | 10 (28) | 7 (17) | 0.392 |
| CS + cyclophosphamide + cyclosporine or tacrolimus | 6 (16) | 16 (39) | 0.056 |
| Sivelestat sodium hydrate | 20 (56) | 26 (63) | 0.639 |
| Mechanical ventilation | 16 (45) | 23 (56) | 0.428 |
| IPPV | 15 (42) | 19 (46) | 0.855 |
| NPPV | 1 (3) | 4 (10) | 0.364 |
Data are expressed as means ± standard deviations or number (%) of patients. The p-values refer to comparisons between historical control group and PMX-DHP group.
CS, corticosteroids; IPPV, invasive positive pressure ventilation; ISAs, immunosuppressive agents; NPPV, noninvasive positive pressure ventilation; PMX-DHP, direct hemoperfusion using polymyxin B-immobilized fiber column.
Effect of treatment on mortality
The Kaplan–Meier estimate of survival function during the 90-day study period is given for both the PMX-DHP and historical control groups in Figure 1. The 90-day mortality rate was 41.5% (17/41) in the PMX-DHP group and 66.7% (24/36) in the control group. There was a significant difference in mortality (p = 0.019 by log-rank test) between the two groups. According to underlying diseases, the 90-day mortality rate in the PMX-DHP and the control group was 60.0% and 57.1% in IPF, 33.3% and 87.5% in idiopathic NSIP, 16.7% and 25.0% in AIP, and 47.6% and 70.6% in CTD-IP, respectively. Based on a classification of the treatment regimen, the 90-day mortality in the combination with CS and immunosuppressive agents was significantly different for the two groups (45.0% in the PMX-DHP group versus 76.0% in the historical control, log-rank test, p = 0.011, Figure 2). Causes of death included respiratory failure due to RPIPs (n = 16) and pulmonary infection (n = 1) in the PMX-DHP group, and respiratory failure due to RPIPs (n = 21), pulmonary infection (n = 2), and unknown cause (n = 1) in the historical control group. We used a Cox proportional hazards model to perform uni- and multivariate analysis to determine the independent factors for mortality. In the univariate analysis, the serum level of CRP and PMX-DHP therapy were significant predictors of mortality (Table 3). Similarly, in the multivariate analysis, the serum level of CRP (HR 0.948; 95% CI, 0.900–0.998; p = 0.042) and PMX-DHP therapy (HR 0.505; 95% CI, 0.270–0.904; p = 0.032) remained independent factors for reduced risk of mortality (after adjusting for age, sex, SOFA score, LDH, and KL-6). Age, sex, diagnosis of IPF, P/F ratio, SOFA score, and serological tests except for CRP levels were not prognostic factors in this study. In subgroup analyses by disease type, PMX-DHP therapy in idiopathic NSIP (HR 0.102; 95% CI, 0.015–0.703; p = 0.021) and CRP levels in CTD-IP (HR 0.879; 95% CI, 0.797–0.970; p = 0.01) were independently associated with mortality (data not shown).
Figure 1.
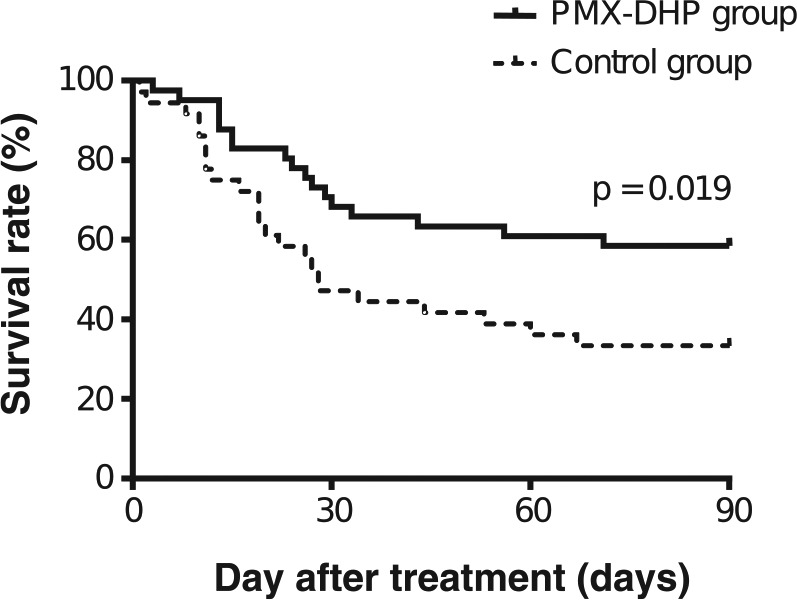
Kaplan–Meier comparison of survival curves in the PMX-DHP group and historical control group. The mortality rate is significantly lower in the PMX-DHP group (solid line) than in the historical control group (dotted line). At 90 days, the mortality rate was significantly lower in the PMX-DHP group than in the control group (41.5% versus 66.7%, p = 0.019, log-rank test).
Figure 2.
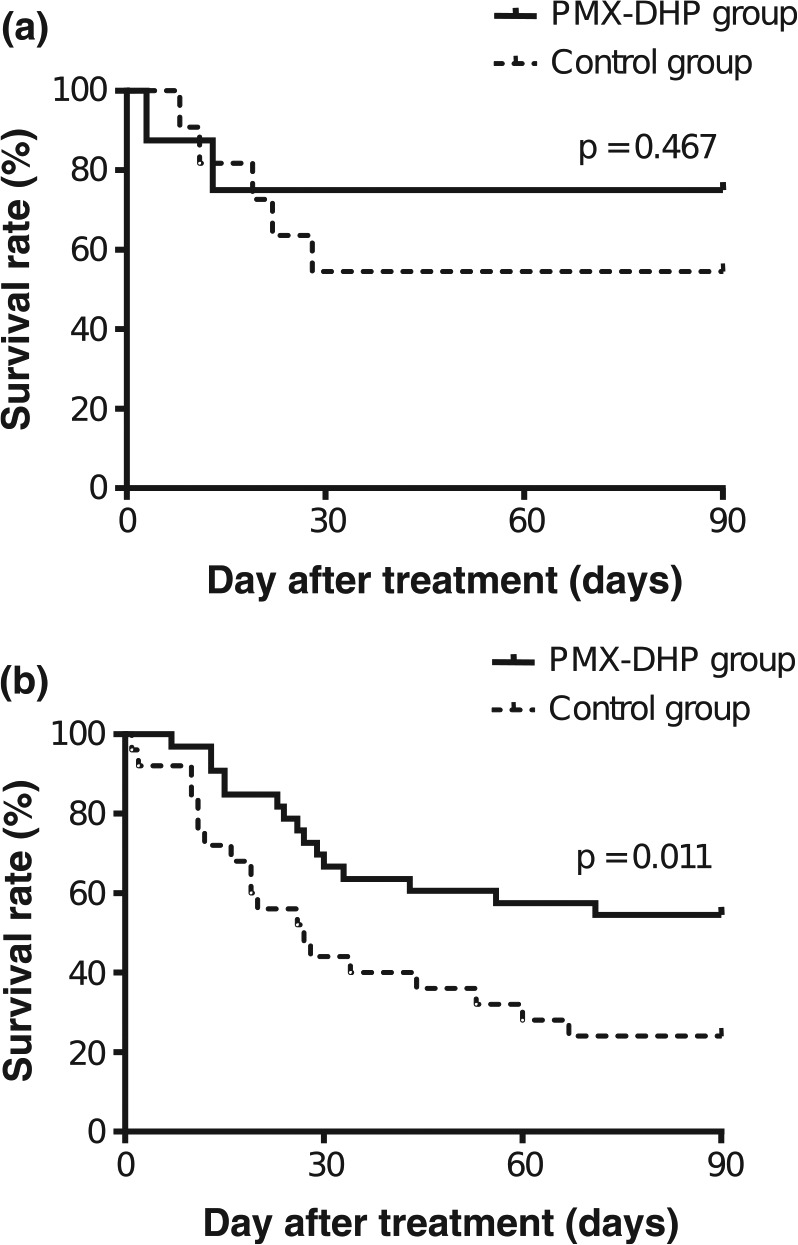
Kaplan–Meier comparison of survival curves according to treatment regimens in the PMX-DHP and historical control group. (a) CS alone. (b) Combination therapy with CS and immunosuppressive agents. The 90-day mortality rate in combination therapy with CS and immunosuppressive agents was significantly lower in the PMX-DHP group (solid line) than in the historical control group (dotted line) (45.0% versus 76.0%, p = 0.011, log-rank test).
Table 3.
Univariate and multivariate predictors of mortality determined by Cox proportional hazards analysis.
| Variables | HR (95% CI) | p-value |
|---|---|---|
| Univariate analysis | ||
| PMX-DHP therapy | 0.485 (0.260–0.904) | 0.023 |
| Age | 1.017 (0.988–1.046) | 0.255 |
| Sex (male) | 0.644 (0.343–1.209) | 0.171 |
| IPF | 1.074 (0.476–2.423) | 0.864 |
| PaO2/FiO2 ratio | 0.998 (0.994–1.003) | 0.508 |
| SOFA score | 1.132 (0.951–1.347) | 0.162 |
| CRP | 0.949 (0.896–0.994) | 0.030 |
| LDH | 1.001 (0.999–1.003) | 0.323 |
| KL-6 | 1.000 (1.000–1.000) | 0.118 |
| Multivariate analysis | ||
| PMX-DHP therapy | 0.505 (0.270–0.904) | 0.032 |
| CRP | 0.948 (0.900–0.998) | 0.042 |
CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; IPF, idiopathic pulmonary fibrosis; KL-6, Krebs von den Lungen-6; LDH, lactate dehydrogenase; PMX-DHP, direct hemoperfusion using polymyxin B-immobilized fiber column; SOFA, Sequential Organ Failure Assessment.
Comparison of the clinical parameters between the survivor and non-survivor groups among the patients with PMX-DHP
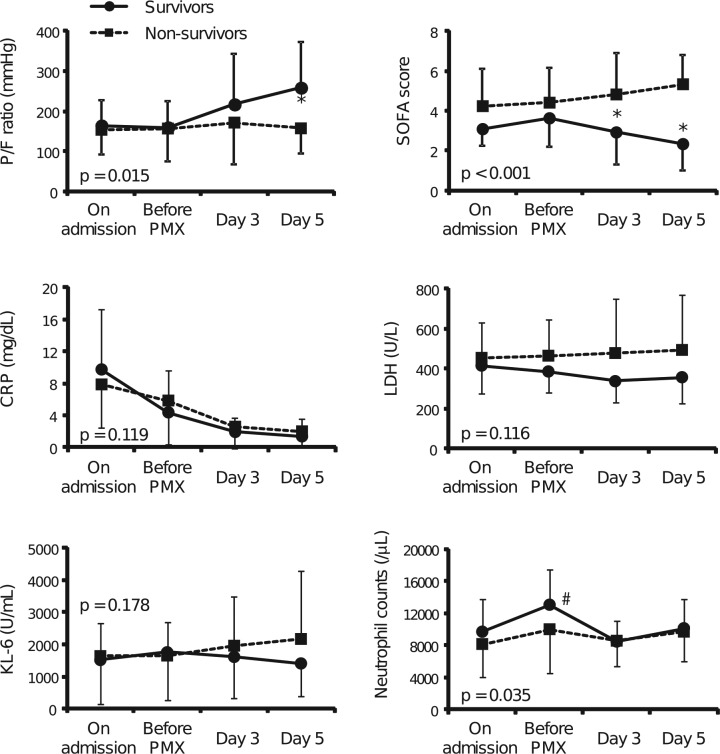
In the PMX-DHP group, the patients were divided into the survivor (24 patients) and non-survivor (17 patients) groups on day 90 after the treatments. A comparison of the demographic and laboratory data of these two groups is shown in Table 4. The mean intervals between the diagnosis of RPIPs and PMX-DHP therapy were 7.0 ± 11.2 days in the survivor group and 5.4 ± 10.9 days in the non-survivor group, but the difference was not significant. The clinical parameters at admission and at the beginning of PMX-DHP therapy were not different between the survivor and non-survivor groups, although the SOFA scores in the survivor group tended to be lower than those in the non-survivor group on admission (3.1 ± 0.8 versus 4.2 ± 1.9, p = 0.051). Figure 3 shows the serial changes in clinical parameters in the survivor and non-survivor groups until 5 days after PMX-DHP therapy. There were significant differences in the serial changes in the P/F ratio, SOFA score, and blood neutrophil counts from initial treatment to day 5 between the two groups (p = 0.015, p < 0.001, p = 0.035, respectively). In the post-hoc test, the P/F ratio in the survivor group was significantly increased on day 5 relative to that in the non-survivor group (p < 0.01). The SOFA score significantly decreased from days 3 to 5 (p < 0.01). Neutrophil counts were increased at the beginning of PMX-DHP therapy in both groups, but the difference was not significant, and the counts were significantly decreased at day 3 after the PMX-DHP therapy in the survivor group relative to those at the beginning. Interestingly, the improved P/F ratio on day 3 after PMX-DHP significantly correlated with the change in neutrophil counts in blood (rs= −0.431, p = 0.006, Figure 4). The serum levels of CRP (p = 0.119), LDH (p = 0.116), and KL-6 (p = 0.178) were not significantly different between the survivor and non-survivor groups.
Table 4.
Comparison of the clinical factors between the survivor and non-survivor groups among the patients with PMX-DHP.
| Survivor group |
Non-survivor group |
p-value | |
|---|---|---|---|
| (n = 24) | (n = 17) | ||
| Age | 66.0 ± 10.3 | 67.8 ± 9.4 | 0.691 |
| Sex (male/female) | 14/10 | 6/11 | 0.256 |
| Time to PMX-DHP (days) | 7.0 ± 11.2 | 5.4 ± 10.9 | 0.266 |
| Data at admission | |||
| PaO2/FiO2 ratio | 164.5 ± 62.1 | 153.9 ± 60.5 | 0.588 |
| SOFA score | 3.1 ± 0.8 | 4.2 ± 1.9 | 0.051 |
| Neutrophil counts (/μL) | 9655 ± 4064 | 8137 ± 4087 | 0.272 |
| CRP (mg/dL) | 9.8 ± 7.5 | 7.8 ± 5.5 | 0.662 |
| LDH (U/L) | 416 ± 141 | 455 ± 175 | 0.525 |
| KL-6 (U/mL) | 1515 ± 1362 | 1637 ± 1029 | 0.288 |
| Data at the beginning of PMX-DHP therapy | |||
| PaO2/FiO2 ratio | 159.9 ± 64.6 | 157.4 ± 81.2 | 0.625 |
| SOFA score | 3.6 ± 1.4 | 4.4 ± 1.7 | 0.082 |
| Neutrophil counts (/μL) | 13,015 ± 4410 | 9980 ± 5404 | 0.053 |
| CRP (mg/dL) | 4.4 ± 5.2 | 5.9 ± 5.5 | 0.420 |
| LDH (U/L) | 386 ± 107 | 465 ± 181 | 0.107 |
| KL-6 (U/mL) | 1756 ± 1476 | 1652 ± 1025 | 0.792 |
Data are expressed as means ± standard deviations or number of patients. The p-values refer to comparisons between the survivor and non-survivor groups.
CRP, C-reactive protein; KL-6, Krebs von den Lungen-6; LDH, lactate dehydrogenase; PMX-DHP, direct hemoperfusion using polymyxin B-immobilized fiber column; SOFA, Sequential Organ Failure Assessment.
Figure 3.
Serial changes from baseline in pulmonary failure indexes and markers in peripheral blood in the survivor and non-survivor groups treated with PMX-DHP therapy. P/F ratio (a), SOFA score (b), CRP (c), LDH (d), KL-6 (e), and neutrophil counts in peripheral blood (f) in the survivor group (solid line and closed circles) and non-survivor group (dotted line and closed squares) among the patients after PMX-DHP therapy. There were significant differences in the serial changes in P/F ratio, the SOFA score, and blood neutrophil counts from initial treatment to day 5 between the two groups (p = 0.015, p < 0.001, p = 0.035, respectively), whereas serial changes in the serum levels of CRP (p = 0.119), LDH (p = 0.116), and KL-6 (p = 0.178) did not show significant differences between the groups. The data are expressed as means ± standard deviations. Comparisons of variables between groups over time were analyzed by repeated measures analysis of variance (ANOVA) adjusted for the baseline values as a covariate, and p-values between groups are illustrated. Additionally, the P/F ratio at day 5 and SOFA scores at days 3 and 5 in the survivor group are significantly better than those in the non-survivor group (*p < 0.01 compared with the non-survivor group). The neutrophil counts in the survivor group at day 3 after PMX-DHP therapy are significantly lower than those at the beginning of PMX-DHP therapy (#p < 0.001 compared with the beginning of PMX-DHP therapy).
Figure 4.
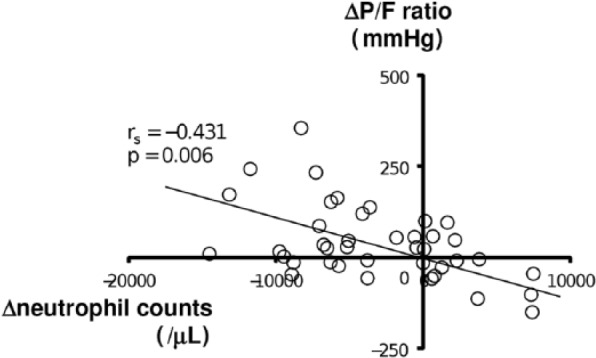
Relationship between changes in the P/F ratio and neutrophil counts before and after PMX-DHP therapy. The change in each variable is defined as a change from just before PMX-DHP to 3 days after the therapy. ΔP/F ratio, changes in the PaO2/FiO2 ratio; Δneutrophil counts, changes in neutrophil counts. The change in the P/F ratio was inversely correlated with the change in neutrophil counts in blood (rs = −0.431, p = 0.006).
Discussion
The results of this study show that PMX-DHP therapy may have a beneficial effect on acute respiratory failure of RPIPs in patients with IIPs and CTD. The 90-day mortality rate was significantly lower in the patients treated with PMX-DHP than in the historical control patients treated without PMX-DHP, and PMX-DHP therapy predicted survival in the univariate and multivariate analyses using a Cox proportional hazards regression model. Our findings suggest that PMX-DHP therapy improves the outcomes in RPIPs.
PMX-DHP therapy has been used for the treatment of endotoxin-induced ARDS in Japan.7,10,11 The early use of polymyxin hemoperfusion in an abdominal septic shock (EUPHAS) trial, a prospective multicenter randomized study, demonstrated significant improvements in hemodynamics and organ dysfunction and reduced 28-day mortality rate.8 The main mechanism of action of PMX-DHP is the direct adsorption of circulating endotoxin.
There are several published clinical studies of small and heterogeneous cohorts, as well as case reports of RPIPs patients mainly from Japan, demonstrating the attractive efficacy of PMX-DHP. The first clinical report on PMX-DHP for RPIPs patients was published in 2006 by Seo and colleagues, who reported that six cases of acute exacerbation of IPF were safely and successfully treated with PMX-DHP.13 Their study showed that PMX-DHP in IPF with acute respiratory failure may be helpful for life-threatening conditions refractory to conventional treatments such as high-dose CS pulse. Since the publication of this interesting case report, this therapy has been tried in a variety of RPIPs, including acute exacerbation of IPF, as summarized in Table 5.14,16,24–29 A multicenter retrospective analysis was done by Abe and colleagues in 2012,16 in which 160 subjects with RPIPs, including 73 with acute exacerbation of IPF, were treated with PMX-DHP. The therapy showed a favorable outcome although control subjects without PMX-DHP therapy had not been set up. Recently, three retrospective studies were conducted with comparative analyses between PMX-DHP and control groups.27–30 In two of these three studies, PMX-DHP therapy showed a significantly favorable prognosis, which was similar to our results. Enomoto and colleagues showed a significant improvement in the 12-month survival rate with PMX-DHP therapy for acute exacerbation of IPF,28 although interstitial lung diseases other than IPF were not examined. However, there are limitations in the interpretation of these results because all of the above studies, including ours, were not randomized. Further studies including randomized controlled trials are necessary to evaluate the efficacy of PMX-DHP in patients with RPIPs.
Table 5.
Summary of clinical studies of PMX-DHP therapy in interstitial pneumonias.
| Reference | Study design | Diseases | No. patients (PMX-DHP/control) | Perfusion duration of PMX-DHP (hours) | Interval between diagnosis and PMX-DHP (days) | Survival rate (%) | Main findings |
|---|---|---|---|---|---|---|---|
| Oishi et al.29 | Retrospective study | IPF-AE | 54 (27/27) | 6 | 1–22 | 90-day; PMX-DHP 63.7%, control 26.1% |
PMX-DHP was an independent prognostic factor of survival (HR 0.442, p = 0.019) |
| Enomoto et al.28 | Retrospective study | IPF-AE | 31 (14/17) | 6–10 | 1 (median) | 12-month; PMX-DHP 48.2%, control 5.9% |
PMX-DHP improved 12-month survival (HR 0.345, p = 0.037) |
| Takada et al.27 | Retrospective study | IP-AE | 26 (13/13) (IPF 6, CTD-IP 13, AIP 2, D-ILD 2, others 3 |
3–24 | 0–6 | N | Simultaneous therapy of PMX-DHP and steroid pulse improved the prognosis |
| Abe et al.16 | Multicenter retrospective study | IP-AE | 160 (160/0) (IPF 73, non-IPF IIP 35, CTD-IP 30, D-ILD 7, cHP 5, others or unknown 10) |
12.5 (mean) | N | 90-day; PMX-DHP 30.1% |
PMX-DHP improved the oxygenation and survival |
| Abe et al.26 | Retrospective study | IPF-AE | 20 (20/0) | 6 | 6.3 (mean) | 30-day; PMX-DHP 70.0% |
PMX-DHP reduced serum HMGB-1 and improved oxygenation |
| Tachibana et al.25 | Retrospective study | IPF-AE | 9 (9/0) | 4–6 | N | 90-day; PMX-DHP 26.3% |
Serum IL-7 is useful prognostic factor of survival |
| Hara et al.14 | Retrospective study | IP-AE | 33 (33/0) (IPF 9, idiopathic NSIP 1, AIP 6, unclassified IP 1, CTD-IP 8, D-ILD 4, asbestosis 3, cHP 1) |
4 (median) | 3 (median) | 90-day; PMX-DHP 51.6% |
PMX-DHP improved the oxygenation and systemic inflammatory response syndrome |
| Kono et al.24 | Retrospective study | IP-AE | 17 (17/0) (IPF 8, non-IPF IIP 5, CTD-IP 2, cHP 2) |
12 (long perfusion) 2–6 (short perfusion) |
0.4 (long perfusion) 1.8 (short perfusion) |
30-day; PMX-DHP 80.0% (long perfusion), 20.0% (short perfusion) |
A long perfusion duration of PMX-DHP was more efficacious than a short perfusion duration |
N, the study did not report the item.
AE, acute exacerbation; AIP, acute interstitial pneumonia; cHP, chronic hypersensitivity pneumonia; CTD, connective tissue disease; D-ILD, drug-induced interstitial pneumonia; HMGB-1, high mobility group box-1; HR, hazard ratio; IIP, idiopathic interstitial pneumonia; IP, interstitial pneumonia; IPF, idiopathic pulmonary fibrosis; NSIP, nonspecific interstitial pneumonia; PMX-DHP, direct hemoperfusion using polymyxin B-immobilized fiber column.
In the present study, we selected patients with IIPs including IPF and CTD-IP with acute respiratory failure and compared the clinical features and outcomes between the patients treated with PMX-DHP and those with conventional therapy without PMX-DHP as a historical control. We showed a significant improvement of mortality in the PMX-DHP group relative to that in the control group. The severity of respiratory failure on initial treatment was not significantly different between the two groups. Approximately half of the patients required mechanical ventilation to ensure sufficient oxygenation in both groups. The factors affecting mortality in patients with acute exacerbation of interstitial pneumonias and acute lung injury have been described, including age, blood biomarkers, scoring systems of organ failure, chest HRCT findings, and presence of systemic inflammatory response syndrome.3,19,30–32 In our study, these demographic findings, blood tests, APACHE II score, and SOFA score showed no significant differences between the PMX-DHP and control groups at baseline, although the analyzed subjects had various types of underlying diseases. Therefore, it was considered reasonable to compare the two groups.
In the present study, the number of neutrophils in blood decreased 3 days after PMX-DHP therapy, and there was a significant difference in the time-dependent changes of peripheral blood neutrophils between the survivor and non-survivor groups (Figure 3). Furthermore, a reduction of neutrophil counts after PMX-DHP may lead to improved pulmonary oxygenation (Figure 4). The major pathological finding of RPIPs is diffuse alveolar damage (DAD).4,5 DAD with chronic fibrotic lung is found in patients with not only IPF but also idiopathic fibrotic NSIP and interstitial pneumonia related to CTD.5,6 Acute lung injury occurring in patients with AIP and CTD at initial presentation also shows a histological feature of DAD without pre-existing interstitial pneumonia.30 Although the mechanism of lung injury in DAD remains unclear, inflammatory cells, including neutrophils and some inflammatory mediators, are thought to have a central role in developing lung damage.4,30 Abe and colleagues demonstrated that neutrophil adsorption by PMX may be of therapeutic value for acute exacerbation of interstitial pneumonias.33
The mechanism of the action of PMX-DHP therapy for RPIPs has not been elucidated. The removal of circulating endotoxin dose has not been shown to be very effective because the blood level of endotoxin was within the normal range in patients with RPIPs of our study and in previous reports.13–17 We observed that blood neutrophil counts were reduced after PMX-DHP and there was a negative relationship between the reduced neutrophil counts and the increased P/F ratio after PMX-DHP, suggesting that the adsorptive removal of neutrophils by PMX-DHP may be a potential mechanism of action, as previously reported.33 Further studies are required to determine the precise mechanism of PMX-DHP therapy for RPIPs.
In our study, the elevated levels of CRP at diagnosis were associated with a decreased hazard of 90-day mortality (Table 3). This finding seems to conflict with previous study findings regarding the role of CRP as an inflammatory marker of predicted risk.3,34 Song and colleagues demonstrated that higher levels of CRP were a significant risk factor of acute exacerbation of IPF and other acute illnesses such as sepsis and pneumonia and were associated with adverse outcome.3 Conversely, a study among patients with ARDS showed an association between higher CRP levels and decreased mortality.35 Thus, the clinical significance of CRP in interstitial lung diseases with acute respiratory failure has been controversial. The significance of CRP as a mortality predictor should be confirmed in future studies.
Acute exacerbation of IPF has been known to be a major risk factor for mortality in patients with interstitial pneumonias. However, in our study, IPF was not associated with increased risk of mortality on univariate analysis. This may contribute to the fact that the majority of patients were diagnosed based on radiographic patterns because of limited availability of histological findings. The non-IPF patients, especially with idiopathic NSIP, may include some cases of misclassified IPF. On the other hand, our results are similar to those of the previous study, which reported that survival rates after acute exacerbation treated by PMX-DHP were comparable between IPF and all interstitial pneumonias, including IPF, in a multicenter retrospective analysis,16 although univariate analysis was not conducted. Taken together, PMX-DHP therapy may have therapeutic benefits not only for acute exacerbation in IPF, but also for RPIPs other than IPF.
The clinical importance of the timing and duration of PMX-DHP therapy has been investigated. In patients with ARDS and RPIPs, including acute exacerbation of IPF, early induction of PMX-DHP therapy gave better effects and was a very important factor affecting survival.25,27 Furthermore, a longer duration of PMX-DHP (12 h) was more effective for acute exacerbation of IP than a shorter duration (⩽6 h).24 Although the starting time and duration of PMX-DHP in our study was somewhat delayed and shorter in comparison with previous studies, there was no difference between the survivor and non-survivor groups (Table 4). Other clinical parameters, including the P/F ratio on admission and just before PMX-DHP therapy, also did not differ between the groups. The SOFA score is a reliable and useful means of classifying the severity of diseases and estimating the outcome in ICU patients.22 Some authors have previously reported that the serial evaluations of the SOFA score also predicted the outcome in critically ill patients.8,36 Recently, Kao and colleagues reported that the sequential assessment of organ dysfunction within the first 3 days of mechanical ventilation predicted the outcome of patients with severe acute respiratory failure.37 A recent randomized controlled study showed that PMX-DHP therapy improved the SOFA scores and mortality rate of sepsis patients.8 In our study, as shown in Figure 3, the SOFA score on days 3 and 5 and the P/F ratio on day 5 after PMX-DHP therapy in the survivors were significantly different from those in the non-survivors. From our results, the changes in the SOFA score after PMX-DHP rather than the initial score may be useful for predicting the clinical outcome in patients with RPIPs, although Hara and colleagues showed that the SOFA score in RPIPs was not affected by PMX-DHP.14
This study had several limitations. First, this was a retrospective historical control and not a randomized controlled study. Temporal trends in the diagnosis and treatment may have affected the results from the use of a historical control group, and there could have been a non-contemporaneous control bias. Because this study included patients from two different time frames within a 13-year period, advances in supportive care such as mechanical ventilation and infection control during the periods may also have influenced the results. Second, this study was conducted in a single institution; therefore, the number of patients was limited. The heterogeneity of underlying diseases also made it difficult to examine prognostic variables. Some negative or positive associations in the statistical analyses may have been due to inadequate power derived from the small sample size. Third, not all the patients in this study were treated according to the same protocol. Takada and colleagues reported that AIP associated with DM/PM patients who were started on immunosuppressive agents simultaneously with CS had significantly better survival than those to whom immunosuppressive agents were added if CS alone did not result in a favorable response.38 In our study, all patients were treated with CS alone or the combination therapy of CS and immunosuppressive agents except for PMX-DHP therapy. The decision regarding the choice of immunosuppressive agents was made by the attending physicians, and the types and amounts of the agents and timing of the administration varied by the individual cases. Those variations in therapeutic regimen may have affected the responses to therapy and outcomes. Finally, because of referral bias, our study group may have been composed of patients who had more severe or complicated diseases; thus, the results of our study may not generally apply to patients in other settings.
Conclusions
In summary, the use of PMX-DHP therapy in combination with conventional therapy, including CS and immunosuppressive agents, gave a 90-day mortality rate of 41.5% in patients with RPIPs, which was an improvement over the 66.7% rate for conventional therapy. Nonetheless, it is more important to note that half of the patients still died of respiratory failure within a few months, despite the use of aggressive combination therapies, including PMX-DHP. Further studies are needed in a large-scale, randomized, controlled study to confirm the effect of PMX-DHP treatment and to develop better therapeutic management for patients with RPIPs.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Hidenori Ichiyasu, Department of Respiratory Medicine, Faculty of Life Sciences, Kumamoto University, 1-1-1 Honjo, Chuo-ku, Kumamoto 860-8556, Japan.
Yuko Horio, Department of Respiratory Medicine, Faculty of Life Sciences, Kumamoto University, 1-1-1 Honjo, Chuo-ku, Kumamoto 860-8556, Japan.
Aiko Masunaga, Department of Respiratory Medicine, Faculty of Life Sciences, Kumamoto University, 1-1-1 Honjo, Chuo-ku, Kumamoto 860-8556, Japan.
Yohei Migiyama, Department of Respiratory Medicine, Faculty of Life Sciences, Kumamoto University, 1-1-1 Honjo, Chuo-ku, Kumamoto 860-8556, Japan.
Yasumiko Sakamoto, Department of Respiratory Medicine, Faculty of Life Sciences, Kumamoto University, 1-1-1 Honjo, Chuo-ku, Kumamoto 860-8556, Japan.
Takayuki Jodai, Department of Respiratory Medicine, Faculty of Life Sciences, Kumamoto University, 1-1-1 Honjo, Chuo-ku, Kumamoto 860-8556, Japan.
Hideharu Ideguchi, Department of Respiratory Medicine, Faculty of Life Sciences, Kumamoto University, 1-1-1 Honjo, Chuo-ku, Kumamoto 860-8556, Japan.
Hiroko Okabayashi, Department of Respiratory Medicine, Faculty of Life Sciences, Kumamoto University, 1-1-1 Honjo, Chuo-ku, Kumamoto 860-8556, Japan.
Shohei Hamada, Department of Respiratory Medicine, Faculty of Life Sciences, Kumamoto University, 1-1-1 Honjo, Chuo-ku, Kumamoto 860-8556, Japan.
Chieko Yoshida, Department of Respiratory Medicine, Faculty of Life Sciences, Kumamoto University, 1-1-1 Honjo, Chuo-ku, Kumamoto 860-8556, Japan.
Susumu Hirosako, Department of Respiratory Medicine, Faculty of Life Sciences, Kumamoto University, 1-1-1 Honjo, Chuo-ku, Kumamoto 860-8556, Japan.
Shinichiro Okamoto, Department of Respiratory Medicine, Faculty of Life Sciences, Kumamoto University, 1-1-1 Honjo, Chuo-ku, Kumamoto 860-8556, Japan.
Hirotsugu Kohrogi, Department of Respiratory Medicine, Faculty of Life Sciences, Kumamoto University, 1-1-1 Honjo, Chuo-ku, Kumamoto 860-8556, Japan.
References
- 1. Kondoh Y, Taniguchi H, Kawabata Y, et al. Acute exacerbation in idiopathic pulmonary fibrosis: analysis of clinical and pathologic findings in three cases. Chest 1993; 103: 1808–1812. [DOI] [PubMed] [Google Scholar]
- 2. Collard HR, Moore BB, Flaherty KR, et al. Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators: acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007; 176: 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song JW, Hong SB, Lim CM, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 2011; 37: 356–363. [DOI] [PubMed] [Google Scholar]
- 4. Churg A, Müller NL, Silva CI, et al. Acute exacerbation (acute lung injury of unknown cause) in UIP and other forms of fibrotic interstitial pneumonias. Am J Surg Pathol 2007; 31: 277–284. [DOI] [PubMed] [Google Scholar]
- 5. Silva CI, Müller NL, Fujimoto K, et al. Acute exacerbation of chronic interstitial pneumonia: high-resolution computed tomography and pathologic findings. J Thorac Imaging 2007; 22: 221–229. [DOI] [PubMed] [Google Scholar]
- 6. Suda T, Kaida Y, Nakamura Y, et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir Med 2009; 103: 846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aoki H, Kodama M, Tani T, et al. Treatment of sepsis by extracorporeal elimination of endotoxin using polymyxin B-immobilized fiber. Am J Surg 1994; 167: 412–417. [DOI] [PubMed] [Google Scholar]
- 8. Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA 2009; 301: 2445–2452. [DOI] [PubMed] [Google Scholar]
- 9. Ronco C, Klein DJ. Polymyxin B hemoperfusion: a mechanistic perspective. Crit Care 2014; 18: e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kushi H, Miki T, Okamaoto K, et al. Early hemoperfusion with an immobilized polymyxin B fiber column eliminates humoral mediators and improves pulmonary oxygenation. Crit Care 2005; 9: 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsushima K, Kubo K, Yoshikawa S, et al. Effects of PMX-DHP treatment for patients with directly induced acute respiratory distress syndrome. Ther Apher Dial 2007; 11: 138–145. [DOI] [PubMed] [Google Scholar]
- 12. Ono S, Tsujimoto H, Matsumoto A, et al. Modulation of human leukocyte antigen-DR on monocytes and CD16 on granulocytes in patients with septic shock using hemoperfusion with polymyxin B-immobilized fiber. Am J Surg 2004; 188: 150–156. [DOI] [PubMed] [Google Scholar]
- 13. Seo Y, Abe S, Kurahara M, et al. Beneficial effect of polymyxin B-immobilized fiber column (PMX) hemoperfusion treatment on acute exacerbation of idiopathic pulmonary fibrosis. Intern Med 2006; 45: 1033–1038. [DOI] [PubMed] [Google Scholar]
- 14. Hara S, Ishimoto H, Sakamoto N, et al. Direct hemoperfusion using immobilized polymyxin B in patients with rapidly progressive interstitial pneumonias: a retrospective study. Respiration 2011; 81: 107–117. [DOI] [PubMed] [Google Scholar]
- 15. Sato N, Kojima K, Horio Y, et al. Successful treatment of severe amiodarone pulmonary toxicity with polymyxin B-immobilized fiber column direct hemoperfusion. Chest 2013; 143: 1146–1150. [DOI] [PubMed] [Google Scholar]
- 16. Abe S, Azuma A, Mukae H, et al. Polymyxin B-immobilized fiber column (PMX) treatment for idiopathic pulmonary fibrosis with acute exacerbation: a multicenter retrospective analysis. Intern Med 2012; 51: 1487–1491. [DOI] [PubMed] [Google Scholar]
- 17. Ichiyasu H, Horio Y, Tsumura S, et al. Favorable outcome with hemoperfusion of polymyxin B-immobilized fiber column for rapidly progressive interstitial pneumonia associated with clinically amyopathic dermatomyositis: report of three cases. Mod Rheumatol 2014; 24: 361–365. [DOI] [PubMed] [Google Scholar]
- 18. American Thoracic Society/European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002; 165: 277–304. [DOI] [PubMed] [Google Scholar]
- 19. Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest 2013; 143: 814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–829. [PubMed] [Google Scholar]
- 22. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 1996; 22: 707–710. [DOI] [PubMed] [Google Scholar]
- 23. Okamoto T, Ichiyasu H, Ichikado K, et al. Clinical analysis of the acute exacerbation in patients with idiopathic pulmonary fibrosis. Nihon Kokyuki Gakkai Zasshi. 2006; 44: 359–367. [PubMed] [Google Scholar]
- 24. Kono M, Suda T, Enomoto N, et al. Evaluation of different perfusion durations in direct hemoperfusion with polymyxin B-immobilized fiber column therapy for acute exacerbation of interstitial pneumonias. Blood Purif 2011; 32: 75–81. [DOI] [PubMed] [Google Scholar]
- 25. Tachibana K, Inoue Y, Nishiyama A, et al. Polymyxin-B hemoperfusion for acute exacerbation of idiopathic pulmonary fibrosis: serum IL-7 as a prognostic marker. Sarcoidosis Vasc Diffuse Lung Dis 2011; 28: 113–122. [PubMed] [Google Scholar]
- 26. Abe S, Hayashi H, Seo Y, et al. Reduction in serum high mobility group box-1 level by polymyxin B-immobilized fiber column in patients with idiopathic pulmonary fibrosis with acute exacerbation. Blood Purif 2011; 32: 310–316. [DOI] [PubMed] [Google Scholar]
- 27. Takada T, Asakawa K, Sakagami T, et al. Effects of direct hemoperfusion with polymyxin B-immobilized fiber on rapidly progressive interstitial lung diseases. Intern Med 2014; 53: 1921–1926. [DOI] [PubMed] [Google Scholar]
- 28. Enomoto N, Mikamo M, Oyama Y, et al. Treatment of acute exacerbation of idiopathic pulmonary fibrosis with direct hemoperfusion using a polymyxin B-immobilized fiber column improves survival. BMC Pulm Med 2015; 15: e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oishi K, Aoe K, Mimura Y, et al. Survival from an acute exacerbation of idiopathic pulmonary fibrosis with or without direct hemoperfusion with a polymyxin B-immobilized fiber column: a retrospective analysis. Intern Med 2016; 55: 3551–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Papanikolaou IC, Drakopanagiotakis F, Polychronopoulos VS. Acute exacerbations of interstitial lung diseases. Curr Opin Pulm Med 2010; 16: 480–486. [DOI] [PubMed] [Google Scholar]
- 31. Fujimoto K, Taniguchi H, Johkoh T, et al. Acute exacerbation of idiopathic pulmonary fibrosis: high-resolution CT scores predict mortality. Eur Radiol 2012; 22: 83–92. [DOI] [PubMed] [Google Scholar]
- 32. Usui Y, Kaga A, Sakai F, et al. A cohort study of mortality predictors in patients with acute exacerbation of chronic fibrosing interstitial pneumonia. BMJ Open 2013; 3: e002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abe S, Seo Y, Hayashi H, et al. Neutrophil adsorption by polymyxin B-immobilized fiber column for acute exacerbation in patients with interstitial pneumonia: a pilot study. Blood Purif 2010; 29: 321–326. [DOI] [PubMed] [Google Scholar]
- 34. Lobo SM, Lobo FR, Bota DP, et al. C-reactive protein levels correlate with mortality and organ failure in critically ill patients. Chest 2003; 123: 2043–2049. [DOI] [PubMed] [Google Scholar]
- 35. Bajwa EK, Khan UA, Januzzi JL, et al. Plasma C-reactive protein levels are associated with improved outcome in ARDS. Chest 2009; 136: 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001; 286: 1754–1758. [DOI] [PubMed] [Google Scholar]
- 37. Kao HC, Lai TY, Hung HL, et al. Sequential oxygenation index and organ dysfunction assessment within the first 3 days of mechanical ventilation predict the outcome of adult patients with severe acute respiratory failure. Scientific World J 2013; 413216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takada K, Kishi J, Miyasaka N. Step-up versus primary intensive approach to the treatment of interstitial pneumonia associated with dermatomyositis/polymyositis: a retrospective study. Mod Rheumatol 2007; 17: 123–130. [DOI] [PubMed] [Google Scholar]