Abstract
Background:
Empirical three-step therapy has been proved in just one hospital. This study aimed to demonstrate applicability of the sequential empirical three-step therapy for chronic cough in different clinical settings.
Methods:
Sequential empirical three-step therapy was given to patients with chronic cough in one tertiary and three secondary care respiratory clinics. Recruiters were initially treated with methoxyphenamine compound as the first-step therapy, followed by corticosteroids as the second-step therapy and the combination of a proton-pump inhibitor and a prokinetic agent as the third-step therapy. The efficacy of the therapy was verified according to the changes in cough symptom score between pre- and post-treatment, and compared among the different clinics.
Results:
In total 155 patients in one tertiary clinic and 193 patients in secondary care clinics were recruited. The total dropout ratio is significantly higher in the secondary care clinics than that in the tertiary clinic (9.3% versus 3.2%, p = 0.023). The therapeutic success rate for cough was 38.7% at first-step therapy, 32.3% at second-step therapy and 20.0% at third-step therapy in the tertiary clinic, and comparable to corresponding 49.7%, 31.1% and 4.1% in secondary care clinics. Furthermore, the overall cough resolution rate was not significantly different (91.0% versus 85.0%, p = 0.091). However, the efficacy of the third-step therapy is much higher (20.0% versus 4.1%, p = 0.001) in the tertiary clinic than in the secondary care clinics.
Conclusions:
Sequential empirical three-step therapy is universally efficacious and useful for management of chronic cough in different clinical settings.
Keywords: antihistamine decongestant, antireflux treatment, corticosteroids, cough, empirical therapy
Introduction
Empirical therapy for chronic cough has been accepted as an alternative to cause-directed therapy, and recommended in several recent guidelines for the management of chronic cough due to its easiness, lower cost and comparable therapeutic efficacy.1–3 Currently, there are three strategies for the implementation of empirical therapy, that is, clinical clue orientation, common cause orientation and integration of diagnostic protocol with empirical therapy.4 Cough can be successfully resolved in most patients, whatever strategy of empirical therapy is used.5
To manage cough efficiently, we developed a sequential three-step empirical therapy that systematically treats the common causes of chronic cough, such as upper airway cough syndrome (UACS), cough variant asthma (CVA), eosinophilic bronchitis (EB) and gastroesophageal reflux-induced chronic cough (GERC).6 The clinical trials have shown the three-step empirical therapy, either in its original or modified form, could resolve cough in 88–92% of patients without an extensive laboratory investigation.6,7 However, the effectiveness of the protocol was only verified in a single specialist cough center, and it remains unclear whether it works in other clinical settings, especially in the primary or secondary care clinics where the empirical therapy for chronic cough is most often employed.
Therefore, we conducted a study in an attempt to compare the efficacy of sequential three-step empirical therapy for chronic cough in the different clinics.
Methods
Patients with chronic cough were consecutively enrolled from the four centers. The allocation numbers were 140 patients for the tertiary clinic and 180 patients for three secondary care clinics, with 60 patients for each center, which was the minimum number of patients required for the comparison of the overall therapeutic success rate between the tertiary and secondary care clinics, and would be sufficient to provide 80% statistical power to demonstrate an efficacy equivalence within 10 percentage points using a 5% two-sided test. The inclusion criteria were: (1) cough persisted for at least 2 months; (2) the absence of wheeze, hemoptysis, fever, dyspnea or adventitious lung sounds on physical examination; (3) normal chest radiography; (4) FEV1 >80% of predicted and ratio of FEV1/FVC >70%; (5) no reported exposure to environmental pollutants or occupational irritants; (6) patients’ ages ⩾18 years; and (7) no known contraindication to the related drugs. The exclusion criteria included: (1) current or ex-smokers for <2 years; (2) the history of upper respiratory tract infection and taking angiotensin-converting enzyme inhibitors during the previous 2 months; and (3) women in pregnancy or lactation. Informed consent was obtained from all participants.
Study design
This was an observation study conducted over 2 years between April 2013 and March 2015 in four respiratory clinics (one tertiary and three secondary care clinics), all located within the urban area of Shanghai, China. The protocol was approved by the Ethics Committee of Tongji Hospital (No. LL(H)-13-170) and registered in the Chinese Clinical Trials Register (www.chictr.org.cn) (ChiCTR-ONC-13003067). During the study, the program monitors (Drs. Yu and Xu) visited each center regularly to collect the data and check the quality of the research work.
Therapeutic protocol
The sequential three-step empirical therapy was given as described previously, with minor modifications.6,7 The treatment commenced with oral methoxyphenamine compound (Asmeton, Daiichi Sankyo Pharmaceutical Shanghai Co., Ltd, Shanghai, China), two capsules, three times daily for 1 week. Each capsule of methoxyphenamine compound contains aminophylline 25 mg, methoxyphenamine 12.5 mg, noscapine 7 mg and chlorpheniramine 2 mg according to the instructions of the manufacturer. The patients responding to this step of the therapy maintained the treatment until their cough resolved. If the cough failed to improve, the treatment with methoxyphenamine compound was discontinued and the next step of the therapy was administrated, which included oral prednisone (25 mg once daily) for 1 week, followed by inhaled budesonide (Pulmicort Turbuhaler, AstraZeneca, Södertälje, Sweden) (300 μg, twice daily) only in the patients with favorable response to prednisone. If the treatment with methoxyphenamine compound attenuated but failed to eliminate the patient’s cough within three weeks, the first step of the therapy was overlapped and combined with the second step. The patients unresponsive to corticosteroids were moved onto the third step of the therapy, which consisted of omeprazole (20 mg twice daily) plus domperidone (10 mg three times daily), and lifestyle modifications including adjustments in diet and sleeping position. Similarly, the third step of therapy was combined with the previous step when the cough improved but failed to be completely resolved within 4 weeks of the second step of the treatment. The therapy was terminated if the cough did not improve after an 8-week course of the third step of the treatment.
Therapeutic endpoints
Cough severity was evaluated using the cough symptom score described and validated by Hsu et al.,8 which rates day-time and night-time cough on a six-point incremental scale, with 0 being best (no cough) and 5 being worst (characterized by a distressing cough). Symptoms were considered to be controlled when the cough disappeared, improved when the combined day-time and night-time cough symptom score decreased by at least 50%, and failed when the cough worsened or was not alleviated to the standard of improvement.9,10 Dropout was defined as a patient not completing the therapeutic course and being lost to follow-up.
Follow-up
The initial assessment included the medical history, physical examination, chest radiography, lung function testing and rating of cough symptom scores. The patients were followed-up weekly during the first and second steps of the therapy, and every 2 weeks during the third step. Patients’ response to the therapy, the changes in cough symptom scores and possible adverse effects were recorded at each follow-up visit.
Analysis
Data are expressed as mean ± standard deviation (SD) except for cough duration and cough symptom score, which are expressed as median (range). Comparisons between groups were made using unpaired t-tests, while gender distribution and efficacy difference between groups were examined using the chi-square test, and the changes in cough symptom score between pre- and post-treatment were analyzed by the Mann–Whitney U-test. SPSS version 10.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. A value of p < 0.05 was considered statistically significant.
Results
General characteristics of patients
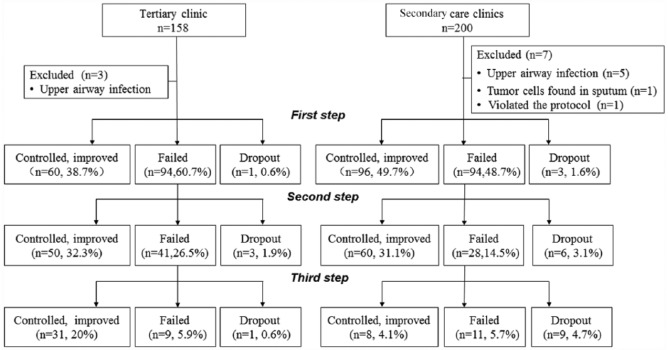
A total of 358 patients met the inclusion criteria, which included 158 patients in the tertiary clinic and 200 patients in the secondary care clinics. Ten patients (three in the tertiary clinic and seven in the secondary care clinics) were excluded because of newly developed upper airway infection in eight patients, positive tumor cells in the sputum of one patient and self-taking antibiotic in one patient. A total of 348 patients were recruited into the study. The demographic characteristics are shown in Table 1. Twenty-three patients (five in the tertiary clinic and eighteen in the secondary care clinics) dropped out during the study (23/348, 6.61%) (Figure 1), and the dropout rate was higher in the secondary care clinics than in the tertiary clinic (9.3% versus 3.2%, p = 0.023).
Table 1.
Demographic and baseline characteristics of patients with chronic cough.
| Characteristic | Tertiary care clinic | Secondary care clinics |
|---|---|---|
| Number of patients | 155 | 193 |
| Male, n (%) | 50 (32.3%) | 62 (32.1%) |
| Age, years (mean ± SD) | 49.3 ± 15.7 | 47.7 ± 13.9 |
| Duration of cough in months, median (range) | 6.0 (5.0, 108.0) | 3.5 (4.0, 72.0) |
| Cough symptom score, median (range) | ||
| Day-time | 3 (1, 5) | 3 (1, 5) |
| Night-time | 1 (0, 4) | 2 (0, 5) |
| FEV1 % predicted (mean ± SD) | 94.1 ± 14.3 | 99.3 ± 12.2 |
| FVC % predicted (mean ± SD) | 95.5 ± 14.0 | 99.7 ± 12.2 |
| FEV1/FVC, % (mean ± SD) | 93.4 ± 11.4 | 89.0 ± 10.3 |
Figure 1.
STROBE diagram showing participant progress through the study, and the comparison of efficacy at each step of the sequential empirical three-step therapy for chronic cough between tertiary and secondary care clinics.
Therapeutic efficacy
The overall therapeutic efficacy was similar between the tertiary and the secondary care clinics, even though it was slightly higher in the tertiary clinic (91.0% versus 85.0%, χ2 = 2.852, p = 0.091). However, the efficacy at the third step of the therapy was much higher in the tertiary clinic than in the secondary care clinics, while the efficacy at the first step of the therapy was marginally lower in the tertiary than in the secondary care clinics (Tables 2 and 3; Figure 1). Among the three secondary care clinics, the total therapeutic success rates were 89.5%, 86.6% and 79.7% respectively, and they were not statistically different (χ2 = 0.998, p = 0.607).
Table 2.
Comparison of efficacy of empirical three-step therapy between the different clinics.
| Tertiary care clinic (n = 155) | Secondary care clinics (n = 193) | χ2 value | p-value | |
|---|---|---|---|---|
| Controlled (n, %) | 65, 41.9 | 67, 34.7 | 0.00672 | 0.966 |
| Improved (n, %) | 76, 49 | 97, 50.3 | ||
| Uncontrolled (n, %) | 9, 5.8 | 11, 5.7 | ||
| Dropout (n, %) | 5, 3.2 | 18, 9.3 | 5.183 | 0.023 |
| Success rate (n, %) | 141, 91.0 | 164, 85.0 | 2.852 | 0.091 |
Table 3.
Difference in the efficacy of sequential empirical three-step therapy between tertiary and secondary care clinics.
| Tertiary care clinic | Secondary care clinics | χ2 value | p-value | |
|---|---|---|---|---|
| First step (n, %) | 60, 38.7 | 96, 49.7 | 4.230 | 0.040 |
| Second step (n, %) | 50, 32.3 | 60, 31.1 | 0.054 | 0.816 |
| Third step (n, %) | 31, 20.0 | 8, 4.1 | 21.716 | 0.001 |
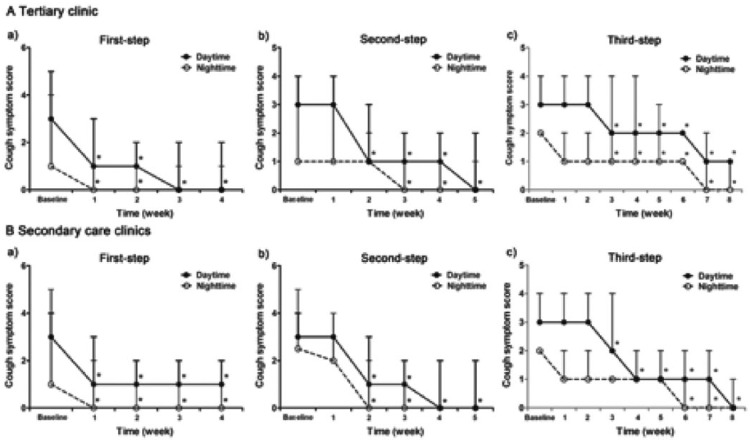
The time for cough relief was comparable at the first two steps of the therapy among all the clinics, while it was longer at the third step of the therapy in the tertiary care clinic than in the secondary care clinics (8.50 ± 0.85 weeks versus 3.63 ± 0.73 weeks, t = 7.828, p = 0.001) (Figures 2 and 3).
Figure 2.
Weekly changes and comparison of cough symptom score at each step of the sequential empirical three-step therapy for chronic cough between the patients visiting tertiary and secondary care clinics (*p < 0.05 when compared with baseline).
Figure 3.
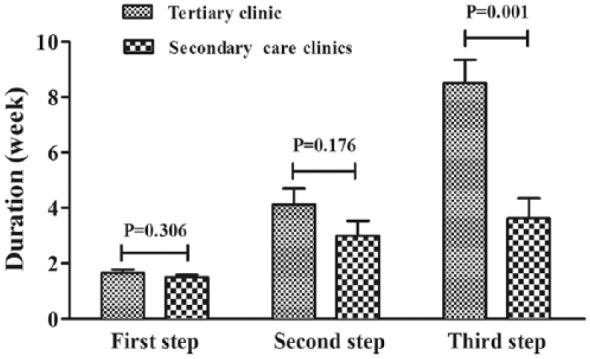
Comparison of the treatment duration required for cough resolution at each step of the sequential empirical three-step therapy for chronic cough between the patients visiting tertiary and secondary care clinics.
Adverse events
Mild dizziness, fatigue and thirst were noted in 19 patients in the tertiary care clinic and 23 patients in the secondary care clinics, respectively, at the first step of the therapy. The symptoms disappeared when the treatment with methoxyphenamine compound was discontinued. Two patients in the tertiary clinic reported stomach discomfort at the second step of the therapy, which resolved automatically after the end of oral prednisone.
Discussion
This clinical trial has reinforced our previous findings that sequential three-step empirical therapy is an efficacious therapeutic option for the management of chronic cough, and can relieve or eliminate the cough in most patients without a previous definite identification of the cause of chronic cough. Moreover, similarly high therapeutic success rates can be achieved by the therapy protocol in tertiary care clinics as well as secondary care clinics.
Several regimens have been developed for the institution of empirical therapy, and their cost-effectiveness has been analyzed.11 Generally, the algorithms for empirical therapy are devised to cover the common causes of chronic cough simultaneously or sequentially, but prioritize the most common diseases in the local district or the most probable etiologies. Pratter and colleagues selected antihistamine decongestant as an initial intervention of the empirical therapy since UACS was the leading cause of chronic cough in the USA.12 Similarly, Shimizu and colleagues started the empirical therapy with a maximal dose of procaterol inhalation, based on the assumption that cough responsive to β2 agonist was the basic characteristic of CVA or cough related to asthma.13 In contrast, Deng and colleagues initiated the empirical therapy pointing to corticosteroid-responsive cough (including CVA, EB and atopic cough), UACS and GERC respectively after figuring out the possible etiologies hinted at by the clinical presentations.14 The reported success rates were 62.5–86.7% at the first step of the empirical therapies and 81.2–95.0% when the algorithms for the empirical treatment were completed.
Our protocol for empirical therapy differs from the above regimens in that it treats CVA and UACS first by administering antihistamine-decongestant and bronchodilators simultaneously, irrespective of clinical clues pertinent to a potential cause.6,7 We designed the protocol this way because these disorders, alone and in combination, account for 61–78% of chronic cough in China,15–17 and the existing clinical manifestations or conditions were not always reliable to predict a favorable response to the therapy specific to the etiologies.18–21 The reported success rates for the protocol were 67.6–82.4% at the initial step and 88.7–91.7% at the completion of the empirical therapy,6,7 which aligns with the observations made by other researchers. In fact, Lu and colleagues have demonstrated that overall therapeutic efficacy was comparable among the various protocols for empirically treating chronic and subacute cough.22
At present, the medical system in China is undergoing vigorous reform, and is far from perfect.23,24 Under the current policy of health insurance in Shanghai, patients are allowed to see a doctor in any clinic without the need for referrals and without a significant difference in fees, just like the ‘health insurance for all’ system in Japan.25 Based on the data from the four centers, >90% of the patients visited tertiary or local clinics without referrals from other doctors. Therefore, the cause profile of chronic cough may not be obviously different among the clinical settings in China, and it may explain why the sequential three-step empirical therapy for chronic cough could achieve a similar high success rate in the tertiary care clinic as well as in the three secondary care clinics. Moreover, the therapy protocol covers the common causes of chronic cough systematically, and the failure of the previous step of the therapy may be compensated by the next step of the therapy specific to the other etiologies. Thus, the similar overall therapeutic efficacy can be predicted, although the cause frequency and distribution of chronic cough varies among individual clinics.4
The significant difference in the therapeutic efficacy among the four clinics lay in the fact that treatment success at the third step of the therapy was significantly higher in the tertiary care clinic than in the secondary care clinics. This may be associated with the patients’ compliance and interventional fidelity to medical antireflux therapy. Cough is an extraesophageal symptom of gastroesophageal reflux disease, and patients with chronic cough visiting a respiratory clinic do not usually easily understand why they are prescribed antireflux drugs.26 Moreover, the antireflux treatment is time-consuming for cough resolution.27 If the doctors cannot convince the patients, it is difficult to ensure their compliance and the strict implementation of the treatment program for GERC. In this situation, the physicians in a tertiary clinic may be better positioned than those in other clinics in China to persuade the patients due to their higher professional reputation, as supported by the higher dropout rate of patients and the lower efficacy at the third step of the therapy in the secondary care clinics.
The success rate at the first step of the empirical therapy in the present study was only 38.7–49.7% within the four centers, much lower than those reported in our previous studies.6,7 This inconsistency may be attributed to several reasons. First, methoxyphenamine compound, the agent for the initial step of the therapy, has been extensively utilized for cough treatment in China because it has been demonstrated to be efficacious in a multicenter clinical trial28 and is recommended in the Chinese guideline for the management of cough.4 Therefore, the cough responsive to methoxyphenamine compound may have been mostly resolved at its acute or subacute stage and has less chance to enter the chronic stage. Moreover, ‘easy to treat’ causes such as UACS may be well-treated in the primary clinical setting as knowledge of the management of cough is widespread, which further decreases patients’ susceptibility to methoxyphenamine compound in the tertiary and secondary care clinics.
There are several drawbacks in this study. The major weakness is its open-intervention nature, which may limit the power of the conclusion. Theoretically, a double-blind and placebo-controlled design is preferred, but it is impossible to execute in the case of the sequential three-step empirical therapy. We do not believe the placebo effect can explain the high efficacy of the therapeutic protocol in multiple centers since the recruited patients had a persistent cough for a median period of at least 3.5 months. However, the true benefit of the therapy is difficult to determine definitively due to the lack of a placebo arm in this study. Furthermore, one can criticize that the cause of chronic cough remains uncertain even though the cough is eliminated by the first two steps of the therapy. It is an inherent defect of the empirical therapy for chronic cough that cannot be overcome.4 Nevertheless, the principal aim of the study was to test whether the empirical therapeutic protocol used in our clinic could be extended to the other clinical settings for general practice, rather than to survey the causes of chronic cough as reported previously.7 In fact, the sequential empirical three-step therapy has been demonstrated to be universally useful for the management of chronic cough in secondary care clinics, as indicated by the similar total efficacy among these three centers.
Conclusion
In conclusion, the sequential empirical three-step therapy is efficacious for management of chronic cough, and can be universally used in the different clinical settings.
Footnotes
Funding: This work was supported by grants from Shanghai Shenkang Hospital Development Center (No. SHDC12012211), the National Natural Science Foundation of China (No. 81170079 and 81470276) and Shanghai Municipal Hospital Frontier Technology Key Project (No: SHDC12014120).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Li Yu, Tongji Hospital, Tongji University School of Medicine, Shanghai, China.
Xianghuai Xu, Tongji Hospital, Tongji University School of Medicine, Shanghai, China.
Jingqing Hang, Putuo People’s Hospital, Shanghai, China.
Kewen Cheng, Renhe Hospital, Shanghai, China.
Xiaoyan Jin, Tongren Hospital, Shanghai, China.
Qiang Chen, Tongji Hospital, Tongji University School of Medicine, Shanghai, China.
Hanjing Lv, Tongji Hospital, Tongji University School of Medicine, Shanghai, China.
Zhongmin Qiu, Department of Respiratory Medicine, Tongji Hospital, Tongji University School of Medicine, No. 389 Xincun Road, Shanghai 200065, China.
References
- 1. Morice AH, Fontana GA, Sovijarvi AR, et al. The diagnosis and management of chronic cough. Eur Respir J 2004; 24: 481–492. [DOI] [PubMed] [Google Scholar]
- 2. Pratter MR, Brightling CE, Boulet LP, et al. An empiric integrative approach to the management of cough: ACCP evidence-based clinical practice guidelines. Chest 2006; 129: 222S–231S. [DOI] [PubMed] [Google Scholar]
- 3. Asthma Workgroup, Chinese Society of Respiratory Diseases (CSRD), Chinese Medical Association. The Chinese national guidelines on diagnosis and management of cough (December 2010). Chin Med J (Engl) 2011; 124: 3207– 3219. [PubMed] [Google Scholar]
- 4. Chummun D, Lu H, Qiu Z. Empiric treatment of chronic cough in adults. Allergy Asthma Proc 2011; 32: 193–197. [DOI] [PubMed] [Google Scholar]
- 5. Ojoo JC, Everett CF, Mulrennan SA, et al. Management of patients with chronic cough using a clinical protocol: a prospective observational study. Cough 2013; 9: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu L, Qiu Z, Lu H, et al. Clinical benefit of sequential three-step empirical therapy in the management of chronic cough. Respirology 2008; 13: 353–358. [DOI] [PubMed] [Google Scholar]
- 7. Wei W, Yu L, Wang Y, et al. Efficacy and safety of modified sequential three-step empirical therapy for chronic cough. Respirology 2010; 15: 830–836. [DOI] [PubMed] [Google Scholar]
- 8. Hsu JY, Stone RA, Logan-Sinclair RB, et al. Coughing frequency in patients with persistent cough: assessment using a 24 hour ambulatory recorder. Eur Respir J 1994; 7: 1246–1253. [DOI] [PubMed] [Google Scholar]
- 9. Xu X, Chen Q, Liang S, et al. Comparison of gastroesophageal reflux disease questionnaire and multichannel intraluminal impedance pH monitoring in identifying patients with chronic cough responsive to antireflux therapy. Chest 2014; 145: 1264–1270. [DOI] [PubMed] [Google Scholar]
- 10. Xu X, Lv H, Yu L, et al. A stepwise protocol for the treatment of refractory gastroesophageal reflux-induced chronic cough. J Thorac Dis 2016; 8: 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin L, Poh KL, Lim TK. Empirical treatment of chronic cough: a cost-effectiveness analysis. Proc AMIA Symp 2001; 383–387. [PMC free article] [PubMed] [Google Scholar]
- 12. Pratter MR, Bartter T, Akers S, et al. An algorithmic approach to chronic cough. Ann Intern Med 1993; 119: 977–983. [DOI] [PubMed] [Google Scholar]
- 13. Shimizu H, Hayashi M, Saito Y, et al. Classification of chronic cough by systematic treatment cascade trial starting with beta agonist. Cough 2013; 9: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deng HY, Luo W, Zhang M, et al. Initial empirical treatment based on clinical feature of chronic cough. Clin Respir J 2016; 10: 622–630. [DOI] [PubMed] [Google Scholar]
- 15. Wei W, Yu L, Lu H, et al. Comparison of cause distribution between elderly and non-elderly patients with chronic cough. Respiration 2009; 77: 259–264. [DOI] [PubMed] [Google Scholar]
- 16. Yu L, Wei WL, Lu HJ, et al. Changes in the spectrum and frequency of causes for chronic cough: a retrospective analysis. Zhonghua Jie He He Hu Xi Za Zhi 2009; 32: 414–417. [PubMed] [Google Scholar]
- 17. Lai K, Chen R, Lin J, et al. A prospective, multicenter survey on causes of chronic cough in China. Chest 2013; 143: 613–620. [DOI] [PubMed] [Google Scholar]
- 18. Mello CJ, Irwin RS, Curley FJ. Predictive values of the character, timing, and complications of chronic cough in diagnosing its cause. Arch Intern Med 1996; 156: 997–1003. [PubMed] [Google Scholar]
- 19. Patterson RN, Johnston BT, Macmahon J, et al. Oesophageal pH monitoring is of limited value in the diagnosis of ‘reflux-cough’. Eur Respir J 2004; 24: 724–727. [DOI] [PubMed] [Google Scholar]
- 20. Yu L, Wei W, Wang L, et al. Upper-airway cough syndrome with latent eosinophilic bronchitis. Lung 2010; 188: 71–76. [DOI] [PubMed] [Google Scholar]
- 21. Yu L, Xu XH, Chen Q, et al. Gastro-esophageal reflux induced cough with airway hyperresponsiveness. Int J Clin Exp Med 2014; 7: 728–735. [PMC free article] [PubMed] [Google Scholar]
- 22. Lu P, Zhou D, Jin C. A novel diagnostic algorithm for chronic and subacute cough. Multidiscip Respir Med 2014; 9: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang J, Kushner K, Frey JJ, III, et al. Primary care reform in the Peoples’ Republic of China: implications for training family physicians for the world’s largest country. Fam Med 2007; 39: 639–643. [PubMed] [Google Scholar]
- 24. Ding D, Pan Q, Shan L, et al. Prescribing patterns in outpatient clinics of township hospitals in China: a comparative study before and after the 2009 health system reform. Int J Environ Res Public Health 2016; pii: ijerph13070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Niimi A. Geography and cough aetiology. Pulm Pharmacol Ther 2007; 20: 383–387. [DOI] [PubMed] [Google Scholar]
- 26. Poelmans J, Tack J. Extraoesophageal manifestations of gastro-oesophageal reflux. Gut 2005; 54: 1492–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kiljander TO, Salomaa ER, Hietanen EK, et al. Chronic cough and gastro-oesophageal reflux: a double-blind placebo-controlled study with omeprazole. Eur Respir J 2000; 16: 633–638. [DOI] [PubMed] [Google Scholar]
- 28. Zhou X, Bao WP, Qu JM, et al. A multicenter study to evaluate the efficacy and safety of compound methoxyphenamine in the treatment of postinfectious cough. Chin J Asthma 2011; 31: 1761–1765. [Google Scholar]