Abstract
Background:
The aim of this study was to compare 1-year outcomes for patients with femoropopliteal in-stent restenosis using directional atherectomy guided by intravascular ultrasound (IVUS) versus directional atherectomy guided by angiography.
Methods and results:
This was a retrospective analysis for patients with femoropopliteal in-stent restenosis treated with IVUS-guided directional atherectomy versus directional atherectomy guided by angiography from a single center between March 2012 and February 2016. Clinically driven target lesion revascularization was the primary endpoint and was evaluated through medical chart review as well as phone call follow up.
Conclusions:
Directional atherectomy guided by IVUS reduces clinically driven target lesion revascularization for patients with femoropopliteal in-stent restenosis.
Keywords: atherectomy, intravascular ultrasound, in-stent restenosis
Background
The United States (US) population is being diagnosed with peripheral artery disease (PAD) at a rate of 1/16,1 with the total number of patients in the US between 8 and 12 million.2 Endovascular therapies have largely replaced open surgery as the first line of treatment for symptomatic femoropopliteal artery stenosis. Stenting in the femoropopliteal vessels have has been shown to improve patencies in superficial femoral and popliteal artery lesions as compared with percutaneous transluminal angioplasty (PTA)3,4 and has been become a mainstay of therapy.5 While the acute improvement over PTA is encouraging, femoropopliteal in-stent restenosis is estimated to be between 19–37%, and identifying the most effective treatment for femoropopliteal in-stent restenosis remains of clinical importance.6–8
Methods
In a study approved by the local institutional review board (IRB), Program for the Protection of Human Subjects, 114 consecutive patients with symptomatic femoropopliteal in-stent restenosis lesions treated with directional atherectomy (DA) at a single center were retrospectively analyzed. Demographic, clinical, angiographic, and follow-up data were retrospectively collected and evaluated. Patients who presented with critical limb ischemia, iliac disease, <1 vessel run-off to the foot, stent fractures/compressions or crashed stents were excluded from the analysis as were overlapped stents. Thrombotic lesions were excluded from the analysis and identified on the following basis: resistance-free passage of the wire, clinical syndromes of sudden onset of symptoms, acute, and sub-acute onset of symptoms were excluded from the study. All in-stent restenosis (ISR) classifications were included in the study.9,10
After informed consent for the interventional procedure was granted, diagnostic peripheral angiogram was performed and deployment of a filter to capture potential embolism was deployed prior to any intervention for all patients, in accordance with the institutional standard operating procedure. An IRB waiver for informed consent for this study was requested and granted as the study is a retrospective data analysis. All lesions were post-dilated following DA using a standard PTA balloon at the reference vessel diameter size, inflated to a minimum of nominal atmospheres.
Atherectomy procedure for DA with adjunctive PTA
DA was performed for all patients by experienced interventionalists after obtaining informed consent for the procedure. Lesion characteristics including (lesion length, ISR classification, pre-stenosis, and degree of calcification) were collected at baseline in accordance with a standard operating procedure. The atherectomy technique utilized a widely accepted approach.11 For femoropopliteal in-stent restenosis lesions, the cutter is first placed laterally, using the femur as a reference. From this position, the cutter is rotated clockwise, in a complete circle using the torqueing device to position the cutter posteriorly, medially, and then anteriorly to make cuts in the lateral, medial, posterior, and anterior wall. The cutter was always returned to the lateral position facing the femur; then clocked into the desired position to avoid repeated cuts in the same plane.11 To ensure that the directional atherectomy cutter did not become entangled in the stent, the operators used tactile, auditory and visual signals mainly through the continued movement of the device. If the device was not moving despite forward pressure, then it was deemed that the device was engaging a stent strut. In this event, the motor unit of the device was turned off, and the cutter head was torqued away from the strut and closed. All lesions were de-bulked to ⩽30% residual stenosis angiographically as determined by the operator. All lesions were post-dilated with a standard PTA balloon to the reference vessel diameter and inflated to adequate pressures to ensure complete balloon expansion. Completion angiography was performed and evaluated for the presence of thrombus, dissection, perforation, embolization into distal protection device, and loss of run-off vessels post PTA.
Atherectomy procedure for intravascular ultrasound-guided DA plus PTA
Following filter deployment, pre-intervention intravascular ultrasound (IVUS) was performed using the following institutional IVUS protocol: advancement of the catheter 1 mm distal to the lesion, data collected at a 1frame/sec using a Trak Back II™ (Volcano Corporation, Rancho Cordova, CA) pullback device set at a motorized pullback rate of 0.1 mm/sec. Raw sequential radiofrequency (RF) IVUS data were then saved and transferred to a workstation for analysis. IVUS images were reconstructed from RF data utilizing IVUS lab software.14 Luminal measurements were collected according to the standard IVUS protocol. DA was performed using widely accepted techniques as previously described.
All lesions were de-bulked to ⩽30% residual stenosis by angiographic assessment as deemed by the operator. Following de-bulking the IVUS catheter was passed through the lesion to confirm the angiographic result. If IVUS determined the lesion to be inadequately de-bulked (>30% stenosis) then repeat DA passes were performed and IVUS repeated until IVUS confirmed residual stenosis <30%.
Data collection
All data were collected at the time of procedure and stored in a research database. The 1-year follow up was obtained through evaluation of medical records for hospital re-admissions for peripheral vascular procedures related to the index limb and phone calls to all patients to ensure accuracy. Major in hospital and 1-year adverse events were collected including amputation (major and minor, planned and unplanned); mortality, distal embolization requiring intervention, vessel perforation, thrombosis, device entrapment, and presence of stent material within the atherectomy specimen. All patients were contacted via telephone follow up.
Endpoints
The primary outcome was freedom from clinically driven target lesion revascularization (CD-TLR) at 12 months. Secondary endpoints included major adverse events defined as perforation, clinically significant embolization, dissection, death, amputation, access site complications, inadvertent device entrapment, and recovery of stent material from atherectomy specimen. All data were collected through medical record review and phone call follow up by dedicated research staff.
Statistical analysis
All patients were dichotomized into two groups: use of IVUS-guided DA with adjunctive PTA and DA guided by angiography with adjunctive PTA (no IVUS). The groups were compared using a Chi-square or Student’s t-test for categorical and continuous variables, respectively. The null hypothesis that the CD-TLR of restenosis at 1 year in patients with IVUS guidance will be equivalent to the proportion of CD-TLR in patients without IVUS guidance. Variables of interest in the current dataset were first explored through visualization and inspection for po-tential outliers and distributional assumptions. Associations were evaluated using logistic regression analysis; the final model included age, high density lipoprotein (HDL), triglycerides and IVUS guidance as covariates. Outcomes of the regression analysis are presented as the odds ratio (OR) and 95% confidence interval (CI). All analyses were performed using SAS software (version 9.3; SAS Institute, Inc, Cary, NC, USA)
Results
A total of 114 consecutive patients with femoropopliteal in-stent restenosis were treated with DA (mean age 69.9: men 67%) at a single site between March 2012 and February 2016. Groups were dichotomized to IVUS-guided DA plus PTA and angiographic-guided DA plus PTA (no IVUS guidance). Baseline demographic and clinical variables were similar between the two groups (DA and IVUS-guided DA). A total of 46 (40%) patients received IVUS-guided DA as compared with 68 (60%) with DA with angiographic guidance. No significant differences were identified in lesion length, ISR classification, vessel run-off, reference vessel diameter, or Rutherford class across groups. Statistically significant differences were found in age (p < .05), HDL (p < .05) and triglycerides (p < .05). Baseline clinical and demographic variables are described in Table 1. IVUS-guided atherectomy patients had a CD-TLR rate of 17.9% compared with angiographic-guided DA with a CD-TLR rate of 51% at 1 year (p = .03).
Table 1.
Clinical, angiographic, and demographic characteristics.
| Variable | Directional atherectomy + IVUS guidance n = 46 |
Directional atherectomy alone n = 68 |
p value |
|---|---|---|---|
| Age | 73.71 ± 9.2 | 67.38 ± 9.3 | .0005 |
| BMI | 27.96 ± 5.1 | 27.33 ± 4.7 | .5 |
| Male | 29 | 47 | .007 |
| DM | 41 | 63 | .51 |
| Smoking | 7 | 8 | .59 |
| CTO | 14 | 15 | .31 |
| CAD | 5 | 4 | .18 |
| Hyperlipidemia | 44 | 63 | .51 |
| Hypertension | 46 | 68 | 1 |
| Cholesterol | 141 ± 30.53 | 131.2 ± 36.8 | .13 |
| HDL | 49.76 ± 14.44 | 38.29 ± 13.33 | .001 |
| LDL | 72.83 ± 22.43 | 70.23 ± 29.8 | .61 |
| Triglycerides | 93.28 ± 52.46 | 116.8 ± 53.3 | .02 |
| Lesion characteristics | |||
| Pre-stenosis | 86.41% ± 10.6 | 86.97% ± 9.12 | .76 |
| Post-DA/PTA Angiographic stenosis | 5.0% ± 4.2.0 | 8.0% ± 3.56 | .80 |
| Lesion length | 166.1 ± 83.0 | 167.5 ± 66.34 | .69 |
| Calcium | 5 | 3 | .8 |
| Directional atherectomy passes | 18 ± 5.4 | 8 ± 3.2 | .02 |
| Reference vessel diameter | 5.8 ± .38 | 5.8 ± .37 | .91 |
| ISR classification | |||
| Class 1 | 10 | 14 | .50 |
| Class 2 | 24 | 37 | .32 |
| Class 3 | 12 | 17 | .24 |
| Directional Atherectomy + IVUS | Directional Atherectomy Alone | p-value | |
| CD-TLR | 8 | 34 | .03 |
BMI, body mass index; CAD, coronary artery disease; CD-TLR, clinically driven target lesion revascularization; CTO, chronic total occlusion; DA, directional atherectomy; DM, diabetes mellitus; HDL, high density lipoprotein; ISR, in-stent restenosis; IVUS, intravascular ultrasound; LDL, low density lipoprotein; PTA, percutaneous transluminal angioplasty
There was no case of inadvertent stent entrapment, or recovery of stent material in the atherectomy specimen in either the IVUS-guided arm or the DA via angiography arm. There were no events of thrombosis, perforation, amputation, access site complications, or death in either group.7
In simple logistic regression analysis, lack of IVUS guidance was found to be a significant predictor of CD-TLR at 12 months with OR 3.5. The final model included age, HDL, triglycerides and IVUS guidance. This model produced ORs of .98 (05% CI: .94–1.039) for age, 1.03 (95% CI: .998–10.69) for HDL, 1.007 (95% CI: .998–1.015) for triglycerides and 3.50 (95% CI: 1.188–10.32) for IVUS guidance.
Discussion
There is no consensus for the treatment of femoropopliteal in-stent restenosis. Multiple available therapies to consider include: PTA, re-stenting, covered stent, drug-eluting balloon (DEB), drug-eluting stent (DES), and atherectomy with adjunctive PTA or DEB. Atherectomy remains a commonly used option and is attractive due to the ability to mechanically de-bulk re-stenotic tissue and increase lumen size. The postulated benefit of performing DA for femoropopliteal in-stent restenosis lesions is the DA catheter’s ability to direct and mechanically remove plaque thereby creating a larger lumen.
DA for the treatment of femoropopliteal in-stent restenosis has not been widely studied due to the current contraindication within the instructions for use (IFU) pamphlet. Alternative atherectomy therapies with PTA such as, excimer laser atherectomy (ELA), and jet stream atherectomy have shown improvements in comparison with PTA for the treatment of femoropopliteal in-stent restenosis. Of the studies completed, 6-month outcomes for the treatment of femoropopliteal in-stent restenosis with jet stream atherectomy and mean lesion length of 16.6 ± 12 cm showed TLR rates of 14.3%.12 The Excite trial using ELA with a mean lesion length 19.6 ± 12 cm reported 6-month TLR rates of 26.5%. Both jet stream atherectomy and ELA are similar to DA in that both are de-bulking plaque, however they are limited by catheter size and the in the ability to maximize luminal gain.
Of the studies completed utilizing DA, CD-TLR rates vary. A study from 2006 (43 patients) showed directional atherectomy for the treatment of femoropopliteal in-stent restenosis to be suboptimal with clinically driven TLR rates published at 47%,13 and a more recent study from 2012 (41 patients) reports CD-TLR rates to be 31.7%.12 A comparative study between ELA and directional SilverHawk atherectomy show 31.77% CD-TLR for SilverHawk patients and 48.7% CD-TLR for ELA.14 The variability seen within these studies may be due to the limitation of angiography in assessing residual plaque burden and thereby resulting in suboptimal de-bulking of the lesion prior to PTA.
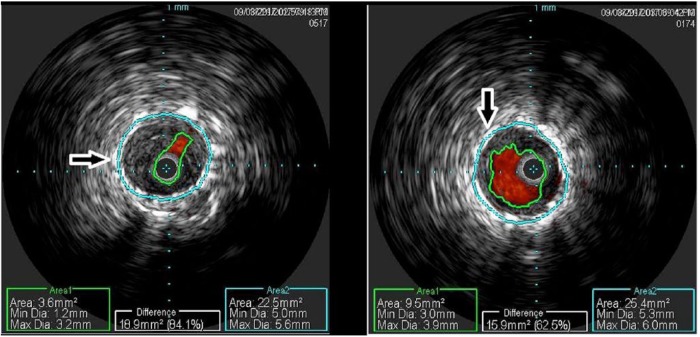
Previously, IVUS use has been shown to be effective at improving outcomes across many lesion types. Adjunctive IVUS imaging allows for greater understanding of lesion morphology, residual stenosis, adventitial injury and results in improved primary patency.15–18 The use of IVUS in the coronary vasculature has been shown to increase luminal diameter by allowing for more accurate sizing of the vessel and thereby allowing for larger balloons to significantly improve luminal diameter without increasing dissection.19 Aggressive de-bulking has been previously postulated to delay re-intervention for these femoropopliteal in-stent restenosis patients.14 IVUS’s ability to inform the operator in real time of more accurate assessment of residual stenosis and vessel characteristics may improve long term CD-TLR rates by allowing for more aggressive de-bulking (see Figure 1).
Figure 1.
Pre-DA intervention IVUS (left) and post-DA intervention IVUS (right).
DA, directional atherectomy; IVUS, intravascular ultrasound.
The 1-year CD-TLR rates (17.9%) observed within the IVUS arm of this study are the lowest reported for any atherectomy femoropopliteal in-stent restenosis study to date. Femoropopliteal in-stent restenosis remains challenging to treat, however IVUS imaging in conjunction with DA and emerging therapies, including DEB, may prove to be the most efficacious option in the treatment of femoropopliteal in-stent restenosis.
Conclusion
IVUS in conjunction with DA may improve CD-TLR rates for femoropopliteal in-stent restenosis patients by allowing the operator the ability to more accurately visualize the lesion and thereby minimize residual stenosis post-directional atherectomy treatment through aggressive de-bulking (see Figure 1).
Limitations
This study is a retrospective, nonrandomized, single center, noncore lab adjudicated study. Follow up data were obtained through phone calls and electronic data records, duplex ultrasonography and ankle brachial index/pulse velocity ratios were not captured for this study. The decision to use IVUS in conjuncture with DA was solely at the operator’s discretion as was the total number of DA passes completed.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Prakash Krishnan, Mount Sinai Medical Center, 1 Gustave l Levy Place, Box 1080, New York, 10026, NY, USA.
Arthur Tarricone, Mount Sinai Hospital, New York, NY, USA.
Purushothaman K-Raman, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Farhan Majeed, Mount Sinai Hospital, New York, NY, USA.
Vishal Kapur, Mount Sinai Hospital, New York, NY, USA.
Karthik Gujja, Mount Sinai Hospital, New York, NY, USA.
Jose Wiley, Mount Sinai Hospital, New York, NY, USA.
Miguel Vasquez, Mount Sinai Hospital, New York, NY, USA.
Rheoneil A. Lascano, Mount Sinai Hospital, New York, NY, USA
Katherine G. Quiles, Mount Sinai Hospital, New York, NY, USA
Tashanne Distin, Mount Sinai Hospital, New York, NY, USA.
Ran Fontenelle, Mount Sinai Hospital, New York, NY, USA.
Farah Atallah-Lajam, Mount Sinai Hospital, New York, NY, USA.
Annapoorna Kini, Mount Sinai Hospital, New York, NY, USA.
Samin Sharma, Mount Sinai Hospital, New York, NY, USA.
References
- 1. Allison MA, Ho E, Denenberg JO, et al. Ethnic-specific prevalence of peripheral arterial disease in the united states. Am J Prev Med 2007; 32: 328–333. [DOI] [PubMed] [Google Scholar]
- 2. Kasapis C, Gurm HS. Current approach to the diagnosis and treatment of femoral-popliteal arterial disease. A systematic review. Curr Cardiol Rev 2009; 5: 296–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muradin GS, Bosch JL, Stijnen T, et al. Balloon dilation and stent implantation for treatment of femoropopliteal arterial disease: meta-analysis. Radiology 2001; 221: 137–145. [DOI] [PubMed] [Google Scholar]
- 4. Mwipatayi BP, Hockings A, Hofmann M, et al. Balloon angioplasty compared with stenting for treatment of femoropopliteal occlusive disease: a meta-analysis. J Vasc Surg 2008; 47: 461–469. [DOI] [PubMed] [Google Scholar]
- 5. Krankenberg H, Tubler T, Ingwersen M, et al. Drug-coated balloon versus standard balloon for superficial femoral artery in-stent restenosis: the randomized femoral artery in-stent restenosis (fair) trial. Circulation 2015; 132: 2230–2236. [DOI] [PubMed] [Google Scholar]
- 6. Laird JR, Katzen BT, Scheinert D, et al. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the resilient randomized trial. Circ Cardiovasc Interv 2010; 3: 267–276. [DOI] [PubMed] [Google Scholar]
- 7. Schillinger M, Sabeti S, Loewe C, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med 2006; 354: 1879–1888. [DOI] [PubMed] [Google Scholar]
- 8. Armstrong EJ, Singh S, Singh GD, et al. Angiographic characteristics of femoropopliteal in-stent restenosis: association with long-term outcomes after endovascular intervention. Catheter Cardiovasc Interv 2013; 82: 1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ho KJ, Owens CD. Diagnosis, classification, and treatment of femoropopliteal artery in-stent restenosis. J Vasc Surg 2017; 65: 545–557. [DOI] [PubMed] [Google Scholar]
- 10. Tosaka A, Soga Y, Iida O, et al. Classification and clinical impact of restenosis after femoropopliteal stenting. J Am Coll Cardiol 2012; 59: 16–23. [DOI] [PubMed] [Google Scholar]
- 11. Tarricone A, Ali Z, Rajamanickam A, et al. Histopathological evidence of adventitial or medial injury is a strong predictor of restenosis during directional atherectomy for peripheral artery disease. J Endovasc Ther 2015; 22: 712–715. [DOI] [PubMed] [Google Scholar]
- 12. Shammas NW, Shammas GA, Jerin M. Differences in patient selection and outcomes between silverhawk atherectomy and laser ablation in the treatment of femoropopliteal in-stent restenosis: a retrospective analysis from a single center. J Endovasc Ther 2013; 20: 844–852. [DOI] [PubMed] [Google Scholar]
- 13. Zeller T, Rastan A, Sixt S, et al. Long-term results after directional atherectomy of femoro-popliteal lesions. J Am Coll Cardiol 2006; 48: 1573–1578. [DOI] [PubMed] [Google Scholar]
- 14. Shammas NW, Shammas GA, Jerin M. Differences in patient selection and outcomes between silverhawk atherectomy and laser ablation in the treatment of femoropopliteal in-stent restenosis: a retrospective analysis from a single center. J Endovasc Ther 2013; 20: 844–852. [DOI] [PubMed] [Google Scholar]
- 15. Krishnan P, Tarricone A, Ali Z, et al. Intravascular ultrasound is an effective tool for predicting histopathology-confirmed evidence of adventitial injury following directional atherectomy for the treatment of peripheral artery disease. J Endovasc Ther 2016; 23: 672–673. [DOI] [PubMed] [Google Scholar]
- 16. Buckley CJ, Arko FR, Lee S, et al. Intravascular ultrasound scanning improves long-term patency of iliac lesions treated with balloon angioplasty and primary stenting. J Vasc Surg 2002; 35: 316–323. [DOI] [PubMed] [Google Scholar]
- 17. Mori S, Hirano K, Nakano M, et al. Intravascular ultrasound measurements after drug-eluting stent placement in femoropopliteal lesions: determining predictors of restenosis. J Endovasc Ther 2015; 22: 341–349. [DOI] [PubMed] [Google Scholar]
- 18. Cioppa A, Stabile E, Popusoi G, et al. Combined treatment of heavy calcified femoro-popliteal lesions using directional atherectomy and a paclitaxel coated balloon: one-year single centre clinical results. Cardiovasc Revasc Med 2012; 13: 219–223. [DOI] [PubMed] [Google Scholar]
- 19. Stone GW, Hodgson JM, St Goar FG, et al. Improved procedural results of coronary angioplasty with intravascular ultrasound-guided balloon sizing: the clout pilot trial. Clinical outcomes with ultrasound trial (clout) investigators. Circulation 1997; 95: 2044–2052. [DOI] [PubMed] [Google Scholar]