Abstract
Background:
This study assessed the ease of use of tobramycin inhalation powder (TIP) administered via T-326 inhaler versus tobramycin inhalation solution (TIS) and colistimethate sodium (COLI), both administered via nebulizers, for the treatment of chronic pulmonary Pseudomonas aeruginosa infection in patients with cystic fibrosis (CF).
Methods:
A real-world, open-label, crossover, interventional phase IV study was conducted in CF patients aged ⩾6 years with forced expiratory volume in 1 second (FEV1) ⩾25% to ⩽90% predicted. Patients were assigned to one of the three treatment arms in Cycle 1; all patients received TIP in Cycle 2. Each cycle consisted of 28 days on and 28 days off the treatment.
Results:
A total of 60 patients [mean (standard deviation) age, 27.6 (8.4) years] were allocated to three treatment arms [TIS/TIP (n = 14); COLI/TIP (n = 28); TIP/TIP (n = 18)] in Cycle 1. The mean total administration time, which included device setup and cleaning, in Cycle 1 versus Cycle 2 for TIS/TIP, COLI/TIP, and TIP/TIP arms were 37.0 versus 5.0 min, 16.4 versus 3.8 min, and 4.2 versus 3.4 min, respectively. The difference in mean total administration time was significantly shorter in Cycle 2 than in Cycle 1 for TIS/TIP (p = 0.0112) and COLI/TIP (p = 0.0016) arms. Overall, 12 patients were found to have contaminated devices across the two treatment cycles. In the TIP/TIP arm, no contamination of the T-326 inhaler was observed in either cycle. Treatment satisfaction, assessed by the Treatment Satisfaction Questionnaire for Medication and ACCEPT® questionnaire, was better overall for TIP compared with TIS and COLI. There were no unexpected adverse events and most were mild or moderate in intensity.
Conclusion:
The T-326 inhaler used to deliver TIP was easy to use, required shorter total administration time, and was much less frequently contaminated than the nebulizers. The safety findings observed for TIP were generally consistent with its established safety profile.
Keywords: device contamination, ease of use, nebulizer, Pseudomonas aeruginosa, T-326 inhaler, tobramycin
Introduction
Cystic fibrosis (CF) patients are generally susceptible to respiratory infection caused by Pseudomonas aeruginosa (Pa), which is associated with progressive lung function decline and increased morbidity and mortality.1,2 Tobramycin inhalation solution (TIS) has been established as an effective inhaled antibiotic for the treatment of chronic Pa pulmonary infection in patients with CF aged ⩾6 years.3,4 Colistimethate sodium (COLI) is also indicated for treating chronic pulmonary infection due to Pa in adult and pediatric CF patients.5 The administration of nebulized TIS and COLI for the treatment of Pa infection is complex, time consuming, and places a high burden on CF patients and their caregivers, posing a significant challenge to treatment adherence.4,6,7 In addition, nebulizers require regular maintenance, consisting of cleaning, disinfection, and drying for each use to minimize microbial contamination.8,9 Moreover, pathogens are commonly isolated from nebulizers and there is a concern that the nebulizer equipment may contribute to bacterial infection in the lower airways of CF patients.2,9 Tobramycin inhalation powder (TIP) has been developed for the suppressive management of pulmonary infection due to Pa in CF patients aged ⩾6 years.10 TIP, delivered via the T-326 inhaler, was reported to have a safety and efficacy profile similar to that of TIS but with a substantially simplified method of administration, which has translated into increased patient convenience and adherence.6,11–13 This study was designed to compare the ease of use of TIP administered via the T-326 inhaler (Novartis Pharma AG, Basel, Switzerland) with TIS and COLI, both administered via nebulizers. Furthermore, the prevalence of microbial contamination of the devices used was also compared in this study.
Methods
This open-label, crossover, interventional, phase IV, 20-week trial was conducted at 22 centers in the United Kingdom (8), Spain (5), Germany (4), Switzerland (3), and Ireland (2). The study protocol was reviewed by an independent ethics committee/institutional review board for each center and was conducted according to the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from each patient or their representative (parent or legal guardian). CF patients aged ⩾6 years with forced expiratory volume in 1 second (FEV1) ⩾25% to ⩽90% predicted were recruited if they had documented use of either COLI, TIS, or TIP for at least one cycle, and two positive cultures for Pa (either sputum, deep cough throat swab, or bronchoalveolar lavage), within the last 6 months. Patients with a current or past history of Burkholderia cepacia complex infection within 2 years prior to screening, hemoptysis >60 ml within 30 days of Visit 2, serum creatinine level ⩾2 mg/dl, or a history of hearing loss or chronic tinnitus deemed clinically significant by the investigator, were excluded from the study.
Study design
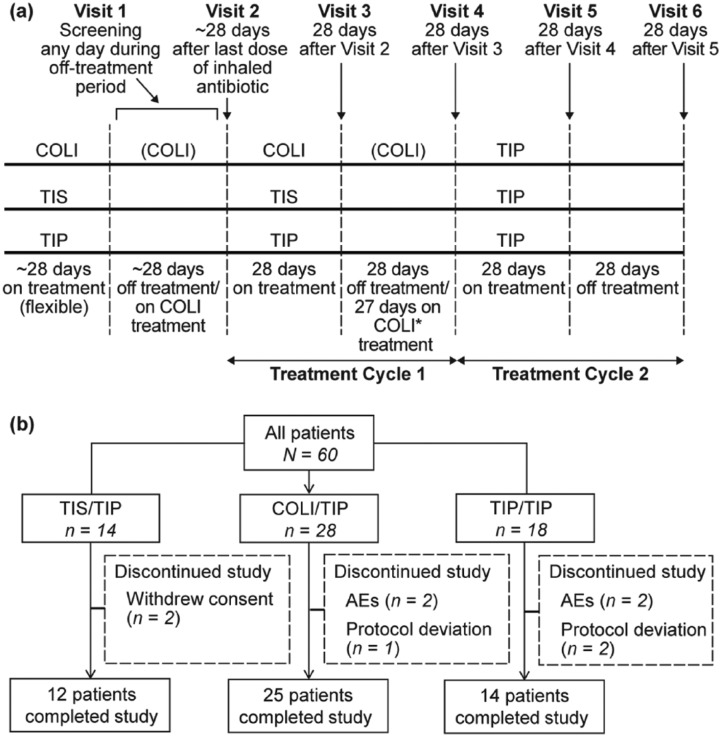
The study consisted of 6 visits (Figure 1(a)) over 20 weeks. Patients were assigned to one of the three treatment arms: COLI/TIP, TIS/TIP, or TIP/TIP.
Figure 1.
(a) Study design, (b) Patient disposition.
*Patients on continuous COLI were to observe a 24-h COLI-free period before the start of TIP at Visit 4.
Note: Patients with COLI could follow a cyclic or noncyclic regimen dependent on local treatment practice.
AEs, adverse events; COLI, colistimethate sodium; TIP, tobramycin inhalation powder; TIS, tobramycin inhalation solution.
In Cycle 1, patients in the COLI/TIP arm received nebulized COLI, 1 or 2 million units two or three times per day depending on local treatment guidelines for 56 days (no off-treatment period), administered using the patient’s usual nebulizer, and patients in the TIS/TIP arm received nebulized TIS, 300 mg twice daily for 28 days administered using the patient’s usual nebulizer, followed by 28 days off treatment. In Cycle 2 of these two arms, and in both Cycles 1 and 2 of the TIP/TIP arm, patients were treated with TIP, 112 mg (4 × 28 mg capsules) twice daily for 28 days followed by 28 days off treatment.
The three inhaled antibiotics were compared with each other with respect to ease of use in Cycle 1 in a real-world setting. Cycle 2 allowed the direct assessment of the ‘switch experience’ from nebulized antibiotics to TIP. The study was open-label as blinding the delivery device for TIP was not feasible, because a double-dummy design would have imposed a great burden on the patients for the feasibility of the study and conflict with measurement of the primary endpoint (ease of use).
Efficacy and patient-reported outcomes
Ease of use was measured by the mean cumulative time required to administer study treatments, including device setup/preparation, drug administration, and device cleaning (including disinfection, where applicable). The readiness of use of the study treatment was assessed as the sum of the time of start to the time of completion of delivery device preparation and the time of start to the time of completion of study treatment preparation. In addition, patient’s satisfaction and acceptance of the treatment were assessed14 by using the Treatment Satisfaction Questionnaire for Medication (TSQM) and Chronic Treatment ACCEPTance (ACCEPT®) questionnaire.15,16 Patients aged ⩾13 years completed the questionnaires by themselves in their local language in a quiet setting, and patients aged <13 years completed the questionnaires with the assistance of their parents or guardians. A patient preference survey was also conducted to evaluate the patients’ experience in switching from COLI or TIS to TIP.
Microbial contamination of the delivery device with Pa and other pathogens was analyzed in terms of light, moderate or heavy growth. The minimum inhibitory concentration (MIC) of tobramycin and other selected antibiotics for Pa isolated from patients’ specimens was assessed. The other efficacy measures evaluated were change in sputum Pa density (log10 colony-forming units (CFU)/g sputum), changes in clinical laboratory results and lung function [FEV1, forced vital capacity (FVC), and forced expiratory flow between 25–75% of FVC (FEF25–75%)].
The delivery devices used by CF patients were assessed for microbial contamination and swabs were collected from four locations on the nebulizer (mouthpiece, reservoir cup, filter, and tubing) and from the mouthpiece of the T-326 inhaler. Microbial contamination of the nebulizers was assessed at Visits 2 and 3 (the start and end of the first treatment period), Visits 4 and 5 (start and end of the second treatment period) and Visit 6 (end of study or discontinuation visit). Patients on TIP brought to their study visits the T-326 device used in the last week of TIP treatment. No assessment was required for the device at Visits 2 and 4, when the patients started the TIP treatment periods. If patients used nebulizers for inhaling any other medications they brought these nebulizers to their study Visits 2 and 6 for testing. Sputum specimens were collected from patients at clinic visits [1 (screening), 2, 3, 4, 5, and 6] for quantitative (CFUs) or semiquantitative culture of Pa (light, moderate, or heavy growth) and semiquantitative culture for non-Pa pathogens. These assessments were performed by a central laboratory. Cultures were performed on a variety of media designed to maximize growth of the pathogens most commonly isolated from the sputum of patients with CF. Cultures were evaluated after 24, 48, and 72 h.
Safety assessments
Safety evaluations consisted of the incidence and intensity of all adverse events (AEs), including cough, and serious AEs (SAEs) during both on- and off-treatment periods; physical condition; hematology and blood chemistry; urinalysis; audiology; and body weight.
Sample size and statistical analysis
A sample size of 15 patients per arm had 91% power to detect a difference in mean total administration time of 14 min as observed between TIS and TIP in the EAGER study,6 assuming a standard deviation (SD) of difference of 15 min or less and using a paired Student’s t-test with a 0.05 two-sided significance level. These calculations were performed a priori.
All data summaries and analyses were performed primarily within the study arms as the characteristics of the patients may vary among these arms. Summary statistics were provided for the mean total administration time per cycle by treatment arm and for within-patient differences in mean total administration time between treatments (Cycle 2−Cycle 1) by treatment arm. Safety analyses were based on descriptive statistics for AEs, cough rates, inhalation-associated cough, SAEs, airway reactivity, laboratory test results, audiology (where assessed), and vital signs that were summarized for each treatment and treatment cycle by arm.
Results
Study population
Of the 60 patients enrolled, the majority (51 patients; 85%) completed the study and nine patients (15%) discontinued the study. The reasons for discontinuation are listed in Figure 1(b). Patient baseline demographic and characteristics were comparable across the treatment arms. The mean age (SD) of the patient population was 27.6 years (±8.40; Table 1). A total of four pediatric patients were enrolled, two patients each in the age groups of 6–12 and 13–17 years.
Table 1.
Baseline demographic and disease characteristics.
| Baseline characteristics* | TIS/TIP N = 14 |
COLI/TIP N = 28 |
TIP/TIP N = 18 |
|---|---|---|---|
| Age (years), mean (SD) | 27.4 (6.82) | 28.4 (9.86) | 26.6 (7.25) |
| Age group (⩾18 years), n (%) | 13 (92.9) | 27 (96.4) | 16 (88.9) |
| Sex (male), n (%) | 10 (71.4) | 18 (64.3) | 11 (61.1) |
| Race, White, n (%) | 14 (100.0) | 28 (100.0) | 18 (100.0) |
| Body mass index (kg/m2), mean (SD) | 21.7 (3.19) | 21.4 (3.12) | 21.2 (3.83) |
| FEV1% predicted, mean (SD)† | 55.0 (17.02) | 59.1 (19.42) | 62.6 (17.78) |
| FVC% predicted, mean (SD)† | 67.7 (18.34) | 80.1 (19.44) | 78.6 (15.45) |
| FEF25–75% predicted, mean (SD)† | 30.6 (21.19) | 32.5 (22.27) | 37.3 (26.79) |
| Sputum density of Pa (log10 CFU/ml) – sum of all biotypes, mean (SD)‡ | 7.8 (1.88) | 6.9 (2.22) | 6.8 (2.46) |
| Pa tobramycin MIC, n (%) | |||
| >8 µg/ml | 6 (42.9) | 6 (21.4) | 6 (33.3) |
| ⩽8 µg/ml | 8 (57.1) | 21 (75.0) | 12 (66.7) |
| Missing | 0 (0.0) | 1 (3.6) | 0 (0.0) |
| Current use of long-acting bronchodilator, n (%) | 1 (7.1) | 5 (17.9) | 10 (55.6) |
| Current use of short-acting bronchodilator, n (%) | 3 (21.4) | 7 (25.0) | 11 (61.1) |
Baseline is defined as the last value before the first dose of study drug.
Recalculated to avoid calculation errors and use of different formulas to calculate % predicted values by local labs, FEV1% predicted/FVC% predicted/FEF25–75% predicted are derived according to Quanjer et al.27
Overall density defined as the sum of biotypes (mucoid, dry and small colony variants).
CFU, colony-forming unit; COLI, colistimethate sodium; FEF, forced expiratory flow; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; MIC, minimum inhibitory concentration; Pa, Pseudomonas aeruginosa; SD, standard deviation; TIP, tobramycin inhalation powder; TIS, tobramycin inhalation solution.
Efficacy results
Ease of use
Ease of use was considered a composite indicator of the speed, simplicity, and convenience of the study treatments. The mean total administration time including the time required to set up the delivery device, administer the drug, and clean the delivery device was significantly shorter in Cycle 2 (TIP treatment) than in Cycle 1 for the TIS/TIP and COLI/TIP arms (Table 2). However, the cumulative administration time between the treatment cycles remained similar for the TIP/TIP arm (Table 2). The mean administration time (excluding the setup, cleaning, and disinfection times) was significantly shorter in Cycle 2 than in Cycle 1 for all three treatment arms [difference in mean administration time, Cycle 2−Cycle 1: TIS/TIP, −9.8 (p = 0.0005); COLI/TIP, −5.3 (p = 0.0001); TIP/TIP, −0.3 (p = 0.0464)].
Table 2.
Analysis of mean total administration* time in minutes.
| TIS/TIP N = 14 |
COLI/TIP N = 28 |
TIP/TIP N = 18 |
|
|---|---|---|---|
| Cycle 1 | |||
| N | 8 | 17 | 14 |
| Mean (SD) | 37.0 (22.06) | 16.4 (9.54) | 4.2 (2.02) |
| Cycle 2 | |||
| N | 10 | 16 | 11 |
| Mean (SD) | 5.0 (2.04) | 3.8 (1.70) | 3.4 (2.06) |
| Time difference between Cycles 1 and 2† | |||
| N | 7 | 11 | 11 |
| Mean (SD) | −32.7 (23.90) | −13.3 (10.35) | −0.2 (0.92) |
| Cycle comparison | |||
| 95% CI | (−54.8, −10.6) | (−20.3, −6.4) | (−0.8, 0.4) |
| p-value‡ | 0.0112 | 0.0016 | 0.4380 |
Note: If the disinfection time was reported at least once in a day, all total administration times of that day were considered for calculating patient’s mean total administration time. This applies for patients on TIS or COLI treatment in Cycle 1.
Data of only patients with available mean total administration time of initial and second cycle were considered.
Total administration time, device setup time + administration time + device cleaning time + disinfection time (if available).
Time difference in the mean total administration time was calculated using within-patient differences, Cycle 2−Cycle 1.
p-values calculated using the paired Student’s t-test and 95% CIs for the mean difference were displayed.
CI, confidence interval; COLI, colistimethate sodium; SD, standard deviation; TIP, tobramycin inhalation powder; TIS, tobramycin inhalation solution.
Microbial contamination of devices
A total of 12 patients were found to have contaminated devices across the treatment cycles; 11 patients had contaminated nebulizers and one patient had a contaminated T-326 inhaler (Table 3): COLI/TIP arm, 9 (32.1%); TIS/TIP arm, 2 (14.3%); and TIP/TIP arm, 1 (5.6%). In the COLI/TIP arm, the majority of pathogens were isolated (only once) from the devices that delivered COLI at Visits 2 and 3. Except for one patient with Staphylococcus aureus infection in the COLI/TIP arm, no patient had the same pathogen isolated from the delivery device and sputum at Visits 2 and 3. In the TIP/TIP arm, no contamination was observed in the T-326 inhaler during either Cycle 1 or 2.
Table 3.
Delivery device cultures (density categories for pathogens including Pa).
| Cycle/visit | Device | Pathogen | N * | Light | Moderate | Heavy |
|---|---|---|---|---|---|---|
| TIS/TIP (N = 14) | ||||||
| Cycle 1 | ||||||
| Visit 2 (BSL) | Nebulizer | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Visit 3 | Nebulizer | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Cycle 2 | ||||||
| Visit 4 | Nebulizer | Pseudomonas aeruginosa biotype 2, dry | 1 | 1 (100.0) | 0 (0.0) | 0 (0.0) |
| Visit 5 | Nebulizer | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| T-326 inhaler† | Staphylococcus aureus | 1 | 1 (100.0) | 0 (0.0) | 0 (0.0) | |
| COLI/TIP (N = 28) | ||||||
| Cycle 1 | ||||||
| Visit 2 (BSL) | Nebulizer | Acinetobacter baumannii | 7 | 0 (0.0) | 0 (0.0) | 1 (14.3) |
| Acinetobacter junii | 7 | 0 (0.0) | 1 (14.3) | 0 (0.0) | ||
| Acinetobacter lwoffi | 7 | 2 (28.6) | 0 (0.0) | 0 (0.0) | ||
| Haemophilus parainfluenza | 7 | 1 (14.3) | 0 (0.0) | 0 (0.0) | ||
| Ochrobactrum anthropi | 7 | 0 (0.0) | 0 (0.0) | 1 (14.3) | ||
| Pseudomonas fluorescens | 7 | 1 (14.3) | 0 (0.0) | 0 (0.0) | ||
| Pseudomonas putida | 7 | 1 (14.3) | 0 (0.0) | 0 (0.0) | ||
| Pseudomonas stutzeri | 7 | 0 (0.0) | 1 (14.3) | 0 (0.0) | ||
| Serratia liquefaciens | 7 | 1 (14.3) | 0 (0.0) | 0 (0.0) | ||
| Sphingobacterium multivorum | 7 | 1 (14.3) | 0 (0.0) | 0 (0.0) | ||
| Stenotrophomonas maltophilia | 7 | 1 (14.3) | 0 (0.0) | 0 (0.0) | ||
| Visit 3 | Nebulizer | Acinetobacter species unspecified | 6 | 1 (16.7) | 0 (0.0) | 0 (0.0) |
| Chryseobacterium indologenes | 6 | 0 (0.0) | 1 (16.7) | 0 (0.0) | ||
| Delftia acidovorans | 6 | 1 (16.7) | 0 (0.0) | 0 (0.0) | ||
| Pseudomonas fluorescens | 6 | 2 (33.3) | 0 (0.0) | 0 (0.0) | ||
| Sphingomonas paucimobilis | 6 | 0 (0.0) | 0 (0.0) | 1 (16.7) | ||
| Staphylococcus aureus | 6 | 1 (16.7) | 0 (0.0) | 0 (0.0) | ||
| Cycle 2 | ||||||
| Visit 4 | Nebulizer | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Visit 5 | Nebulizer | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| T-326 inhaler | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| TIP/TIP (N = 18) | ||||||
| Cycle 1 | ||||||
| Visit 2 (BSL) | Nebulizer‡ | Pseudomonas aeruginosa biotype 2, dry | 1 | 0 (0.0) | 1 (100.0) | 0 (0.0) |
| Visit 3 | Nebulizer | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| T-326 inhaler | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Cycle 2 | ||||||
| Visit 4 | Nebulizer | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Visit 5 | Nebulizer | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| T-326 inhaler | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
Note: Commercial packs of TIP were used at Visit 4 (start of Cycle 2) by each patient.
All nebulizers used by the patients were analyzed, including those on other inhaled medications (e.g. mucolytics).
Number of patients with any contaminated delivery device.
S. aureus isolated (light growth) from one T-326 inhaler was not present in the patient’s sputum.
Pa (moderate growth) was isolated from the nebulizer (medication not specified) at Visit 2.
BSL, baseline; COLI, colistimethate sodium; TIP, tobramycin inhalation powder; TIS, tobramycin inhalation solution.
Pseudomonas aeruginosa sputum density and MIC
The absolute change in the number of Pa CFUs in the sputum was assessed after a period of up to 28 days of treatment. At Visit 3 (Cycle 1), the mean log reduction in Pa for the sum of all biotypes was 1.4 log10 CFU in the TIS/TIP arm, 0.6 log10 CFU in the COLI/TIP arm, and 1.7 log10 CFU in the TIP/TIP arm. At Visit 5 (Cycle 2), the mean log reduction was slightly lower for the TIS/TIP arm and was similar for the other two arms. In general, the result of each biotype was comparable with the result observed for the sum of all biotypes.
Tobramycin MIC50 and MIC90 values showed that there was a 1-fold dilution increase in the tobramycin MICs at Visit 5 compared with Visit 3 for the TIS/TIP arm (MIC50: 4 μg/ml versus 2 μg/ml; MIC90: 512 μg/ml versus 256 μg/ml) and the COLI/TIP arm (MIC50: 4 μg/ml versus 2 μg/ml; MIC90: 32 μg/ml versus 16 μg/ml). In the TIP/TIP arm, the MIC50 and MIC90 tobramycin values were stable up to Visit 5 (2 μg/ml and 64 μg/ml, respectively) and were further decreased to 1 μg/ml and 32 μg/ml, respectively, at the end of Visit 6.
Lung function
The assessment of lung function was an exploratory efficacy endpoint. At Visit 3, the mean FEV1% predicted showed a relative increase from baseline in the TIS/TIP (2.2%) and COLI/TIP (3.9%) arms and a slight decrease from baseline in the TIP/TIP arm (−2.8%). However, at Visit 5, the FEV1% predicted remained stable across the treatment arms, as compared with Visit 4. No notable difference was observed (p >0.05) in absolute change from pre-dose value to the end of on-/off-treatment periods of any visits across all treatment arms. Similarly, the pre-dose FVC% and FEF25–75% predicted relative change from start to end of on- and off-treatment periods at each visit showed no significant difference in any treatment arm.
TSQM, ACCEPT® and patient preference questionnaire results
The median scores for the TSQM questionnaire were high in Cycle 1 and were either sustained or further improved in Cycle 2 for the majority of domains, indicating treatment satisfaction in patients receiving TIP (Table 4). Improvements were reported in Cycle 2 in the COLI/TIP arm for the effectiveness, convenience, and global satisfaction domains; improvements were also reported for convenience in the TIS/TIP arm, although the scores for effectiveness and global satisfaction decreased in Cycle 2 from Cycle 1. Similar scores were reported between cycles for the TIP/TIP arm with the exception of a slight decrease in global satisfaction in Cycle 2 for the TIP/TIP arm.
Table 4.
Summary of the TSQM and ACCEPT® questionnaire results.
| TIS/TIP N = 14 |
COLI/TIP N = 28 |
TIP/TIP N = 18 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycle 1 Visit 3 |
Cycle 2 Visit 5 |
Cycle 1 Visit 3 |
Cycle 2 Visit 5 |
Cycle 1 Visit 3 |
Cycle 2 Visit 5 |
|||||||
| n | Median | n | Median | n | Median | n | Median | n | Median | n | Median | |
| TSQM | ||||||||||||
| Effectiveness | 13 | 66.7 | 12 | 55.6 | 26 | 63.9 | 25 | 72.2 | 17 | 72.2 | 15 | 72.2 |
| Side effects* | 12 | 100.0 | 11 | 100.0 | 25 | 100.0 | 24 | 100.0 | 17 | 100.0 | 15 | 100.0 |
| Convenience | 12 | 63.9 | 11 | 77.8 | 25 | 61.1 | 24 | 83.3 | 17 | 77.8 | 15 | 77.8 |
| Global satisfaction | 12 | 71.4 | 11 | 64.3 | 25 | 64.3 | 24 | 75.0 | 17 | 78.6 | 15 | 71.4 |
| ACCEPT®† | ||||||||||||
| Medication inconvenience | 12 | 80.0 | 11 | 90.0 | 25 | 70.0 | 24 | 90.0 | 16 | 80.0 | 14 | 85.0 |
| Long-term treatment | 12 | 66.7 | 10 | 75.0 | 25 | 66.7 | 24 | 79.2 | 16 | 66.7 | 14 | 66.7 |
| Regime constraints | 11 | 80.0 | 10 | 90.0 | 25 | 70.0 | 23 | 80.0 | 16 | 70.0 | 14 | 72.5 |
| Numerous medications, m/M (%)‡ | ||||||||||||
| Yes, not easy to accept | 2/11 | 18.2 | 3/9 | 33.3 | 0/25 | 0.0 | 2/23 | 8.7 | 4/16 | 25.0 | 3/14 | 21.4 |
| Yes, easy to accept | 4/11 | 36.4 | 5/9 | 55.6 | 20/25 | 80.0 | 16/23 | 69.6 | 10/16 | 62.5 | 9/14 | 64.3 |
| No | 5/11 | 45.5 | 1/9 | 11.1 | 5/25 | 20.0 | 5/23 | 21.7 | 2/16 | 12.5 | 2/14 | 14.3 |
| Side effects | 11 | 100.0 | 10 | 100.0 | 25 | 100.0 | 21 | 90.0 | 16 | 90.0 | 14 | 90.0 |
| Effectiveness | 11 | 83.3 | 10 | 58.3 | 24 | 66.7 | 23 | 66.7 | 16 | 75.0 | 14 | 91.7 |
| General | 11 | 66.7 | 10 | 50.0 | 25 | 66.7 | 23 | 66.7 | 16 | 75.0 | 14 | 83.3 |
Each domain score ranges between 0–100, with a higher score indicating a higher treatment satisfaction.
All values expressed as median values, mean scores in the side effect domain ranged from 84.2–96.8 across treatment arms.
Data are n, median unless otherwise specified.
Percentages are based on M.
COLI, colistimethate sodium; m, number of patients with the score; M, number of patients with data of the domain at the visit; n, number of patients with data of the domain at the visit; TIP, tobramycin inhalation powder; TIS, tobramycin inhalation solution; TSQM, Treatment Satisfaction Questionnaire for Medication.
Regarding the ACCEPT® questionnaire, in Cycle 2 the median scores for most of the domains were mostly improved or sustained from Cycle 1. At Visit 2 in the TIS/TIP arm, the median scores were improved from Cycle 1 for the domains of medication inconvenience (10 units improvement), long-term treatment (8.3 units improvement) and regime constraints (10 units improvement). The exception was that median scores decreased in Cycle 2 for the domains of effectiveness and general. In the COLI/TIP arm, the median scores were improved from Cycle 1 for the domains of medication inconvenience (20 units improvement), long-term treatment (12.5 units improvement) and regime constraints (10 units improvement). In the TIP/TIP arm, the median scores for most of the domains were high and comparable between the treatment cycles. Improvements were observed in Cycle 2 for the domains of medication inconvenience (5 units improvement), regime constraints (2.5 unit improvement), effectiveness (16.7 units improvement) and general (8.3 units improvement) (Table 4). Using a separate patient preference questionnaire, the majority of the patients showed either ‘strong’ or ‘somewhat’ preference to use TIP in the TIS/TIP (9 of 12 patients, 75.0%) and COLI/TIP (18 of 23 patients, 78.3%) arms. ‘Strong’ or ‘somewhat’ preference for TIS and COLI in the TIS/TIP and COLI/TIP arms were 16.7% (2 of 12 patients) and 8.7% (2 of 23 patients), respectively (Table 5).
Table 5.
Adverse events, reported by at least two patients, regardless of study drug relationship, by preferred term and cycle.
| TIS/TIP N = 14 |
COLI/TIP N = 28 |
TIP/TIP N = 18 |
|
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Cycle 1 | |||
| Number of patients treated in cycle in the analysis set | 14 (100.0) | 28 (100.0) | 18 (100.0) |
| Patients* with AE(s) | 6 (42.9) | 19 (67.9) | 11 (61.1) |
| Infective pulmonary exacerbation of cystic fibrosis | 5 (35.7) | 10 (35.7) | 2 (11.1) |
| Cough | 2 (14.3) | 2 (7.1) | 2 (11.1) |
| Nasopharyngitis | 0 (0.0) | 3 (10.7) | 2 (11.1) |
| Headache | 1 (7.1) | 0 (0.0) | 3 (16.7) |
| Sputum increased | 1 (7.1) | 0 (0.0) | 3 (16.7) |
| Hemoptysis | 0 (0.0) | 0 (0.0) | 2 (11.1) |
| Pyrexia | 0 (0.0) | 0 (0.0) | 2 (11.1) |
| Cycle 2 | |||
| Number of patients treated in cycle in the analysis set | 12 (100.0) | 25 (100.0) | 15 (100.0) |
| Patients* with AE(s) | 8 (66.7) | 12 (48.0) | 10 (66.7) |
| Infective pulmonary exacerbation of cystic fibrosis | 3 (25.0) | 7 (28.0) | 1 (6.7) |
| Cough | 1 (8.3) | 0 (0.0) | 2 (13.3) |
| Hemoptysis | 1 (8.3) | 2 (8.0) | 0 (0.0) |
| Headache | 0 (0.0) | 0 (0.0) | 3 (20.0) |
| Nasopharyngitis | 0 (0.0) | 0 (0.0) | 3 (20.0) |
| Sputum increased | 0 (0.0) | 0 (0.0) | 2 (13.3) |
Patients with multiple incidences of the same AE are counted only once for the preferred term.
AE, adverse events; CI, confidence interval; COLI, colistimethate sodium; SD, standard deviation; TIP, tobramycin inhalation powder; TIS, tobramycin inhalation solution.
Safety results
Treatment-emergent AEs (on and off treatment) were reported by 36 patients in Cycle 1 [(n/N, %); TIS/TIP: 6/14, 42.9%; COLI/TIP: 19/28, 67.9%; TIP/TIP: 11/18, 61.1%] and by 30 patients (57.7%) overall in Cycle 2 (Table 6).
The mean post-inhalation cough rates were comparable between the cycles for the COLI/TIP arm (0.56 at Visit 2 and 0.47 at Visit 3 versus 0.65 at Visit 4 and 0.51 at Visit 5). However, for the TIP/TIP arm, the post-inhalation cough rate was reduced over time, with a low cough rate in Cycle 2 than in Cycle 1 (0.40 at Visit 4 and 0.33 at Visit 5 versus 0.53 at Visit 2 and 0.56 at Visit 3, respectively).
Treatment-related AEs were reported in four patients (one each in the TIS/TIP and COLI/TIP arms and two in the TIP/TIP arm) in Cycle 1; none was considered severe. In Cycle 2, eight patients (15.4%) reported AEs that were suspected to be treatment related; three were severe (each with decreased FEV1 and an upper respiratory tract infection, abnormal acoustic stimulation tests and tinnitus, and cough). Overall, four patients discontinued the study treatment due to SAEs; however, none of these events were suspected to be treatment related.
The frequency of airway reactivity (i.e. decrease of ⩾20% in post-dose FEV1% predicted compared with the pre-dose value) from pre-dose to 15–45 min post-dose was low: one patient each at Visits 2 and 3 in the COLI/TIP arm and one patient at Visit 4 in the TIP/TIP arm.
Discussion
Despite recent advances in therapy and improvements in median survival age, the treatment burden of CF patients has increased, which is primarily associated with the number of nebulized medications.17–19 The complexity of nebulized therapy leads to reduced adherence.20,21 Moreover, lack of adherence with therapy has been considered an important cause for the increased hospitalization and pulmonary exacerbations in CF patients.17,22 The ease of use of inhalers is an important factor in encouraging patient adherence with therapy, minimizing the handling errors and improving satisfaction.17,23 The present study showed that patients took significantly lower cumulative time to administer TIP than COLI or TIS. This resulted in approximately 13–33 min of time saving per administration with the T-326 inhaler used for TIP compared with nebulizers used for TIS and COLI. Ease of T-326 inhaler use for administration of TIP and reduction in administration time have been associated with improved adherence and clinical outcomes.6 In general, treatment satisfaction, as assessed by TSQM and ACCEPT® questionnaires showed better results for TIP, with higher scores for ‘convenience’ and greater acceptance for TIP, although there were decreases in scores reported in Cycle 2 compared with Cycle 1 in some of the domains for both the TSQM and ACCEPT® questionnaires. These patient-reported outcome measures were supported by the shorter cumulative administration time for TIP and a comparable efficacy profile as reported in other studies.11 Furthermore, when assessing the patient’s preference of inhaled therapy, the majority of patients showed either ‘strong’ or ‘somewhat’ treatment preference for TIP in the TIS/TIP and COLI/TIP arms over the nebulizers, which emphasizes the ease of use of TIP over other nebulized drugs.
Several published studies have described frequent contamination of home nebulizers with microorganisms and reported that Pa constitutes the major contaminant.2,8 In the present study, the majority of pathogens were isolated from the nebulizer used to administer COLI in Cycle 1. The microbial contamination assessment suggested that the T-326 inhaler used for TIP was much less frequently contaminated, thus potentially reducing the sources of infection in CF patients compared with the nebulizers used for TIS or COLI. These results advocate the use of T-326 inhaler over nebulizers.
Consistent with previous studies,11,12 sputum Pa densities decreased in all the treatment arms. Although the mean FEV1% predicted at the end of the on-treatment period in Cycle 1 showed a decrease in the TIP arm, and increases in the COLI and TIS arms, the mean FEV1% predicted by the end of the on-treatment period in Cycle 2 remained stable across the three arms. Overall, TIP showed comparable efficacy with TIS and COLI, which is in accordance with previous publications.6,17,24,25
In addition, this study demonstrated that AEs were comparable in all three treatment arms and no unexpected safety events were reported. Although cough is a common AE with inhaled therapies,25,26 a clear trend was observed in the decreased frequency of post-inhalation cough over time from Cycle 1 to Cycle 2 in the TIP/TIP arm. Moreover, most cough events were of mild or moderate intensity in all the arms. Overall, TIP was well tolerated and the safety findings observed for TIP in this study were generally consistent with its established safety profile.6,12,26
These results provide clinicians with further guidance on the relative differences between the speed and ease of use of inhalers and nebulizers as well as evidence on the prevalence of microbial contamination of the inhalation devices in the real-world setting. However, this was an open-label, non-randomized study, which carries the risk of reporting bias, particularly for the patient-reported outcomes. In addition, data on the nebulizer type were not collected, and therefore, results are applicable to nebulizers in general but not necessarily to any specific nebulizer.
Conclusions
In summary, the T-326 inhaler used to deliver TIP showed significantly shorter administration and cleaning times compared with nebulizers used for COLI and TIS, suggesting that TIP is easy to use in CF patients treated for pulmonary Pa infection. Ease of use was also supported by the outcomes of the TSQM and ACCEPT® questionnaires. Furthermore, the T-326 inhaler was much less frequently contaminated than the nebulizers, thus potentially reducing the sources of pathogenic bacteria in CF patients. In addition, this study provides further evidence on the efficacy of TIP as shown by the sustained suppression of Pa and stability in FEV1% predicted.
Acknowledgments
The authors acknowledge Anupama Tamta (Novartis Healthcare Pvt. Ltd., Hyderabad, India) for providing medical writing assistance for this manuscript. This research was registered at ClinicalTrials.gov [ClinicalTrials.gov identifier: NCT01844778].
Footnotes
Conflict of interest statement: JG received honorarium for participation in a Novartis-sponsored advisory board and received educational grants to attend conferences from Novartis. CS received financial support from Novartis for an investigator-initiated trial. US, EFN, and MT have nothing to disclose. WC, PM, LD, and KH are full-time employees of Novartis Pharmaceuticals Corporation (East Hanover, NJ, USA).
Funding: The study was sponsored by Novartis Pharma AG (Basel, Switzerland).
Contributor Information
James Greenwood, Liverpool Heart and Chest Hospital NHS Foundation Trust, Thomas Drive, Liverpool, UK.
Carsten Schwarz, Department of Pediatric Pneumology and Immunology, Cystic Fibrosis Centre Berlin, Charité–University Medicine Berlin, Berlin, Germany.
Urte Sommerwerck, Department of Pneumology, Ruhrlandklinik, West German Lung Center, University Hospital of Essen, University Duisburg-Essen, Essen, Germany.
Edward F Nash, West Midlands Adult Cystic Fibrosis Centre, Heart of England NHS Foundation Trust, Birmingham, UK.
Michael Tamm, Clinic of Pneumology and Respiratory Cell Research, University Hospital, Basel, Switzerland.
Weihua Cao, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Paul Mastoridis, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Laurie Debonnett, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Kamal Hamed, Novartis Pharmaceuticals Corporation, East Hanover, NJ 07936-1080, USA.
References
- 1. Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med 1999; 340: 23–30. [DOI] [PubMed] [Google Scholar]
- 2. Della Zuana A, Garcia Dde O, Juliani RC, et al. Effect that an educational program for cystic fibrosis patients and caregivers has on the contamination of home nebulizers. J Bras Pneumol 2014; 40: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. TOBI (tobramycin inhalation solution). Prescribing information. East Hanover, NJ: Novartis Pharmaceuticals Corp, 2015. [Google Scholar]
- 4. Flume PA, O’Sullivan BP, Robinson KA, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med 2007; 176: 957–969. [DOI] [PubMed] [Google Scholar]
- 5. Promixin®. Summary of product characteristics. Profile Pharma Limited: Chichester; (WestSussex), 2015. [Google Scholar]
- 6. Konstan MW, Flume PA, Kappler M, et al. Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: the EAGER trial. J Cyst Fibros 2011; 10: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Briesacher BA, Quittner AL, Saiman L, et al. Adherence with tobramycin inhaled solution and health care utilization. BMC Pulm Med 2011; 11: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blau H, Mussaffi H, Mei Zahav M, et al. Microbial contamination of nebulizers in the home treatment of cystic fibrosis. Child Care Health Dev 2007; 33: 491–495. [DOI] [PubMed] [Google Scholar]
- 9. Lester MK, Flume PA, Gray SL, et al. Nebulizer use and maintenance by cystic fibrosis patients: a survey study. Respir Care 2004; 49: 1504–1508. [PubMed] [Google Scholar]
- 10. TOBI Podhaler (tobramycin inhalation powder). Prescribing information. East Hanover, NJ: Novartis Pharmaceuticals Corp, 2015. [Google Scholar]
- 11. Galeva I, Konstan MW, Higgins M, et al. Tobramycin inhalation powder manufactured by improved process in cystic fibrosis: the randomized EDIT trial. Curr Med Res Opin 2013; 29: 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Konstan MW, Geller DE, Minic P, et al. Tobramycin inhalation powder for P. aeruginosa infection in cystic fibrosis: the EVOLVE trial. Pediatr Pulmonol 2011; 46: 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lam J, Vaughan S, Parkins MD. Tobramycin Inhalation Powder (TIP): an efficient treatment strategy for the management of chronic pseudomonas aeruginosa infection in cystic fibrosis. Clin Med Insights Circ Respir Pulm Med 2013; 7: 61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patrick DL, Burke LB, Powers JH, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health 2007; 10(Suppl 2): S125–S137. [DOI] [PubMed] [Google Scholar]
- 15. Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2004; 2: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Regnault A, Balp MM, Kulich K, et al. Validation of the treatment satisfaction questionnaire for medication in patients with cystic fibrosis. J Cyst Fibros 2012; 11: 494–501. [DOI] [PubMed] [Google Scholar]
- 17. Harrison MJ, McCarthy M, Fleming C, et al. Inhaled versus nebulised tobramycin: a real-world comparison in adult cystic fibrosis (CF). J Cyst Fibros 2014; 13: 692–698. [DOI] [PubMed] [Google Scholar]
- 18. Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. J Cyst Fibros 2009; 8: 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Geller DE, Weers J, Heuerding S. Development of an inhaled dry-powder formulation of tobramycin using PulmoSphere technology. J Aerosol Med Pulm Drug Deliv 2011; 24: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Agent P, Parrott H. Inhaled therapy in cystic fibrosis: agents, devices and regimens. Breathe (Sheff) 2015; 11: 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Latchford G, Duff A, Quinn J, et al. Adherence to nebulised antibiotics in cystic fibrosis. Patient Educ Couns 2009; 75: 141–144. [DOI] [PubMed] [Google Scholar]
- 22. Sawicki GS, Heller KS, Demars N, et al. Motivating adherence among adolescents with cystic fibrosis: youth and parent perspectives. Pediatr Pulmonol 2015; 50: 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Von Schantz S, Katajavuori N, Antikainen O, et al. Evaluation of dry powder inhalers with a focus on ease of use and user preference in inhaler-naive individuals. Int J Pharm 2016; 509: 50–58. [DOI] [PubMed] [Google Scholar]
- 24. Konstan MW, Flume PA, Galeva I, et al. One-year safety and efficacy of tobramycin powder for inhalation in patients with cystic fibrosis. Pediatr Pulmonol 2016; 51: 372–378. [DOI] [PubMed] [Google Scholar]
- 25. Schuster A, Haliburn C, Doring G, et al. Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (Colobreathe DPI) in patients with cystic fibrosis: a randomised study. Thorax. 2013; 68: 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geller DE, Konstan MW, Smith J, et al. Novel tobramycin inhalation powder in cystic fibrosis subjects: pharmacokinetics and safety. Pediatr Pulmonol 2007; 42: 307–313. [DOI] [PubMed] [Google Scholar]
- 27. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]