Abstract
Background:
The purpose of this study was to examine the anti-atherogenic and anti-inflammatory effect of supplemental taurine prior to and following incremental exercise in patients with heart failure (HF).
Methods:
Patients with HF and left ventricle ejection fraction less than 50%, and placed in functional class II or III according to the New York Heart Association classification, were randomly assigned to two groups: (1) taurine supplementation; or (2) placebo. The taurine group received oral taurine (500 mg) 3 times a day for 2 weeks, and performed exercise before and after the supplementation period. The placebo group followed the same protocol, but with a starch supplement (500 mg) rather than taurine. The incremental multilevel treadmill test was done using a modified Bruce protocol.
Results:
Our results indicate that inflammatory indices [C-reactive protein (CRP), platelets] decreased in the taurine group in pre-exercise, post-supplementation and post-exercise, post-supplementation as compared with pre-exercise, pre-supplementation (p < 0.05) whereas these indices increased in pre-exercise, post-supplementation and post-exercise, post-supplementation as compared with pre-exercise, pre-supplementation in the placebo group (p < 0.05). Our results also show that atherogenic indices [Castelli’s Risk Index-I (CRI-I), Castelli’s Risk Index-II (CRI-II) and Atherogenic Coefficient (AC)] decreased in the taurine group in pre-exercise, post-supplementation and post-exercise, post-supplementation as compared with pre-exercise, pre-supplementation (p < 0.05). No such changes were noted in the placebo group (p > 0.05).
Conclusions:
our results suggest that 2 weeks of oral taurine supplementation increases the taurine levels and has anti-atherogenic and anti-inflammatory effects prior to and following incremental exercise in HF patients.
Keywords: anti-atherogenic, anti-inflammatory, taurine, exercise, heart failure
Introduction
It is estimated that by 2030 roughly 20% of the global population will be aged 65 or older.1 Aging is a positive risk factor for cardiovascular disease and related morbidity and mortality.1 It is also well known that aging results in a decline in physical performance due in part to alterations in skeletal muscles such as reduction in the relative number of type II fibers and in capillary density,2 as well as reduced physical capacity, which is also an independent risk factor for cardiovascular disease (CVD).3
Heart failure (HF), commonly occurring during the seventh decade, is characterized by either structural or functional cardiac abnormalities impairing left ventricular filling.4 It is well established that HF is related to prothrombotic shift, predisposing thrombi formation within blood vessels and cardiac chambers.5 Furthermore, it is also related to inflammation which is an important pathogenic mechanism in HF.6 Reina-Couto and colleagues6 have suggested that the inflammatory response in HF may result either from excessive activation of proinflammatory cascades or disturbances in the resolution of inflammation. However, the mechanisms responsible for inflammation associated with this disease remain largely disregarded.6 Thus, investigating these mechanisms may be of benefit in inflammatory conditions and CVD, especially in HF. In addition to that, Kitzman and colleagues2 reported that the HF patients are suffering multiple skeletal muscle abnormalities, including a reduced percentage of type I (oxidative) fibers and a decreased number of capillaries per fiber, and that these contribute dramatically to their exercise intolerance. Nevertheless, treatment advances have increased prognosis and treatment options, with more patients now living with advanced HF.7
There is a growing body of literature describing various exercise modalities and drugs/supplements (e.g. phosphodiesterase-5 inhibition, ivabradine) which can potentially improve clinical status and exercise capacity in HF.8–10 Exercise training is widely used as a cost-effective method of rehabilitation from or prevention of CVD. Kitzman and colleagues10 found that 16 weeks of endurance training improved peak VO2 in HF patients without altering endothelial function or arterial stiffness, suggesting that noncardiac mechanisms including enhanced skeletal muscle perfusion or oxygen utilization may be responsible for the endurance exercise-mediated increase in peak VO2 in HF. Further acute exercise in HF patients can improve circulating angiogenic cells migratory capacity, and restore these to levels similar to healthy controls.11 A single bout of exercise does have anti-inflammatory effects in healthy patients, mainly through subsequent increase in plasma levels of the cytokines interleukin (IL)-10, IL-1 receptor antagonist (ILra) and the soluble tumor necrosis factor (TNF) receptors I and II, an effect induced by IL-6.12 However, thrombotic and inflammation responses of HF patients to exercise are poorly described.
In terms of a pharmacological treatment, phosphodiesterase-5 inhibition with sildenafil has been shown to reverse exercise oscillatory breathing in chronic HF.9 However, this drug does not significantly improve exercise capacity nor clinical status.8 Thus, there is a need to develop effective treatment options, such as diet and exercise, which can easily be prescribed for HF patients.
Taurine (2-aminoethanesulfonic acid) is the most abundant free amino acid in humans. It plays an important role in various biological processes including bile acid conjugation, maintenance of calcium homeostasis, osmoregulation and membrane stabilization, as well as having a protective effect against acute cardiovascular events.13,14 Although the effects of taurine are largely attributed to its free radical scavenging (antioxidant) ability, the exact mechanisms of the anti-thrombotic and anti-inflammatory effects of taurine are still unknown. Moreover, in humans, taurine supplementation is reported to significantly increase exercise performance as measured by exercise time-to-exhaustion, VO2max, and maximal workload.15 Rahman and colleagues16 have also reported that taurine supplementation prevents hypertension and increases exercise capacity in rats with fructose-induced hypertension.16 Similarly, Da Silva and colleagues17 contest that taurine supplementation can improve exercise performance by: decreasing muscle damage, reducing oxidative stress,17 increasing cardiac contractility, and increasing whole body fat oxidation.18 Taken together, we can hypothesize that there is a relationship between the effectiveness of taurine supplementation on exercise performance and its protective effects against oxidative stress and CVDs, especially in HF patients.
To the best of our knowledge, no published study has previously described an investigation of taurine supplementation on the inflammatory and thrombotic status of HF patients. Thus, the purpose of this study was to examine the anti-atherogenic and anti-inflammatory effect of taurine following incremental exercise in patients with HF. It was hypothesized that taurine supplementation would have positive effects on inflammatory and thrombotic statues of HF patients prior to and following incremental exercise.
Methods
Participants and ethical approval
Patients with HF and a left ventricle ejection fraction <50% were invited to participate in this study. Exclusion criteria included: systolic blood pressure <90 mmHg or diastolic pressure more than 95 mmHg; hemodynamically important stenotic valvular heart disease; active myocarditis or pericarditis; automatic implanted cardioverter defibrillators; coronary angioplasty before 1 week of enrolment; coronary artery bypass graft surgery, acute myocardial infarction, or unstable angina pectoris before 2 weeks of enrolment; cerebrovascular accident or transient ischemic attack within 6 weeks of enrolment; documented or suspected significant renal artery stenosis; hematuria; serum creatinine concentrations higher than 220 µmol/l; and orthopedic injury that preventing successful completion of the exercise protocol. After providing informed written consent, participants were randomly divided into two groups: (1) taurine supplementation group and (2) placebo group. Additionally, participants performed a modified Bruce treadmill protocol in order to determine their VO2max, with all stress testing conducted under the close supervision of a physician. All procedures in this study were approved by the University of Mazandaran Human Research Ethics Committee, and were conducted in accordance with the Declaration of Helsinki.
Taurine supplementation and incremental exercise protocols
The taurine group participants were supplemented for 2 weeks, 500 mg three times a day with taurine (oral capsule, Solgar, Leonia, NJ, USA).19 The placebo group participants received the same amount of capsulated starch in a similar period. Participants performed exercise pre- and post-supplementation. The exercise consisted of a modified Bruce protocol and the point of termination was as previously described.20 The exercise was performed twice (the day before the supplementation period began and the day after the supplementation period finished). All exercise testing was done in the morning between 8 a.m. and 12 noon with the participants in a post-absorptive (fasted) state.
Atherogenic and inflammatory indices, and taurine measurements
Blood samples were collected prior to and following each Bruce protocol test via venipuncture of the antecubital vein so that blood collection was performed in an overnight fasted state. Following collection into a blood (clot) tube, serum was separated by centrifugation at 3000 rpm for 15 min. Fresh serum was used for estimation of total cholesterol (TC), triglyceride, high-density lipoprotein cholesterol (HDL-c), and platelet concentrations. The tests were carried out in an automated clinical auto analyzer. Additionally, low-density lipoprotein (LDL-c), and very low-density lipoprotein (VLDL-c) were calculated by using Friedewald’s formula.21 Selected atherogenic indices including Castelli’s Risk Index-I (CRI-I) = TC/HDL-c, Castelli’s Risk Index-II (CRI-II) = LDL-c/HDL-c, and Atherogenic Coefficient (AC) = triglyceride/HDL-c ratio were measured. Further, taurine and C-reactive protein (CRP) levels were measured by high-performance liquid chromatography and sandwich enzyme-linked immunosorbent assay methods respectively. Platelet and CRP levels were considered as inflammatory indices.
Statistical analyses
All statistical analyses were performed using the statistical package SPSS for windows, version 21.0 (IBM Corp, Armonk, North Castle, NY, USA). After confirmation of normality of data by one-sample Kolmogrov–Smirnov test, a two-way repeated measures analysis of variance was used to assess the differences between pre- and post-supplementation for all groups. The independent Student’s t-test also was used to assess the differences of anthropometric characteristics of participants in taurine and placebo groups. Statistical significance was set at p < 0.05.
Results
The baseline characteristics of participants are summarized in Table 1. No significant difference was noted between the taurine and placebo groups at baseline (Table 1). Atherogenic and inflammatory indices as well as serum taurine levels are shown in Table 2. There were no significant differences between taurine and placebo groups at baseline (all, p > 0.05). There was a significant time effect for time-to-exhaustion (TTE) in the taurine group (p = 0.007). TTE significantly increased in post-exercise, post-supplementation as compared with post-exercise, pre-supplementation (10.67 ± 1.57 versus 13.34 ± 1.49 min, p = 0.007). However, no significant time effect was noted for TTE in post-exercise, post-supplementation as compared with post-exercise, pre-supplementation in the placebo group (8.43 ± 1.31 versus 9.13 ± 1.65min, p = 0.476).
Table 1.
Mean and standard deviation of baseline characteristics of participants.
| Groups | Age (years) | Weight (kg) | Height (cm) | BMI (kg/m2) | VO2max (ml/kg/min) | EF (%) |
|---|---|---|---|---|---|---|
| Taurine group | 60.12 ± 5.4 | 66.5 ± 3.11 | 165.6 ± 4.3 | 24.22 ± 1.05 | 28.21 ± 7.25 | 29.75 ± 4.97 |
| Placebo group | 60.13 ± 8.3 | 60.12 ± 5.35 | 165 ± 4.1 | 24.23 ± 1.10 | 29.54 ± 6.29 | 28.13 ± 7.53 |
| p-values | 0.999 | 0.994 | 0.993 | 0.999 | 0.865 | 0.59 |
BMI, body mass index; EF, ejection fraction.
Table 2.
Mean and standard deviation of atherogenic, inflammatory indices, and serum taurine levels of participants in different phases.
| Variables | Taurine |
Placebo | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre-Exer, Pre-Suppl | Post-Exer, Pre-Suppl | Pre-Exer, Post-Suppl | Post-Exer, Post-Suppl | Pre-Exer, Pre-Suppl | Post-Exer, Pre-Suppl | Pre-Exer, Post-Suppl | Post-Exer, Post-Suppl | |
| Atherogenic indices | ||||||||
| CRI-I (TC/HDL-c) | 4.9339 ± 0.15369 | 4.8451 ± 0.19662 | 3.5096 ± 0.13026 | 3.5230 ± 0.14851 | 4.3843 ± 0.13291 | 4.3499 ± 0.17403 | 4.6915 ± 0.22184 | 4.7464 ± 0.21539 |
| CRI-II (LDL-c/HDL-c) | 3.5398 ± 0.26571 | 3.5961 ± 0.26849 | 2.4201 ± 0.08174 | 2.5271 ± 0.6772 | 3.4126 ± 0.16529 | 3.5175 ± 0.38527 | 3.7971 ± 0.26640 | 3.9813 ± 0.20190 |
| AC (triglyceride/HDL-c) | 4.0034 ± 0.13330 | 3.9840 ± 0.19288 | 3.0680 ± 0.14558 | 3.0491 ± 0.09942 | 3.4925 ± 0.11901 | 3.5157 ± 0.12589 | 4.2010 ± 0.22570 | 4.2635 ± 0.14096 |
| Inflammatory indices | ||||||||
| CRP (pg/ml) | 617.5 ± 12.35 | 647.25 ± 14.38 | 491.88 ± 17.22 | 521 ± 13.18 | 647.87 ± 14.11 | 681.5 ± 11.61 | 709.25 ± 15.17 | 733.87 ± 14.44 |
| Platelet (µmol/l) | 185.75 ± 19.53 | 186.75 ± 19.38 | 175.87 ± 22.27 | 174.62 ± 14.76 | 162.75 ± 6.29 | 173.87 ± 21.83 | 190.75 ± 20.82 | 192 ± 17.53 |
| Taurine (µmol/l) | 58.88 ± 19.64 | 59.87 ± 12.97 | 140.5 ± 20.12 | 111 ± 25.37 | 44.13 ± 9.19 | 42 ± 8.14 | 61.63 ± 13.29 | 49.1 ± 10.42 |
AC, atherogenic coefficient; CRI-I, Castelli’s Risk Index-I; CRI-II, Castelli’s Risk Index-II; CRP, C-reactive protein; Exer, exercise; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; TC, total cholesterol; Suppl, supplementation.
Atherogenic indices
CRI-I (TC/HDL-c)
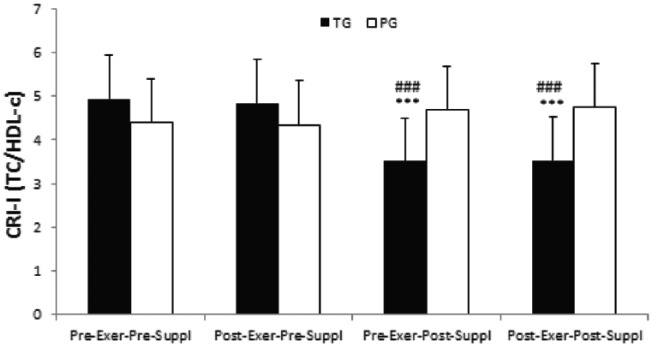
There was a significant time effect for CRI-I index in the taurine group (p = 0.000). CRI-I index significantly decreased in pre-exercise, post-supplementation and post-exercise, post-supplementation as compared with pre-exercise, pre-supplementation in the taurine group (both, p = 0.000, Figure 1). Similarly, CRI-II index significantly decreased in pre-exercise, post-supplementation and post-exercise, post-supplementation as compared with post-exercise, pre-supplementation in the taurine group (both, p = 0.0000, Figure 1). However, no significant time effects were noted for CRI-I index in the placebo group (all, p > 0.05, Figure 1).
Figure 1.
CRI-I index in the taurine group and placebo group. Data are expressed as means ± SD.
CRI-I, Castelli’s Risk Index-I; Exer, exercise; HDL-c, high-density lipoprotein cholesterol; PG, placebo group; SD, standard deviation; Suppl, supplementation; TC, total cholesterol; TG, taurine group.
***p < 0.001 versus Pre-Exer, Pre-Suppl in TG.
###p < 0.001 versus Post-Exer, Pre-Suppl in TG.
CRI-II (LDL-c/HDL-c)
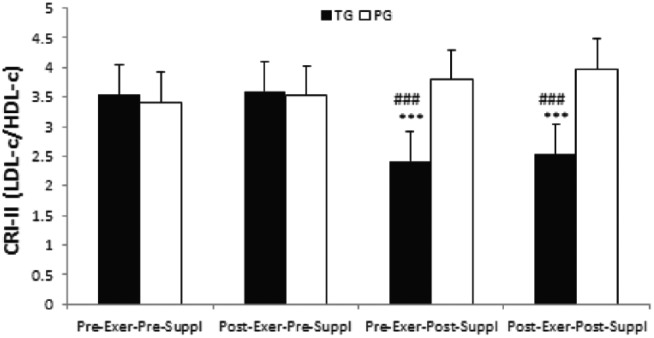
There was a significant time effect for CRI-II index in the taurine group (p = 0.000). CRI-II index significantly decreased in pre-exercise, post-supplementation and post-exercise, post-supplementation as compared with pre-exercise, pre-supplementation in the taurine group (both, p = 0.000,Figure 2). Similarly, CRI-II index significantly decreased in pre-exercise, post-supplementation and post-exercise, post-supplementation as compared with post-exercise, pre-supplementation in the taurine group (both, p = 0.0000, Figure 2). However, no significant time effects were noted for CRI-II index in the placebo group (all, p > 0.05).
Figure 2.
CRI-II index in TG and PG. Data are expressed as means ± SD.
CRI-II, Castelli’s Risk Index-II; Exer, exercise; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; PG, placebo group; SD, standard deviation; Suppl, supplementation; TG, taurine group.
***p < 0.001 versus Pre-Exer, Pre-Suppl in TG.
###p < 0.001 versus Post-Exer, Pre-Suppl in TG.
AC (triglyceride/HDL-c)
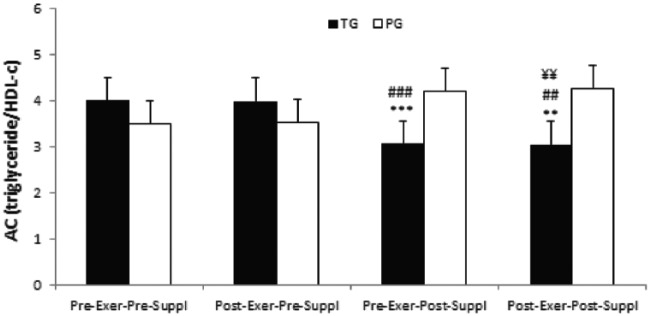
There was a significant time effect for AC index in the taurine group (p = 0.001). AC index significantly decreased in pre-exercise, post-supplementation and post-exercise, post-supplementation as compared with pre-exercise, pre-supplementation in the taurine group (p-values were 0.000 and 0.001 respectively, Figure 3). Similarly, AC index significantly decreased in pre-exercise, post-supplementation and post-exercise, post-supplementation as compared with post-exercise, pre-supplementation in the taurine group (p-values were 0.000 and 0.003 respectively, Figure 3). Furthermore, AC index significantly decreased in post-exercise, post-supplementation as compared with pre-exercise, post-supplementation in the taurine group (p = 0.008, Figure 3). However, no significant time effects were noted for AC index in PG (all, p > 0.05).
Figure 3.
AC index in TG and PG. Data are expressed as means ± SD.
AC, atherogenic coefficient; Exer, exercise; HDL-c, high-density lipoprotein cholesterol; PG, placebo group; SD, standard deviation; Suppl, supplementation; TG, taurine group.
***p < 0.001
**p < 0.01 versus Pre-Exer, Pre-Suppl in TG
###p < 0.001
##p < 0.01 versus Post-Exer, Pre-Suppl in TG
¥¥p < 0.01 versus Pre-Exer, Post-Suppl in TG
Inflammatory indices
CRP
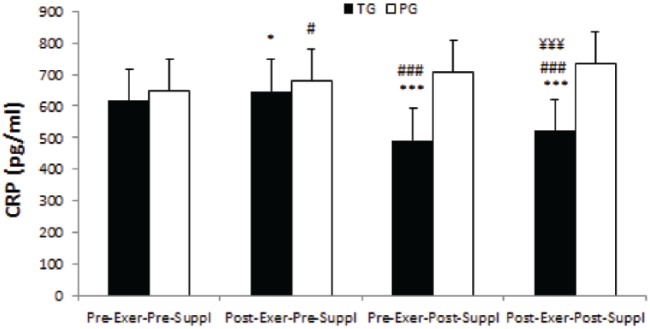
There was a significant time effect for CRP in the taurine and placebo groups (p-values were 0.000 and 0.001 respectively). CRP significantly increased in post-exercise, pre-supplementation as compared with pre-exercise, pre-supplementation in the taurine and placebo groups (p-values were 0.015 and 0.016 respectively, Figure 4). Conversely, CRP significantly decreased in pre-exercise, post-supplementation and post-exercise, post-supplementation as compared with pre-exercise, pre-supplementation in the taurine group (both, p = 0.000, Figure 4) whereas CRP significantly increased in pre-exercise, post-supplementation and post-exercise, post-supplementation as compared with pre-exercise, pre-supplementation in the placebo group (both, p = 0.000, Figure 4). Further, CRP significantly decreased in post-exercise, post-supplementation as compared with post-exercise, pre-supplementation in the taurine group (p = 0.000, Figure 4).
Figure 4.
CRP in TG and PG. Data are expressed as means ± SD.
CRP, C-reactive protein; Exer, exercise; PG, placebo group; SD, standard deviation; Suppl, supplementation; TG, taurine group.
*p < 0.05 versus Pre-Exer, Pre-Suppl in TG.
#p < 0.05 versus Pre-Exer, Pre-Suppl in PG.
***p < 0.001 versus Pre-Exer, Pre-Suppl in TG.
###p < 0.001 versus Pre-Exer, Pre-Suppl in PG.
¥¥¥p < 0.001 versus Post-Exer, Pre-Suppl in TG.
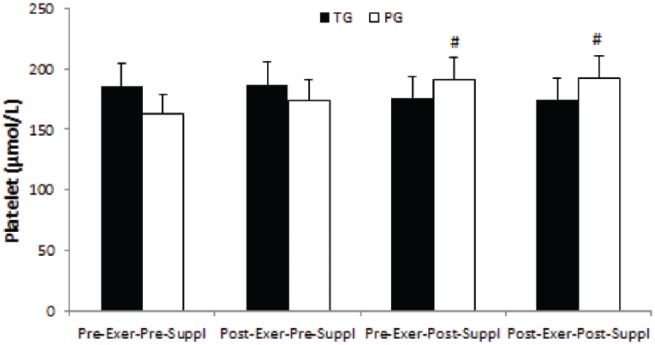
Platelet
Although there were decrease trends in platelet in pre-exercise, post-supplementation and post-exercise, post-supplementation as compared with pre-exercise, pre-supplementation and post-exercise, pre-supplementation in the taurine group, they were not significant (all, p > 0.05). However, a significant time effect was noted for platelets in the placebo group (p = 0.001). Platelets significantly increased in pre-exercise, post-supplementation and post-exercise, post-supplementation as compared with pre-exercise, pre-supplementation in the placebo group (p-values were 0.032 and 0.012 respectively, Figure 5).
Figure 5.
Platelet in TG and PG. Data are expressed as means ± SD.
Exer, exercise; PG, placebo group; SD, standard deviation; Suppl, supplementation; TG, taurine group.
#p < 0.05 versus Pre-Exer, Pre-Suppl in PG.
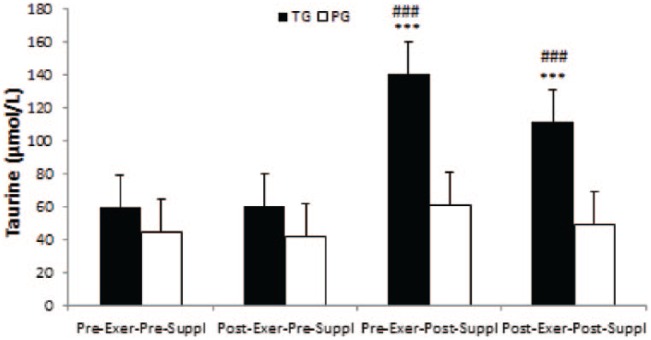
Taurine
There was a significant time effect for taurine in the taurine group (p = 0.000). Taurine significantly increased in pre-exercise, post-supplementation and post-exercise, post-supplementation as compared with pre-exercise, pre-supplementation and post-exercise, pre-supplementation in TG (all, p = 0.000, Figure 6). However, no significant time effects were noted for taurine in the placebo group (all, p > 0.05).
Figure 6.
Taurine in TG and PG. Data are expressed as means ± SD.
Exer, exercise; PG, placebo group; Suppl, supplementation; TG, taurine group.
***p < 0.001 versus Pre-Exer, Pre-Suppl in TG.
###p < 0.001 versus Post-Exer, pre-Suppl in PG.
Discussion
This study was designed to examine the influence of 2 weeks of oral taurine supplementation on inflammatory and thrombotic states in HF patients prior to and following incremental exercise. To the best of our knowledge, this study is unique in that it is the first to rigorously compare the inflammatory and thrombotic responses of HF patients to acute exercise with emphasis on taurine supplementation; a nutrient gaining popularity for its protective effect against CVD.
Atherogenic indices
Our data show that atherogenic indices (CRI-I, CRI-II, and AC) decreased in the taurine group in pre-exercise, post-supplementation and post-exercise, post-supplementation as compared with pre-exercise, pre-supplementation. No such changes were noted in the placebo group. Our results are in line with previous studies examining taurine administration on serum lipids in humans and rodents.15,22
Taurine is a sulfur-containing nonprotein amino acid that is well known for its conjugation with bile acids, thus changing cholesterol’s solubility and enabling its excretion.23 It is also involved in the coordination of nerve function, stabilization of the cell membrane, detoxification, antioxidant reactions and modulation of osmotic pressure and also it is a protective nutritional supplement in CVD.21
The upregulation of 7-alpha-hydroxylase (CYP7A1), the rate-limiting enzyme in the production of bile acids, can dramatically accelerate the production of bile.24 It is reported that the expression of CYP7A1 is significantly greater 4 h after taurine supplementation, and continues to significantly increase at 24 h and 48 h.25 Therefore, the primary reason for decreased atherogenic indices found in the present study could be due in part to taurine effects on CYP7A1 activity (upregulation), increased rates of bile synthesis, and thus cholesterol utilization. In addition, another explanation could be related to this fact that taurine may also decrease cholesterol levels through an upregulation of the hepatic low-density lipoprotein receptor (LDLR) or through an improvement in the binding of LDL to LDLR.23 Lastly, taurine has an antioxidant effect which may reduce the oxidation of LDL,26 and thereby attenuate the process of atherosclerosis.
Our results, however, are inconsistent with the observations of previous studies27 which indicate that neither serum lipid nor biliary lipid composition is altered following 3.2 g/day taurine supplementation for 2 weeks. But when cholesterol was administered along with taurine, serum LDL-C and biliary cholesterol levels increased significantly.27 The reasons for these contradictory results are not completely clear. Although the supplementation time was similar between studies, the present study used HF patients as participants and supplemented them with taurine only.
Inflammatory indices
Our data indicates that inflammatory indices (CRP and platelets) decreased in the taurine group in pre-exercise, post-supplementation and post-exercise, post-supplementation compared with pre-exercise and pre-supplementation whereas these indices all increased in the placebo group. These results confirm the anti-inflammatory effect of oral taurine. Our results are supported by previous studies reporting that taurine is protective against oxidative stress under various conditions,15,17 and acts as an anti-inflammatory agent.17,28,29 Silva and colleagues17 found that taurine affects skeletal muscle function by decreasing oxidative stress in association with decreased superoxide radical production. Similarly, Kim and Cha29 reported that taurine chloramine produced from taurine under inflammation provided anti-inflammatory and cytoprotective effects. It suggested that the anti-inflammatory action of taurine could be due in part to the antioxidant ability of taurine to neutralize hypochlorous acid by forming taurine chloramine. Subsequently, taurine chloramine can be produced at the site of inflammation and regulates expression and secretion of cytokines such as nitric oxide, IL-6, IL-8, and TNF-α.30 The mechanism involves the inhibition of nuclear factor κB activation by oxidation of the inhibitory protein IκB-α.30 Studies evaluating the effects of taurine supplementation in HF are rare, and the protective anti-inflammatory activity of taurine in humans is still poorly known. Clearly more research is needed to investigate the anti-inflammatory properties of taurine.
Conclusion
The present study indicates that 2 weeks of oral taurine supplementation is able to ease decreasing atherogenic (CRI-I,CRI-II, and AC) and inflammatory indices (CRP and platelets) in HF patients. Further research is required to informclinical practice. Nevertheless, this study provides some evidence that taurine may offer a relatively inexpensive agent to treat some forms of CVD, especially when prescribed alongside exercise.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Mehdi Ahmadian, Department of Physical Education and Sport Sciences, Aliabad Katoul Branch, Islamic Azad University, Aliabad Katoul, Iran.
Valiollah Dabidi Roshan, Department of Sport Physiology, College of Physical Education and Sport Sciences, University of Mazandaran (UMZ), Babolsar, Iran.
Elaheh Aslani, Department of Sport Physiology, College of Humanities, Sari Branch, Islamic Azad University, Sari, Iran.
Stephen R Stannard, School of Sport and Exercise, Massey University, Palmerston North, New Zealand.
References
- 1. North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circul Res 2012; 110: 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kitzman DW, Nicklas B, Kraus WE, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol 2014; 306: H1364–H1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wisløff U, Najjar SM, Ellingsen Ø, et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 2005; 307: 418–420. [DOI] [PubMed] [Google Scholar]
- 4. Jurgens CY, Goodlin S, Dolansky M, et al. Heart failure management in skilled nursing facilities: a scientific statement from the American Heart Association and the Heart Failure Society of America. Circ Heart Fail 2015; 8: 655–687. [DOI] [PubMed] [Google Scholar]
- 5. Shantsila E, Wrigley BJ, Blann AD, et al. A contemporary view on endothelial function in heart failure. Eur J Heart Fail 2012; 14: 873–881. [DOI] [PubMed] [Google Scholar]
- 6. Reina-Couto M, Vale L, Carvalho J, et al. Resolving inflammation in heart failure: novel protective lipid mediators. Curr Drug Targets 2016; 17: 1206–1223. [DOI] [PubMed] [Google Scholar]
- 7. Kane P, Murtagh F, Ryan K, et al. The gap between policy and practice: a systematic review of patient-centred care interventions in chronic heart failure. Heart Fail Rev 2015; 20: 673–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. Jama 2013; 309: 1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guazzi M, Vicenzi M, Arena R. Phosphodiesterase 5 inhibition with sildenafil reverses exercise oscillatory breathing in chronic heart failure: a long-term cardiopulmonary exercise testing placebo-controlled study. Eur J Heart Fail 2012; 14: 82–90. [DOI] [PubMed] [Google Scholar]
- 10. Kitzman DW, Brubaker PH, Herrington DM, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J Am Coll Cardiol 2013; 62: 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Craenenbroeck EM, Beckers PJ, Possemiers NM, et al. Exercise acutely reverses dysfunction of circulating angiogenic cells in chronic heart failure. Eur Heart J 2010; 31: 1924–1934. [DOI] [PubMed] [Google Scholar]
- 12. Júnior MB, Lopes RD, Seelaender MCL, et al. Anti-inflammatory effect of physical training in heart failure: role of TNF-α and IL-10. Arq Bras Cardiol 2009; 93: 643–651. [PubMed] [Google Scholar]
- 13. Ijiri Y, Ikarugi H, Tamura Y, et al. Antithrombotic effect of taurine in healthy Japanese people may be related to an increased endogenous thrombolytic activity. Thromb Res 2013; 131: 158–161. [DOI] [PubMed] [Google Scholar]
- 14. Marcinkiewicz J, Kontny E. Taurine and inflammatory diseases. Amino Acids 2014; 46: 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang M, Izumi I, Kagamimori S, et al. Role of taurine supplementation to prevent exercise-induced oxidative stress in healthy young men. Amino Acids 2004; 26: 203–207. [DOI] [PubMed] [Google Scholar]
- 16. Rahman MM, Park H-M, Kim S-J, et al. Taurine prevents hypertension and increases exercise capacity in rats with fructose-induced hypertension. Am J Hypertens 2011; 24: 574–581. [DOI] [PubMed] [Google Scholar]
- 17. Silva LA, Silveira PC, Ronsani MM, et al. Taurine supplementation decreases oxidative stress in skeletal muscle after eccentric exercise. Cell Biochem Funct 2011; 29: 43–49. [DOI] [PubMed] [Google Scholar]
- 18. Ishikura K, Miyazaki T, Ra S-G, et al. Effect of taurine supplementation on the alterations in amino acid content in skeletal muscle with exercise in rat. J Sport Sci Med 2011; 10: 306–314. [PMC free article] [PubMed] [Google Scholar]
- 19. Miyazaki T, Matsuzaki Y, Ikegami T, et al. Optimal and effective oral dose of taurine to prolong exercise performance in rat. Amino Acids 2004; 27: 291–298. [DOI] [PubMed] [Google Scholar]
- 20. Pinkstaff S, Peberdy MA, Kontos MC, et al. Quantifying exertion level during exercise stress testing using percentage of age-predicted maximal heart rate, rate pressure product, and perceived exertion. Mayo Clin Proc 2010; 85: 1095–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502. [PubMed] [Google Scholar]
- 22. Choi MJ. Effects of dietary taurine supplementation on plasma and liver lipids in OVX rats fed calcium-deficient diet. Nutr Res Pract 2008; 2: 13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wójcik OP, Koenig KL, Zeleniuch-Jacquotte A, et al. The potential protective effects of taurine on coronary heart disease. Atherosclerosis 2010; 208: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamori Y, Murakami S, Ikeda K, et al. Fish and lifestyle-related disease prevention: experimental and epidemiological evidence for anti-atherogenic potential of taurine. Clin Exp Pharmacol Physiol 2004; 31: S20–S23. [DOI] [PubMed] [Google Scholar]
- 25. Lam N, Chen W, Suruga K, et al. Enhancing effect of taurine on CYP7A1 mRNA expression in Hep G2 cells. Amino Acids 2006; 30: 43–48. [DOI] [PubMed] [Google Scholar]
- 26. Xu Y, Arneja AS, Tappia PS, et al. The potential health benefits of taurine in cardiovascular disease. Exp Clin Cardiol 2008; 13: 57. [PMC free article] [PubMed] [Google Scholar]
- 27. Tanno N, Oikawa S, Koizumi M, et al. Effect of taurine administration on serum lipid and biliary lipid composition in man. Tohoku J Exp Med 1989; 159: 91–99. [DOI] [PubMed] [Google Scholar]
- 28. Miyamoto TA, Ueno T, Iguro Y, et al. Taurine-mediated cardioprotection is greater when administered upon reperfusion than prior to ischemia. Adv Exp Med Biol 2009; 643: 27–36. [DOI] [PubMed] [Google Scholar]
- 29. Kim C, Cha YN. Taurine chloramine produced from taurine under inflammation provides anti-inflammatory and cytoprotective effects. Amino Acids 2014; 46: 89–100. [DOI] [PubMed] [Google Scholar]
- 30. Rosa FT, Freitas EC, Deminice R, et al. Oxidative stress and inflammation in obesity after taurine supplementation: a double-blind, placebo-controlled study. Eur J Nutr 2014; 53: 823–830. [DOI] [PubMed] [Google Scholar]