Abstract
Objectives:
Asynchrony between patient and ventilator breaths is associated with increased duration of mechanical ventilation (MV). Neurally Adjusted Ventilatory Assist (NAVA) controls MV through an esophageal reading of diaphragm electrical activity via a nasogastric tube mounted with electrode rings. NAVA has been shown to decrease asynchrony in comparison to pressure support ventilation (PSV). The objective of this study was to conduct a health economic evaluation of NAVA compared with PSV.
Methods:
We developed a model based on an indirect link between improved synchrony with NAVA versus PSV and fewer days spent on MV in synchronous patients. Unit costs for MV were obtained from the Swedish intensive care unit register, and used in the model along with NAVA-specific costs. The importance of each parameter (proportion of asynchronous patients, costs, and average MV duration) for the overall results was evaluated through sensitivity analyses.
Results:
Base case results showed that 21% of patients ventilated with NAVA were asynchronous versus 52% of patients receiving PSV. This equals an absolute difference of 31% and an average of 1.7 days less on MV and a total cost saving of US$7886 (including NAVA catheter costs). A breakeven analysis suggested that NAVA was cost effective compared with PSV given an absolute difference in the proportion of asynchronous patients greater than 2.5% (49.5% versus 52% asynchronous patients with NAVA and PSV, respectively). The base case results were stable to changes in parameters, such as difference in asynchrony, duration of ventilation and daily intensive care unit costs.
Conclusion:
This study showed economically favorable results for NAVA versus PSV. Our results show that only a minor decrease in the proportion of asynchronous patients with NAVA is needed for investments to pay off and generate savings. Future studies need to confirm this result by directly relating improved synchrony to the number of days on MV.
Keywords: asynchrony, economic model, intensive care, mechanical ventilation, Neurally Adjusted Ventilatory Assist, pressure support ventilation
Objectives
The 1952 polio epidemic triggered the construction of a new reliable ventilator for positive pressure ventilation [Bang, 1953]. The Engström ventilator from 1950, the ventilator predecessor to Servo 900, Servo 300 and Servo-i was introduced for this purpose [Engstrom, 1954]. Introducing this treatment into standard clinical practice required the development of special intensive care units (ICUs) which in the 1960s increased in numbers and contributed to impressive reductions in mortality. When ventilatory care became more widespread questions arose on how the patients should be weaned from the ventilatory support.
With the introduction of spontaneous breaths added to the mandatory breaths and these breaths being either assisted or unassisted, the delicate patient–ventilator interaction was introduced as an important issue. The ventilator can be classified as a pressure, volume or flow controller and the individual breath is shaped by phase variables that determine how the breath is triggered (started), limited (sustained) and cycled (stopped) and is either mandatory or spontaneous. Information from all these entities is needed to describe all the different modes of ventilation which currently are available from different manufacturers [Sassoon et al. 1989].
The perfect trigger should be in synchrony with the electrical impulses from the brain to the breathing muscles to increase synchrony between ventilator and patient. Today all ventilators can trigger from the pressure and or flow signal measured within the patient circuit. A number of factors affect the time delay during pressure triggering as described by Sassoon [Sassoon et al. 1989]. The inspiratory phase ends when a set time, pressure, flow or set delivered volume is reached.
Asynchrony between patient and machine breaths is very common, especially in patients with high intrinsic respiratory rates, low drive or muscle weakness, or in patients with airway obstruction [Parthasarathy et al. 1998; Sassoon and Foster, 2001; Tobin et al. 2001]. Asynchrony is caused by either missed breaths due to ineffective efforts (IEEs) or triggering delays due to set sensitivity and ventilator response time. The neural inspiratory time may be shorter or longer than the inflation time of the ventilator. If set volume is reached before the end of neural inspiratory time the patient continues to make an inspiratory effort which can lead to double triggering. Autotriggering is a problem which can be due to water and debris accumulating on the flow-measuring device. All flow triggering also has the potential problem that leakage is recognized as a patient effort which is especially common during neonatal and pediatric ventilation with uncuffed tubes. Delayed off cycling can be due to reduced expiratory flow (e.g. slow time constant as in chronic obstructive pulmonary disease) when mechanical inflation persists into the neural expiration. In summary, there are four different types of asynchrony:
missed breaths: due to IEEs or triggering delays which are caused by settings in the ventilator or strength in the activity of the patient to trigger a breath;
double triggering: if set volume or time is reached before end of neural inspiratory time the patients still inhales, which leads to a new cycle staggered on the first;
autotriggering: is a problem due to water and debris or leakage which is mistakenly recognized as a patient effort;
delayed off cycling: can be due to reduced expiratory flow which prolongs the mechanical inflation into the neural expirations phase.
Asynchrony has been studied and shown to be associated with increased length of stay on the ventilator if the percentage of asynchrony is high [asynchrony index (AI) > 10%] and could prolong the stay in the ICU by 4–8 days and hospital length of stay (HLOS) by 8–21 days [Chao et al. 1997; de Wit et al. 2009; Thille et al. 2006]. The capability of different systems to evaluate asynchrony has been described in two recent articles. Colombo and colleagues reported that the ability to properly recognize patient–ventilator asynchronies by visual inspection of flow and pressure tracings was low; ICU staff physicians were able to identify just one-third of asynchronies and residents 16%. In a computerized system (Better Care, Barcelona, Spain) Blanch and colleagues showed sensitivity values of 65% to identify IEEs [Blanch et al. 2012; Colombo et al. 2011].
In 1999 a new ventilatory mode Neurally Adjusted Ventilatory Assist (NAVA, Solna, Sweden) was presented by Sinderby [Sinderby et al. 1999]. This mode uses the neural excitation of the diaphragm to estimate the respiratory center output. During NAVA an electromyogram of the diaphragm is sensed by electrodes mounted on a regular nasogastric tube. The esophageal/gastric reading of the diaphragm electrical activity (Edi) is shown on the ventilator screen as a curve and is used to control a mechanical ventilator. The signal from the brain is used to trigger and off cycle the ventilator breaths and provide pressure support proportional to the Edi signal and is totally independent of leaks. The assist (NAVA level) is set by the user and support is proportional to the Edi signal with the goal to have a balanced offloading of the patient effort. The goal is to offload and sedate but still have the Edi signal active and avoid disuse atrophy in the diaphragm. The steps to transform central respiratory drive into an inspiration are explained in an adapted figure from the original article (see Figure 1).
Figure 1.
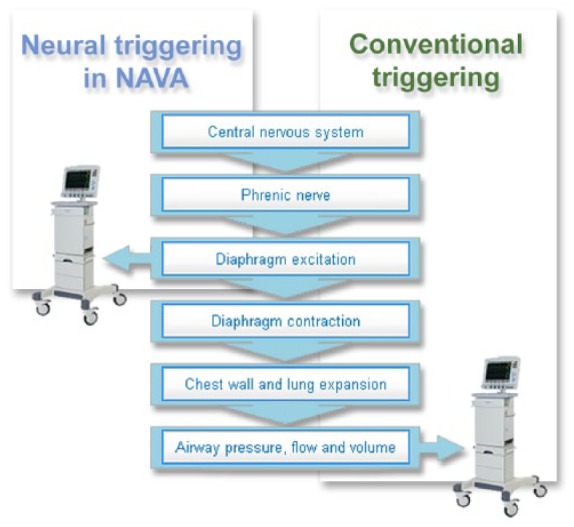
Principle of Neurally Adjusted Ventilator Assist (NAVA) in relation to ventilation physiology. Steps necessary to transform central respiratory drive into an inspiration with NAVA (neuroventilatory coupling, left), and with conventional pressure/flow triggering (pneumatic coupling, right).
This new mode, NAVA, was implemented into the Servo-i ventilator (Maquet Critical Care AB, Solna, Sweden) in 2007 and in 2010 as noninvasive NAVA (NIV NAVA). In these modes the triggering will be according to Edi or to the set pneumatic trigger (pressure or flow) on a first come first serve basis. The off cycling is when the Edi has peaked and decreased to 70% of the peak value.
NAVA versus other means of mechanical ventilation (MV) has been researched in several comparative studies, in which a clear emphasis has been put on physiological outcomes, such as patient–ventilator asynchrony, tidal volumes, peak airway pressure and respiratory rates. NAVA has primarily been compared with pressure support ventilation (PSV), which is the most commonly used support mode of ventilation. Based on the physiological outcomes measured, the most common study design has been within patient crossover, when outcomes have been measured for patients, during the course of sequential switching between different ventilation modes (e.g. PSV followed by NAVA, followed by PSV).
In adults, NAVA has been shown to give similarly low average tidal volumes compared with PSV [Colombo et al. 2008; Spahija et al. 2010; Terzi et al. 2010]. A difference has been demonstrated only for high assist levels, when NAVA tidal volumes plateau, based on a negative feedback loop, and lower tidal volumes are observed for NAVA compared with PSV [Colombo et al. 2008; Spahija et al. 2010; Terzi et al. 2010]. However, NAVA is never fixed on tidal volumes and the breathing pattern represents a more chaotic/natural breathing when an average tidal volume can still represent a large spread of values.
In line with the expectations of its fundamental principle, NAVA has in over 10 clinical studies been shown to decrease (and in several studies almost eradicate) patient–ventilator asynchrony, in comparison to other modes of ventilation [Alander et al. 2012; Beck et al. 2009; Bengtsson and Edberg, 2010; Breatnach et al. 2010; Cammarota et al. 2011; Clement et al. 2011; Colombo et al. 2008; de la Oliva et al. 2012; Moerer et al. 2008; Piquilloud et al. 2011; Spahija et al. 2010; Terzi et al. 2010]. Based on these results, the relationship between NAVA and improved synchrony is well established. Moreover, asynchrony has been shown to be associated with increased duration of MV [de Wit et al. 2009; Thille et al. 2006]. However, there is limited comparative clinical trial evidence demonstrating a reduction in MV time with NAVA. To date there is no trial evidence in adult populations, with the exception of an abstract from King’s College in London, which refers to an increase in ventilator-free days in survivors when using Edi monitoring. The median ventilated days were significantly reduced from 9 (3–82) in the NAVA/Edi monitoring group compared with 12 (3–91) in the no NAVA group [Hadfield et al. 2013]. In the pediatric setting, Kallio and colleagues showed in a recent randomized controlled study, in a per protocol analysis, that the stay in the pediatric ICU was significantly shorter in the NAVA group compared with conventional MV [Kallio et al. 2015].
The adoption of a new technology in the healthcare sector is not only dependent on the clinical benefit it provides to patients. Healthcare payers and reimbursement bodies are becoming more and more interested in understanding whether a new health technology could reduce overall healthcare costs, which would balance the extra cost imposed by the new treatment. In situations like this, health economic models are often used to synthesize indirect evidence chains to evaluate the economic value of a health technology based on a number of assumptions [Hjelmgren et al. 2001]. The cost effectiveness or value for money of NAVA could be expressed as an assumed reduction in the number of days spent on MV as a result of improved patient–ventilator synchrony. The purpose of this study was to develop such a health economic model based on the published literature, which attempted to answer the overarching question: what is the potential cost saving of decreasing asynchrony through NAVA? In the model we used for this analysis we aimed to establish an indirect link between improved synchrony with NAVA [Cammarota et al. 2011; Colombo et al. 2008; de la Oliva et al. 2012; Moerer et al. 2008; Piquilloud et al. 2011; Spahija et al. 2010; Terzi et al. 2010] and fewer days spent on MV in synchronous patients [de Wit et al. 2009; Thille et al. 2006].
Methods
The outline of the economic model
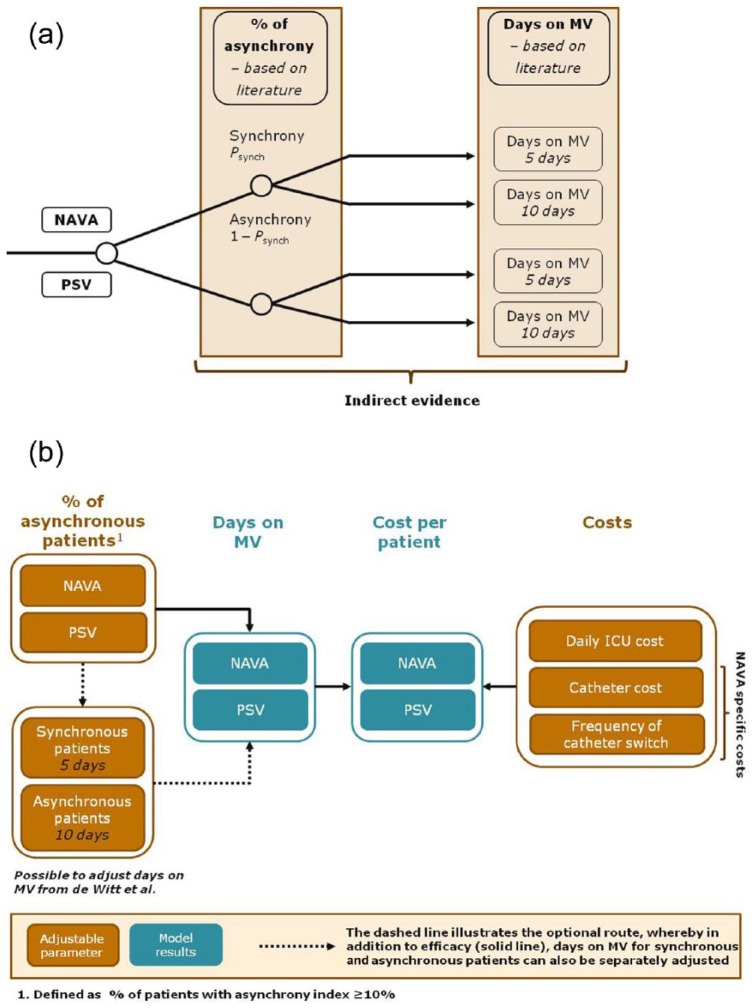
We developed a model with two decision nodes, where the first node represents: ‘treat with NAVA’ or ‘treat with PSV’, and for the second node, each arm of ventilated patients has a probability of having an AI less than 10% (Psync) or an AI of at least 10% (1 – Psync) (see Figure 2). In the model, each state of synchrony/asynchrony is associated with a specified duration of MV (in days) and a corresponding cost (daily ICU cost). Further, NAVA incremental costs are implemented in the model and the effect of each model parameter on the overall results are evaluated through sensitivity analyses.
Figure 2.
Two-node decision tree model. (a) Describes the two nodes of the decision model: (1) treat with Neurally Adjusted Ventilator Assist (NAVA) or pressure support ventilation (PSV), and (2) patient is synchronous or asynchronous. (b) Described the adjustable model input parameters (shown in orange) and the model output parameters (shown in blue). In addition to adjusting the proportion of patients with asynchrony index of at least 10% and costs, the duration of mechanical ventilation (MV) for synchronous and asynchronous patients can be adjusted from the default, originating from the de Wit et al. (2009) study. ICU, intensive care unit.
Data used in the economic model
An overview of the data used in the economic model base case is presented in Table 1. The following sections describe the methods used to arrive at these data.
Table 1.
Base case parameters.
| Parameter | Base case |
|---|---|
| Proportion of patients with AI ⩾10% | NAVA: 21%PSV: 52%Difference: 31% |
| Days on mechanical ventilation | Synchronous patients: 5 daysAsynchronous patients:* 10 daysBase case population:**a 7.1 days |
| Cost per day on mechanical ventilation | Lund: US$5007 |
| Cost per NAVA catheter | US$351 |
| Average number of catheters per patient | Catheter switch every fifth day: 1.76 |
With AI ⩾10%. **Assuming 50% of patients on NAVA and 50% on PSV.
AI, asynchrony index; NAVA, Neurally Adjusted Ventilator Assist; PSV, pressure support ventilation.
Difference in asynchrony between NAVA and PSV
Based on the nodes of the decision tree model, a cutoff for defining patient–ventilator asynchrony had to be implemented, and this was set to an AI of 10%, based on the studies by de Wit and Thille and colleagues [de Wit et al. 2009; Thille et al. 2006]. Through using the AI 10% cutoff, patients could be stratified and an associated duration of MV (see below) could be assigned.
We evaluated the published literature on NAVA versus PSV comparisons by building a search strategy for Medline, to identify which studies used the AI as an outcome, and identified seven studies (see Table 2). The literature review and evaluation were categorized by clinical/physiological search terms (sedation, sleep, synchrony/asynchrony, spontaneous breathing, diagram atrophy); mode of MV (any MV, PSV, NAVA); and long-term health outcomes (cost, days in ICU, days on ventilator, ventilator-induced lung injury). Medical subject heading terms were used in the literature search, when available. When a search generated over 40 hits, the search terms were used in combination so that a manageable number of articles were retrieved for abstract relevance screening. Articles were required to be written in English to be considered for further evaluation. The search was originally performed in 2010 and later updated during 2011. The result included studies with adult, but also few with pediatric patients, and both invasive and noninvasive (helmet) NAVA. The largest study constituted a within-patient crossover design in which invasive NAVA was compared with PSV in 22 adult ICU patients [Piquilloud et al. 2011]. This study provided the observed median and 25th and 75th percentiles of the AI for NAVA 4.5 (2.6–9.9) and PSV 12.4 (4.8–26.4), respectively, and from there the proportion of patients with AI at least 10% had to be inferred. This was done by fitting two exponential functions to the published data, one for PSV and NAVA, respectively. These functions were used to identify the percentile at which AI equaled 10% for each alternative. The corresponding proportion of asynchronous patients (AI ⩾ 10%) was 21% for NAVA and 52% for PSV. This difference in asynchrony of NAVA versus PSV was used as the base case. It should be noted that the study by Piquilloud and colleagues is based on data from Switzerland; to our knowledge there is no published literature using Swedish data. However, Swedish data presented in oral presentations indicate that the data reported by Piquilloud are also representative of Swedish clinical practice.
Table 2.
Identified studies comparing NAVA with PSV.
| Base case | NAVA AI ⩾ 10% | PSV AI ⩾ 10% | Incremental difference |
|---|---|---|---|
| Piquilloud et al. [2010] | 21% | 52% | 31% |
| Sensitivity | |||
| Terzi et al. CCM [2010] | 0% | 32% | 32% |
| Colombo et al. [2008] | 0% | 36% | 36% |
| De la Oliva et al. [2012] | 0% | 44% | 44% |
| Moerer et al. ICM [2008] | 9% | 68% | 59% |
| Spahija et al. CCM [2010] | 7% | 78% | 71% |
| Cammarota et al. [2011] | 0% | 75% | 75% |
AI, asynchrony index; CCM, Critical Care Medicine; ICM, Intensive Care Medicine; NAVA, Neurally Adjusted Ventilator Assist; PSV, pressure support ventilation.
Further, few of the other studies reported the proportion of patients with AI at least 10% and therefore this often had to be inferred. Where medians and percentiles were reported, the proportion of patients with AI at least 10% was inferred in the same way as described above, while a normal distribution was assumed when mean ± standard deviation (SD) had been reported, and the normal distribution was used to work out the proportion of patients with AI ⩾ 10%, by using the reported mean and SD (see Table 2).
Based on the corresponding estimated difference between NAVA and PSV, sensitivity analyses were performed. For two studies, several different ventilation modes had been explored during the course of the trial, and here sensitivity analyses were performed for the weighted average of all comparative results [Moerer et al. 2008; Terzi et al. 2010].
In summary, the various differences in asynchrony between NAVA and PSV in all identified studies were used as the reference points in the sensitivity analyses to estimate their effect on the overall results.
Duration of MV
We used the latest publication describing the association between patient–ventilator asynchrony and duration of MV to extract an estimate of the number of days on MV for asynchronous (AI ⩾ 10%) and synchronous patients (AI < 10%), respectively [de Wit et al. 2009]. This study did not provide mean days on MV, but these were estimated based on plotting the information provided in Figure 3 of the publication into an Excel sheet. The estimated weighted average was calculated and this corresponded to 4.6 days for synchronic patients and 10.3 days for asynchronous patients. However, to make the model flexible for adjusting the duration on MV, we needed a manageable distribution of days on MV, not just average values. The Poisson distribution is a discrete probability distribution that only returns whole numbers and was used to generate two random number distributions based on λ = 5 (mean value 5 days) and λ = 10 (mean value 10 days) for synchronous and asynchronous patients, respectively. These distributions were used to estimate the average days on MV in the base case analysis based on the difference in asynchrony results from the study by Piquilloud and colleagues (assuming half of the patients were on NAVA and half of the patients were on PSV), which corresponded to 7.1 days [Piquilloud et al. 2011].
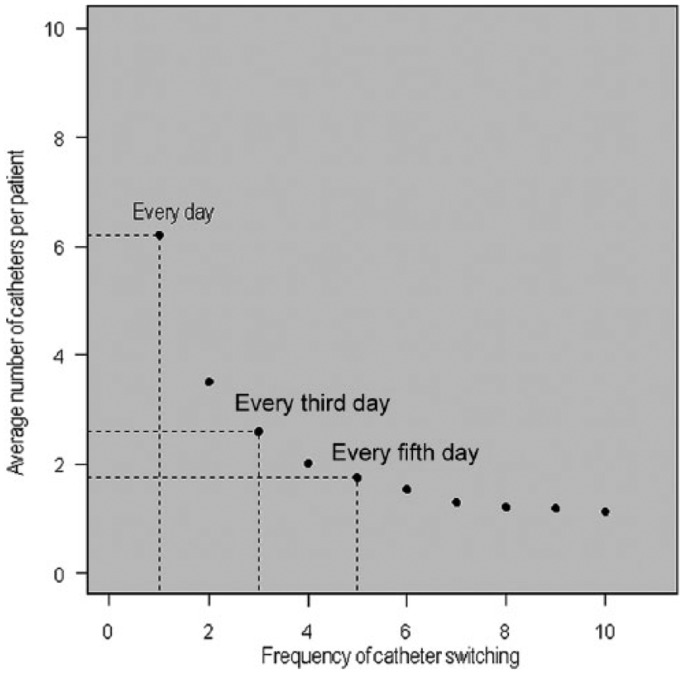
Figure 3.
Average number of catheters per Neurally Adjusted Ventilator Assist (NAVA) patient, in relation to switch frequency. The model was used to estimate the average number of catheters per NAVA patient, given alternative catheter switch frequencies. The frequencies used for the sensitivity analyses are indicated by dashed lines; catheter switch every fifth day (base case), every third day, and every day, corresponded to an average 1.76 catheters, 2.59 catheters, and 6.20 catheters per patient.
The average duration of MV can differ between countries, hospitals, ICUs and over time. We therefore searched the Swedish Intensive Care Unit Register (www.icuregswe.org/) for updated and comprehensive statistics for the Swedish setting. Based on the 2010 report, the average duration of MV was 88.5, corresponding to 3.7 days. This weighted average duration and a hypothetical duration of 1.45 days were used as lower bounds for the sensitivity analysis.
Costs
NAVA catheter costs
The unit cost for one catheter was assumed to be US$351 (SEK 2500), which was used for the base case analysis. Further, costs of US$700 (SEK 5000), US$1050 (SEK 7500) and US$1400 (SEK 10,000) were evaluated in the sensitivity analysis to explore the effect of catheter cost on the overall cost effectiveness of NAVA.
Frequency of NAVA catheter switching
According to the user instructions, a NAVA catheter should be exchanged every fifth day. We used the weighted average of the two Poisson distributions (described above) to create a distribution of the number of exchanges per patient. Assuming an exchange every fifth day, the average number of switches per patient amounted to 1.76 (see Figure 3). Thus the total cost for NAVA catheters used was US$615 per patient (US$351 × 1.76), which corresponded to the base case. However, given that catheters can be displaced (which requires more frequent switching), in the sensitivity analyses, the effect on the NAVA cost effectiveness of switching every day and every third day was also explored.
Other NAVA-specific costs and savings
The incremental cost of NAVA over PSV is dependent on the cost of catheters, as discussed above, and the potential impact of NAVA on sedation, the cost for buying the NAVA module or renting NAVA ventilators (capital costs), and potential differences in the need to monitor patients on NAVA versus PSV. The developed decision tree model contains functionality for evaluating the effect of these costs on the overall results; however, none of these potential NAVA-specific costs and savings were included in the base case of the model. No references were identified that described a difference in sedation (potential NAVA cost savings) or monitoring need between NAVA and PSV. The capital cost per NAVA use is very small [approximately US$56; Edi module and software approximately US$14,000, assuming a 10-year lifespan and 25 patients per year (US$14,000/10 years/25 patients); Maquet Critical Care, data on file] in relation to the overall ICU cost and would on a patient level have a minor impact on the overall findings. For this reason, the cost of capital was omitted.
Daily ICU costs
The daily ICU cost is used in the model to calculate the cost associated with time (days) on MV. We attempted to implement a source for the daily ICU cost which was up to date, and which could be continuously updated on a regular basis, and therefore used information from the Svenska Intensivvårdsregistrets (SIR) 2010 (www.icuregswe.org/). Since 2002, Sweden has presented yearly reports from ICUs in Sweden. Associated ICUs report their average VTS points per day (VårdTyngd Sverige) to SIR, which is a measure that takes into account the case mix and the intensity of care at the specific clinic [Hjortso et al. 1992; Walther et al. 2004]. The reported daily VTS reflects the basic cost of a day’s care (i.e. clinics might have additional costs) and score can be translated into a cost/reimbursement level, based on available regional price lists. For the base case, we selected the ICU at the Lund University hospital, where the NAVA technology is used. Further, the cost per VTS score was derived from the Southern hospital district price list, to which Lund belongs; US$85 (SEK 606), amounting to a daily cost of US$5007 (Södra regionvårdsnämnden, regional priser 2010; www.srvn.org).
For the sensitivity analyses, the effect of the average daily cost was evaluated by using the minimum, median and maximum VTS score per day in the ICU, reported across Sweden.
Model output
The model output was the average number of days spent on MV and the associated costs for NAVA and PSV, respectively. The incremental cost was extracted by taking the difference in overall cost (ICU and catheter costs) between NAVA and PSV. The difference in cost was referred to as the budget impact per patient and expressed as the average days or cost per patient. All cost results are presented in US$ 2010.
Currency conversion rate
Costs that were provided in Swedish Krona were converted to US$ based on the average conversion rate during 2010, provided by the Sweden’s central bank (Sveriges Riksbank; www.riksbanken.se. The conversion rate for U$1 amounted to SEK 7.1411, and the corresponding conversion rate to EUR was SEK 10.1948.
Results
Base case
Table 3 shows the results per patient from the base case analysis in terms of days on MV and the associated cost per patient, for patients ventilated on NAVA compared with PSV. Patients in our model ventilated with NAVA would spend on average 1.7 days less on MV compared with PSV, as derived from the lower patient–ventilator asynchrony. The reduced days on MV would amount to a cost saving of US$8501 for patients on NAVA, and the total cost saving of NAVA compared with PSV was in total US$7886, after having accounted for the NAVA catheter cost.
Table 3.
Base case results.
| NAVA | PSV | Difference at patient level | |
|---|---|---|---|
| Days on MV per patient | 6.23 | 7.93 | −1.70 |
| Cost per patient (US$) | |||
| ICU cost | 31,198 | 39,699 | −8 501 |
| NAVA catheter cost | 615 | – | 615 |
| Total cost (US$) | 31,813 | 39,699 | −7886 |
ICU, intensive care unit; NAVA, Neurally Adjusted Ventilator Assist; PSV, pressure support ventilation.
Sensitivity analyses
Altogether, 16 sensitivity analyses were performed to explore the effect of all model parameters on the cost effectiveness of NAVA versus PSV. The sensitivity analyses are summarized in Table 4, and the results are presented in the subsections, below.
Table 4.
Summary of sensitivity analyses.
| Scenario | Description | Budget impact per patient (US$) | Change versus base case (absolute/%) | Reference |
|---|---|---|---|---|
| Base case | See Table 2 | −7886 | ||
| 1. Efficacy | NAVA: 0% | −8160 | 274/3% | Terzi et al. [2010] |
| PSV: 32% | ||||
| 2. Efficacy | NAVA: 0% | 9257 | 1371/17% | Colomboet al. [2008] |
| PSV: 36% | ||||
| 3. Efficacy | NAVA: 0% | −11,451 | 3565/45% | De la Olivaet al. [2012] |
| PSV: 44% | ||||
| 4. Efficacy | NAVA: 9% | −15,427 | 7541/96% | Moerer et al. [2008] |
| PSV: 67.5% | ||||
| 5. Efficacy | NAVA: 7% | −18,855 | 10,969/139% | Spahija et al. [2010] |
| PSV: 78% | ||||
| 6. Efficacy | NAVA: 0% | −19,952 | 12,066/153% | Cammarotaet al. [2011] |
| PSV: 75% | ||||
| 7. Catheter cost | US$700 | −7271 | −615/–8% | |
| 8. Catheter cost | US$1050 | −6656 | −1230/–16% | |
| 9. Catheter cost | US$1400 | −6041 | −1845/–23% | |
| 10. Frequency of catheter switch | Every third day | −7593 | −239/–4% | |
| 11. Frequency of catheter switch | Every day | −6329 | −1557/–20% | |
| 12. Daily ICU cost min, Sweden | NU Trollhättan: US$2461 | −3563 | −4323/–55% | Swedish ICU register |
| 13. Daily ICU cost median, Sweden | Kristianstad/Gävle: US$4370 | −6805 | −1081/–14% | Swedish ICU register |
| 14. Daily ICU cost max, Sweden | K Solna CIVA: US$5516 | −8750 | 864/11% | Swedish ICU register |
| 15. Days on MV | 3.7 days | −4579 | −3307/–42% | Swedish ICU register |
| 16. Days on MV | 1.45 days | −1532 | −6354/–81% | Hypothetical |
ICU, intensive care unit; MV, mechanical ventilation; NAVA, Neurally Adjusted Ventilator Assist; PSV, pressure support ventilation.
Sensitivity analyses of difference in asynchrony between NAVA and PSV
The observed difference in asynchrony (as measured through the proportion of patients with AI ⩾ 10%), for the seven comparative studies of NAVA versus PSV were implemented in a sensitivity analysis (see Table 2). In comparison to the base case, the six identified studies all showed larger differences between NAVA and PSV, and the modeled differences in days on MV and total cost were consequently larger. The largest difference was observed in the study by Cammarota and colleagues [Cammarota et al. 2011], which corresponded to a total cost saving of close to US$20,000, while the smallest difference from the study by Terzi and colleagues corresponded to a total cost saving of US$8000 [Terzi et al. 2010], which was still slightly higher compared with the base case (see Table 4).
Sensitivity analyses of duration of MV
Since the base case analysis was built using the average days on MV as estimated in the study by de Witt and colleagues, we wanted to explore the effect of changing the average duration on MV to more recent observations. In the sensitivity analyses, the average time on MV in the Swedish ICU setting (3.7 days) was evaluated, in which case the total cost saving of NAVA ventilation amounted to US$4600 compared with US$7900 in the base case (see Table 4). To make the analysis more comprehensive we also tested an average 1.45 days on MV, which resulted in a per patient cost saving of US$1532.
Sensitivity analyses of costs
Further, the costs that were used to build the model were explored in sensitivity analyses, evaluating the effects of the daily ICU cost, the NAVA catheter cost and the frequency of switching the NAVA catheter.
The daily ICU cost had a relatively large effect on the results, whereby the lowest observed ICU cost in Sweden (Trollhättan, US$2461) corresponded to a reduction in costs of US$3560 and the largest observed ICU cost (Karolinska Solna, US$5500) corresponded to a reduction in costs of US$8750, when ventilating a patient on NAVA compared with PSV (see Table 4). The NAVA catheter cost and the frequency of switching had a small effect on the overall model results, whereby the largest change observed in cost, when increasing the NAVA catheter cost by 400%, was a 30% reduction in the cost saving (see Table 4). For frequency of switching, the base case scenario of switching every fifth day resulted in an average 1.8 catheters per patient, and switching every third day or every day resulted in an average of 2.6 and 6.2 catheters per patient, respectively (see Figure 3). However, when relating the additional cost of switching the NAVA catheter every day instead of every fifth, to the overall budget impact of ventilating patients on NAVA, the increased switch frequency only corresponded to a 25% reduction in the potential cost saving of NAVA (see Table 4).
Breakeven analyses
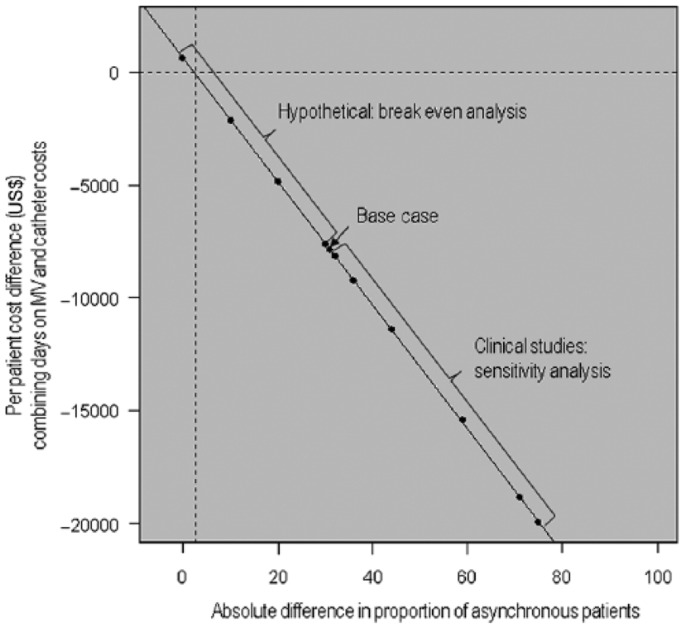
Given that the difference in asynchrony of NAVA versus PSV can vary between clinics, and that the published references identified for the sensitivity analyses might not capture the full range of that variation, we performed a breakeven analysis searching for the level of relative difference in asynchrony when NAVA is no longer cost effective in relation to PSV (from an absolute difference in the proportion of asynchronous patients of 31% in the base case, down to no difference). The results are illustrated in Table 5. Further, these results from hypothetical scenarios were combined with that from the clinical studies to perform a regression analysis that demonstrated at which incremental efficacy level the breakeven is reached. In this analysis, an absolute difference greater than 2.5% between NAVA and PSV in the proportion of asynchronous patients with an AI at least 10% (49.5% versus 52% asynchronous patients with NAVA and PSV, respectively) was needed in order for NAVA to be cost effective (see Figure 4).
Table 5.
Summary of breakeven analyses.
| Scenario | Description | Budget impact per patient (US$) | Change versus base case (absolute/%) | Reference |
|---|---|---|---|---|
| Base case | See Table 2 | −7886 | ||
| 1. Efficacy | NAVA: 22% | −7612 | −274/–3% | Hypothetical |
| PSV: 52% | ||||
| Absolute difference: 30% | ||||
| 2. Efficacy | NAVA: 32% | −4870 | −3017/–38% | Hypothetical |
| PSV: 52% | ||||
| Absolute difference: 20% | ||||
| 3. Efficacy | NAVA: 42% | −2127 | −5759/–73% | Hypothetical |
| PSV: 52% | ||||
| Absolute difference: 10% | ||||
| 4. Efficacy | NAVA: 49.5% | −71 | −8501/–108% | Hypothetical |
| PSV: 52% | ||||
| Absolute difference: 2.5% | ||||
| 5. Efficacy | NAVA: 52% | 615 | −8501/–108% | Hypothetical |
| PSV: 52% | ||||
| Absolute difference: 0% |
In addition to the base case, five scenarios were tested in order to define the required difference in proportion of asynchronous patients with asynchrony index at least 10% for breakeven, where the incremental cost of NAVA equals the economic gain from reducing asynchrony.
NAVA, Neurally Adjusted Ventilator Assist; PSV, pressure support ventilation.
Figure 4.
Incremental cost in relation to the efficacy of Neurally Adjusted Ventilator Assist (NAVA) versus pressure support ventilation (PSV). The relation between the incremental cost (model output) and the absolute difference in proportion of asynchronous patients with asynchrony index (AI) at least 10% between PSV and NAVA (model input) is illustrated. The base case, with efficacy taken from Piquilloud and colleagues [Piquilloud et al. 2011] is indicated, and the results of the clinical studies applied in the sensitivity analyses are shown in the following order from the base case: [Terzi et al. 2010; Colomboet al. 2008; de la Oliva et al. 2012; Moereret al. 2008; Spahija et al. 2010; Cammarota et al. 2011]. In addition, four hypothetical scenarios were added, to illustrate breakeven; that is, at which point the difference in proportion of asynchronous patients with AI at least 10% between NAVA and PSV is so small that the additional cost of the NAVA catheter balances the gain from reducing the number of days on mechanical ventilation (MV). In this analysis, an absolute efficacy difference larger than 2.5% is needed in order for NAVA to be cost effective.
Discussion
In this study we developed a decision analytical model evaluating the economic effects of using the NAVA technology, which reduces patient ventilator asynchrony. Extrapolating the de Wit and Thille data [de Wit et al. 2009; Thille et al. 2006] and demonstrating a decrease in ventilator days in patients with less asynchrony (AI < 10%), our results show that only a minor decrease (2.5%) in the proportion of asynchronous ICU patients (AI ⩾ 10%) with NAVA is needed for investments to pay off in terms of reduced days on MV. The base case results were generally very stable and robust to changes in important parameters, such as proportion of asynchronous patients with an AI at least 10%, duration of ventilation and daily ICU costs. Decision analytic modeling allows a feasible, scientific and timely approach to measure the cost effectiveness of new medical technologies in healthcare by using the best available evidence of different sources to produce detailed estimates of the clinical and economic consequences of different healthcare interventions. To the best of our knowledge, no studies have yet reported health economic outcomes of the NAVA technology, so we see the positive results of the model as an encouragement to start designing and performing studies that also evaluate this important aspect.
This model is based on the direct link between NAVA and improved patient–ventilator asynchrony and the association between patient ventilator asynchrony and long duration of mechanical ventilation. The link between NAVA and synchrony is well established and had by 2012 been reported in over 10 peer-reviewed publications, investigating both adult and pediatric populations [Alander et al. 2012; Beck et al. 2009; Bengtsson and Edberg, 2010; Breatnach et al. 2010; Cammarota et al. 2011; Clement et al. 2011; Colombo et al. 2008; de la Oliva et al. 2012; Moerer et al. 2008; Piquilloud et al. 2011; Spahija et al. 2010; Terzi et al. 2010]. Three studies have reported on an association between asynchrony and days on MV; all have been observational [Chao et al. 1997; de Wit et al. 2009; Thille et al. 2006]. We note however that the NAVA technology enables an intervention study on reducing asynchrony for the first time, while other parameters that were previously potential subject to intervention, for example fixed tidal volumes and sedation levels, have already been explored in randomized controlled trials for evaluation of health economic outcomes. For example, decreased tidal volumes have been shown to decrease both mortality and days on MV [ARDSNet, 2012; Petrucci and Iacovelli, 2007], and reduced sedation and improved sedation protocols have been shown to decrease days on ventilation [Brattebo et al. 2002; Breen et al. 2005; de Wit et al. 2008; Strom et al. 2010].
This health economic model is built based on clinical data that have been gathered from several different sources, including both clinical studies and the Swedish ICU register, and further it is based on a number of assumptions and simplifications. Therefore, in our sensitivity analyses, we have evaluated the effect on the results when considering different extreme scenarios for the parameters of the model to illustrate potential applicability to various scenarios, complementary to the base case. In order to do this, we chose upper and lower limits of the parameters to be evaluated that reflect a range of relevant scenarios in the real-world setting. When evaluating the efficacy of NAVA versus PSV in reducing patient–ventilator asynchrony, we included all identified studies that report outcomes as an AI. This gives us potential incremental efficacies for different patient groups, different NAVA modes and different study sizes. The study chosen for the base case included the largest population, all adults, and on invasive NAVA, and the difference (compared with PSV) in the proportion of asynchronous patients proved to be the most conservative estimate since all additional studies showed larger differences between NAVA and PSV, and hence a larger cost-saving potential. The sensitivity analyses we performed based on the other studies indicate that differences in the proportion of asynchronous patients had a large impact on results, and that the data used in the base case corresponded to a conservative estimate.
In the sensitivity analyses of daily ICU cost, we evaluated the full scenario of costs observed across Swedish hospitals and note that even at the lowest daily ICU cost, NAVA was clearly cost saving. As in many other countries, Sweden has over a 20-year period been trying to control the escalating cost in healthcare. Hence, the number of ICU beds (ventilator beds) in Sweden has been reduced and was 8/100,000 inhabitants in 2011 (extrapolated from occupancy statistics in SIR 2011 annual report; www.icuregswe.org/). When comparing this to the situation in other countries [Brattebo et al. 2002; Breen et al. 2005; de Wit et al. 2008; Strom et al. 2010], it is in the lower end of the number of ICU beds/100,000, for example, the UK is the only European country with fewer number of ICU beds (3/100,000 population) while Germany has as many as 25 ICU beds/100,000 population. A high occupancy of beds means a high demand for effective treatment of ICU patients to move the patients to intermediate care or regular wards as soon as possible. Thus we believe that overall cost per ICU patient in Sweden could be on the lower side among comparable countries. In our analysis of various Swedish hospitals, the total range of incremental cost savings spanned from US$3560 to 8750, representing a 245% difference between the highest and lowest values, while all were well within the limits of NAVA being less costly.
The average duration of MV can also differ between countries, hospitals, wards and also over time. We therefore explored the effects of implementing an average duration of MV from 2010, national Swedish statistics (3.7 days) and a hypothetical value of 1.45 days, compared with that of the study by Thille and colleagues (7.1 days). This resulted in a 42% and 81% reduction in the incremental patient budget impact, respectively. Further, we could illustrate that the cost of the NAVA catheter had negligible effects on the total results, when a fourfold cost increase only resulted in a 23% reduction in the incremental cost saving. A similar trend was observed when evaluating the effect of changing the catheter every day instead of changing the catheter every fifth day (as per recommendations), which only resulted in a 25% reduction in the incremental cost saving at the patient level, even though the average number of NAVA catheters per patient increased from 1.8 to 6.2.
The key limitation of these results is the lack of randomized controlled studies in adults with which to compare the results; however, decision tree models like the one used in this study are frequent in situations when the availability of data is limited. The aim of these models is to synthesize data from different sources, as well as assumptions. Decision tree models are widely used and typically accepted by reimbursement authorities and payers when evaluating health technologies.
Another potential limitation with this model is that we have not evaluated all costs that might differ between NAVA and PSV, but have focused on daily ICU costs, and NAVA catheter costs. Several studies have suggested that the sedation costs are likely to be reduced with NAVA compared with PSV, since the improved patient–ventilator synchrony results in a higher comfort level for the patient. No study has yet been performed with this as an end point in an adult population. In the recent pediatric study published by Kallio and colleagues it was reported that when postoperative patients were excluded from the analysis the amount of sedation needed was significantly lower in the NAVA group [Kallio et al. 2015]. However, since no adult studies have measured and reported on comparative sedation levels, this potential cost saving of NAVA was not incorporated. The two ventilation modes have more or less equal amounts of parameters to set, implement and support, and no studies have reported that either method is more labor intensive than the other. Since the same ventilator can be used for both NAVA and PSV ventilation, the only additional incremental investment, on top of the catheter cost, is that of a NAVA module and software, which can be considered low in comparison to catheter costs. The same conclusion applies for the capital cost for the NAVA-enabled ventilator.
In the current model, the economic effects of reducing days on MV have been evaluated by assigning a daily ICU cost to the duration. The cost for a day on MV in the ICU is most likely higher than the average daily ICU cost, which we have implemented here. Previous studies have estimated the incremental cost of MV in the ICU to be on average 13–38% higher than intensive care without MV [Dasta and Kane-Gill, 2009; Tan et al. 2008]. In summary, we decided to use a more conservative estimate of the daily costs by focusing on the average daily ICU cost. Bearing this in mind, the results point to an even larger cost-saving potential.
Moreover, differences in patient characteristics and context should be considered when the results from the model are interpreted and validated with real-world data; the fact that potential subgroups of patients can benefit differently from treatment should be taken into account. For example, patients with a longer average duration of MV might have a larger cost saving potential, or from the clinics perspective, the case mix at specific clinics can have a large impact on the cost-saving potential. The studies performed in neonates show that triggering of ventilator support is difficult due to leakage using uncuffed tubes. This makes it difficult for the ventilator to identify patient efforts and has been shown to increase patient ventilator asynchrony extensively. Most recently, Piastra and colleagues published results from a nested study comparing retrospective data on neonates recovering from acute respiratory distress syndrome treated with NAVA with a control group not treated with NAVA. The study found that the duration of MV with NAVA (41 ± 17 h) was significantly lower than that of PSV (72.5 ± 44 h, p = 0.011) [Piastra et al. 2014]. This corresponds to a difference of 1.3 days which, despite being based on a different patient population, is in line with our model results of 1.7 days. Future studies in this patient group might also, beside the important impact on time on ventilator, show an even more important decrease in morbidity as this is linked to time on MV. Thus future research should investigate the efficacy of NAVA in different patient populations. Clearly, it would be of great interest to conduct an intervention study that would be designed to validate the results in a real-world setting at clinics that ventilate their patients with both NAVA and PSV.
The potential effects of an increased patient flow through ICUs could also generate interesting and important health economic data.
Conclusion
This economic study showed economically favorable results for NAVA compared with PSV. Our results show that only a minor decrease in the proportion of asynchronous patients (AI ⩾ 10%) with NAVA is needed for investments to pay off in terms of reduced days on MV. Future studies need to confirm this result by directly relating improved synchrony to the number of days on MV.
Acknowledgments
JH conceived of the study, developed and validated the model, participated in interpretation of results and critically reviewed the manuscript. SBW carried out the literature searches used as the evidence base for the model and drafted the manuscript. PH participated in drafting the manuscript. KJM participated in the design of the study. SG participated in designing the literature searches, designing the model, and helped to draft the manuscript. All authors read and approved the final manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Maquet Critical Care is manufacturer of Edi-catheters.
Funding: This study was supported by Maquet Critical Care AB. Jonas Hjelmgren, Sara Bruce Wirta, Pernilla Huetson, and Karl-Johan Myrén are HEOR (Health Economics Outcomes Research) consultants to Maquet Critical Care AB. Sylvia Göthberg is employed by Maquet Critical Care AB. Maquet Critical Care AB funded the development of the health economic model, but has not taken an active part in the data collection or model calculations.
Contributor Information
Jonas Hjelmgren, IMS Health HEOR, Sveavägen 155, Stockholm, Sweden Amgen (Europe) GmbH, Dammstrasse 23, Zug, Switzerland.
Sara Bruce Wirta, IMS Health HEOR, Sveavägen 155, Stockholm, Sweden.
Pernilla Huetson, IMS Health HEOR, Sveavägen 155, Stockholm, Sweden.
Karl-Johan Myrén, IMS Health HEOR, Sveavägen 155, Stockholm, Sweden SOBI, Tomtebodavägen 23A, Solna, Sweden.
Sylvia Göthberg, Maquet Critical Care AB, Röntgenvägen 2, Solna, Sweden.
References
- Alander M., Peltoniemi O., Pokka T., Kontiokari T. (2012) Comparison of pressure-, flow-, and NAVA-triggering in pediatric and neonatal ventilatory care. Pediatr Pulmonol 47: 76–83. [DOI] [PubMed] [Google Scholar]
- ARDSNet (2012) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. New Engl J Med 342: 1301–1308. [DOI] [PubMed] [Google Scholar]
- Bang C. (1953) A new respirator. Lancet 1: 723–726. [DOI] [PubMed] [Google Scholar]
- Beck J., Reilly M., Grasselli G., Mirabella L., Slutsky A., Dunn M., et al. (2009) Patient-ventilator interaction during neurally adjusted ventilatory assist in low birth weight infants. Pediatr Res 65: 663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson J., Edberg K. (2010) Neurally adjusted ventilatory assist in children: an observational study. Pediatr Crit Care Med 11: 253–257. [DOI] [PubMed] [Google Scholar]
- Blanch L., Sales B., Montanya J., Lucangelo U., Garcia-Esquirol O., Villagra A., et al. (2012) Validation of the Better Care(R) system to detect ineffective efforts during expiration in mechanically ventilated patients: a pilot study. Intensive Care Med 38: 772–780. [DOI] [PubMed] [Google Scholar]
- Brattebo G., Hofoss D., Flaatten H., Muri A., Gjerde S., Plsek P. (2002) Effect of a scoring system and protocol for sedation on duration of patients’ need for ventilator support in a surgical intensive care unit. BMJ 324: 1386–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breatnach C., Conlon N., Stack M., Healy M., O’Hare B. (2010) A prospective crossover comparison of neurally adjusted ventilatory assist and pressure-support ventilation in a pediatric and neonatal intensive care unit population. Pediatr Crit Care Med 11: 7–11. [DOI] [PubMed] [Google Scholar]
- Breen D., Karabinis A., Malbrain M., Morais R., Albrecht S., Jarnvig I., et al. (2005) Decreased duration of mechanical ventilation when comparing analgesia-based sedation using remifentanil with standard hypnotic-based sedation for up to 10 days in intensive care unit patients: a randomised trial [ISRCTN47583497]. Crit Care 9: R200–R210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota G., Olivieri C., Costa R., Vaschetto R., Colombo D., Turucz E., et al. (2011) Noninvasive ventilation through a helmet in postextubation hypoxemic patients: physiologic comparison between neurally adjusted ventilatory assist and pressure support ventilation. Intensive Care Med 37: 1943–1950. [DOI] [PubMed] [Google Scholar]
- Chao D., Scheinhorn D., Stearn-Hassenpflug M. (1997) Patient-ventilator trigger asynchrony in prolonged mechanical ventilation. Chest 112: 1592–1599. [DOI] [PubMed] [Google Scholar]
- Clement K., Thurman T., Holt S., Heulitt M. (2011) Neurally triggered breaths reduce trigger delay and improve ventilator response times in ventilated infants with bronchiolitis. Intensive Care Med 37: 1826–1832. [DOI] [PubMed] [Google Scholar]
- Colombo D., Cammarota G., Alemani M., Carenzo L., Barra F., Vaschetto R., et al. (2011) Efficacy of ventilator waveforms observation in detecting patient-ventilator asynchrony. Crit Care Med 39: 2452–2457. [DOI] [PubMed] [Google Scholar]
- Colombo D., Cammarota G., Bergamaschi V., de Lucia M., Corte F., Navalesi P. (2008) Physiologic response to varying levels of pressure support and neurally adjusted ventilatory assist in patients with acute respiratory failure. Intensive Care Med 34: 2010–2018. [DOI] [PubMed] [Google Scholar]
- Dasta J., Kane-Gill S. (2009) Pharmacoeconomics of sedation in the ICU. Crit Care Clin 25: 571–583, ix. [DOI] [PubMed] [Google Scholar]
- De la Oliva P., Schuffelmann C., Gomez-Zamora A., Villar J., Kacmarek R. (2012) Asynchrony, neural drive, ventilatory variability and COMFORT: NAVA versus pressure support in pediatric patients. A non-randomized cross-over trial. Intensive Care Med 38: 838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit M., Gennings C., Jenvey W., Epstein S. (2008) Randomized trial comparing daily interruption of sedation and nursing-implemented sedation algorithm in medical intensive care unit patients. Crit Care 12: R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit M., Miller K., Green D., Ostman H., Gennings C., Epstein S. (2009) Ineffective triggering predicts increased duration of mechanical ventilation. Crit Care Med 37: 2740–2745. [DOI] [PubMed] [Google Scholar]
- Engstrom C. (1954) Treatment of severe cases of respiratory paralysis by the Engstrom universal respirator. Brit Med J 2: 666–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield D., Skorko A., Vercueil A., Bell C., Feehan A., Peters K., et al. (2013) An observational retrospective review of utilisation and outcomes of diaphragmatic EMG monitoring and neurally adjusted ventilatory assist in a central London teaching hospital over a three year period. 33rd ISICEM (International Symposium on Intensive Care and Emergency Medicine). Poster 146 Brussels, Belgium: ISICEM. [Google Scholar]
- Hjelmgren J., Berggren F., Andersson F. (2001) Health economic guidelines – similarities, differences and some implications. Value Health 4: 225–250. [DOI] [PubMed] [Google Scholar]
- Hjortso E., Buch T., Ryding J., Lundstrom K., Bartram P., Dragsted L., et al. (1992) The nursing care recording system. A preliminary study of a system for assessment of nursing care demands in the ICU. Acta Anaesthesiol Scand 36: 610–614. [DOI] [PubMed] [Google Scholar]
- Kallio M., Peltoniemi O., Anttila E., Pokka T., Kontiokari T. (2015) Neurally Adjusted Ventilatory Assist (NAVA) in pediatric intensive care – a randomized controlled trial. Pediatr Pulmonol 50: 55–62. [DOI] [PubMed] [Google Scholar]
- Moerer O., Beck J., Brander L., Costa R., Quintel M., Slutsky A., et al. (2008) Subject-ventilator synchrony during neural versus pneumatically triggered non-invasive helmet ventilation. Intensive Care Med 34: 1615–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Jubran A., Tobin M. (1998) Cycling of inspiratory and expiratory muscle groups with the ventilator in airflow limitation. Am J Resp Crit Care Med 158: 1471–1478. [DOI] [PubMed] [Google Scholar]
- Petrucci N., Iacovelli W. (2007) Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev CD003844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piastra M., de Luca D., Costa R., Pizza A., de Sanctis R., Marzano, et al. (2014) Neurally adjusted ventilatory assist vs pressure support ventilation in infants recovering from severe acute respiratory distress syndrome: nested study. J Crit Care 29: 312.e1–5. [DOI] [PubMed] [Google Scholar]
- Piquilloud L., Vignaux L., Bialais E., Roeseler J., Sottiaux T., Laterre P., et al. (2011) Neurally adjusted ventilatory assist improves patient-ventilator interaction. Intensive Care Med 37: 263–271. [DOI] [PubMed] [Google Scholar]
- Sassoon C., Foster G. (2001) Patient-ventilator asynchrony. Curr Opin Crit Care 7: 28–33. [DOI] [PubMed] [Google Scholar]
- Sassoon C., Giron A., Ely E., Light R. (1989) Inspiratory work of breathing on flow-by and demand-flow continuous positive airway pressure. Crit Care Med 17: 1108–1114. [DOI] [PubMed] [Google Scholar]
- Sinderby C., Navalesi P., Beck J., Skrobik Y., Comtois N., Friberg S., et al. (1999) Neural control of mechanical ventilation in respiratory failure. Nat Med 5: 1433–1436. [DOI] [PubMed] [Google Scholar]
- Spahija J., de Marchie M., Albert M., Bellemare P., Delisle S., Beck J., et al. (2010) Patient-ventilator interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med 38: 518–526. [DOI] [PubMed] [Google Scholar]
- Strom T., Martinussen T., Toft P. (2010) A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet 375: 475–480. [DOI] [PubMed] [Google Scholar]
- Tan S., Hakkaart-van R., Al M., Bouwmans C., Hoogendoorn M., Spronk P., et al. (2008) A microcosting study of intensive care unit stay in the Netherlands. J Intensive Care Med 23: 250–257. [DOI] [PubMed] [Google Scholar]
- Terzi N., Pelieu I., Guittet L., Ramakers M., Seguin A., Daubin C., et al. (2010) Neurally adjusted ventilatory assist in patients recovering spontaneous breathing after acute respiratory distress syndrome: physiological evaluation. Crit Care Med 38: 1830–1837. [DOI] [PubMed] [Google Scholar]
- Thille A., Rodriguez P., Cabello B., Lellouche F., Brochard L. (2006) Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med 32: 1515–1522. [DOI] [PubMed] [Google Scholar]
- Tobin M., Jubran A., Laghi F. (2001) Patient-ventilator interaction. Am J Resp Crit Care Med 163: 1059–1063. [DOI] [PubMed] [Google Scholar]
- Walther S., Jonasson U., Karlsson S., Nordlund P., Johansson A., Malstam J. (2004) Multicentre study of validity and interrater reliability of the modified Nursing Care Recording System (NCR11) for assessment of workload in the ICU. Acta Anaesthesiol Scand 48: 690–696. [DOI] [PubMed] [Google Scholar]