Abstract
Background:
We investigated the effects of neoadjuvant chemotherapy administered via bronchial arterial infusion (BAI) on unresectable stage III lung squamous cell carcinoma (SCC).
Methods:
This was a single-arm retrospective study of chemotherapy with gemcitabine plus cisplatin (GP) administered via BAI to patients with unresectable lung SCC. Data regarding the post-treatment response rate, downstage rate, and surgery rate, as well as progression-free survival (PFS), overall survival (OS), quality of life, and post-BAI side effects were collected.
Results:
A total of 36 patients were enrolled in this study between August 2010 and May 2014. The response rate was 72.2%, and the downstage rate was 22.2%. Among the patients who were downstaged, 16 (44.4%) patients were because of their T stage, and 5 (13.9%) patients were downstaged due to to their N stage. The surgery rate was 52.8%, the 1-year survival rate was 75.4%, and the 2-year survival rate was 52.1%. The median PFS was 14.0 months [95% confidence interval (CI): 8.6–19.4], and the median OS was 25.0 months (95% CI: 19.1–30.9). The quality of life was significantly improved, and the chemotherapy was well tolerated.
Conclusions:
Compared with intravenous neoadjuvant chemotherapy, BAI chemotherapy significantly improved the surgery rate, prolonged PFS and OS, and improved the quality of life in patients with unresectable stage III lung SCC.
Keywords: bronchial arterial infusion, chemotherapy, neoadjuvant, squamous cell carcinoma, unresectable
Introduction
Lung cancer represents the leading cause of cancer-related mortality worldwide, accounting for 1.2 million deaths each year. Locally advanced or stage III non-small cell lung cancer (NSCLC) is the most advanced stage at which cure may be achieved. Whether the disease is treated by surgery or chemoradiotherapy is determined based on tumor pathology and the presence of tumor extension and lymph node invasion. For patients with resectable NSCLC, randomized trials have shown that treating the disease with the combination of adjuvant or neoadjuvant chemotherapy and surgery improves survival compared with surgery alone.1
Neoadjuvant chemotherapy can theoretically reduce tumor volumes and control micrometastatic diffusion, which may eventually improve tumor resectability.
Lung cancers are supplied primarily by the bronchial artery,2 as well as by the subclavian artery. Compared with conventional intravenous chemotherapy, bronchial arterial infusion (BAI) chemotherapy increases the local concentrations of chemotherapeutic agents by delivering these drugs directly into lung cancer tissues via the bronchial artery, making maximum reductions in tumor size possible and thus increasing the treatment options available for patients with NSCLC.
In this study, we administered neoadjuvant BAI chemotherapy to stage III lung squamous cell carcinoma (SCC) patients whose tumors were deemed unresectable by their surgeons before treatment and investigated whether these patients’ tumors were resectable after BAI. Treatment side effects, survival, and the patients’ quality of life were also assessed.
Materials and methods
Patient population
A total of 39 patients with unresectable stage III SCC who may have been able to undergo radical resection after two cycles of BAI chemotherapy and who were treated in Shanghai Pulmonary Hospital, Tongji University School of Medicine between August 2010 and May 2014 were enrolled in this study. This study was approved by the Ethics Committee of Shanghai Pulmonary Hospital, Tongji University School of Medicine (ethics approval no. K16-309). All the patients had signed informed consent (informed consent of study FK1210). All the patients underwent thoracic computed tomography angiography (CTA) prior to beginning chemotherapy. The patients also underwent additional routine pretreatment evaluations such as abdominal ultrasonography or computed tomography (CT), brain magnetic resonance imaging (MRI) or head CT, cardiopulmonary function testing and whole-body radionuclide bone scans. The patients were staged in accordance with the seventh edition of the TNM classification system.
The following patients were included in the study: patients who were treatment naïve; patients with an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score 0–2; patients with an ability to tolerate BAI chemotherapy; patients with pathologically or cytologically confirmed stage IIIa or IIIb SCC; patients deemed to have unresectable disease at the time of diagnosis because their tumor exhibited bulky lymph nodes on the ipsilateral side of the mediastinum (N2) disease (as determined by endobronchial ultrasonography-guided transbronchial needle aspiration biopsy, n = 3; or by CT, in which the tumor was found to have a short-axis diameter >1.5 cm, n = 1) or vital organ invasion; patients with normal blood test results, as well as patients with normal liver and kidney function, normal ECG results, and normal blood gas analysis results; patients with measurable solid lung lesion(s) (as demonstrated by lung radiographic imaging) that could be used to assess treatment efficacy; and patients expected to survive more than 6 months.
A total of 39 patients were enrolled in this study (sample size justification: because this was a retrospective study, the sample size was based on the number of incoming patients that met the inclusion criteria. As it took longer to identify patients who met the eligibility requirements, 39 patients between August 2010 and May 2014 were enrolled in this study). Three of them were excluded because of pre-existing heart disease or an inability to complete chemotherapy. Thus, 36 patients were eligible for the study, whose population comprised one female and 35 males with the median age of 61 (39–78 years). Of these, 25 patients had stage IIIa disease, and 11 patients had stage IIIb disease (Table 1).
Table 1.
Clinical characteristics of the patients.
| Gender | Male | 35 (97.2%) |
| Female | 1 (2.8%) | |
| Age | ⩽70 years | 29 (80.6%) |
| >70 years | 7 (19.4%) | |
| Smoking history | Current or former smoker | 32 (88.9%) |
| Never smoked | 4 (11.1%) | |
| ECOG score | 0 | 4 (11.1%) |
| 1 | 28 (77.8%) | |
| 2 | 4 (11.1%) | |
| Disease stage | IIIa | 25 (69.4%) |
| IIIb | 11 (30.6%) | |
| T1 | 2 (5.5%) | |
| T2 | 2 (5.5%) | |
| T3 | 1 (2.8%) | |
| T4 | 31 (86.2%) | |
| N0 | 7 (19.4%) | |
| N1 | 13 (36.1%) | |
| N2 | 16 (44.5%) |
ECOG, Eastern Cooperative Oncology Group.
Treatment regimen
The chemotherapy treatment regimen, which consisted of gemcitabine plus cisplatin (GP), was administered in accordance with the following schedule: gemcitabine (1000 mg/m2 BAI on day 1 and intravenous infusion on day 8) and cisplatin (75 mg/m2 BAI on day 1 and intravenous infusion on day 2) every 3 weeks. Each patient was scheduled to receive two cycles of BAI chemotherapy, after which treatment efficacy was evaluated to determine whether the patient should undergo surgery or continue receiving chemotherapy or radiotherapy.
BAI chemotherapy was administered under local anesthesia. Femoral artery catheterization was performed using a modified Seldinger percutaneous technique. After successful selective bronchial artery intubation and bronchial arteriography, the chemotherapy drugs were slowly injected.
Follow up
Treatment-related side effects and ECOG PS scores were recorded during chemotherapy. After two cycles of chemotherapy, chest CT, brain MRI, an isotope bone scan and an abdominal ultrasound were performed, and treatment efficacy was defined based patient responsiveness and disease status according to the Response Evaluation Criteria in Solid Tumors (RECIST) scale, which is categorized as follows: complete response (CR): disappearance of all target lesions, any pathological lymph nodes (whether target or nontarget) must have reduction in short axis to <10 mm; partial response (PR): at least a 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum diameters; stable disease (SD): neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for progressive disease (PD), taking as reference the smallest sum diameters while on study; and PD: at least a 20% increase in the sum of diameters of target lesions, taking as reference the smallest sum on study (this includes the baseline sum if that is the smallest on study). In addition to the relative increase of 20%, the sum must also demonstrate an absolute increase of at least 5 mm (note: the appearance of one or more new lesions is also considered progression). Based on patient outcomes, the downstage rate, surgery rate, progression-free survival (PFS), overall survival (OS), and ECOG PS were determined, and treatment toxicity was documented.
Statistical analysis
Descriptive statistics were used for the baseline clinical characteristics. Enumeration data were described by percentage or rate. OS was defined from the day of diagnosis to the day of death from any cause, and PFS was defined from the day of diagnosis to the day of progression or death. Kaplan–Meier estimates were used to obtain the median survival time and the corresponding 95% confidence interval (CI). All statistical analyses were conducted using SPSS software for Windows (version 21.0, SPSS Inc., United States of America).
Results
Bronchial arterial infusion response rate and treatment follow up
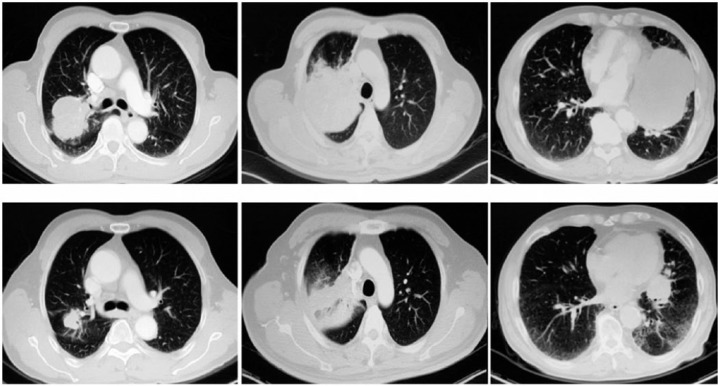
A total of 36 patients were enrolled in this study. The post-BAI chemotherapy response evaluation showed that disease control was achieved in 91.7% of patients (Figures 1 and 2), including 26 (72.2%) patients who displayed a PR to treatment and 7 (19.5%) patients who were found to have SD. Three patients (8.3%) displayed PD (with local progression). The response rate was 72.2%. BAI chemotherapy attenuated vascular invasion, shrank lymph nodes and resulted in eight patients’ (22.2%) diseases being downstaged. Sixteen (44.4%) patients were downstaged with respect to their T stage, and five (13.9%) patients were downstaged with respect to their N stage. We discussed the above cases with the appropriate surgeons, after which a total 19 patients (52.8%) underwent surgery (lobectomy: n = 18; pneumonectomy: n = 1) (Tables 2 and 3). Patients who were not suitable for surgery continued to receive intravenous chemotherapy with or without radiotherapy.
Figure 1.
Computed tomography contrast of three patients before and after bronchial arterial infusion chemotherapy.
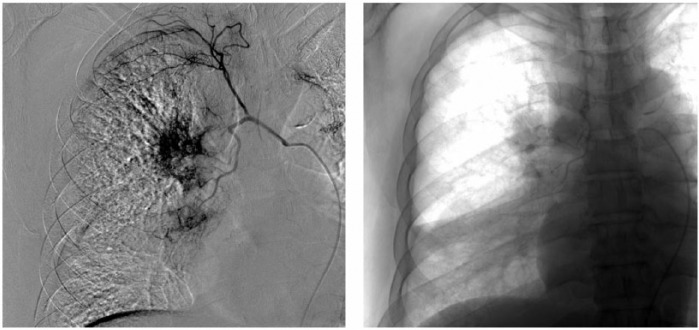
Figure 2.
Procedure of bronchial arterial infusion chemotherapy of one patient. (Right bronchial–intercostal arteriography showed tumor staining in the bronchial artery. A microcatheter was placed in the bronchial artery for perfusion.)
Table 2.
Staging of lung cancer before and after bronchial arterial infusion chemotherapy.
| Before |
After |
||
|---|---|---|---|
| Staging | n (%) | n (%) | |
| IIa | 0 (0.0%) | 2 (5.5%) | |
| IIb | 0 (0.0%) | 4 (11.1%) | |
| IIIa | 25 (69.4%) | 21 (58.4%) | |
| T1-3N2M0 | Bulky N2 | 4 (11.1%) | 1 (2.8%) |
| Skipping N2 | 1 (2.8%) | 1 (2.8%) | |
| T3N1M0 | 0 (0.0%) | 12 (33.4%) | |
| T4N0–1M0 | Vascular invasion | 17 (47.2%) | 4 (11.1%) |
| Heart invasion | 2 (5.5%) | 2 (5.5%) | |
| Vertebral invasion | 1 (2.8%) | 1 (2.8%) | |
| With different lobes of lung metastasis | 0 (0.0%) | 0 (0.0%) | |
| IIIb | 11 (30.6%) | 9 (25.0%) | |
| T4N2M0 | Vascular invasion | 9 (25.0%) | 7 (19.4%) |
| Heart invasion | 1 (2.8%) | 1 (2.8%) | |
| Vertebral invasion | 0 (0.0%) | 0 (0.0%) | |
| With different lobes of lung metastasis | 1 (2.8%) | 1 (2.8%) | |
Table 3.
Staging of lung cancer after bronchial arterial infusion chemotherapy and follow-up treatment.
| Patient | Staging before BAI | Staging after BAI | Follow-up treatment | Downstage (yes/no) |
|---|---|---|---|---|
| 1 | T1N2M0-IIIa | T1N2M0-IIIa | Lobectomy and chemotherapy | N |
| 2 | T1N2M0-IIIa | T1N1M0-IIa(N↓) | Lobectomy and chemotherapy | Y |
| 3 | T2N2M0-IIIa | T2N1M0-IIb(N↓) | Lobectomy and chemotherapy | Y |
| 4 | T2N2M0-IIIa | T2N2M0-IIIa | Chemoradiotherapy | N |
| 5 | T3N2M0-IIIa | T1N1M0-IIa(T↓)(N↓) | Lobectomy and chemotherapy | Y |
| 6 | T4N0M0-IIIa | T4N0M0-IIIa | Chemoradiotherapy | N |
| 7 | T4N0M0-IIIa | T4N0M0-IIIa | Chemoradiotherapy | N |
| 8 | T4N0M0-IIIa | T3N0M0-IIb(T↓) | Lobectomy and chemotherapy | Y |
| 9 | T4N0M0-IIIa | T4N0M0-IIIa | Chemoradiotherapy | N |
| 10 | T4N0M0-IIIa | T3N0M0-IIb(T↓) | Lobectomy and chemotherapy | Y |
| 11 | T4N0M0-IIIa | T3N0M0-IIb(T↓) | Lobectomy and chemotherapy | Y |
| 12 | T4N0M0-IIIa | T4N0M0-IIIa | Chemoradiotherapy | N |
| 13 | T4N1M0-IIIa | T3N1M0-IIIa(T↓) | Lobectomy and chemotherapy | N |
| 14 | T4N1M0-IIIa | T4N1M0-IIIa | Chemoradiotherapy | N |
| 15 | T4N1M0-IIIa | T3N1M0-IIIa(T↓) | Lobectomy and chemotherapy | N |
| 16 | T4N1M0-IIIa | T3N1M0-IIIa(T↓) | Lobectomy and chemotherapy | N |
| 17 | T4N1M0-IIIa | T3N1M0-IIIa(T↓) | Lobectomy and chemotherapy | N |
| 18 | T4N1M0-IIIa | T4N1M0-IIIa | Chemoradiotherapy | N |
| 19 | T4N1M0-IIIa | T3N1M0-IIIa(T↓) | Lobectomy and chemotherapy | N |
| 20 | T4N1M0-IIIa | T3N1M0-IIIa(T↓) | Lobectomy and chemotherapy | N |
| 21 | T4N1M0-IIIa | T3N1M0-IIIa(T↓) | Lobectomy and chemotherapy | N |
| 22 | T4N1M0-IIIa | T4N1M0-IIIa | Chemoradiotherapy | N |
| 23 | T4N1M0-IIIa | T3N1M0-IIIa(T↓) | Pneumonectomy and chemotherapy | N |
| 24 | T4N1M0-IIIa | T3N1M0-IIIa(T↓) | Lobectomy and chemotherapy | N |
| 25 | T4N1M0-IIIa | T3N1M0-IIIa(T↓) | Lobectomy and chemotherapy | N |
| 26 | T4N2M0-IIIb | T4N2M0-IIIb | Chemoradiotherapy | N |
| 27 | T4N2M0-IIIb | T4N2M0-IIIb | Chemoradiotherapy | N |
| 28 | T4N2M0-IIIb | T3N1M0-IIIa(T↓) (N↓) | Lobectomy and chemotherapy | Y |
| 29 | T4N2M0-IIIb | T4N2M0-IIIb | Chemotherapy | N |
| 30 | T4N2M0-IIIb | T4N2M0-IIIb | Chemoradiotherapy | N |
| 31 | T4N2M0-IIIb | T4N2M0-IIIb | Chemoradiotherapy | N |
| 32 | T4N2M0-IIIb | T3N1M0-IIIa(T↓) (N↓) | Lobectomy and chemotherapy | Y |
| 33 | T4N2M0-IIIb | T4N2M0-IIIb | Chemotherapy | N |
| 34 | T4N2M0-IIIb | T4N2M0-IIIb | Chemoradiotherapy | N |
| 35 | T4N2M0-IIIb | T4N2M0-IIIb | Chemotherapy | N |
| 36 | T4N2M0-IIIb | T4N2M0-IIIb | Chemoradiotherapy | N |
BAI, bronchial arterial infusion.
The date of the last follow up was 31 December 2016 (mean follow-up time 47 months), and none of the patients were lost to follow up. At the end of the follow up, 23 patients had died.
Survival time
The median PFS and OS for all patients were 14.0 months (95% CI: 8.6–19.4) and 25.0 months (95% CI: 19.1–30.9), respectively. The 1-year survival rate was 75.4%, and the 2-year survival rate was 52.1%. At the end of the follow-up period, 11 patients (30.6%) were not found to have PD, and 13 (36.1%) patients were still alive. The median OS for patients with subsequent surgery and nonsurgery were 29.1 months and 21.4 months (p = 0.032), respectively.
Changes in symptoms
After treatment, ECOG PS scores improved in 21 (58.3%) patients, did not change in 14 (38.9%) patients, and worsened in 1 (2.8%) patient. Regarding symptom improvement, 31 (86.1%) patients showed improvements in cough and sputum production, 10 (27.8%) patients showed improvements in shortness of breath, two (5.5%) patients showed improvement in upper extremity edema and eight (22.2%) patients showed improvement in bloody sputum production. In contrast, one (2.8%) patient displayed worsening symptoms. These results demonstrate that BAI chemotherapy significantly improved patient quality of life.
Safety
In general, the toxicity and side effects of BAI chemotherapy were mild (Table 4), and no grade IV toxic effects were observed. The most common side effects were grade I leukopenia (6/36, 16.7%) and anorexia (6/36, 16.7%). Moreover, one patient experienced grade III leukopenia, neutropenia, and nausea. The overall incidence of side effects was low, and BAI chemotherapy was well tolerated. Neither BAI-related bleeding episodes or deaths nor BAI-related paraplegia was noted.
Table 4.
Toxicity in all patients (n = 36).
| Toxicities | Number of patients |
|||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Leukopenia | 6 | 2 | 1 | 0 |
| Neutropenia | 4 | 2 | 1 | 0 |
| Anemia | 2 | 1 | 0 | 0 |
| Thrombocytopenia | 3 | 2 | 0 | 0 |
| Febrile | 2 | 0 | 0 | 0 |
| Nausea | 5 | 2 | 1 | 0 |
| anorexia | 6 | 1 | 0 | 0 |
| Alopecia | 1 | 0 | 0 | 0 |
| Diarrhea | 1 | 0 | 0 | 0 |
| Erythra | 2 | 2 | 0 | 0 |
| Bloody phlegm | 3 | 0 | 0 | 0 |
| Pneumonitis | 1 | 0 | 0 | 0 |
| Constipation | 5 | 2 | 0 | 0 |
| Fatigue | 5 | 2 | 0 | 0 |
| Neuropathy | 0 | 0 | 0 | 0 |
Discussion
Because metastasis of adenocarcinomas is more common,3 subsequent treatments using small molecular-targeted drugs could be implemented,4 and the drug for SCC was less and the drug for SCC was less than for adenocarcinoma; in this study, we chose SCC but did not include adenocarcinoma. Concurrent chemoradiotherapy could be used in stage III lung cancer, but in the Arrieta et al.5 study, only 21.1% of the patients underwent surgery after concurrent chemoradiotherapy. Considering which treatment caused radioactive pneumonia and mortality, we chose the BAI as a preoperative induction method, anticipating the increased probability of surgery. The regimen consisting of GP is a standard combination used for the treatment of SCC6,7 and has a longer PFS than other cytotoxic platinum regimens. Thus, we used GP as the BAI chemotherapy regimen in this study.
BAI chemotherapy significantly prolonged PFS and OS in patients with stage III lung SCC. An OS of at least 25 months is clinically significant. Pless et al.8 showed that the PFS was 11.6 months, and the OS was 26.2 months in patients with stage IIIa lung cancer (no patients with stage IIIb lung cancer were enrolled in the study) after neoadjuvant chemotherapy, results similar to those of our study.
WJTOG99039 (WJTOG 9903 is the abbreviation of a clinical trial which compared concurrent chemoradiotherapy with chemotherapy along before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer) displayed a 21% of downstage rate in cases in which induction chemotherapy was administered to NSCLC patients with N2 invasion. In our study, the downstage rate was 22.2% after two cycles of BAI chemotherapy. Specifically, 16 (44.4%) patients were downstaged with respect to their T stage and five (13.9%) patients were downstaged with respect to their N stage. Thus, 19 (52.8%) patients who were previously thought to have unresectable or marginally resectable disease underwent complete resections. The surgery rate in our study was 52.8%, a rate much higher than those of other neoadjuvant treatment studies. At the end of the 5-year follow-up period, 11 patients had SD, and 13 patients were alive. In Kocher et al.’s study,10 the surgery rate was 34% for patients with unresectable stage IIIa and IIIb lung cancer after neoadjuvant chemotherapy and radiotherapy. Notably, BAI chemotherapy resulted in more T-stage downstaging than N-stage downstaging, suggesting that T4 disease with tumor extension into vital organs or blood vessel involvement may be an indication for neoadjuvant BAI chemotherapy.
Pless et al.11 showed that radiotherapy did not enhance the benefits of induction chemotherapy followed by surgery, so we did not use BAI chemotherapy followed by radiotherapy in our study. During chemotherapy with BAI, blood vessels supplying the tumor must be precisely located,12 as blood vessel location is a prerequisite for effective BAI treatment. Nakanishi13 conducted a study involving patients with stage III–IV NSCLC who were unable to tolerate conventional chemotherapy. Those authors found the PFS was 6.5 months and the OS was 17.4 months after BAI chemotherapy. Those survival times were shorter than the PFS and OS of our study because that study enrolled patients with stage IV disease. Our study demonstrated that all the patients who displayed significantly prolonged OS were those who were referred for surgery after BAI and those who underwent radical tumor resection, indicating that surgery played a vital role in extending OS. Hence, we believe that an important consideration with respect to BAI chemotherapy is whether the therapy will improve a patient’s chances of being able to undergo surgery. We advise against administering simple BAI chemotherapy in patients with stage IV lung cancer because tumor shrinkage alone had little effect on survival in these patients. Wang et al.14 showed that BAI plus hyperfractionated radiotherapy was effective for the treatment of advanced cancer. Adjuvant chemotherapy after surgery is effective, but radiotherapy does not increase the efficacy of this treatment.15
Among patients who underwent BAI chemotherapy, their quality of life was improved and 58.3% of patients showed improvement in their ECOG PS score, a change related to significant tumor shrinkage. Symptoms induced by local tumor compression and other systemic symptoms (including symptoms induced by tumor-related superior vena cava, recurrent laryngeal nerve, trachea, and heart compression) also subsequently improved. BAI chemotherapy even showed efficacy in the treatment of metastatic lung tumors.16 However, as is the case with other types of chemotherapy, BAI chemotherapy also resulted in worsening symptoms in some patients (2.8%), although symptom worsening associated with BAI occurred less often than symptom worsening associated with conventional intravenous chemotherapy.
BAI chemotherapy was well tolerated. None of the patients in this study required suspensions or delays of their BAI chemotherapy because of treatment side effects, and no grade IV toxicity was observed. These findings are probably attributable to the fact that the local concentrations of chemotherapy drugs, that is, the concentrations of chemotherapy drugs in the tumor, were high, which resulted in reduced systemic concentrations of the drugs. Nakanishi et al.17 also showed that patients with advanced NSCLC who were unable to tolerate intravenous chemotherapy may tolerate BAI chemotherapy. Furthermore, advances in surgical techniques have reduced the incidence of surgical complications after BAI chemotherapy. No BAI-related side effects were noted in this study. All patients underwent CTA before beginning BAI to ensure that they experienced the best possible treatment outcome. Paraplegia is considered a severe side effect of BAI. However, because much attention was devoted to the position of the catheter, there were no cases of paraplegia in our study. Therefore, adequate preoperative preparation and experienced operators can improve the efficacy and safety of the BAI procedure. If other cancer centers also have much experience of chemotherapy, and interventional departments employ operators who are experienced with selective bronchial artery intubation and bronchial arteriography, then BAI can be performed reliably.
This study revealed that BAI chemotherapy significantly increased the surgery rate, prolonged PFS and OS, and improved quality of life in patients with unresectable stage III lung SCC compared with intravenous neoadjuvant chemotherapy in the same group of patients. BAI resulted in more T-stage downstaging than N-stage downstaging, suggesting that we must choose more suitable patients when considering BAI chemotherapy as a neoadjuvant treatment.
However, further research is needed to investigate whether BAI chemotherapy is as effective as neoadjuvant therapy with respect to NSCLC treatment. The role of radiotherapy in neoadjuvant therapy is still unclear. NSCLC patients can usually tolerate the toxicity of BAI combined with other treatments, such as radiotherapy.18 The effect of the combination of radiotherapy and BAI chemotherapy must also be studied further.
In addition, the sample size in our study was small, in part because BAI chemotherapy requires a specific route of administration and is more expensive than conventional intravenous chemotherapy. Studies with a larger sample size and longer follow-up period are needed for the performance of a better efficacy evaluation and to determine the survival benefits offered by BAI chemotherapy.
Footnotes
Funding: This work was supported by the Shanghai Municipal Science and Technology Commission (grant no. 134119a6500).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Jun Zhu, Department of Oncology, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai 200433, China.
Hai-ping Zhang, Department of Oncology, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai 200433, China.
Sen Jiang, Department of Radiology, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China.
Jian Ni, Department of Oncology, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai 200433, China.
References
- 1. NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014; 383: 1561–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Milne EN. Circulation of primary and metastatic pulmonary neoplasms. A postmortem microarteriographic study. Am J Roentgenol Radium Ther Nucl Med 1967; 100: 603–619. [DOI] [PubMed] [Google Scholar]
- 3. Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011; 6: 244–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004; 350: 2129–2139. [DOI] [PubMed] [Google Scholar]
- 5. Arrieta O, Gallardo-Rincón D, Villarreal-Garza C, et al. High frequency of radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with concurrent radiotherapy and gemcitabine after induction with gemcitabine and carboplatin. J Thorac Oncol 2009; 4: 845–852. [DOI] [PubMed] [Google Scholar]
- 6. Van Putte BP, Grootenboers M, van Boven WJ, et al. Selective pulmonary artery perfusion for the treatment of primary lung cancer: improved drug exposure of the lung. Lung Cancer 2009; 65: 208–213. [DOI] [PubMed] [Google Scholar]
- 7. Osaki T, Hanagiri T, Nakanishi R, et al. Bronchial arterial infusion is an effective therapeutic modality for centrally located early-stage lung cancer: results of a pilot study. Chest 1999; 115: 1424–1428. [DOI] [PubMed] [Google Scholar]
- 8. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet 2015; 386: 1049–1056. [DOI] [PubMed] [Google Scholar]
- 9. Katakami N, Tada H, Mitsudomi T, et al. A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903). Cancer 2012; 118: 6126–6135. [DOI] [PubMed] [Google Scholar]
- 10. Kocher F, Pircher A, Mohn-Staudner A, et al. Multicenter phase II study evaluating docetaxel and cisplatin as neoadjuvant induction regimen prior to surgery or radiochemotherapy with docetaxel, followed by adjuvant docetaxel therapy in chemonaive patients with NSCLC stage II, IIIA and IIIB (TAX-AT 1.203 Trial). Lung Cancer 2014; 85: 395–400. [DOI] [PubMed] [Google Scholar]
- 11. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet 2015; 386: 1049–1056. [DOI] [PubMed] [Google Scholar]
- 12. Nakanishi M, Demura Y, Umeda Y, et al. Multi-arterial infusion chemotherapy for non-small cell lung carcinoma–significance of detecting feeding arteries and tumor staining. Lung Cancer 2008; 61: 227–234. [DOI] [PubMed] [Google Scholar]
- 13. Nakanishi M, Yoshida Y, Natazuka T. Prospective study of transarterial infusion of docetaxel and cisplatin to treat non-small-cell lung cancer in patients contraindicated for standard chemotherapy. Lung Cancer 2012; 77: 353–358. [DOI] [PubMed] [Google Scholar]
- 14. Wang G, Song M, Xu H, et al. Prospective trial of combined hyperfractionated radiotherapy and bronchial arterial infusion of chemotherapy for locally advanced nonsmall cell lung cancer. Int J Radiat Oncol Biol Phys 1996; 34: 309–313. [DOI] [PubMed] [Google Scholar]
- 15. NSCLC Meta-analyses Collaborative Group, Arriagada R, Auperin A, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 2010; 375: 1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoshida T, Kamada K, Miura K, et al. Successful treatment of hepatocellular carcinoma with lung metastasis using hepatic and bronchial artery infusion chemotherapy. Intern Med 2014; 53: 2493–2497. [DOI] [PubMed] [Google Scholar]
- 17. Nakanishi M, Umeda Y, Demura Y, et al. Effective use of multi-arterial infusion chemotherapy for advanced non-small cell lung cancer patients: four clinical specified cases. Lung Cancer 2007; 55: 241–247. [DOI] [PubMed] [Google Scholar]
- 18. Rieber A, Brambs HJ, Kauffmann G, et al. Combined intra-arterial chemotherapy and radiotherapy in inoperable non-small cell bronchial carcinoma. Strahlenther Onkol 1991; 167: 14–18. [PubMed] [Google Scholar]