Abstract
Eosinophils have long been implicated as playing a central role in the pathophysiology of asthma in many patients, and eosinophilic asthma is now recognized as an important asthma endotype. Eosinophil differentiation, maturation, migration, and survival are primarily under the control of interleukin-5 (IL-5). Reslizumab is a humanized monoclonal (immunoglobulin G4/κ) antibody that binds with high affinity to circulating human IL-5 and downregulates the IL-5 signaling pathway, potentially disrupting the maturation and survival of eosinophils. In 2016, an intravenous formulation of reslizumab was approved in the USA, Canada, and Europe as add-on maintenance treatment for patients aged ⩾18 years with severe asthma and with an eosinophilic phenotype. The efficacy of reslizumab as add-on intravenous therapy has been reported in several phase III studies in patients with inadequately controlled moderate-to-severe asthma and elevated blood eosinophil counts (⩾400 cells/µl). Compared with placebo, reslizumab was associated with significant improvements in clinical exacerbation rate, forced expiratory volume in 1 s, asthma symptoms and quality of life, and significant reductions in blood eosinophil counts. Reslizumab also demonstrated a favorable tolerability profile similar to that of placebo, with reported adverse events being mostly mild to moderate in severity. Ongoing studies are focusing on the evaluation of a subcutaneous formulation of reslizumab in patients with asthma and elevated eosinophil levels. This review discusses the preclinical and clinical trial data available on reslizumab, potential opportunities for predicting an early response to reslizumab, and future directions in the field of anti-IL-5 antibody therapy.
Keywords: antibodies, asthma endotype, eosinophilic asthma, interleukin-5, monoclonal, reslizumab, safety, treatment outcome
Introduction
Asthma is a complex chronic disease with a heterogeneous presentation, having many different phenotypes. A phenotype describes the observable characteristics of a disease and in the context of asthma describes clinical and morphologic characteristics, such as clinical presentation, triggers, and treatment response, but without reference to the underlying pathogenesis. For this reason, the elucidation of asthma phenotypes has been further refined with the emergence of endotypes, which categorize asthma into distinct subtypes according to underlying functional or pathophysiologic mechanisms.1,2 Each asthma endotype can be present in various phenotypes, just as a specific phenotype may be associated with more than one endotype. The identification of asthma phenotypes and endotypes is an ongoing process, and it has been proposed that stratifying patients with asthma on the basis of specific phenotypes and endotypes could result in improved treatment outcomes through the use of individualized therapy.1–3
Inflammation is the most important pathophysiological mechanism underlying the development of asthma, involving a complex interplay between lymphocytes of the adaptive immune system and multiple cell types of the innate immune system, including innate lymphoid cells, mast cells, basophils, neutrophils, eosinophils, and dendritic cells. If inadequately treated, chronic inflammation of the airways leads to mucus hypersecretion, airway hyperresponsiveness, and bronchial remodeling, including airway thickening, fibrosis, and angiogenesis.4,5
Inhibition of IL-5 and eosinophil-mediated inflammation in asthma
Eosinophils have long been implicated as playing a central role in the pathophysiology of asthma in many patients. Eosinophilic asthma is now recognized as an important asthma endotype and assessment of eosinophilia in patients with severe asthma is an important tool for monitoring asthma control and guiding therapeutic decisions (Table 1).6–8 Increased numbers of eosinophils in the airways, peripheral blood, and bronchoalveolar lavage (BAL) fluid have been widely documented in patients with chronic asthma.8 This increase correlates with asthma severity9 and has been implicated in asthma exacerbation.10–12 Furthermore, loss of asthma control following cessation of inhaled corticosteroid (ICS) therapy can be predicted by the presence of eosinophils in the airway lumen, as identified by sputum cell counts.13,14
Table 1.
Typical clinical profile of patients with late-onset eosinophilic asthma.6
| Characteristic |
|---|
| Adult onset of asthma |
| Equal distribution between sexes |
| Few or no allergies to common allergens |
| Elevated eosinophil counts in peripheral blood |
| At risk of severe exacerbations |
| Normal or moderately elevated IgE level |
| Low FEV1 and often persistent airflow limitation |
| Air trapping and dynamic hyperinflation |
| Chronic rhinosinusitis with nasal polyposis |
| Aspirin sensitivity |
| Good response to systemic corticosteroids |
| Good response to anti-IL-5 treatment |
FEV1, forced expiratory volume in 1 s; Ig, immunoglobulin; IL, interleukin.
Reproduced with permission of the European Respiratory Society ©: ERJ Open Research May 2015, 1 (1) 00024-2015; DOI: 10.1183/23120541.00024-2015.
Eosinophils are multifunctional leukocytes that play an important role in the mediation of allergic and asthmatic type 2 immune responses. During an asthma exacerbation, eosinophils are activated to secrete granule-derived proteins including major basic protein, eosinophil cationic protein, eosinophil peroxidase, and eosinophil-derived neurotoxin, all of which are cytotoxic to airway epithelial cells. In addition, eosinophils release a plethora of inflammatory mediators including cytokines (interleukin [IL]-13 and IL-5), growth factors (transforming growth factor-α and -β), cysteinyl leukotrienes, platelet-activating factor, thromboxane, and prostaglandins. Collectively, the release of these compounds leads to augmentation of the inflammatory process, damage to the respiratory epithelium, and bronchospasm.15,16
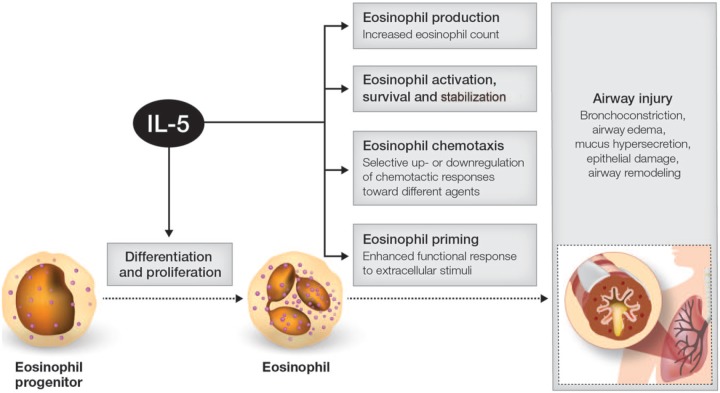
Although a number of bioactive proteins, including IL-3 and granulocyte-macrophage colony-stimulating factor, regulate and control the life cycle of eosinophils, eosinophils respond primarily to IL-5. Together with its specific receptor (IL-5R) on target cells, IL-5 has a central role in eosinophil growth, differentiation, recruitment, activation, and survival (Figure 1).17 T-helper type 2 (Th2) lymphocytes are the main source of IL-5 in the lung, although type 2 innate lymphoid cells (ILC2), eosinophils, mast cells, and other cell lines also contribute to the levels of this cytokine.18–21 Both allergic and non-allergic mechanisms may drive eosinophilic inflammation in the lungs; non-allergic pathways, driven mainly by ILC2 cells producing IL-5 and IL-13, appear to be the more important in late-onset (adult) eosinophilic severe asthma.21,22
Figure 1.
Schematic depicting role of IL-5 in promoting eosinophilic asthma.
IL, interleukin.
Data from a number of studies confirm the pivotal role played by IL-5 in the pathogenesis of asthma. IL-5 expression is increased in BAL fluid and bronchial biopsies from patients with asthma compared with non-asthmatic controls,23 and a correlation has been reported between IL-5 mRNA levels in bronchial mucosa and disease severity in patients with atopic asthma.24 Furthermore, inhalation of recombinant human IL-5 by patients with allergic bronchial asthma is associated with increased airway sensitivity, significant eosinophilia, and elevated levels of eosinophil cationic protein in induced sputum.25 In severe eosinophilic asthma, in situ eosinophilopoiesis – promoted by locally derived IL-5 and IL-13 from ILC2 cells – may also arise and has been reported to be associated with resistance to high-dose oral corticosteroid (OCS) therapy.26 As a central mediator of eosinophilic inflammation, IL-5 therefore represents an important potential target and treatment strategy for patients with poorly controlled asthma.27
Reslizumab
Reslizumab (CINQAIR®; formerly SCH-55700) is a humanized monoclonal (immunoglobulin [Ig] G4/κ) antibody that targets IL-5. It is currently available as an intravenous (IV) formulation and development of a subcutaneous (SC) formulation is ongoing. The development of reslizumab has followed a complex path involving several different pharmaceutical companies. Reslizumab was originally developed by Celltech, and initial development of the drug was jointly undertaken by Celltech and Schering-Plough (now Merck and Co., Inc.). In 2004 Ception Therapeutics acquired the license for reslizumab after Schering-Plough discontinued development. Reslizumab then attracted the interest of Cephalon, which acquired the drug in 2010 following its acquisition of Ception Therapeutics. Reslizumab subsequently passed to Teva, following its acquisition of Cephalon in 2011.
Pharmacology
Reslizumab binds with high affinity to circulating human IL-5 and downregulates the IL-5 signaling pathway, potentially disrupting maturation and survival of eosinophils. Reslizumab has a dissociation constant (Kd) of 81 pM, and inhibits the bioactivity of IL-5 by blocking its binding to the alpha chain of the IL-5R complex expressed on the surface of eosinophils.28,29
Reslizumab demonstrated good inhibition of eosinophilia in a number of early animal models of eosinophilic inflammation.30–32 A single IV dose of reslizumab 0.3 mg/kg achieved an approximate 75% reduction in lung lavage eosinophilia in monkeys when administered 1 h before antigen challenge, and this effect persisted following rechallenge 6 months later.30,32 In ovalbumin-sensitized guinea pigs, intraperitoneal administration of reslizumab (0.03–30 mg/kg) 2 h before ovalbumin challenge reduced eosinophilia, airway hyperreactivity and bronchoconstriction.32 Reslizumab 5 mg/kg IV also reduced eosinophil influx into the skin of ovalbumin-sensitized rabbits, without affecting the number of total cells and neutrophils in the skin, and reslizumab reduced the number of total cells and eosinophils in BAL fluid when administered intraperitoneally (1 mg/kg) to ovalbumin-sensitized mice.32
When characterized in healthy adults, patients with asthma, and other patient populations, the pharmacokinetic profile of reslizumab was similar across groups, with an interindividual variability in peak and overall exposure of approximately 20–30%. Plasma concentrations of reslizumab were dose proportional and serum concentrations of reslizumab accumulated by approximately 1.5- to 1.9-fold following multiple-dose administration. Peak serum concentrations of reslizumab are typically achieved at the end of IV infusion and then decline in a biphasic manner.29 In patients with asthma who received reslizumab 1 mg/kg IV, the mean maximal plasma concentration of reslizumab was 30.3 µg/ml at 6.9 h post-dose, which declined to 0.87 µg/ml by day 90 and 0.43 µg/ml by day 120.33 Reslizumab’s volume of distribution is approximately 5 l, clearance is approximately 7 ml/h, and its elimination half-life is 25–30 days. Systemic exposure to reslizumab appears to be unaffected by the presence of treatment-emergent anti-reslizumab antibodies and in vitro data indicate that IL-5 and reslizumab are unlikely to affect cytochrome P450 1A2, 2B6, or 3A4 enzyme activity.29 According to population pharmacokinetic analyses, mild hepatic impairment, mild or moderate renal impairment, or the concomitant use of leukotriene antagonists or corticosteroids do not significantly affect the pharmacokinetic profile of reslizumab.29
Clinical trials of reslizumab in eosinophilic asthma
Because of a lack of patient selection in terms of baseline eosinophilia, initial data from the early stages of the reslizumab clinical development program were discouraging. A phase I pilot trial of reslizumab in a small number of patients with severe persistent asthma (n = 26) failed to demonstrate a significant improvement in asthma symptoms and lung function, despite achieving a reduction in blood eosinophilia.33 Eligible patients with asthma inadequately controlled by high-dose ICS or OCS, who were not preselected for eosinophils in sputum or blood, were randomized to receive either placebo or a single IV dose of reslizumab 0.03, 0.1, 0.3, or 1 mg/kg. Aside from a short-lived transient increase in baseline forced expiratory volume in 1 s (FEV1) in patients treated with reslizumab 0.3 mg/kg (p = 0.019 versus placebo at 24 h post-dose), no significant differences in FEV1, FEV1/forced vital capacity (FVC) ratio, peak flow recordings, symptom score, or physician-evaluated overall condition were observed between reslizumab versus placebo. However, reslizumab dose dependently reduced the circulating blood eosinophil count from baseline, with a significant reduction reported up to day 30 with reslizumab 1 mg/kg [mean 0.07 × 109/l (day 30) versus 0.23 × 109/l (baseline); p = 0.05].
Reslizumab was well tolerated at the administered doses. Headache and fatigue were the most common treatment-related adverse events (AEs) and occurred with the same frequency in the reslizumab and placebo groups. The incidence of aggravation of asthma was not significantly different between the two groups: 25% (3/12) with reslizumab 1 mg/kg and 12.5% (1/8) with placebo. This study highlighted the importance of adequately preselecting patients whose asthma is dependent on the eosinophilic inflammatory pathway, and this led to the incorporation of appropriate patient selection (i.e. enrollment of patients with eosinophilia) in the design of phase II/III reslizumab clinical studies.
In the randomized, double-blind, phase II, proof-of-concept study (ClinicalTrials.gov identifier: NCT00587288), reslizumab 3 mg/kg IV was compared with placebo administered once every 4 weeks for 15 weeks in adult patients with eosinophilic asthma (n = 106).34 Eligible patients had confirmed airway hyperreactivity (20% reduction in FEV1 after methacholine challenge) or airway reversibility (⩾12% improvement in FEV1 after β-agonist administration); had poorly controlled asthma [Asthma Control Questionnaire (ACQ) score ⩾1.5]; were receiving high-dose ICS; and had induced sputum eosinophils of ⩾3%. Mean change from baseline to end of therapy in ACQ score (primary endpoint) was similar in patients receiving reslizumab and placebo (−0.7 and −0.3, respectively; p = 0.0541) but was statistically significantly in favor of reslizumab in a subgroup of patients with nasal polyps (–1.0 versus −0.1, respectively; p = 0.0119) and in patients with a baseline ACQ score >2 (−0.9 versus −0.4; p = 0.0505).
In the overall patient population, a significant improvement from baseline was noted in FEV1 with reslizumab versus placebo (0.18 versus −0.08l, respectively; p = 0.0023). In addition, patients in the reslizumab group achieved a significantly greater median percentage reduction from baseline in sputum eosinophil count (−95.4% versus placebo −38.7%; p = 0.0068) and blood eosinophil count (−0.40 versus 0.00 × 103 cells/µl; p < 0.0001). Asthma exacerbations were reported in 8% (4/53) of patients in the reslizumab group and in 19% (10/53) of patients in the placebo group [odds ratio 0.33; 95% confidence interval (CI), 0.10–1.15; p = 0.0833].
The incidence and profile of AEs were generally similar in the two study groups. The majority of AEs were mild or moderate in severity; nasopharyngitis was the most common AE (reslizumab 21% versus placebo 9%). Serious AEs (SAEs) were reported in two patients in the reslizumab group (pneumonia and worsening of asthma) and in one patient in the placebo group (hypertension). Only one reslizumab-treated patient discontinued treatment because of an AE and this was the patient with worsening of asthma.
The efficacy and safety data from the phase II study subsequently supported the initiation of the phase III BREATH program evaluating reslizumab in the treatment of eosinophilic asthma (Table 2). BREATH is an extensive development program and comprises four completed placebo-controlled phase III reslizumab studies in patients with inadequately controlled, moderate-to-severe asthma. Three of the studies were conducted in patients with elevated eosinophil counts and one study enrolled patients unselected for baseline blood eosinophil levels.
Table 2.
Overview of completed phase III, randomized, double-blind studies of reslizumab in patients with asthma.
| Study | Design | Patients | No. pts | Study treatment | Efficacy | Safety |
|---|---|---|---|---|---|---|
| Study 3081 (ClinicalTrials.gov identifier: NCT01270464)35 |
mc, r (1:1:1), db, pc, pll | EA, aged ⩾12 years, uncontrolled asthma (ACQ-7 score ⩾1.5), airway reversibility (⩾12% to SABA), at least medium-dose ICS, BE ⩾400 cells/µl | 104 | Res 0.3 mg/kg IV q4w × 16 weeks (4 doses) | FEV1 change (Wk 16) = 0.242 l [Δ 0.115 (95% CI,
0.016, 0.215); p = 0.0237] FVC change (Wk 16) = 0.220 l [Δ 0.048 (95% CI, –0.058, 0.155); p = 0.3731] FEF25–75% change (Wk 16) = −0.114 l/s [Δ 0.030 (95% CI, −0.209, 0.270); p = 0.8020] SABA-use change (Wk 16) = −1.0 puffs/day [Δ −0.648 (95% CI −1.152, −0.144); p = 0.0119] ACQ change (Wk 16) = −0.732 l [Δ −0.238 (95% CI −0.456, −0.019); p = 0.0329] AQLQ change (Wk 16) = 1.057 [Δ 0.278 (95% CI −0.036, 0.591); p = 0.0822] BE change (Wk 16) = −358 cells/µl [Δ −323 (95% CI –370, –275); p = 0.0000] |
(n = 103) AEs (any grade) = 57%; most common headache (8%), asthma worsening (6%), nasopharyngitis (6%) Treatment-related AEs = 6% SAEs = 0% Discontinuations due to AEs = <1% |
| 106 | Res 3 mg/kg IV q4w × 16 weeks (4 doses) | FEV1 change (Wk 16) = 0.286 l [Δ 0.160 (95% CI,
0.060, 0.259); p = 0.0018] FVC change (Wk 16) = 0.301 l [Δ 0.130 (95% CI, 0.023, 0.237); p = 0.0174] FEF25–75% change (Wk 16) = 0.089 l/s [Δ 0.233 (95% CI –0.005, 0.472); p = 0.0552] SABA-use change (Wk 16) = −0.9 puffs/day [Δ −0.624 (95% CI, −1.126, −0.121); p = 0.0151] ACQ change (Wk 16) = −0.853 l [Δ −0.359 (95% CI, −0.577, −0.140); p = 0.0014] AQLQ change (Wk 16) = 1.138 [Δ 0.359 (95% CI, 0.047, 0.670); p = 0.0241] BE change (Wk 16) = −529 cells/µl [Δ −494 (95% CI, −542, −447); p = 0.0000] |
(n = 103) AEs (any grade) = 59%; most common asthma worsening (16%), headache (11%), nasopharyngitis (6%) Treatment-related AEs = 12% SAEs = 4% (2 pts asthma exacerbation, 1 pt sinusitis, 1 pt pneumonia, RTA, rib fracture, and asthma exacerbation) Discontinuations due to AEs = 6% |
|||
| 105 | PL q4w × 16 weeks (4 doses) | FEV1 change (Wk 16) = 0.126 l FVC change (Wk 16) = 0.172 l FEF25–75% change (Wk 16) = −0.145 l/s SABA-use change (Wk 16) = −0.3 puffs/day ACQ change (Wk 16) = −0.494 AQLQ change (Wk 16) = 0.779 BE change (Wk 16) = −35 cells/µl |
(n = 105) AEs (any grade) = 63%; most common asthma worsening (19%), headache (6%), nasopharyngitis (4%) Treatment-related AEs = 8% SAEs = <1% (1 acute MI) Discontinuations due to AEs = 10% |
|||
| Study 3082 (ClinicalTrials.gov identifier: NCT01287039)36 |
mc, r (1:1:1), db, pc, pll | EA, aged ⩾12 years, uncontrolled asthma (ACQ-7 score ⩾1.5), airway reversibility (⩾12% to SABA), at least medium-dose ICS, history of asthma exacerbations, BE ⩾400 cells/µl | 245 | Res 3 mg/kg IV q4w × 1 year | CAE rate (events per pt per year) = 0.90 [RR = 0.50 (95% CI,
0.37, 0.67); p <
0.0001] FEV1 change (Wk 52) = 0.235 l [Δ 0.126 (95% CI, 0.06, 0.188); p < 0.0001] AQLQ total score change (Wk 52) = 1.09 [Δ 0.30 (95% CI, 0.14, 0.47); p = 0.0004] ACQ-7 score change (Wk 52) = −1.02 [Δ −0.26 (95% CI, –0.39, −0.12); p = 0.0002] SABA-use change (Wk 52) = −0.58 puffs/day [Δ −0.15 (95% CI, −0.47, 0.16); p = 0.3435] BE change (Wk 52) = −582 cells/µl [Δ –455 (95% CI, –491, –419); p < 0.0001] |
AE (any grade) = 80% (most common asthma worsening 40%, URTI
16%, nasopharyngitis 11%) SAEs = 10% (11 pts asthma, 2 pts pneumonia) Discontinuation due to AEs = 2% Deaths = 0% |
| 244 | PL q4w × 1 year | CAE rate (events per pt per year) =
1.80 FEV1 change (Wk 52) = 0.109 AQLQ total score change (Wk 52) = 0.79 ACQ-7 score change (Wk 52) = −0.76 SABA-use change (Wk 52) = −0.42 puffs/day BE change (Wk 52) = −127 cells/µl |
AE (any grade) = 85% (most common asthma worsening 52%,
nasopharyngitis 14%, URTI 13%) SAEs = 14% (13 pts asthma) Discontinuation due to AEs = 3% Deaths = <1% |
|||
| Study 3083 (ClinicalTrials.gov identifier: NCT01285323)36 |
mc, r (1:1:1), db, pc, pll | EA, aged ⩾12 years, uncontrolled asthma (ACQ-7 score ⩾1.5), airway reversibility (⩾12% to SABA), history of asthma exacerbations, BE ⩾400 cells/µl | 232 | Res 3 mg/kg IV q4w × 1 year | CAE rate (events per pt per year) = 0.86; RR = 0.41 (95% CI,
0.28, 0.59; p <
0.0001) FEV1 change (Wk 52) = 0.201 l [Δ 0.090 (95% CI, 0.003, 0.153); p = 0.0057] AQLQ total score change (Wk 52) = 1.12 [Δ 0.23 (95% CI, 0.07, 0.40); p = 0.0052] ACQ-7 score change (Wk 52) = −1.04 [Δ −0.24 (95% CI, –0.37, −0.11); p = 0.0003] SABA-use change (Wk 52) = −0.73 puffs/day [Δ −0.18 (95% CI, −0.50, 0.14); p = 0.2732] BE change (Wk 52) = −565 cells/µl [Δ –489 (95% CI, –525, –453); p < 0.0001] |
AE (any grade) = 76% (most common asthma worsening 29%,
nasopharyngitis 19%, headache 14%) SAEs = 8% (3 pts asthma, 2 pts pneumonia, 1 pt RTA) Discontinuation due to AEs = 3% Deaths = 0% |
| 232 | PL q4w × 1 year | CAE rate (events per pt per year) =
2.11 FEV1 change (Wk 52) = 0.111 l AQLQ total score change (Wk 52) = 0.89 ACQ-7 score change (Wk 52) = −0.80 SABA-use change (Wk 52) = −0.55 puffs/day BE change (Wk 52) = −76 cells/µl |
AE (any grade) = 87% (most common asthma worsening 51%,
nasopharyngitis 24%, URTI 7%, headache 7%) SAEs = 10% (6 pts asthma, 6 pts pneumonia, 3 pts RTA) Discontinuation due to AEs = 4% Deaths = 0% |
|||
| Study 3084 (ClinicalTrials.gov identifier: NCT01508936)37 |
mc, r (4:1), db, pc | Moderate/severe uncontrolled asthma (ACQ-7 ⩾1.5), aged ⩾18 years, airway reversibility (⩾12% to SABA), at least medium-dose ICS, asthma, unselected for blood eosinophil count | 394 | Res 3 mg/kg IV q4w × 16 weeks (4 doses) |
Overall population
FEV1 change (Wk 16) = 0.255 l [Δ 0.068 (95% CI, –0.030, 0.165); p = 0.1719 versus PL] FVC change (Wk 16) = 0.247 l [Δ 0.012 (95% CI, −0.098, 0.122); p = 0.8361 versus PL] ACQ-7 change (Wk 16) = −0.844 [Δ −0.195 (95% CI, –0.387, −0.004); p = 0.0457 versus PL] SABA-use change (Wk 16) = −0.3 puffs/day [Δ 0.063 (95% CI, −0.340, 0.466); p = 0.7589 versus PL] Linear regression (BL BE level versus FEV1 change) = 0.0229 (slope) (slope difference versus PL = 0.3; p = 0.24) Pts with BE ⩾400 cells/µl (n = 77) FEV1 change (Wk 16) = 0.272 l [Δ 0.270 (95% CI 0.008, 0.532); p = 0.0436 versus PL] FVC change (Wk 16) = 0.230 l [Δ 0.175 (95% CI, −0.137, 0.487); p = 0.2675 versus PL] ACQ-7 change (Wk 16) = −0.858 [Δ −0.490 (95% CI, –1.010, 0.030); p = 0.0643 versus PL] SABA-use change (Wk 16) = −0.8 puffs/day [Δ −0.708 (95% CI, –1.619, 0.204); p = 0.1264 versus PL] |
AE (any grade) = 55% (most common asthma 13%, URTI 11%,
sinusitis 6%) AE (treatment-related) = 7% Discontinuations due to AEs = 8% (asthma 5%) |
| 97 | PL q4w × 16 weeks (4 doses) |
Overall population
FEV1 change (Wk 16) = 0.187 l FVC change (Wk 16) = 0.236 l ACQ-7 change (Wk 16) = −0.648 SABA change (Wk 16) = 0.4 puffs/day Linear regression (BL BE level versus FEV1 change) = –0.2778 (slope) Pts with BE ⩾400 cells/µl (n = 19) FEV1 change (Wk 16) = 0.002 l FVC change (Wk 16) = 0.055 l ACQ-7 change (Wk 16) = −0.368 SABA-use change (Wk 16) = −0.1 puffs/day |
AE (any grade) = 74% (most common asthma 20%, URTI 11%,
sinusitis 7%) AE (treatment-related) = 16% Discontinuations due to AEs = 12% (asthma 8%) |
Δ, difference versus PL (Res – PL); ACQ, asthma control questionnaire; AE, adverse event; AQLQ, asthma quality of life questionnaire; BE, blood eosinophil; BL, baseline; db, double-blind; CAE, clinical asthma exacerbation; CI, confidence interval; EA, eosinophilic asthma; FEF25–75%, forced expiratory flow at 25–75% of the pulmonary volume; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; ICS, inhaled corticosteroid; IV, intravenous; mc, multicenter; MI, myocardial infarction; PL, placebo; pll, parallel group; pt, patient; q4w, every 4 weeks; r, randomized; Res, reslizumab; RR, rate ratio, which represents the ratio of adjudicated CAE rates between the reslizumab and placebo groups; RTA, road traffic accident; SABA, short-acting b-agonist; SAE, serious adverse event; URTI, upper respiratory tract infection.
The first study (Study 3081; ClinicalTrials.gov identifier: NCT01270464) evaluated two different doses of reslizumab in patients (n = 315) aged 12–75 years with asthma inadequately controlled by at least a medium-dose ICS and a blood eosinophil count of ⩾400 cells/μl.35 Patients were randomized to receive reslizumab 0.3 or 3 mg/kg or placebo once every 4 weeks for 16 weeks. Reslizumab 0.3 and 3 mg/kg significantly improved FEV1 over 16 weeks (primary efficacy endpoint) by 0.115 l (p = 0.0237) and 0.160 l (p = 0.0018), respectively, compared with placebo. Significant improvements in short-acting β-agonist (SABA) use and ACQ score were also reported with both doses of reslizumab compared with placebo; however, significant improvements (versus placebo) in other secondary endpoints including FVC and asthma quality of life questionnaire (AQLQ) score were only achieved with the 3 mg/kg dose of reslizumab. A higher proportion of patients treated with reslizumab than those receiving placebo achieved a minimal important difference of ⩾0.5 improvement in the AQLQ total score at week 16 [59% (0.3 mg/kg) and 64% (3 mg/kg) versus 48% (placebo); p = 0.0189 for reslizumab 3 mg/kg versus placebo]. Significant reductions in blood eosinophil concentrations from baseline were observed for both the 0.3 mg/kg and 3 mg/kg reslizumab doses compared with placebo and were greatest with reslizumab 3 mg/kg (difference −323 cells/µl and −494 cells/µl, respectively; both p < 0.0001 versus placebo). The incidence and profile of AEs were similar across the three study groups. The most common AEs were asthma worsening, headache, and nasopharyngitis; most were mild to moderate in severity.
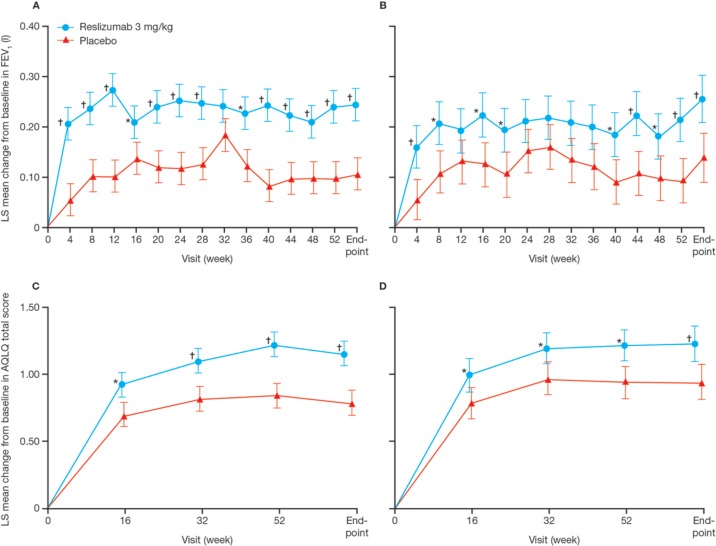
Castro and colleagues subsequently conducted two identical phase III trials using the same design [Study 3082 (ClinicalTrials.gov identifier: NCT01287039) and Study 3083 (ClinicalTrials.gov identifier: NCT01285323)] in patients aged ⩾12 years (n = 953) with inadequately controlled asthma, elevated blood eosinophils (⩾400 cells/μl) and a history of asthma exacerbation (⩾1 exacerbation during the previous year).36 Patients were randomized to receive reslizumab 3 mg/kg IV or placebo every 4 weeks for 1 year. In both studies patients treated with reslizumab experienced a significant reduction in clinical asthma exacerbations (primary endpoint) compared with patients receiving placebo [Study 3082: 50% reduction (95% CI, 0.37–0.67); Study 3083: 59% reduction (95% CI, 0.28–0.59); both p < 0.0001] and this effect was independent of which concomitant drug treatments were being received at baseline. Significant improvements in FEV1, AQLQ score, and ACQ-7 score were also observed at week 16 and week 52 in favor of reslizumab (Figure 2), together with a significant reduction in blood eosinophil count, which was apparent by the first on-treatment assessment at week 4 and was sustained for the duration of the studies. There was no significant change in SABA use between reslizumab and placebo in either study. Worsening asthma symptoms was the most common AE, followed by upper respiratory tract infection (URTI), nasopharyngitis, and headache in both the reslizumab and placebo groups. SAEs were reported more frequently in the placebo group than in the reslizumab group in both studies. Two reslizumab-treated patients experienced an anaphylactic reaction considered to be treatment related; both patients responded to standard treatment and were withdrawn from the study.
Figure 2.
Changes in FEV1 and AQLQ over 52 weeks in patients receiving reslizumab or placebo in Study 3082 (A, C) and Study 3083 (B, D).36
AQLQ, asthma quality of life questionnaire; FEV1, forced expiratory volume in 1 s; LS, least-squares; *p < 0.05. †p < 0.01 versus placebo. Reproduced with permission of Elsevier, from Castro and colleagues;36 permission conveyed through Copyright Clearance Center, Inc.
Indirect confirmation of the efficacy of reslizumab was provided by a fourth phase III study in which patients aged ⩾18 years (n = 491) with poorly controlled asthma who were unselected for eosinophil count were randomized to treatment with IV reslizumab 3 mg/kg or to placebo every 4 weeks for 16 weeks (Study 3084; ClinicalTrials.gov identifier: NCT01508936).37 Predictably, because of the lack of patient selection in terms of baseline eosinophilia, reslizumab did not achieve statistically significant improvements in FEV1 (primary endpoint), FVC, or SABA use compared with placebo in the overall population. Furthermore, linear regression analysis failed to demonstrate a significant relationship between baseline blood eosinophils and change in FEV1. In a subgroup of patients with blood eosinophils ⩾400 cells/μl at baseline (n = 96) reslizumab achieved a significantly greater improvement in FEV1 compared with placebo (0.272 versus 0.002 l, respectively; p = 0.0436), and although not statistically significant, improvements in ACQ-7 score, rescue SABA use, and FVC were considerably larger with reslizumab compared with placebo in this subgroup. However, it should be noted that the study was not designed nor statistically powered to specifically test this group of patients.
Reslizumab was well tolerated in this study and the incidence of AEs was lower in reslizumab-treated patients versus those receiving placebo (55% versus 74%, respectively). One patient in the reslizumab group had treatment-related anaphylaxis, but recovered fully after standard treatment at the study site. A total of 3% of reslizumab-treated patients tested positive for anti-drug (reslizumab) antibodies (ADAs) at screening (before drug exposure) and 5% of reslizumab-treated patients were classified as ADA-positive during treatment. However, the majority of patients had low and transient ADA titers and, consistent with a lack of neutralizing ability, ADA positivity did not affect the safety profile or eosinophil-depleting ability of reslizumab.
Eligible patients who had participated in one of the phase III reslizumab studies (specifically Studies 3081/82/83) were given the option to enter a long-term, open-label extension study in which they received reslizumab 3 mg/kg IV once every 4 weeks for up to 2 years (Study 3085; ClinicalTrials.gov identifier: NCT01290887). Endpoints included evaluation of long-term safety and efficacy (including FEV1, percentage predicted FEV1, and FVC). The study was terminated when enrollment had reached 1052 patients (740 planned); preliminary data were reported at a recent meeting of the American Thoracic Society38 and a full publication is imminent. Of the 1052 patients who were enrolled in the study, 480 were reslizumab naïve (i.e. had received placebo in a prior reslizumab study); 1051 patients received at least one dose of reslizumab during the extension study. Median (range) duration of treatment was 337 (1.0–833.0) days for prior reslizumab-experienced patients and 312 (1.0–858.0) days for prior reslizumab-naïve patients; overall 237 patients received ⩾24 months of reslizumab treatment. Long-term (up to 24 months) safety data indicated that reslizumab was generally well tolerated by patients with asthma. The most common AEs (occurring in >5% of patients) were nasopharyngitis, URTI, sinusitis, bronchitis, and headache. SAEs were reported in 7% of prior reslizumab-naïve and 7% of prior reslizumab-experienced patients; study withdrawal due to an AE occurred in 1% and 2% of patients, respectively. Headache was the only AE assessed by investigators to be treatment related that occurred in >1% of patients (incidence 2%). Three deaths were reported during the study but all were considered unrelated to study treatment. Baseline lung function, measured by spirometry, was more favorable in patients who had previously been randomized to reslizumab and this was maintained throughout the extension study. FEV1 was also improved by week 4 in prior reslizumab-naïve patients and improvements were maintained throughout the extension study period.
Regulatory
Based on efficacy and safety data from phase II/III clinical trials, the US Food and Drug Administration granted approval of reslizumab IV (CINQAIR®) in March 2016 as add-on maintenance treatment for patients aged ⩾18 years with severe asthma and with an eosinophilic phenotype. Approval, with similar indications, was also granted a few months later in Canada (July 2016) and Europe (August 2016). The recommended dosing regimen for reslizumab is 3 mg/kg administered once every 4 weeks as an IV infusion over 25–50 min.29
Ongoing studies
Two ongoing phase III studies are evaluating SC administration of reslizumab in patients with asthma and elevated blood eosinophil levels. One study (ClinicalTrials.gov identifier: NCT02452190) is similar in design to the IV reslizumab studies (3082 and 3083) and is comparing the effect of treatment every 4 weeks with reslizumab 110 mg SC versus placebo for 52 weeks on the frequency of asthma exacerbations in patients aged ⩾12 years with uncontrolled asthma and elevated blood eosinophils. The second study (ClinicalTrials.gov identifier: NCT02501629) is assessing the percentage reduction in daily OCS dose with SC reslizumab 110 mg every 4 weeks versus placebo for 24 weeks in patients aged ⩾12 years with OCS-dependent asthma and elevated blood eosinophils.
A third study (phase II/III; NCT02559791) is evaluating monthly reslizumab 3 mg/kg IV for 16 weeks in patients aged ⩾18 years with prednisone-dependent eosinophilic asthma previously treated with an SC administered IL-5 antagonist (Table 3).
Table 3.
Summary of ongoing reslizumab studies in patients with eosinophilic asthma.
| ClinicalTrials.gov identifier and study status | Design | Patients | No. pts* | Study treatment | Study endpoints |
|---|---|---|---|---|---|
|
NCT02452190 (ongoing) |
Ph III, r, db, pll | Aged ⩾12 years, uncontrolled asthma, elevated BE | 400 | Res 110 mg SC q4w or PL q4w × 52 weeks | Primary: frequency of asthma exacerbations Secondary†: FEV1, AQLQ, ACQ, AEs |
|
NCT02501629 (ongoing) |
Ph III, r, db, pll | Aged ⩾12 years, OCS-dependent, elevated BE | 152 | Res 110 mg SC q4w or PL q4w × 24 weeks | Primary: percentage reduction in daily OCS dose
versus BL Secondary†: Pts with ⩾50% reduction in OCS dose, pts with OCS dose reduction to ⩽5 mg/day, annualized rate of CAE, AQLQ |
|
NCT02559791 (final data collection October 2016) |
Ph II/III, sb (pt) | Aged 18–75 years, prednisone-dependent EA, MP pretreated (100 mg SC, ⩾6 mo) | 15 | PL q4w × 8 weeks then Res 3 mg/kg IV q4w × 16 weeks | Primary: blood and sputum eosinophils Secondary†: ILC2, CD4+, CD8+, CD34+, FEV1, ACQ |
Estimated. †Not exhaustive.
ACQ, asthma control questionnaire; AE, adverse event; AQLQ, asthma quality of life questionnaire; BE, blood eosinophils; BL, baseline; CAE, clinical asthma exacerbations; db, double-blind; EA, eosinophilic asthma; FEV1, forced expiratory volume in 1 s; ILC2, type 2 innate lymphoid cells; IV, intravenous; MP, mepolizumab; OCS, oral corticosteroid; Ph, phase; PL, placebo; pll, parallel; pt, patient; q4w, every 4 weeks; r, randomized; Res, reslizumab; sb single-blind; SC, subcutaneous.
Predicting response to reslizumab
Among patients with eosinophilic asthma there are some specific subgroups in whom reslizumab has been shown to be particularly effective and for whom reslizumab may be considered as the preferred add-on option, based on response rates in clinical trials.
Nasal polyposis is a hallmark of eosinophilic disease in patients with asthma, and available evidence suggests that the presence of nasal polyps may aid identification of a subset of patients with uncontrolled, eosinophilic asthma who are highly likely to benefit from anti-IL-5 therapy. IL-5 is the predominant cytokine in nasal polyposis associated with tissue eosinophilia, promoting the activation and prolonged survival of eosinophils39,40 IL-5 is increased in nasal polyp tissue compared with nasal tissue in healthy controls, and correlates with the degree of tissue eosinophilia, strongly suggesting a rationale for anti-IL-5 therapy in this condition.41–43 In a small pilot study (n = 24), administration of a single IV dose of reslizumab (1 mg/kg) achieved a significant reduction in polyp size in patients with large bilateral nasal polyps or recurrent nasal polyps after surgery. Furthermore, post-hoc analysis indicated that elevated levels of IL-5 (>40 pg/ml) in nasal secretions were predictive of response to reslizumab.44 In addition to the improvement in ACQ score associated with reslizumab treatment in patients with asthma and nasal polyps reported by Castro and colleagues,34 preliminary data have been presented by Weinstein and colleagues from a post-hoc analysis to evaluate the effect of reslizumab on clinical asthma exacerbations in patients with chronic sinusitis (CS) with or without nasal polyps enrolled in two of the phase III reslizumab BREATH studies (Studies 3082 and 3083).45 Of 953 patients randomized to the two trials [all of whom were preselected for baseline eosinophilia (blood eosinophils ⩾400 cells/µl)], 16% (150/953) had CS with nasal polyps and 26% (252/953) had CS without nasal polyps. Reslizumab therapy was associated with a reduction of 83% in the annual rate of clinical asthma exacerbations versus placebo in patients with nasal polyps [RR 0.17 (95% CI, 0.10, 0.32); p = 0.0002] and a reduction of 70% in patients with CS without nasal polyps [RR 0.30 (95% CI, 0.20, 0.44); p = 0.0103]. In addition, both groups of patients experienced substantial improvements in FEV1 over 52 weeks.
Asthma severity as defined by the American Thoracic Society/European Respiratory Society (ATS/ERS)46 or Global Initiative for Asthma (GINA) guidelines47 may also be a predictor of response to reslizumab. Preliminary data from post-hoc subgroup analyses of data from patients enrolled in the phase III studies (Studies 3082 and 3083) have reported a highly beneficial effect of reslizumab in terms of improvement compared with placebo in asthma exacerbation rate, lung function, asthma symptoms, and patient-reported asthma control and quality of life in subgroups of patients with an ATS/ERS definition of inadequately controlled asthma48 or with GINA Step 4 and Step 5 categories of asthma severity.49
Older age may also be a predictor of good response. In another analysis of the pooled data, Bernstein and colleagues evaluated the efficacy of reslizumab in older (age ⩾65 years, n = 77) versus younger adults (age 18–64 years, n = 851). Reductions in the frequency of asthma exacerbations per patient during the treatment period were numerically larger with reslizumab versus placebo in older compared with younger adults [older: 67% reduction (RR 0.33, 95% CI, 0.15, 0.71); younger: 53% reduction (RR 0.47, 95% CI, 0.36, 0.60)], with similar findings also reported for other endpoints, including FEV1.50 There are few reports concerning the efficacy of asthma drugs in older patients; these data provide reassurance about the efficacy of reslizumab across the age spectrum of adult asthma.
Preliminary data presented recently from a further post-hoc analysis of the phase III trials (Studies 3082 and 3083) also provide potential opportunities for the development of strategies to enable early prediction of the long-term beneficial effect of reslizumab therapy. This analysis investigated the rate of asthma exacerbations in patients who demonstrated an early FEV1 response (defined as ⩾100 ml) or early ACQ response up to week 16 (n = 953). In this population, patients who demonstrated a response to reslizumab before 16 weeks (FEV1 and/or ACQ) had a greater improvement in clinical asthma exacerbation rate relative to placebo after 52 weeks than patients who did not respond according to either criterion by week 16 (59–76% versus 26–31% reduction relative to placebo).51 An algorithm using changes in clinical variables from baseline to week 16 of reslizumab treatment has been developed recently to predict response at week 52 and guide the continuation of therapy for patients with inadequately controlled eosinophilic asthma.52
Future directions
Currently approved therapies directed against the type 2 immune response include the anti-IgE antibody omalizumab, and the anti-IL-5 antibodies mepolizumab and reslizumab. In addition, many other compounds are still under development, including antibodies directed against IL-4Ra, IL-5R, IL-13, IL-33, and anti-thymic stromal lymphopoietin, and type 2 prostaglandin D2 receptor inhibitors. The introduction to the clinic of anti-IL-5 treatments represents a new direction in asthma treatment, with reslizumab and other similar therapies demonstrating good efficacy and safety profiles in appropriately selected patient populations.
Strategies to enable identification of patients most responsive to IL-5 pathway inhibitors, and thereby ensuring assignment of treatment to the correct patients, are highly dependent on the validation of existing biomarkers, and also the development of new biomarkers for the IL-5 pathway. Such biomarkers may ultimately replace the existing gold standard of increased sputum eosinophil count, a test which is currently only performed at highly specialized centers and is not available in general practice. A high blood eosinophil count (i.e. ⩾400 cells/µl35–37 or >450 cells/µl53) appears to be a specific marker for eosinophilic airway inflammation and is a very specific marker to identify patients more likely to respond to reslizumab.
Many healthcare systems will likely challenge the concept of correct patient selection before agreeing to pay for IL-5 pathway inhibitor treatment. Therefore, the ability to predict a positive response to treatment based on a combination of blood eosinophil count and the presence of a clinical biomarker, such as CS (which may imply an exacerbation reduction of between 70% and 83%),45 represents a valuable tool for the clinician when selecting the best candidates for reslizumab treatment. Attainment of an early FEV1 and ACQ response may further aid the prediction of efficacy and exacerbation-rate reduction.51
An important question that is still unanswered at present is the clinical relevance of differences between anti-IL-5 treatments, such as mepolizumab and reslizumab, and the anti-IL-5R-alpha monoclonal antibody benralizumab. In a recent meta-analysis, the authors concluded that all anti-IL-5 treatments achieved significant clinical benefits in patients with severe asthma characterized by frequent exacerbations and evidence of eosinophilic inflammation.54 Reslizumab appeared to be the most effective monoclonal antibody in terms of exacerbation-rate reduction and improvement in FEV1, although mepolizumab 100 mg and benralizumab 20 mg also appeared to be excellent alternatives. Similar findings were also reported in a network meta-analysis conducted by Wang and colleagues.55 The authors reported significant improvements in FEV1, quality of life, blood sputum eosinophils, and asthma exacerbations with reslizumab, mepolizumab, and benralizumab versus placebo, with reslizumab generally demonstrating the greatest efficacy.55 However, the limited number of studies available for inclusion in both meta-analyses precluded the identification of any clear significant differences between treatments in terms of efficacy and safety.
Controversy also exists as to whether a patient with severe eosinophilic asthma and atopy should be treated with anti-IL-5 therapy or with an anti-IgE monoclonal antibody. Although to date there have been no head-to-head comparisons of these therapies in this setting, there is evidence to support the use of reslizumab or mepolizumab regardless of the presence or absence of atopy.56,57 Furthermore, there is a growing body of data to suggest that such anti-IL-5 treatments may also be effective in patients who have not responded to prior omalizumab therapy.58
Additional questions surrounding the use of anti-IL-5 treatments include time to relapse, optimal duration of treatment, effects on airway remodeling, and clinical profile in children and teenagers with asthma. Hopefully continued investigations over the next few years will provide answers to at least some of these questions.
Acknowledgments
Editorial assistance was provided by Julie Adkins of Anthemis Consulting Ltd., funded by Teva Pharmaceuticals USA, Frazer, PA. Teva provided a single medical-accuracy review of the final draft. The author was not compensated and retained full editorial control over the content of the paper.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: Dr. Máspero has been an investigator for three Teva-sponsored reslizumab clinical trials (Studies 3081, 3082, and 3085) and has participated as a speaker at Teva-sponsored symposia. He has also participated as an investigator in clinical trials of mepolizumab, benralizumab, and dupilumab and has been part of national/international advisory boards and steering committees for Teva, Sanofi, Astra Zeneca, and Novartis.
References
- 1. Lötvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol 2011; 127: 355–360. [DOI] [PubMed] [Google Scholar]
- 2. Agache I, Akdis C, Jutel M, et al. Untangling asthma phenotypes and endotypes. Allergy 2012; 67: 835–846. [DOI] [PubMed] [Google Scholar]
- 3. Desai M, Oppenheimer J. Elucidating asthma phenotypes and endotypes: progress towards personalized medicine. Ann Allergy Asthma Immunol 2016; 116: 394–401. [DOI] [PubMed] [Google Scholar]
- 4. Bergeron C, Tulic MK, Hamid Q. Airway remodelling in asthma: from benchside to clinical practice. Can Respir J 2010; 17: e85–e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schuijs MJ, Willart MA, Hammad H, et al. Cytokine targets in airway inflammation. Curr Opin Pharmacol 2013; 13: 351–361. [DOI] [PubMed] [Google Scholar]
- 6. de Groot JC, Ten Brinke A, Bel EH. Management of the patient with eosinophilic asthma: a new era begins. ERJ Open Res 2015; 1: 00024-2015. Erratum in: ERJ Open Res 2015; 1: 00024-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ray A, Oriss TB, Wenzel SE. Emerging molecular phenotypes of asthma. Am J Physiol Lung Cell Mol Physiol 2015; 308: L130–L140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aleman F, Lim HF, Nair P. Eosinophilic endotype of asthma. Immunol Allergy Clin North Am 2016; 36: 559–568. [DOI] [PubMed] [Google Scholar]
- 9. Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med 1990; 323: 1033–1039. [DOI] [PubMed] [Google Scholar]
- 10. Restrepo RD, Peters J. Near-fatal asthma: recognition and management. Curr Opin Pulm Med 2008; 14: 13–23. [DOI] [PubMed] [Google Scholar]
- 11. Malinovschi A, Fonseca JA, Jacinto T, et al. Exhaled nitric oxide levels and blood eosinophil counts independently associate with wheeze and asthma events in National Health and Nutrition Examination Survey subjects. J Allergy Clin Immunol 2013; 132: 821–827. [DOI] [PubMed] [Google Scholar]
- 12. Price D, Wilson AM, Chisholm A, et al. Predicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practice. J Asthma Allergy 2016; 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jatakanon A, Lim S, Barnes PJ. Changes in sputum eosinophils predict loss of asthma control. Am J Respir Crit Care Med 2000; 161: 64–72. [DOI] [PubMed] [Google Scholar]
- 14. Deykin A, Lazarus SC, Fahy JV, et al. ; Asthma Clinical Research Network, National Heart, Lung, and Blood Institute/NIH. Sputum eosinophil counts predict asthma control after discontinuation of inhaled corticosteroids. J Allergy Clin Immunol 2005; 115: 720–727. [DOI] [PubMed] [Google Scholar]
- 15. Fulkerson PC, Rothenberg ME. Targeting eosinophils in allergy, inflammation and beyond. Nat Rev Drug Discov 2013; 12: 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol 2013; 13: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mukherjee M, Sehmi R, Nair P. Anti-IL5 therapy for asthma and beyond. World Allergy Organ J 2014; 7: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ying S, Humbert M, Barkans J, et al. Expression of IL-4 and IL-5 mRNA and protein product by CD4+ and CD8+ T cells, eosinophils, and mast cells in bronchial biopsies obtained from atopic and nonatopic (intrinsic) asthmatics. J Immunol 1997; 158: 3539–3544. [PubMed] [Google Scholar]
- 19. Walker C, Checkel J, Cammisuli S, et al. IL-5 production by NK cells contributes to eosinophil infiltration in a mouse model of allergic inflammation. J Immunol 1998; 161: 1962–1969. [PubMed] [Google Scholar]
- 20. Hogan MB, Piktel D, Landreth KS. IL-5 production by bone marrow stromal cells: implications for eosinophilia associated with asthma. J Allergy Clin Immunol 2000; 106: 329–336. [DOI] [PubMed] [Google Scholar]
- 21. Nussbaum JC, Van Dyken SJ, von Moltke J, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013; 502: 245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walford HH, Doherty TA. Diagnosis and management of eosinophilic asthma: a US perspective. J Asthma Allergy 2014; 7: 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamid Q, Azzawi M, Ying S, et al. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest 1991; 87: 1541–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Humbert M, Corrigan CJ, Kimmitt P, et al. Relationship between IL-4 and IL-5 mRNA expression and disease severity in atopic asthma. Am J Respir Crit Care Med 1997; 156: 704–708. [DOI] [PubMed] [Google Scholar]
- 25. Shi HZ, Xiao CQ, Zhong D, et al. Effect of inhaled interleukin-5 on airway hyperreactivity and eosinophilia in asthmatics. Am J Respir Crit Care Med 1998; 157: 204–209. [DOI] [PubMed] [Google Scholar]
- 26. Smith SG, Chen R, Kjarsgaard M, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol 2016; 137: 75–86. [DOI] [PubMed] [Google Scholar]
- 27. Molfino NA, Gossage D, Kolbeck R, et al. Molecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptor. Clin Exp Allergy 2012; 42: 712–737. [DOI] [PubMed] [Google Scholar]
- 28. Zhang J, Kuvelkar R, Murgolo NJ, et al. Mapping and characterization of the epitope(s) of Sch 55700, a humanized mAb, that inhibits human IL-5. Int Immunol 1999; 11: 1935–1944. [DOI] [PubMed] [Google Scholar]
- 29. Teva Respiratory LLC. CINQAIR® (reslizumab) injection, for intravenous use. Highlights of prescribing information, http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761033lbl.pdf (2016, accessed 17 January 2017).
- 30. Egan RW, Athwahl D, Chou CC, et al. Inhibition of pulmonary eosinophilia and hyperreactivity by antibodies to interleukin-5. Int Arch Allergy Immunol 1995; 107: 321–322. [DOI] [PubMed] [Google Scholar]
- 31. Egan RW, Athwahl D, Chou CC, et al. Pulmonary biology of anti-interleukin 5 antibodies. Mem Inst Oswaldo Cruz 1997; 92(Suppl. 2): 69–73. [DOI] [PubMed] [Google Scholar]
- 32. Egan RW, Athwal D, Bodmer MW, et al. Effect of Sch 55700, a humanized monoclonal antibody to human interleukin-5, on eosinophilic responses and bronchial hyperreactivity. Arzneimittelforschung 1999; 49: 779–790. [DOI] [PubMed] [Google Scholar]
- 33. Kips JC, O’Connor BJ, Langley SJ, et al. Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. Am J Respir Crit Care Med 2003; 167: 1655–1659. [DOI] [PubMed] [Google Scholar]
- 34. Castro M, Mathur S, Hargreave F, et al. ; Res-5-0010 Study Group. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med 2011; 184: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 35. Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest 2016; 150: 789–798. [DOI] [PubMed] [Google Scholar]
- 36. Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 2015; 3: 355–366. [DOI] [PubMed] [Google Scholar]
- 37. Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest 2016; 150: 799–810. [DOI] [PubMed] [Google Scholar]
- 38. Murphy K, Jacobs J, Bjermer L, et al. Long-term safety and efficacy of reslizumab in patients with inadequately controlled moderate-to-severe asthma and elevated blood eosinophil counts: an open-label extension study. Am J Respir Crit Care Med 2015; 191: abstract 6455. [Google Scholar]
- 39. Bachert C, Wagenmann M, Rudack C, et al. The role of cytokines in infectious sinusitis and nasal polyposis. Allergy 1998; 53: 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bachert C, Zhang N. Chronic rhinosinusitis and asthma: novel understanding of the role of IgE ‘above atopy’. J Intern Med 2012; 272: 133–143. [DOI] [PubMed] [Google Scholar]
- 41. Bachert C, Wagenmann M, Hauser U, et al. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol 1997; 99: 837–842. [DOI] [PubMed] [Google Scholar]
- 42. Bachert C, Zhang N, Holtappels G, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol 2010; 126: 962–968. [DOI] [PubMed] [Google Scholar]
- 43. Håkansson K, Bachert C, Konge L, et al. Airway inflammation in chronic rhinosinusitis with nasal polyps and asthma: the united airways concept further supported. PLoS One 2015; 10: e0127228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gevaert P, Lang-Loidolt D, Lackner A, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol 2006; 118: 1133–1141. [DOI] [PubMed] [Google Scholar]
- 45. Weinstein SF, Germinaro M, Bardin P, et al. Efficacy of reslizumab with asthma, chronic sinusitis with nasal polyps and elevated blood eosinophils. J Allergy Clin Immunol 2016; 137(Suppl.): AB86 (abstract 283). [Google Scholar]
- 46. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. [DOI] [PubMed] [Google Scholar]
- 47. Global Initiative for Asthma. Global strategy for asthma management and prevention, www.ginasthma.org (2016, accessed 14 February 2017).
- 48. Virchow JC, Zangrilli J, Weiss S, et al. Reslizumab (RES) in patients (pts) with inadequately controlled asthma and elevated blood eosinophils (EOS): analysis of two phase 3, placebo-controlled trials. Eur Respir J 2016; 48: OA1797. [Google Scholar]
- 49. Brusselle G, McElhattan J, Canvin J, et al. Reslizumab (RES) in asthma patients (pts) with severe eosinophilic asthma stratified by GINA asthma steps 4 and 5: analysis of two phase 3, placebo (PBO)-controlled trials. Eur Respir J 2016; 48: PA4107. [Google Scholar]
- 50. Bernstein D, Mansfield L, Zangrilli J, et al. Efficacy of reslizumab in older patients (⩾65 years) with asthma and elevated blood eosinophils: results from a pooled analysis of two phase 3, placebo-controlled trials. Oral presentation at the American Academy of Allergy, Asthma and Immunology, 4–7 March 2016, Los Angeles, CA. [Google Scholar]
- 51. Bateman ED, Zangrilli J, Germinaro M, et al. Association between early improvements in lung function and asthma control with reslizumab and the annual rate of asthma exacerbations. Poster presented at the American Thoracic Society (ATS) International Congress, 13–18 May 2016, San Francisco, CA. [Google Scholar]
- 52. Canvin J, Noble R, Djukanovic R, et al. Early identification of responders to reslizumab at 16 weeks using an algorithm derived from the pivotal clinical studies of severe eosinophilic asthma (SEA) patients. Eur Respir J 2016; 48: OA2998. [DOI] [PubMed] [Google Scholar]
- 53. Fowler SJ, Tavernier G, Niven R. High blood eosinophil counts predict sputum eosinophilia in patients with severe asthma. J Allergy Clin Immunol 2015; 135: 822–824. [DOI] [PubMed] [Google Scholar]
- 54. Cabon Y, Molinari N, Marin G, et al. Comparison of anti-interleukin-5 therapies in patients with severe asthma: global and indirect meta-analyses of randomized placebo-controlled trials. Clin Exp Allergy 2017; 47: 129–138. [DOI] [PubMed] [Google Scholar]
- 55. Wang FP, Liu T, Lan Z, et al. Efficacy and safety of anti-interleukin-5 therapy in patients with asthma: a systematic review and meta-analysis. PLoS One 2016; 11: e0166833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ortega H, Chupp G, Bardin P, et al. The role of mepolizumab in atopic and nonatopic severe asthma with persistent eosinophilia. Eur Respir J 2014; 44: 239–241. [DOI] [PubMed] [Google Scholar]
- 57. Katial R, Hoyte F, Germinaro M, et al. Efficacy of reslizumab in asthma patients with aspirin sensitivity and elevated blood eosinophils. J Allergy Clin Immunol 2017; 139(Suppl.): abstract 26. [Google Scholar]
- 58. Magnan A, Bourdin A, Prazma CM, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy 2016; 71: 1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]