Abstract
For many years, management of cystic fibrosis (CF) lung disease was focused on symptomatic treatment of chronic lung infection, which is characterized by cough and sputum production, leading to progressive lung damage. With increasing survival and better knowledge of the pathogenesis of CF lung disease, it has become clear that treatment has to start very early because lung damage occurs in young patients, often before obvious symptoms appear. The arrival of new cystic fibrosis transmembrane conductance-regulator (CFTR)-correcting therapies will bring more opportunities to prevent the disease, apart from only treating chronic lung infection.
In this review, a summary of the current knowledge of early CF lung disease is provided, based on animal model studies, as well as on data obtained from well structured follow-up programs after newborn screening (NBS). The most important clinical guidelines for treating young CF patients are also summarized.
Keywords: animal models, cystic fibrosis, early therapy, lung disease, newborn screening
Introduction
Cystic fibrosis (CF) is an autosomal-recessive disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) [Riordan et al. 1989]. CF was long considered a disease of young children with few children surviving beyond school age. Thanks to improved insight in this complex disease and ensuing better treatment, survival has improved dramatically over the last decades. For a newborn baby with CF, the predicted survival is at least 40 years (see http://www.cff.org). This prediction will most likely further improve as soon as therapies with small molecules correcting the CFTR function become more widely available.
CF affects many organs (including lung, pancreas, liver, intestines and reproductive tract), but the respiratory manifestation is often the most recognized clinical feature and is the leading cause of mortality and morbidity of this disease. Lung disease is characterized by persistent airway infection and chronic inflammation, causing irreversible lung damage.
Although the clinical face of CF has changed, one should not forget that the disease process still starts very early in life, often even before symptoms appear. Therefore, early diagnosis [preferably by newborn screening (NBS)], followed by appropriate treatment remains of utmost importance [Elborn, 2016].
In this overview, the literature is reviewed on early stage CF lung disease from animal model data to clinical research. Additionally, how to adequately treat early CF lung disease is discussed, with reference to the highlights of existing consensus documents on this topic.
Lessons from the cystic fibrosis pig model
CF mouse models, developed shortly after the CFTR gene was discovered, lack the characteristic lung disease present in CF patients [Ahmed and Mukherjee, 2016]. To study the origins of CF lung disease, the CF pig, however, proved to be an ideal model [Meyerholz, 2016; Rogers et al. 2008]. At birth, CF-pig lungs have no features of inflammation but were often less sterile than controls. Within the first weeks to months of life, they develop infection, inflammation, airway remodelling as well as mucus plugs in the respiratory tract [Stoltz et al. 2010]. A wide range of bacteria is isolated from the CF-pig airway, including Pseudomonas aeruginosa in older pigs, a similar pattern being observed in human CF disease. A defective bacterial clearance after a pulmonary challenge with Staphylococcus aureus has been demonstrated in the CF pigs compared with wild-type pigs.
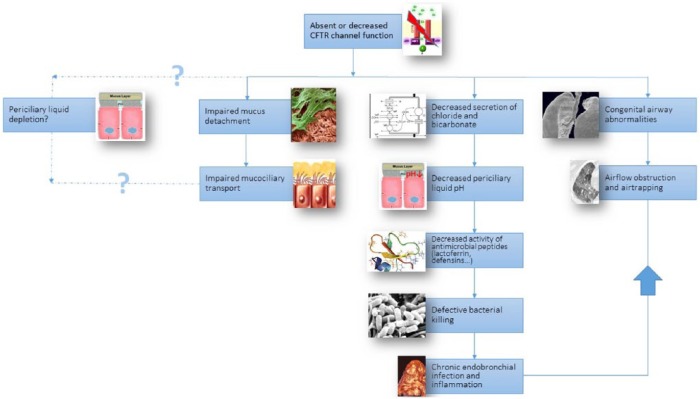
Figure 1.
Pathogenesis of early CF lung disease.
CFTR, cystic fibrosis transmembrane conductance regulator.
Due to the CFTR channel dysfunction, chloride and bicarbonate secretion are reduced, leading to a lower airway surface liquid (ASL) pH. The antimicrobial substances secreted by the lung in the ASL (including lactoferrin, defensins and lysozyme) have a reduced bactericidal activity in this more acid environment. This could partially explain the observed failing bacterial clearance in CF. The hypothesized sodium and liquid hyperabsorption [due to loss of negative feedback regulation by CFTR on epithelial sodium channel (ENAC)] leading to ASL dehydration, was not confirmed in the pig model and is still a controversial issue. Finally, decreased mucociliary clearance is present in the CF lung and this is at least in part the consequence of tethering of abnormal strands of mucus (secreted by the submucosal glands) to the airway wall. In newborn CF piglets, impaired mucociliary transport was caused by tethering of mucus strands to the gland ducts [Hoegger et al. 2014] and not by periciliary liquid depletion.
Apart from the acquired airway pathology, the CF pig has congenital airway anomalies such as underdevelopment of the sinuses and tracheal conformational defects (smaller and less circular conformation). The former was already known in CF patients, but the tracheal abnormalities were found only on reviewing older autopsy material confirming the same findings in CF babies. A recent study confirmed what CF clinicians already knew: trachea−malacia is common in CF patients and is associated with more severe lung disease [Fischer et al. 2014]. These structural airway abnormalities may be the cause of airflow obstruction and airtrapping on computed tomography (CT) scan in newborn piglets even before infection and inflammation settles in.
Translating the cystic fibrosis pig findings to human disease, one may conclude the following:
(1) CF lung disease starts from birth and, thus, CF should be treated as early as possible. Part of the airway anomalies are even congenital.
(2) Due to defective host defense, a variety of bacteria enter the airway early in life. Clinical studies are to evaluate the best treatment approach. This may involve interven-tions like airway clearance techniques and prophylactic antibiotic treatment, even before the ‘usual suspects appear’ (Staphylococcus aureus, Pseudomonas aeruginosa).
Lessons learned from follow-up studies after newborn screening
Since CF disease starts early in life, timely diagnosis and institution of treatment could prevent irreversible lung damage prediagnosis. Based on CF register data, the median age of diagnosis in countries without NBS, such as Belgium, is around 6 months of age with a wide range. This is not ‘early enough’ since a prognostic advantage has been documented if the diagnosis is made before the age of 2 months [Sims et al. 2007]. The latter is only achievable by means of NBS. NBS for CF has a positive effect on short-term and long-term clinical outcomes [Castellani et al. 2016] and is a cost-effective public health strategy. Not surprisingly, a worldwide expansion of screening for CF has been reported. Thanks to two structured and extensive clinical follow-up programs, much has been learned about the early course of CF disease (Australian AREST-CF study and the London CF collaborative) [Bush and Sly, 2015]. Although there are some discrepancies in the findings between the two cohorts, some important data can be summarized. In the London group, by 2 years of age, babies with CF diagnosed on clinical grounds had airflow obstruction on lung function and signs of infection and inflammation on bronchoscopy, even in asymptomatic babies, and this despite specialist treatment. From 2007 onwards, the London cohort started follow up of an NBS CF cohort. Although at 3 months of age, lung clearance index (LCI) was elevated and remained elevated at 1 year, lung function [forced expiratory volume (FEV) 0.5] had improved. At 2 years of age, lung function indices were the same as at 1 year of age [Brennan and Thia, 2013; Nguyen et al. 2014]. Chest CT abnormalities in this cohort were mild.
In the AREST CF cohort, babies with CF diagnosed after NBS were followed up with lung function, bronchoscopy and chest CT. Although early nutritional outcomes were excellent, lung function was abnormal by 6 months of age and lung function decline correlated with isolation of S. aureus and P. aeruginosa. At school age, lung function (FEV 0.75) was, however, only modestly decreased. As early as by median age of 3.6 months, significant bacterial infection was found on broncho alveolar lavage (BAL) in 21% of patients; the majority of these children being asymptomatic [Sly et al. 2009]. Chest CT at that time point showed mostly airtrapping (67%) and bronchial wall thickening (45%); again, most children with abnormal CTs were asymptomatic [Sly et al. 2009]. On serial chest CTs, bronchiectasis (defined as bronchial dilatation) was found early and did resolve in 12.5% from 3 months to 1 year. By 3 years of age, the point prevalence of bronchiectasis was as high as 60% [Stick et al. 2009]. Factors associated with bronchiectasis were BAL absolute neutrophil count, BAL neutrophil elastase and infection with P. aeruginosa.
Together, these data illustrate that although early diagnosis by NBS improves prognosis, our current treatment strategies are insufficient to prevent early lung damage. If CFTR modulators become available, these data support studying the effect of these new molecules also in the youngest age groups [Davies et al. 2016].
New insights in gut and lung microbiota
Microbial colonization patterns in infancy are influenced by environment (including diet and medication), human genetics and immune function. CF has been associated with atypical microbial colonization; not only of the respiratory tract, but also of the gut.
A prospective longitudinal analysis of the upper respiratory tract and gastrointestinal (GI) tract was performed in a cohort of 13 CF babies followed from birth [Hoen et al. 2015]. Significant changes in bacterial abundance were found in the GI as well as the respiratory tract prior to Pseudomonas infection, with significant decrease in Parabacteroides in the gut, and an increase in Salmonella species in the airway. Significant associations were found between gut microbiome and CF exacerbations. Breastmilk feeding was an important determinant of microbial diversity in the respiratory tract, and there was a trend towards longer time to first exacerbation in breastfed babies. The same trend was found between GI microbial diversity and longer time to first exacerbation.
In a prospective cohort of 30 babies with CF followed up from birth, with nasal swabs every 2 weeks, nasal microbiota were compared with the same in a healthy cohort [Mika et al. 2016]. There was a significant different bacterial community composition even in CF babies naïve for antibiotics (AB) treatment, with an increase in relevant abundance of S. aureus (as well as coagulase negative). After AB treatment, S. aureus decreases with a relative increase of coagulase-negative staphylococci.
Early cystic fibrosis therapy: consensus guidelines and beyond
An evidence-based guideline for management of infants with CF was published in 2009 [Cystic Fibrosis Foundation et al. 2009]. For further reading, we can also refer to the Standards of Care Guidelines of the european cystic fibrosis society (ECFS) [Smyth et al. 2014]. Very recently, clinical practice guidelines have been published by the CF foundation, specifically for preschoolers [Lahiri et al. 2016]. From these extensive reviews, important items concerning treatment of respiratory disease will be highlighted.
Clinical follow up
Lung function
The respiratory follow up of preschoolers is a challenge since standard testing, such as spirometry, cannot easily be performed in this age group. The guidelines advise to attempt spirometry as early as 3 years of age using FEV-0.5 seconds instead of FEV-1 second as follow up. Since performing spirometry in this age group is very time consuming and associated with low success, many CF centers typically postpone this age to 5–6 years. The use of multiple-breath washout (MBW) testing measures regional ventilation heterogeneity and appears to be more sensitive than spirometry in detecting lung disease in young children with CF [Aurora et al. 2011; Gustafsson et al. 2008]. The LCI, one of the MBW indices, is also being used as an outcome parameter in clinical trials in preschoolers [Amin et al. 2010, 2011; Davies et al. 2013; Subbarao et al. 2013]. The usefulness of the LCI may, however, be age dependent. In a recent study on LCI versus chest CT in children with CF, it was shown that in the group 0–2 years, LCI is too insensitive to detect structural disease and cannot replace CT chest for detection of bronchiectasis [Ramsey et al. 2016]. In preschool children, LCI correlated with total disease extent on CT, and in the oldest cohort studied (7–16 years), LCI also correlated with bronchiectasis and airtrapping. The utility of MBW in clinical follow up and decision making is not fully clear yet; that is, how does the LCI respond to antibiotic treatment and possibly other treatment interventions? Recent studies did not show clear improvement of LCI after intravenous (IV) AB treatment [Sonneveld et al. 2015; Yammine et al. 2014]. Therefore, the guidelines do not recommend routine use of LCI in the clinical setting for the moment.
Imaging
Chest X-rays are relatively insensitive and nonspecific in early CF disease. They can, however, detect aggravation of lung disease and should be used for clinical guidance, if important signs and symptoms appear. Chest CT is a more sensitive tool for monitoring early CF lung disease [de Jong et al. 2004]. Although radiation exposure continues to decrease with technical innovations, some concerns remain. Therefore, some centers evaluate the use of lung magnetic resonance imaging as an alternative, but this is still in a research setting because of cost and time constraints [Wielputz and Mall, 2015]. Because no valid surrogates are currently in place, the guidelines allow chest CT to be performed every 2–3 years, preferably using a scoring system and standardized interpretation of repeated scans over time [Kuo et al. 2014].
Microbiology
Quarterly respiratory cultures are the standard of care for all CF patients. Despite the limited diagnostic accuracy of oropharyngeal (OP) swabs, an RCT in infants and preschoolers with CF found no difference in outcome at 5 years between therapy based on OP cultures or based on BAL [Jain et al. 2013; Wainwright et al. 2011]. Induced sputum has a greater yield than OP culture [Al-Saleh et al. 2010; De Boeck et al. 2000; Mussaffi et al. 2008], but is often considered time consuming and impractical in the routine clinical setting. In my opinion it is underused and should be attempted before deciding for bronchoscopical sampling. Bronchoscopy with BAL is advised by the guidelines if OP-guided AB treatment does not resolve the respiratory exacerbation.
Pulmonary therapies
The aim of early diagnosis and early treatment is to prevent lung damage with development of bronchiectasis early in the course.
Airway clearance techniques and inhaled therapies
Airway clearance therapy (ACT) is recommended for all individuals with CF. This guideline statement also includes presymptomatic babies diagnosed through NBS. However, in daily practice, some physicians only prescribe ACT once symptoms appear. The reason may be that the scientific basis for this statement is still low and the expected benefit moderate [Cystic Fibrosis Foundation et al. 2009]. Our current knowledge and understanding of early CF disease fully supports the statement on ACT made in the guidelines, although firm proof of efficacy is still lacking.
Whether a nebulized mucolytic drug or hypertonic saline should be instigated at diagnosis is less straightforward. Although hypertonic saline is safe in this young age group, efficacy has not been proven [Rosenfeld et al. 2012]. A new trial is under way, with the aim of further resolving this issue. Recombinant human DNAse (rhDNAse) is safe and well tolerated and has many potential, but not proven benefits, being a strong mucolytic [Quan et al. 2001]. Because of its cost, the CFF guidelines advise to commence it on indication [Cystic Fibrosis Foundation et al. 2009]. Since inhaled steroids and bronchodilators have no proven effect in CF, prescription should be limited to children with a combined asthma phenotype [Balfour-Lynn and Welch, 2014].
Infection control
It cannot be stressed enough that after early diagnosis, all the necessary precautions should be taken to prevent cross infection and hospital-acquired infection. We can refer to the CFF guidelines on this topic. Additionally, annual influenza vaccination is recommended [Cystic Fibrosis Foundation et al. 2009] from 6 months of age. Although there is no proven benefit, palivizumab is recommended by the guideline under age 2 [Cystic Fibrosis Foundation et al. 2009]. However, in many countries, reimbursement restrictions will not allow to follow this guideline. With new evolution in respiratory syncytial virus (RSV) antiviral treatments and vaccine development, new treatment and prophylaxis opportunities will most likely arise in the near future [Mazur et al. 2015a, 2015b].
Antibiotic treatment
Antibiotic treatment should be started similarly, as agreed upon, in older children with CF [Doring et al. 2012; Smyth et al. 2014], treating exacerbations, as well as eradicating first or new P. aeruginosa infections [Mogayzel et al. 2014]. Although methicillin-sensitive S. aureus is an important and common cause of lung infection in CF, there is no agreement on treating long-term infection due to lack of appropriate data [Ahmed and Mukherjee, 2016]. Prophylactic use of flucloxacillin is still routine in some countries, like UK, but is not recommended. Studies on this topic are currently being performed. For azithromycin therapy [Southern et al. 2012], again, data are missing in the recommendation for or against in this young age group.
Cystic fibrosis transmembrane conductance-regulator-correcting therapies
Kalydeco (Ivacaftor, Vertex Pharmaceutical, USA) is a CFTR potentiator improving the CFTR function for a range of so called ‘class 3’ or ‘gating mutations’. It has a robust clinical response on lung function, decreases the frequency of respiratory exacerbations [Ramsey et al. 2011] and reduced the rate of FEV1 decline over 3 years compared with matched controls homozygous for F508 del mutation [Sawicki et al. 2015]. After proof of efficacy in older patients, a study has documented the safety in preschoolers [Davies et al. 2016], with some concern on rise in liver function tests (LFT). In the latter study, the effect on sweat chloride was largely comparable with adult studies. The CFF guidelines recommend the use of Kalydeco in preschoolers starting from age 2 years. Interestingly, fecal elastase [a marker of pancreatic insufficient (PI)] increased with 100 mcg/g feces [standard deviation (SD) 138] after 24 weeks of treatment leading to values in the pancreatic sufficient (PS) range for some children. This suggests that correcting therapies could even reverse pancreatic insufficiency in the very young. Ideally, these CFTR-correcting therapies should thus be started after diagnosis, at least if safety is sufficiently documented. Because of the extreme high cost of this medication, ethical consideration to the decision of when to start remains and in many countries, where prescription is not possible due to limited reimbursement.
Unanswered questions
Determining optimal care pathways for preschool children with CF is a challenge, since evidence-based research in this age group is scarce. Many guidelines are based on expert advice, small trials, and experience with well established clinical follow-up programs after NBS. Additional research, however difficult it may be, is necessary to resolve the many unanswered questions. Important issues are, amongst others, choosing the best methods to monitor early disease, and efficacy of chronic medication established in older age groups [like DNAse, hypertonic saline (HS) and azithromycin]. Promising CFTR modulators should be studied in the very young, first on safety and the efficacy. Studies should aim at establishing the best timing to start the CFTR modulators, in order to prevent irreversible damage, not only in the lung, but possibly to prevent PI and CF-related diabetes mellitus.
Footnotes
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Ahmed M., Mukherjee S. (2016). Treatment for chronic methicillin-sensitive staphylococcus aureus pulmonary infection in people with cystic fibrosis. Cochrane Database Syst Rev 3: CD011581. [DOI] [PubMed] [Google Scholar]
- Al-Saleh S., Dell S., Grasemann H., Yau Y., Waters V., Martin S., et al. (2010). Sputum induction in routine clinical care of children with cystic fibrosis. J Pediatr 157: 1006–1011. [DOI] [PubMed] [Google Scholar]
- Amin R., Subbarao P., Jabar A., Balkovec S., Jensen R., Kerrigan S., et al. (2010). Hypertonic saline improves the LCI in paediatric patients with CF with normal lung function. Thorax 65: 379–383. [DOI] [PubMed] [Google Scholar]
- Amin R., Subbarao P., Lou W., Jabar A., Balkovec S., Jensen R., et al. (2011). The effect of dornase alfa on ventilation inhomogeneity in patients with cystic fibrosis. Eur Respir J 37: 806–812. [DOI] [PubMed] [Google Scholar]
- Aurora P., Stanojevic S., Wade A., Oliver C., Kozlowska W., Lum S., et al. (2011). Lung clearance index at 4 years predicts subsequent lung function in children with cystic fibrosis. Am J Respir Crit Care Med 183: 752–758. [DOI] [PubMed] [Google Scholar]
- Balfour-Lynn I., Welch K. (2014). Inhaled corticosteroids for cystic fibrosis. Cochrane Database Syst Rev CD001915. [DOI] [PubMed] [Google Scholar]
- Brennan L.C., Thia L.P. (2013). Evolution of lung function during the first two years of life in infants with cystic fibrosis diagnosed by newborn screening. Thorax 68(Suppl 3): A1–A220.24288777 [Google Scholar]
- Bush A., Sly P. (2015). Evolution of cystic fibrosis lung function in the early years. Curr Opin Pulm Med 21: 602–608. [DOI] [PubMed] [Google Scholar]
- Castellani C., Massie J., Sontag M., Southern K. (2016). Newborn screening for cystic fibrosis. Lancet Respir Med 4: 653–661. [DOI] [PubMed] [Google Scholar]
- Cystic Fibrosis Foundation, Borowitz D., Parad R., Sharp J., Sabadosa K., Robinson K., et al. (2009). Cystic Fibrosis Foundation practice guidelines for the management of infants with cystic fibrosis transmembrane conductance regulator-related metabolic syndrome during the first two years of life and beyond. J Pediatr 155: S106–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Cunningham S., Harris W., Lapey A., Regelmann W., Sawicki G., et al. (2016). Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2–5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm study. Lancet Respir Med 4: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Sheridan H., Bell N., Cunningham S., Davis S., Elborn J., et al. (2013). Assessment of clinical response to ivacaftor with lung clearance index in cystic fibrosis patients with a G551D-CFTR mutation and preserved spirometry: a randomised controlled trial. Lancet Respir Med 1: 630–638. [DOI] [PubMed] [Google Scholar]
- De Boeck K., Alifier M., Vandeputte S. (2000). Sputum induction in young cystic fibrosis patients. Eur Respir J 16: 91–94. [DOI] [PubMed] [Google Scholar]
- De Jong P., Nakano Y., Lequin M., Mayo J., Woods R., Pare P., et al. (2004). Progressive damage on high resolution computed tomography despite stable lung function in cystic fibrosis. Eur Respir J 23: 93–97. [DOI] [PubMed] [Google Scholar]
- Doring G., Flume P., Heijerman H., Elborn J.: Consensus Study Group. (2012). Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros 11: 461–79. [DOI] [PubMed] [Google Scholar]
- Elborn J. (2016). Cystic fibrosis. Lancet pii: S0140-6736(16)00576-6. [Google Scholar]
- Fischer A., Singh S., Adam R., Stoltz D., Baranano C., Kao S., et al. (2014). Tracheomalacia is associated with lower FEV1 and Pseudomonas acquisition in children with CF. Pediatr Pulmonol 49: 960–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson P., de Jong P., Tiddens H., Lindblad A. (2008). Multiple-breath inert gas washout and spirometry versus structural lung disease in cystic fibrosis. Thorax 63: 129–134. [DOI] [PubMed] [Google Scholar]
- Hoegger M., Fischer A., McMenimen J., Ostedgaard L., Tucker A., Awadalla M., et al. (2014). Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 345: 818–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoen A., Li J., Moulton L., O’Toole G., Housman M., Koestler D., et al. (2015). Associations between gut microbial colonization in early life and respiratory outcomes in cystic fibrosis. J Pediatr 167: 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain K., Wainwright C., Smyth A. (2013). Bronchoscopy-guided antimicrobial therapy for cystic fibrosis. Cochrane Database Syst Rev: CD009530. [DOI] [PubMed] [Google Scholar]
- Kuo W., Ciet P., Tiddens H., Zhang W., Guillerman R., van Straten M. (2014). Monitoring cystic fibrosis lung disease by computed tomography. Radiation risk in perspective. Am J Respir Crit Care Med 189: 1328–1336. [DOI] [PubMed] [Google Scholar]
- Lahiri T., Hempstead S., Brady C., Cannon C., Clark K., Condren M., et al. (2016). Clinical practice guidelines from the cystic fibrosis foundation for preschoolers with cystic fibrosis. Pediatrics 137(4): 1–28. [DOI] [PubMed] [Google Scholar]
- Mazur N., Martinon-Torres F., Baraldi E., Fauroux B., Greenough A., Heikkinen T., et al. (2015a). Lower respiratory tract infection caused by respiratory syncytial virus: current management and new therapeutics. Lancet Respir Med 3: 888–900. [DOI] [PubMed] [Google Scholar]
- Mazur N., van Delden J., Bont L. (2015b). Respiratory syncytial virus trials and beyond. Lancet Infect Dis 15: 1363–1365. [DOI] [PubMed] [Google Scholar]
- Meyerholz D. (2016). Lessons learned from the cystic fibrosis pig. Theriogenology 86: 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika M., Korten I., Qi W., Regamey N., Frey U., Casaulta C., et al. (2016). The nasal microbiota in infants with cystic fibrosis in the first year of life: a prospective cohort study. Lancet Respir Med 4: 627–635. [DOI] [PubMed] [Google Scholar]
- Mogayzel P., Jr., Naureckas E., Robinson K., Brady C., Guill M., Lahiri T., et al. (2014). Cystic Fibrosis Foundation pulmonary guideline. pharmacologic approaches to prevention and eradication of initial Pseudomonas aeruginosa infection. Ann Am Thorac Soc 11: 1640–1650. [DOI] [PubMed] [Google Scholar]
- Mussaffi H., Fireman E., Mei-Zahav M., Prais D., Blau H. (2008). Induced sputum in the very young: a new key to infection and inflammation. Chest 133: 176–182. [DOI] [PubMed] [Google Scholar]
- Nguyen T., Thia L., Hoo A., Bush A., Aurora P., Wade A., et al. (2014). Evolution of lung function during the first year of life in newborn screened cystic fibrosis infants. Thorax 69: 910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan J., Tiddens H., Sy J., McKenzie S., Montgomery M., Robinson P., et al. (2001). A two-year randomized, placebo-controlled trial of dornase alfa in young patients with cystic fibrosis with mild lung function abnormalities. J Pediatr 139: 813–20. [DOI] [PubMed] [Google Scholar]
- Ramsey B., Davies J., McElvaney N., Tullis E., Bell S., Drevinek P., et al. (2011). A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 365: 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey K., Rosenow T., Turkovic L., Skoric B., Banton G., Adams A., et al. (2016). Lung Clearance Index and Structural Lung Disease on Computed Tomography in Early Cystic Fibrosis. Am J Respir Crit Care Med 193: 60–67. [DOI] [PubMed] [Google Scholar]
- Riordan J., Rommens J., Kerem B., Alon N., Rozmahel R., Grzelczak Z., et al. (1989). Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245: 1066–1073. [DOI] [PubMed] [Google Scholar]
- Rogers C., Stoltz D., Meyerholz D., Ostedgaard L., Rokhlina T., Taft P., et al. (2008). Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 321: 1837–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M., Ratjen F., Brumback L., Daniel S., Rowbotham R., McNamara S., et al. (2012). Inhaled hypertonic saline in infants and children younger than 6 years with cystic fibrosis: the ISIS randomized controlled trial. JAMA 307: 2269–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki G., McKone E., Pasta D., Millar S., Wagener J., Johnson C., et al. (2015). Sustained Benefit from ivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data. Am J Respir Crit Care Med 192: 836–842. [DOI] [PubMed] [Google Scholar]
- Sims E., Clark A., McCormick J., Mehta G., Connett G., Mehta A., et al. (2007). Cystic fibrosis diagnosed after 2 months of age leads to worse outcomes and requires more therapy. Pediatrics 119: 19–28. [DOI] [PubMed] [Google Scholar]
- Sly P., Brennan S., Gangell C., de Klerk N., Murray C., Mott L., et al. (2009). Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med 180: 146–152. [DOI] [PubMed] [Google Scholar]
- Smyth A., Bell S., Bojcin S., Bryon M., Duff A., Flume P., et al. (2014). European Cystic Fibrosis Society Standards of Care: Best Practice guidelines. J Cyst Fibros 13: S23–S42. [DOI] [PubMed] [Google Scholar]
- Sonneveld N., Stanojevic S., Amin R., Aurora P., Davies J., Elborn J., et al. (2015). Lung clearance index in cystic fibrosis subjects treated for pulmonary exacerbations. Eur Respir J 46: 1055–1064. [DOI] [PubMed] [Google Scholar]
- Southern K., Barker P., Solis-Moya A., Patel L. (2012). Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev 11: CD002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stick S., Brennan S., Murray C., Douglas T., von Ungern-Sternberg B., Garratt L., et al. (2009). Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J Pediatr 155: 623–628 e1. [DOI] [PubMed] [Google Scholar]
- Stoltz D., Meyerholz D., Pezzulo A., Ramachandran S., Rogan M., Davis G., et al. (2010). Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med 2: 29ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao P., Stanojevic S., Brown M., Jensen R., Rosenfeld M., Davis S., et al. (2013). Lung clearance index as an outcome measure for clinical trials in young children with cystic fibrosis. A pilot study using inhaled hypertonic saline. Am J Respir Crit Care Med 188: 456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright C., Vidmar S., Armstrong D., Byrnes C., Carlin J., Cheney J., et al. (2011). Effect of bronchoalveolar lavage-directed therapy on Pseudomonas aeruginosa infection and structural lung injury in children with cystic fibrosis: a randomized trial. JAMA 306: 163–171. [DOI] [PubMed] [Google Scholar]
- Wielputz M., Mall M. (2015). Imaging modalities in cystic fibrosis: emerging role of MRI. Curr Opin Pulm Med 21: 609–616. [DOI] [PubMed] [Google Scholar]
- Yammine S., Bigler A., Casaulta C., Singer F., Latzin P. (2014). Reasons for heterogeneous change in LCI in children with cystic fibrosis after antibiotic treatment. Thorax 69: 183. [DOI] [PubMed] [Google Scholar]