Abstract
The renin–angiotensin system (RAS) plays a central role in the control of blood pressure in the body and the way this interacts with other systems is widely recognized. This has not always been the case and this review summarizes how our knowledge has evolved from the initial discovery of renin by Tigerstedt and Berman in 1898. This includes the identification of angiotensin in the 1950s to the proposed relationship between this system, hypertension and ultimately cardiovascular disease. While the RAS is far more complex than originally thought, much is now known about this system and the wide ranging effects of angiotensin in the body. This has enabled the development of therapies that target the various proteins in this pathway and hence are implicated in disease. The first of these treatments was the angiotensin converting enzyme inhibitors (ACE-Is), followed by the angiotensin receptor blockers (ARBs), and more recently the direct renin inhibitors (DRIs). Clinical outcome trials have shown these drugs to be effective, but as they act at contrasting points in the RAS, there are differences in their efficacy and safety profiles. RAS blockade is the foundation of modern combination therapy with a calcium channel blocker and/or a diuretic given to reduce blood pressure and limit the impact of RAS activation. Other options that complement these treatments may be available in the future and will offer more choice to clinicians.
Keywords: angiotensin II, cardiovascular remodelling, renin–angiotensin system (RAS)
Discovery of renin and milestones in the history of the renin–angiotensin system
Many investigators have made major contributions to our knowledge of the renin–angiotensin system (RAS), and also the renin–angiotensin aldosterone system (RAAS), during an era of discovery and translational medicine that began in 1898. This started with Tigerstedt and Bergman who showed how a crude saline renal extract from a rabbit increased blood pressure when infused into a different rabbit [Tigerstedt and Bergman, 1898]. This pressor substance, which they named renin, was a key discovery as it indicated a link between the kidneys and hypertension. There was then little progress until 1934 when Goldblatt and colleagues demonstrated that renal artery constriction with a clip caused renal ischaemia and induced hypertension in dogs [Goldblatt et al. 1934]. This was followed in 1939/40 by Braun-Menendez and colleagues in Argentina, and Page and Helmer in the USA, who independently and simultaneously discovered a crystalline pressor substance capable of causing renal hypertension. This was originally named hypertensin in Argentina and angiotonin in the USA [Braun-Menendez et al. 1940; Page and Helmer, 1940]; it was later renamed as angiotensin to reflect its discovery by both groups [Skeggs et al. 1976].
Skeggs and colleagues purified angiotensin and identified that it existed in two forms, with the precursor angiotensin I differing from angiotensin II only in terms of the histidine and leucine moiety at the C-terminus (Asp-Arg-Val-Tyr-IIe-His-Pro-Phe-His-Leu and Asp-Arg-Val-Tyr-IIe-His-Pro-Phe, respectively [Skeggs et al. 1954a, 1954b, 1955]. It was subsequently discovered that angiotensinogen (Asp-Arg-Val-Tyr-IIe-His-Pro-Phe-His-Leu-Leu-Val-Tyr), the substrate for renin in the RAS, contains an additional leucine, valine and tyrosine at the C-terminus [Skeggs et al. 1954a, 1954b, 1955]. Two groups synthesized angiotensin II, which was distributed by Ciba Pharmaceuticals to research groups [Bumpus et al. 1957; Rittel et al. 1957]. Subsequent research initiatives transformed our understanding of this protein and its effects on different tissues, prompting its experimental application.
By 1958, Gross and colleagues had proposed a hypothetical relationship between renin, angiotensin and aldosterone, and were the first to speculate that angiotensin promotes the release of aldosterone. Together these two molecules promote sodium retention in the kidney. Other scientists such as Davis, Laragh, Genest, Ganong and Mulrow added to this research and also demonstrated aldosterone secretion in response to angiotensin II. In 1969, Bakhle and colleagues showed that a bradykinin-potentiating factor, originally described by Ferreira and colleagues, inhibited the conversion of angiotensin I into angiotensin II and so a potential drug target was identified [Bakhle et al. 1969; Ferreira et al. 1970]. Rapid progress in this area enabled assay development to test for renin along with peptide antagonists for angiotensin II. This discovery of angiotensin converting enzyme (ACE) inhibition was the start of a new era of experimental intervention that provided considerable information on the RAS and its role in the pathogenesis of cardiovascular disease.
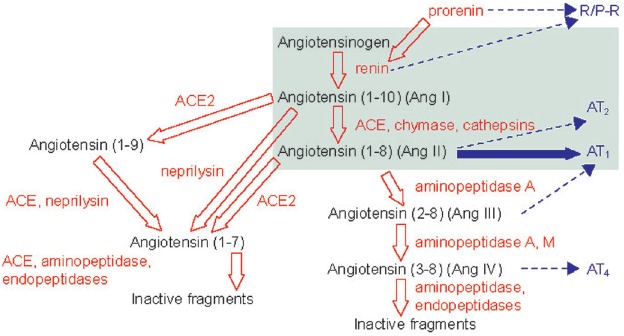
Between the late 1980s and the present day, significant work has been undertaken to determine the complexity of the RAS and the interaction of multiple enzymes and receptors involved in this process. This has been aided by endpoint trials as well as genetic models and highly sophisticated experimental techniques. Collectively, this information has allowed the molecular dissection of this system and the identification of other targets and new therapies. Furthermore, the system was shown to be more complex than originally thought (see Figure 1) [Schmieder et al. 2007]. Much is now known about the RAS and the involvement of other proteins such as ACE-2 and angiotensin-(1-7) and various receptors, but the function of some of these components is still widely unknown.
Figure 1.
Expanded view of the complexity of the renin–angiotensin system (RAS).
Reproduced with permission from Schmieder et al. [2007].
ACE, angiotensin converting enzyme; Ang, angiotensin; AT1, angiotensin II type 1; AT2, angiotensin II type 2; AT4, angiotensin II type 4; R/P-R, renin/prorenin receptor.
RAS and cardiovascular disease, friend or foe
While angiotensin is known to raise blood pressure and affect sodium balance, the full extent of its influence and wide-ranging effects have only recently been understood. Together these processes mediate many of the damaging effects of angiotensin. These include cardiac remodelling, increased oxidative stress and inflammation, direct atherothrombotic effects, lipid deposition in the vascular wall, accelerating the development of atherosclerosis, cardiovascular fibrosis, and influencing glomerular haemodynamics and permeability, thereby causing proteinuria and the progression of chronic kidney disease. Many of these effects appear independent of blood pressure per se, but are enhanced and magnified when hypertension is present.
It is likely the RAS originally existed as a repair mechanism and, in early evolution, activation of this was fundamental for the preservation of life, particularly for volume regulation in the face of trauma and/or significant blood loss [Fournier et al. 2012]. As part of this repair mechanism, the pressor action maintained blood pressure, its action on the kidney retained sodium, and the ability to stimulate coagulation, fibrosis and tissue repair promoted wound healing. Today, as trauma is less common, the RAS plays an important role in blood pressure regulation and its overactivation can be problematic; the pressor action becomes hypertension, the sodium retention a predisposition to volume overload and heart failure, and the benefits on tissue repair and coagulation lead to structural change, fibrosis and thrombosis. Inappropriate activation of this system, even at a low grade, contributes to the development of cardiovascular disease and other related conditions.
RAS as a therapeutic target to prevent cardiovascular disease
Teprotide was the first drug to target the RAS, and this synthetic nonapeptide inhibitor induced a decrease in blood pressure via inhibition of the conversion of angiotensin I to II [Cushman et al. 1973]. This was followed by the discovery of captopril, a first-in-class, orally active angiotensin converting enzyme inhibitor (ACE-I) [Cushman et al. 1979, 1980; Mylan Pharmaceuticals, 2015], which was the first treatment shown to enzymatically block the conversion of angiotensin I to angiotensin II. Angiotensin receptor blockers (ARBs), such as candesartan, were subsequently developed and these specifically block the binding of angiotensin II to the angiotensin II type 1 (AT1) receptor [AstraZeneca, 1998]. This increases their selectivity and improves tolerability compared with ACE-Is [Smith, 2002]. A more recent treatment addition includes direct renin inhibitors (DRIs) such as aliskiren [Novartis, 2015]. The clinical development and positioning of DRIs has been challenging, due in part, to the effectiveness of the ACE-Is and ARBs. Anticipated new developments include the angiotensin receptor/neprilysin inhibitor (ARNI) [Bloch and Basile, 2010; McMurray et al. 2014] in addition to angiotensin II type 2 (AT2) receptor agonists [Kaschina et al. 2008; Bosnyak et al. 2010; Rehman et al. 2012] and a possible anti-RAS vaccine [Ambuhl et al. 2007].
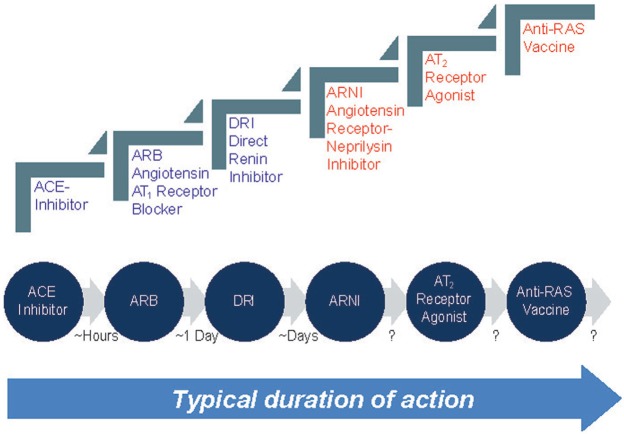
One important difference between the RAS-blocking drugs is their duration of action (Figure 2); the original ACE-I captopril requires twice daily or more frequent dosing [Mylan Pharmaceuticals, 2015], while ARBs usually need to be taken once daily [Novartis, 1996; AstraZeneca, 1998] and DRIs have been shown to be effective for a number of days once steady state blood levels are achieved after 7–8 days of dosing [Novartis, 2015]. This differing duration of action between ACE-Is and ARBs was originally shown in a study by Hermida and colleagues [Hermida et al. 2008] who investigated loss of efficacy after 16 weeks of treatment with the ACE-I enalapril or the ARB valsartan in patients with previously untreated mild to moderate hypertension. Results indicated valsartan was associated with a sustained blood pressure lowering effect beyond the initial 24 hours after dosing. Furthermore, there was no significant change in the efficacy of valsartan in the 24 hours after a missed dose. This indicated less rebound with the ARB compared with the ACE-I. The same trend is also observed with the DRIs compared with ARBs, where the DRIs show less rebound than the ARBs. In addition to duration of action, other differences in specificity and hence tolerability are seen among these treatments and these can have important consequences for patient outcomes.
Figure 2.
Evolution of renin–angiotensin system (RAS) inhibition strategies and their duration of action.
Note: Therapies included in blue are currently available in the clinic, while those in red are in only available in clinical trials or under development. This figure shows the typical duration of action of the various drug classes; however, some drugs will have longer or shorter durations of action than the typical value given in this figure. For example, some ACE inhibitors will have a longer duration of action than hours and are dosed daily, while some AT2 agonists may have a halflife of hours and require frequent dosing. It is thought the duration of action for these new therapies is likely to be longer than the action of currently available therapies.
ACE, angiotensin converting enzyme; AT1, angiotensin II type 1; AT2; angiotensin II type 2; RAS, renin–angiotensin system.
Comparison of current antihypertensive treatments and clinical outcome trials
As the first treatment to target the RAS was the ACE-I captopril, the pivotal and registration trials for this drug compared it with placebo [Mylan Pharmaceuticals, 2015]. A key challenge when introducing a new drug into a market in which there is already an effective treatment is the ethical requirement that all patients should receive appropriate treatment if available and tolerated [D’agostino et al. 2003]. This meant pivotal trials for ARBs required an ACE-I active comparator as this was the current standard of care for patients with hypertension. These trials were also powered to show noninferiority or equivalence rather than superiority and required the enrolment of a large number of patients. For DRIs, the situation is even more complex as ACE-Is or ARBs are already available for patients with hypertension and most will have already been treated with one of these classes of drug. Given that high risk patients are likely to be enrolled in these trials, there is an inherently high risk of adverse events being reported for these treatments.
Monotherapy and RAS blockade
While there is the perception ACE-Is are more effective than ARBs in reducing morbidity and mortality, noninferiority trial data are not powered to show this and instead indicate that ARBs are at least as effective as ACE-Is. One such study is the Valsartan in Heart Failure Trial (Val-HeFT), which compared valsartan (40 mg twice daily titrated to 160 mg twice daily) with placebo in 5010 patients with heart failure [New York Heart Association (NYHA) class II–IV, ejection fraction <40% and left ventricular internal diastolic diameter (LVIDd) >2.9 cm/m2) [Cohn and Tognoni, 2001]. This treatment was given in addition to standard of care, with 93% receiving an ACE-I, 86% receiving treatment with a diuretic, 67% being treated with digoxin and 35% receiving a beta-blocker. After a mean follow up of 23 months, valsartan significantly (p = 0.009) reduced the risk of the combined primary endpoint of mortality and heart failure morbidity (cardiac arrest with resuscitation, hospitalization for worsening heart failure or treatment with intravenous inotropes or vasodilators) by 13% compared with placebo. Although it was not possible to compare ARBs and placebo as the main purpose of the study, data are available for a subgroup of Val-HeFT patients who were unable to take ACE-Is due to tolerability issues (e.g. cough). In this subgroup analysis of 366 patients who took valsartan, the risk of the combined primary endpoint (all-cause mortality, cardiac arrest with resuscitation, hospitalization for worsening heart failure or treatment with intravenous inotropes or vasodilators) was significantly reduced by 44% (p < 0.001) compared with placebo [Maggioni et al. 2002].
Diabetes and RAS blockade
Hypertension and diabetes are closely linked and tend to be seen in the same person more often than would be expected by chance alone [Cheung, 2010]. While increased body weight is commonly observed in people with hypertension and/or diabetes, this alone does not fully explain the inverse relationship between hypertension and insulin sensitivity [Sironi et al. 2004]. An increased deposition of visceral fat in individuals with hypertension has been implicated in this process, which occurs as a result of the antagonism of angiotensin on insulin signalling [Motley et al. 2003]. As this antagonism occurs through the AT1 receptor, there is a biological basis for the hypothesis that blocking the RAS will improve insulin sensitivity and also offer some level of protection against renal damage [Cheung, 2010].
Some studies, including a meta-analysis, compared the effect of different antihypertensive drugs on the development of new-onset diabetes [Elliott and Meyer, 2007]. Results showed that in comparison with a diuretic, the rank order for reducing the risk of developing new-onset diabetes was ARB [odds ratio (OR): 0.57; p < 0.001], ACE-I (OR: 0.67; p < 0.0001), placebo (OR: 0.77; p = 0.009), calcium channel blocker (OR: 0.75; p = 0.002) and beta-blockers (OR: 0.90; p = 0.30). When compared with placebo rather than the diuretic, only the ARB (OR: 0.75; p = 0.003) and the diuretic (OR: 1.30; p = 0.009) maintained significance. While not a powerful effect, clinicians can conclude that ARBs, unlike beta-blockers and diuretics, will not worsen the risk of diabetes.
Combination therapy and dual blockade of the RAS
The VALsartan In Acute myocardial iNfarcTion Trial (VALIANT) directly compared valsartan 160 mg twice daily (n = 4,909) with captopril 50 mg 3 times daily (n = 4,909) and combination therapy with valsartan plus captopril (n = 4,885) [Pfeffer et al. 2003]. This study enrolled high risk patients who had clinical or radiological signs of heart failure and/or evidence of left ventricular systolic dysfunction and received treatment in the 12 hours to 10 days following an acute myocardial infarction (MI). Data show the risk of death with valsartan was similar to that observed with captopril, with no additional benefits from combination therapy with valsartan and captopril in this population (the lack of benefit for combination therapy is discussed in full later in this article).
The findings of VALIANT regarding ARB and ACE-I combination therapy were confirmed by ON-TARGET. This study investigated combination therapy with the ACE-I ramipril and the ARB telmisartan compared with ramipril or telmisartan monotherapy [Yusuf et al. 2008]. This study showed there was no advantage in giving combination therapy over monotherapy and the risk of adverse events was higher with an ACE-I and an ARB. Furthermore, it confirmed the ARB was replicating the benefit of the ACE-I, although the ARB was better tolerated.
The ALTITUDE study investigated the benefits of the DRI aliskiren as an add-on to an ACE-I or an ARB in patients with high cardiovascular or renal risk and type 2 diabetes [Parving et al. 2012]. This study showed that dual RAS blockade in patients who had diabetes and renal complications was associated with increased hyperkalaemia, hypotension and general side effects compared with monotherapy. In addition, DRI add-on therapy was not associated with any benefit and the authors concluded that this may even do some harm. ALTITUDE therefore ended the concept of dual RAS blockade for high risk patients, but its findings do not mean the DRI was ineffective, rather aliskiren as an add-on to an ACE-I was not appropriate and DRIs are effective when given as monotherapy [Novartis, 2015].
Due to the limitations of dual blockade of the RAS with an ARB and ACE-I combination therapy or a DRI as add-on to an ACE-I or ARB, other options are needed as many patients with hypertension require more than a single drug to achieve blood pressure targets [James et al. 2014]. RAS blockade, irrespective of its benefits in reducing target organ damage, is fundamental to improving the efficacy of other blood pressure lowering drugs [Mancia et al. 2014]. Indeed, modern combination therapy typically includes a RAS blocker as part of the treatment regimen alongside a diuretic or calcium channel blocker, and other options may be possible in the future.
Future antihypertensive treatments
Several new therapies are under investigation as antihypertensive treatments and include vaccination with an angiotensin II-derived peptide conjugated to an adjuvant [Ambuhl et al. 2007]. While this concept is attractive in terms of treatment compliance, many questions remain over the durability of the effect and the safety of the approach. Indeed, concerns exist over the development of off-target effects, including immune complex disease or irreversibly blocking the RAS in patients who subsequently become volume depleted or go into systemic shock. In addition, vaccination is unlikely to control blood pressure on its own, and as most people will need to take antihypertensives, the requirement for daily dosing will remain.
An alternative approach has investigated the use of an oral AT2 agonist, a receptor which is upregulated in response to damage and tissue remodelling. Additionally, AT2 has been shown to release nitric oxide, and has antiproliferative as well as anti-inflammatory properties. An AT2 receptor agonist, C21, is currently in preclinical testing and this has shown the potential to reduce hypertension when given with an ARB [Bosnyak et al. 2010], as well as improve endothelial function and vascular composition [Rehman et al. 2012], left ventricular function, remodelling post-MI and reduce the infarct size [Kaschina et al. 2008]. Furthermore, C21 has demonstrated antifibrotic and neuroprotective effects [Unger and Dahlof, 2010] and is anti-inflammatory [Kaschina et al. 2008; Rompe et al. 2010]. This treatment therefore has the potential to become a useful add-on therapy to an ARB and/or may have benefit in other clinical indications.
Another exciting development is LCZ696, a first-in-class ARNI. LCZ696 potentiates the benefits of natriuretic peptides and induces a physiological response in addition to blocking the RAS and reducing the pathological effects of this system. This treatment has been tested in proof of concept trials [Bloch and Basile, 2010; Gu et al. 2010], in addition to the PARADIGM study [McMurray et al. 2014]. Here it was shown to be superior to the ACE-I enalapril in terms of reducing morbidity and mortality over standard care in patients with systolic heart failure (class II, III or IV heart failure) and an ejection fraction of 40%. Further investigations are currently underway in the PARAMETER (Prospective comparison of Angiotensin Receptor neprilysin with Angiotensin receptor blocker Measuring arterial sTiffness in the elderly) study [Williams et al. 2014]. It is anticipated these results will be published in 2016 and it is hoped this treatment has the potential to inhibit cardiac remodelling and fibrosis, arterial stiffness and renal fibrosis, as well as improve renal haemodynamics. LCZ696 may also reduce vascular aging and aortic stiffness; this is discussed elsewhere in this special issue.
Conclusion
Our knowledge of the RAS has evolved over the past decades and the blockade of this system is an important foundation for the treatment for hypertension, heart failure and chronic renal disease. Many different therapeutic options are available that target the RAS and these treatments have been shown to improve patient outcomes. While data exist to confirm the benefits of an ARB over an ACE-I in terms of blood pressure lowering and duration of effect, similar benefits are seen in terms of other clinical outcomes and both classes are better than diuretics at lowering diabetes risk. The adverse event rates, however, are lower with ARBs, and these drugs are better tolerated as they are associated with lower rates of cough and angioedema. RAS blockade therefore remains the foundation of modern combination therapy with a calcium channel blocker and/or a diuretic given to reduce blood pressure and limit the impact of RAS activation.
While considerable research is ongoing into potential new therapies that target the RAS, it is unlikely we will see the emergence of a new mass treatment RAS inhibition target. These new therapies are more likely to target niche applications or new treatment modalities such as vaccines, which can be used alongside existing therapies, or include complementary treatment strategies like combining an ARB and a neprilysin inhibitor. Clinical data indicate that these new therapeutic targets have shown promise and may enhance what is already delivered by highly effective and well-established treatments, such as ACE-Is and ARBs.
Acknowledgments
This manuscript is based on a transcript of a lecture delivered by B.W. at a Renin–angiotensin System Masterclass Medical meeting held in Shanghai, China, in June 2015 and sponsored by Beijing Novartis Pharma Co. Ltd. Editorial assistance was provided by Elaine O’Prey of Novartis Ireland Ltd., Dublin, Ireland.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: B.W. has received travel grants and speaker fees from Novartis, Boehringer Ingelheim, Servier, Daiichi Sankyo and Pfizer.
References
- Ambuhl P., Tissot A., Fulurija A., Maurer P., Nussberger J., Sabat R., et al. (2007) A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. J Hypertens 25: 63–72. [DOI] [PubMed] [Google Scholar]
- AstraZeneca (1998) Atacand® (candesartan cilexetil) prescribing information. Wilmingont, DE: AstraZeneca. [Google Scholar]
- Bakhle Y., Reynard A., Vane J. (1969) Metabolism of the angiotensins in isolated perfused tissues. Nature 222: 956–959. [DOI] [PubMed] [Google Scholar]
- Bloch M., Basile J. (2010) Combination angiotensin receptor blocker-neutral endopeptidase inhibitor provides additive blood pressure reduction over angiotensin receptor blocker alone. J Clin Hypertens 12: 809–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnyak S., Welungoda I., Hallberg A., Alterman M., Widdop R., Jones E. (2010) Stimulation of angiotensin AT2 receptors by the non-peptide agonist, compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br J Pharmacol 159: 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun-Menendez E., Fasciolo J., Leloir L., Muñoz J. (1940) The substance causing renal hypertension. J Physiol 98: 283–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus F., Schwarz H., Page I. (1957) Synthesis and pharmacology of the octapeptide angiotonin. Science 125: 886–887. [DOI] [PubMed] [Google Scholar]
- Cheung B. (2010) The hypertension-diabetes continuum. J Cardiovasc Pharmacol 55: 333–339. [DOI] [PubMed] [Google Scholar]
- Cohn J., Tognoni G. (2001) A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 345: 1667–1675. [DOI] [PubMed] [Google Scholar]
- Cushman D., Cheung H., Sabo E., Rubin B., Ondetti M. (1979) Development of specific inhibitors of angiotensin I converting enzyme (kininase II). Fed Proc 38: 2778–2782. [PubMed] [Google Scholar]
- Cushman D., Ondetti M., Cheung H., Antonaccio M., Murthy V., Rubin B. (1980) Inhibitors of angiotensin-converting enzyme. Adv Exp Med Biol 130: 199–225. [DOI] [PubMed] [Google Scholar]
- Cushman D., Pluscec J., Williams N., Weaver E., Sabo E., Kocy O., et al. (1973) Inhibition of angiotensin-coverting enzyme by analogs of peptides from Bothrops jararaca venom. Experientia 29:1032–1035. [DOI] [PubMed] [Google Scholar]
- D’agostino R., Sr, Massaro J., Sullivan L. (2003) Non-inferiority trials: design concepts and issues – the encounters of academic consultants in statistics. Stat Med 22: 169–186. [DOI] [PubMed] [Google Scholar]
- Elliott W., Meyer P. (2007) Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet 369: 201–207. [DOI] [PubMed] [Google Scholar]
- Ferreira S., Greene L., Alabaster V., Bakhle Y., Vane J. (1970) Activity of various fractions of bradykinin potentiating factor against angiotensin I converting enzyme. Nature 225: 379–380. [DOI] [PubMed] [Google Scholar]
- Fournier D., Luft F., Bader M., Ganten D., Andrade-Navarro M. (2012) Emergence and evolution of the renin–angiotensin-aldosterone system. J Mol Med 90: 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblatt H., Lynch J., Hanzal R., Summerville W. (1934) Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med 59: 347–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross F. (1958) Renin und Hypertensin, physiologische oder pathologische Wirkstoffe? Klin Wschr 36: 693. [DOI] [PubMed] [Google Scholar]
- Gu J., Noe A., Chandra P., Al-Fayoumi S., Ligueros-Saylan M., Sarangapani R., et al. (2010) Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNI). J Clin Pharmacol 50: 401–414. [DOI] [PubMed] [Google Scholar]
- Hermida R., Ayala D., Khder Y., Calvo C. (2008) Ambulatory blood pressure-lowering effects of valsartan and enalapril after a missed dose in previously untreated patients with hypertension: a prospective, randomized, open-label, blinded end-point trial. Clin Ther 30: 108–120. [DOI] [PubMed] [Google Scholar]
- James P., Oparil S., Carter B., Cushman W., Dennison-Himmelfarb C., Handler J., et al. (2014) 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311: 507–520. [DOI] [PubMed] [Google Scholar]
- Kaschina E., Grzesiak A., Li J., Foryst-Ludwig A., Timm M., Rompe F., et al. (2008) Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin–angiotensin system in myocardial infarction? Circulation 118: 2523–2532. [DOI] [PubMed] [Google Scholar]
- Maggioni A., Anand I., Gottlieb S., Latini R., Tognoni G., Cohn J. (2002) Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J Am Coll Cardiol 40: 1414–1421. [DOI] [PubMed] [Google Scholar]
- Mancia G., Fagard R., Narkiewicz K., Redon J., Zanchetti A., Bohm M., et al. (2014) 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press 23: 3–16. [DOI] [PubMed] [Google Scholar]
- McMurray J., Packer M., Desai A., Gong J., Lefkowitz M., Rizkala A., et al. (2014) Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- Motley E., Eguchi K., Gardner C., Hicks A., Reynolds C., Frank G., et al. (2003) Insulin-induced AKT activation is inhibited by angiotensin II in the vasculature through protein kinase C-alpha. Hypertension 41: 775–780. [DOI] [PubMed] [Google Scholar]
- Mylan Pharmaceuticals (2015) Captopril prescribing information. Canonsburg, PA: Mylan Pharmaceuticals Inc. [Google Scholar]
- Novartis (1996) Diovan (valsartan) prescribing information. East Hanover, NJ: Novartis. [Google Scholar]
- Novartis (2015) Tekturna (Aliskiren) prescribing information. East Hanover, NJ: Novartis. [Google Scholar]
- Page I., Helmer O. (1940) A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. J Exp Med 71: 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parving H., Brenner B., McMurray J., De Zeeuw D., Haffner S., Solomon S., et al. (2012) Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 367: 2204–2213. [DOI] [PubMed] [Google Scholar]
- Pfeffer M., McMurray J., Velazquez E., Rouleau J., Kober L., Maggioni A., et al. (2003) Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 349: 1893–1906. [DOI] [PubMed] [Google Scholar]
- Rehman A., Leibowitz A., Yamamoto N., Rautureau Y., Paradis P., Schiffrin E. (2012) Angiotensin type 2 receptor agonist compound 21 reduces vascular injury and myocardial fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension 59: 291–299. [DOI] [PubMed] [Google Scholar]
- Rittel W., Iselin I., Kappeler H., Riniker B., Schwyzer R. (1957) Synthese eines hochwirksamen hypertensin II-amids. Helv Chim Acta 40: 614–624. [Google Scholar]
- Rompe F., Artuc M., Hallberg A., Alterman M., Stroder K., Thone-Reineke C., et al. (2010) Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappaB. Hypertension 55: 924–931. [DOI] [PubMed] [Google Scholar]
- Schmieder R., Hilgers K., Schlaich M., Schmidt B. (2007) Renin–angiotensin system and cardiovascular risk. Lancet 369: 1208–1219. [DOI] [PubMed] [Google Scholar]
- Sironi A., Gastaldelli A., Mari A., Ciociaro D., Positano V., Buzzigoli E., et al. (2004) Visceral fat in hypertension: influence on insulin resistance and beta-cell function. Hypertension 44: 127–133. [DOI] [PubMed] [Google Scholar]
- Skeggs L., Dorer F., Kahn J., Lentz K., Levine M. (1976) The biochemistry of the renin–angiotensin system and its role in hypertension. Am J Med 60: 737–748. [DOI] [PubMed] [Google Scholar]
- Skeggs L., Jr., Marsh W., Kahn J., Shumway N. (1954a) The existence of two forms of hypertensin. J Exp Med 99: 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeggs L., Jr., Marsh W., Kahn J., Shumway N. (1954b) The purification of hypertensin I. J Exp Med 100: 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeggs L., Jr., Marsh W., Kahn J., Shumway N. (1955) Amino acid composition and electrophoretic properties of hypertensin I. J Exp Med 102: 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. (2002) Strategies to meet lower blood pressure goals with a new standard in angiotensin II receptor blockade. Am J Hypertens 15: 108s–114s. [DOI] [PubMed] [Google Scholar]
- Tigerstedt R., Bergman P. (1898) Niere und kreislauf. Skandinavisches Archiv Physiolgie 8:223–271. [Google Scholar]
- Unger T., Dahlof B. (2010) Compound 21, the first orally active, selective agonist of the angiotensin type 2 receptor (AT2): implications for AT2 receptor research and therapeutic potential. J Renin–angiotensin Aldosterone Syst 11: 75–77. [DOI] [PubMed] [Google Scholar]
- Williams B., Cockcroft J., Kario K., Zappe D., Cardenas P., Hester A., et al. (2014) Rationale and study design of the prospective comparison of angiotensin receptor neprilysin inhibitor with angiotensin receptor blocker measuring arterial stiffness in the elderly (PARAMETER) study. BMJ Open 4: e004254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S., Teo K., Pogue J., Dyal L., Copland I., Schumacher H., et al. (2008) Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 358: 1547–1559. [DOI] [PubMed] [Google Scholar]