Abstract
This review discusses the relationship between elevated blood pressure, hypertension, arterial stiffness and hence vascular ageing. This is a complex process and the majority of treatments target the consequences of this, rather than the pathophysiology of ageing itself. This is because preventing vascular ageing from occurring is complex and would require very early intervention and lifelong treatment. The process of arteriosclerosis is known to result from reversible and irreversible functional components, and, together, these are responsible for the increased systolic and decreased diastolic blood pressure seen with advancing age. Indeed, hypertension develops as it becomes more difficult for the heart to drive blood flow around the body, as a result of poor ventricular coupling and increased arterial stiffness. Elevated blood pressure is therefore a clinical manifestation of ageing that continues to increase with advancing years, and is also linked with an increased risk of cardiac, cerebrovascular and chronic kidney disease. These manifestations arise due to changing haemodynamics associated with ageing, and therefore treatments that reduce the development of these conditions or delay their progression have the potential to improve patient outcomes. This may be possible with existing therapies as well as new treatments currently under investigation.
Keywords: angiotensin II, cardiovascular remodelling, renin–angiotensin system
Arteriosclerosis and vascular ageing
The majority of treatments for vascular ageing target the consequences, rather than the underlying pathophysiology, of ageing itself. While considerable information on atherosclerosis is available, arteriosclerosis (or stiffening and ageing of the arteries) has received less attention. Such stiffening is responsible for increased systolic and pulse pressure observed in patients, with both acting as major risk factors for stroke and heart failure. Stiffening is frequently measured as an increase in pulse wave velocity and pulsatile flow to the target organs. In diabetic patients, an increase in microvascular pressure is also commonly seen. This arteriosclerosis may result from a reversible functional component, such as endothelial dysfunction, or changes in smooth muscle tone. More commonly, such arteriosclerosis is due to an irreversible structural component like elastin loss, collagen deposition, glycation of proteins in the vascular cell wall, or calcification of the vessels (especially in people with chronic kidney disease) [Williams, 2009].
At a cellular level, ageing can be determined by the length of telomeres: small segments of DNA occurring at the end of chromosomes that are essential for maintaining DNA integrity during replication. As cells age, telomeres shorten and their length can be used to age the cell [Herbert et al. 2008]. An association between vascular disease and telomere shortening in leucocytes isolated from the aorta has previously been shown [Wilson et al. 2008]. This is reflected systemically, rather than confined to the aorta, and leucocyte DNA is a good indicator of vascular age. Even when corrected for age and gender, leucocytes from healthy individuals have longer telomeres than those isolated from individuals with vascular disease. Accelerated telomere shortening is also observed in patients at increased cardiovascular risk due to the increased vascular age of the cell. It is likely this vascular ageing develops as a result of oxidative stress, a finding confirmed in preclinical studies with angiotensin II [Herbert et al. 2008]. Exposure to angiotensin II, a powerful pro-inflammatory molecule, was linked with the generation of oxidative free radicals in vascular smooth muscle cells, and damage to cellular DNA, particularly in the telomere region. Treatment with an angiotensin receptor blocker (ARB) can, however, reduce this vascular damage to below baseline values and reduce cellular damage. By selectively blocking the effect of angiotensin II at the angiotensin II receptor, type 1 (AT1), ARBs may prevent cellular-mediated ageing effects.
Preclinical studies with angiotensin-converting enzyme inhibitors (ACE-Is) also show these drugs have an effect on ageing, with ACE-Is shown to increase life span in mice [Ferder et al. 2002; Basso et al. 2005]. This effect is due to the direct acceleration of ageing by the renin–angiotensin system (RAS) and not simply a lowering of blood pressure (BP). Other antihypertensive treatments, such as calcium channel blockers and diuretics did not increase life span. Beta-blockers were, however, associated with some increase in life span, likely due to suppression of plasma renin activity and hence angiotensin II. [Blumenfeld et al. 1999; Ferder et al. 2002] In rats, treatment with an ARB or ACE-I was shown to prevent age-related increases in heart weight and left ventricular enlargement, while also reducing collagen and fibrosis in the aorta (and, hence, reduced aortic mass). These preclinical studies indicate drugs that target the RAS protect against vascular ageing and allow the maintenance of function. This provides a rationale for targeting the RAS to reduce vascular ageing.
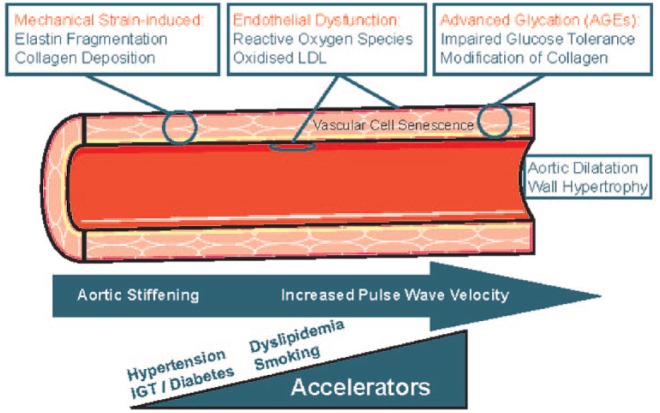
Arterial pressure primarily exists to drive blood flow around the body. In young individuals, arteries are often relaxed, there is a good coupling with the ventricle, and little pressure is needed to drive the blood around the body. However, as the body ages, arteries stiffen, and this creates a highly resistive and low conductive system that requires higher pulse pressure to drive the flow, ultimately resulting in hypertension. Should a treatment be available to reduce this resistance and limit the stiffness in the aorta, the pathology behind much of the hypertension we see clinically would be reversed. While many factors and processes may represent potential targets (Figure 1), it is the mechanical strain present in the vessel wall that is crucial. This is because it causes the irreversible fragmentation of elastin and the deposition of collagen and hence leads to stiffening [Williams, 2009]. Endothelial dysfunction also plays a role in this process and the smooth muscle in the vascular wall will stiffen, but this is potentially a reversible process. Here, the stiffening is due to oxidative stress and the presence of reactive oxygen species (ROS) and oxidized low-density lipoprotein (LDL) in the subintima. The third process implicated in the pathophysiology of arterial ageing involves post-translational modification of collagen with advanced glycation endproducts (AGEs). This is particularly relevant in patients with diabetes [Williams, 2009]. Together these processes lead to aortic stiffening, dilatation and wall hypertrophy and manifest as an increase in pulse wave velocity. Indeed, pulse wave velocity is typically 5 or 6 m/s at age 20 years, but will double throughout life and can be as high as 10–15 m/s at 70–80 years. This increase in pulse wave velocity can be predicted by age due to the accumulation of damage and elevated BP, as this stiffens and distends the aorta [Cecelja and Chowienczyk, 2009].
Figure 1.
Pathophysiology of arterial ageing.
Reproduced with permission from Williams [2009].
Blood pressure and ageing
BP is a clinical manifestation of ageing that increases with advancing age. The majority of cases of hypertension occur in those aged over 40 years, and from about 50 years systolic blood pressure (SBP) increases while diastolic blood pressure (DBP) decreases. This divergence leads to a widening of pulse pressure. It is this pulse pressure that is a manifestation of stiffening of the aorta and arterial ageing. Pulse pressure also increases pulsatile flow and causes stress in target organs. Most cases of hypertension seen in the clinic are a result of ageing of the vascular cell wall. The majority of these individuals will typically present with isolated systolic hypertension or systolic–diastolic hypertension [Franklin et al. 2001; Williams et al. 2008]. The incidence of isolated systolic hypertension increases from around 20% in untreated people with hypertension aged up to 40 years to 54% in those aged 50–59 years, to 87% in those 60–69 years, and accounts for almost 95% of cases in those aged over 70 years [Franklin et al. 2001]. Similar incidences of systolic hypertension were also seen with increasing age in patients with inadequately treated hypertension [Franklin et al. 2001]. As most disease is caused by systolic hypertension, which is often inadequately treated, this remains an unresolved problem and an unmet treatment need. Targeting the processes responsible for the increased SBP observed as individuals age, may prevent or delay the development of hypertension.
Identifying the direct effects of a drug on reducing stiffness in the aorta wall can be challenging, particularly if the drug also alters the patient’s BP, as this can affect stiffness and distension of the aorta. One option to measure flow is magnetic resonance imaging (MRI). This can be used to assess compliance in the aorta and calculate impedance. Impedance is a useful indicator, as it indicates the pressure required to drive flow in the aorta and is a measure of the coupling of the ventricle to the aortic root. As such, increased impedance is fundamental to the development of heart failure in patients with hypertension and contributes to increased left ventricular afterload and left ventricular hypertrophy [Gottdiener et al. 1985; Krzesinski et al. 2015].
By the age of 50 years, the accelerated loss of aortic root compliance is almost complete, with very little change seen after 50 years [Redheuil et al. 2010]. This is because most damage occurs in the first 50 years of life, so the aorta has already stiffened to the point at which systolic hypertension can develop. Therefore, any intervention that aims to reduce vascular age should be given before 50 years of age as this may prevent progressive aortic stiffening. Due to these age-related differences, hypertension can be thought of as two different entities; the first in younger patients and the second in older patients. The steady component (mean pressure disease) is seen in younger patients and predominantly results from mean arterial pressure and DBP, that increase cardiac output and systemic vascular resistance. A pulsatile component (pulse pressure disease) develops in older patients due to increased pulse pressure (systolic pressure), aortic stiffening, increased pulse wave velocity and pulsatile flow. A transition from the first state into the second occurs as a result of mean pressure increases and age-related damage to the aortic wall. This transition can be accelerated by inflammation, the presence of vascular risk factors and the effect of angiotensin II, which can increase cellular senescence, fibrosis and ageing.
Age-related changes in pulse rate also occur and, while the radial pulse pressure will double between 18 and 97 years, the aortic pulse pressure will quadruple. This means that once the vessels begin to age, the pressure that is experienced centrally increases dramatically. This rapid increase in pulse pressure may explain the increased rate of stroke and other pressure-dependent conditions seen in patients over 50 years.
Consequences of uncontrolled hypertension
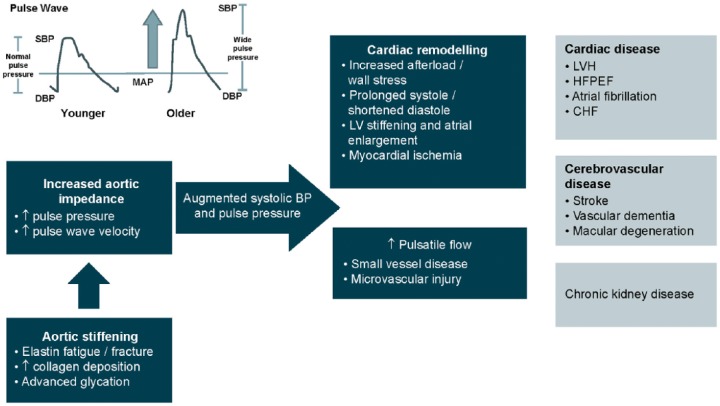
The impact of aortic ageing and hypertension and the consequences of lack of treatment are summarized in Figure 2. If untreated, aortic impedance and stiffening continue to increase systolic and pulse pressure. Together these processes augment the ageing process and lead to a range of deleterious effects such as cardiac remodelling and impaired ventricular and vascular coupling [Kass, 2005; Lantelme et al. 2008]. Arterial stiffness has also been linked with damage to the brain microvasculature, including subcortical and white matter infarcts, small vessel disease, and microvascular injury [Mitchell et al. 2011]. Ultimately hypertension will result in end-organ damage manifested as cardiac disease (including heart failure), cerebrovascular disease and chronic kidney disease. As these manifestations arise due to changing haemodynamics associated with ageing, they represent potential therapeutic targets. This may reduce the development of these conditions or delay their progression and therefore improve patient outcomes.
Figure 2.
Disease impact of aortic ageing and hypertension.
The overall pathophysiology of arterial ageing can be accelerated by known risk factors for cardiovascular and heart disease, such as hypertension, impaired glucose tolerance, diabetes, dyslipidaemia and smoking. Reducing these risk factors has the potential to delay arterial ageing, but this needs further investigation.
Current treatment options for hypertension
Ideally, treatment of hypertension should prevent vascular ageing from occurring; however, this is difficult as it requires very early intervention and lifelong treatment. An alternative is to treat the consequences of vascular ageing, primarily through lowering systolic pressure and pulse pressure. Such treatment requires vasodilation and diuresis to offload the volume and reduce the wall stress on the aorta as well as RAS blockade. In addition, RAS blockade exerts effects that are independent of BP control, with improvements in the compliance of the aorta reported as well as evidence that targeting the RAS with an ARB can delay development of hypertension [Julius et al. 2006]. This was confirmed in the trial of preventing hypertension (TROPHY) study in which treatment of prehypertension with candesartan 16 mg daily (n = 391) was associated with a relative risk reduction of 66% compared with placebo in delaying the development of hypertension in people aged 30–65 years [Julius et al. 2006].
Currently available antihypertensive treatments include ACE-Is and ARBs as well as calcium channel blockers, diuretics, and beta-blockers. Despite this wide range of options, many patients continue to have inadequately controlled hypertension, and in particular, isolated systolic hypertension. This lack of control of systolic hypertension indicates the challenging nature of this condition and a continued unmet need.
Clinical studies comparing different antihypertensive treatments
A small study (n = 32) demonstrated how different antihypertensive treatments cause varied and disproportionate changes in pressure in the brachial artery compared with the aorta [Morgan et al. 2004]. For ACE-Is, the reduction in brachial pressure was lower than expected based on the significant reduction in pressure seen in the ascending aorta. This suggests ACE-I have a favourable effect on the performance of the vascular system. The results seen with ACE-Is contrast with the findings for beta-blockers. These have an exaggerated pressure lowering benefit in the brachial artery compared with their effect in the aorta. Calcium channel blockers and diuretics have similar BP lowering effects on both the brachial artery and aorta and so the fall in these pressures parallels the fall seen in SBP and DBP. These findings were confirmed in the large-scale conduit artery function evaluation (CAFE) study (n = 2199) [Williams et al. 2006], which compared a beta-blocker–diuretic combination of atenolol ± a thiazide with the calcium channel blocker-ACE-I combination of amlodipine ± perindopril. In this study, similar reductions in brachial SBPs were seen, but there was a substantially greater reduction in central aortic pressure with the amlodipine-based regimen (p<0.0001 for both central aortic systolic pressure and central aortic pulse pressure). This suggests the amlodipine-based treatment has a beneficial effect on vascular function. This treatment was also associated with a 25% additional reduction in stroke. This finding was confirmed in a study that compared the ARB valsartan with the beta-blocker atenolol when both were given in combination with the calcium channel blocker amlodipine [Boutouyrie et al. 2010]. Brachial systolic and pulse pressure were similar with these treatments, but a greater reduction in aortic systolic and aortic pulse pressures were seen with the ARB.
More recently, the differential effect of antihypertensive drugs has been assessed by determining the cardio-ankle vascular index (CAVI), which is an independent measure of arterial stiffness [Miyashita et al. 2009]. Miyashita and colleagues compared treatment with the ARB olmesartan with the calcium channel blocker amlodipine in patients with hypertension and diabetes (n = 70). Data obtained confirmed the findings from earlier studies and, after 12 months of treatment, similar effects on BP were seen with both treatments. However, the ARB was able to improve arterial stiffness more than the calcium channel blocker. This confirms a differential impact of antihypertensive drugs on the ageing process even in patients who already have stiffened arteries.
Future therapies
While omapatrilat has now been withdrawn from the market due to reports of serious angioedema, its development provided a rationale for dual blockage with the inhibition of the RAS and inactivation of natriuretic peptides (NPs). Omapatrilat, containing an ACE-I and neutral endopeptidase (NEP) activity, was investigated in 167 patients with systolic hypertension who received either omapatrilat or the ACE-I enalapril for 12 weeks [Mitchell et al. 2002]. Omapatrilat was twice as effective as enalapril at reducing brachial pulse pressure (p < 0.05). In addition, omapatrilat administration directly affected the function of the aorta by significantly reducing central pulse pressure (p < 0.01) and impedance (p < 0.001 and p < 0.05 after adjusting for mean pressure). NEP inhibition likely potentiates brain natriuretic peptide (BNP) and atrial natriuretic peptide (ANP), which are released when the heart is under stress [Bonow, 1996] These peptides act as diuretics to reduce volume and reduce wall stress, as well as acting directly on the aortic route to improve coupling with the ventricle. This improved coupling translates into a requirement for less pressure to eject blood and is a natural destiffening mechanism. Inhibition of NEP, in addition to RAS blockade, potentially improves performance in the ventricle by reducing central pulse pressure and impedance and should be of benefit to patients with heart failure (including those with preserved ejection fraction).
This hypothesis was investigated with LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor (ARNI) [Ruilope et al. 2010]. Patients (n = 1328) with mild to moderate hypertension were randomized to eight weeks of treatment with different doses of LCZ696, valsartan, AHU337 (a NEP), or placebo. Data showed treatment with LCZ696 was almost twice as effective in reducing mean sitting SBP and DBP and ambulatory pulse pressure compared with valsartan monotherapy. This indicates there is additive and complementary mechanism of action for the ARB and NEP in the ARNI. Treatment was well tolerated, with no cases of angioedema.
LCZ696 has been investigated in the prospective comparison of angiotensin receptor neprilysin with angiotensin receptor blocker measuring arterial stiffness in the elderly (PARAMETER) study. This 52-week study enrolled patients aged 60 or over with a mean sitting SBP ⩾150 to <180 mmHg and a pulse pressure of >60 mmHg to treatment with either LCZ696 (200 mg) or the ARB olmesartan (20 mg) for 4 weeks. This was followed by a forced titration to double the initial doses over the next 8 weeks. After this time period, patients who had not achieved their BP target were able to add in the calcium channel blocker amlodipine or a thiazide diuretic to achieve control. The aim of this study is to evaluate the mechanisms associated with BP lowering in elderly patients. It is anticipated results will be published in early 2016. Furthermore, this study aims to confirm the benefit of LCZ696 on central aortic systolic and central aortic pulse pressure. It will also be interesting to see if the effect is related to a BP-independent reduction and therefore due to a novel mechanism of action [Williams et al. 2014] An MRI study to investigate the mode of action of LCZ696 is also ongoing.
Conclusion
The RAS has been implicated in the underlying biological ageing of cells, and data are available to show that in small animals, blockade of the RAS is able to increase life span. One explanation for this is the association between vascular ageing, arterial stiffening, and increased systolic blood and pulse pressure. Targeting vascular stiffness effectively may therefore require much earlier intervention than current treatment strategies allow.
Current antihypertensive therapies such as RAS blockade with ACE-Is or ARBs, calcium channel blockers or diuretics (or a combination of these interventions) are effective at lowering systolic blood pressure, which develops as a consequence of arterial stiffness. In contrast to currently available therapies, future treatments may have a different role to play as these may be directed at improving ventricular to vascular coupling so providing a more effective reduction in pulse pressure, and hence are better able to prevent the development of heart failure in patients with hypertension.
Acknowledgments
This manuscript is based on a transcript of a lecture delivered by Professor Bryan Williams. This was originally presented at a Renin–Angiotensin System Masterclass Medical meeting held in Shanghai, China in June 2015, sponsored by Beijing Novartis Pharma Co. Ltd. Editorial assistance was provided by Elaine O’Prey of Novartis Ireland Ltd, Dublin, Ireland.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Professor Bryan Williams has received travel grants and speaker fees from Novartis, Boehringer Ingelheim, Servier, Daiichi Sankyo and Pfizer.
References
- Basso N., Paglia N., Stella I., De Cavanagh E., Ferder L., Del Rosario Lores Arnaiz M., et al. (2005) Protective effect of the inhibition of the renin–angiotensin system on aging. Regul Pept 128: 247–252. [DOI] [PubMed] [Google Scholar]
- Blumenfeld J., Sealey J., Mann S., Bragat A., Marion R., Pecker M., et al. (1999) Beta-adrenergic receptor blockade as a therapeutic approach for suppressing the renin–angiotensin-aldosterone system in normotensive and hypertensive subjects. Am J Hypertens 12: 451–459. [DOI] [PubMed] [Google Scholar]
- Bonow R. (1996) New insights into the cardiac natriuretic peptides. Circulation 93: 1946–1950. [DOI] [PubMed] [Google Scholar]
- Boutouyrie P., Achouba A., Trunet P., Laurent S. (2010) Amlodipine-valsartan combination decreases central systolic blood pressure more effectively than the amlodipine-atenolol combination: the explorer study. Hypertension 55: 1314–1322. [DOI] [PubMed] [Google Scholar]
- Cecelja M., Chowienczyk P. (2009) Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension 54: 1328–1336. [DOI] [PubMed] [Google Scholar]
- Ferder L., Inserra F., Basso N. (2002) Advances in our understanding of aging: role of the renin–angiotensin system. Curr Opin Pharmacol 2: 189–194. [DOI] [PubMed] [Google Scholar]
- Franklin S., Jacobs M., Wong N., L’italien G., Lapuerta P. (2001) Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on national health and nutrition examination survey (NHANES) IIII. Hypertension 37: 869–874. [DOI] [PubMed] [Google Scholar]
- Gottdiener J., Gay J., Maron B., Fletcher R. (1985) Increased right ventricular wall thickness in left ventricular pressure overload: echocardiographic determination of hypertrophic response of the ‘nonstressed’ ventricle. J Am Coll Cardiol 6: 550–555. [DOI] [PubMed] [Google Scholar]
- Herbert K., Mistry Y., Hastings R., Poolman T., Niklason L., Williams B. (2008) Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ Res 102: 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius S., Nesbitt S., Egan B., Weber M., Michelson E., Kaciroti N., et al. (2006) Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med 354: 1685–1697. [DOI] [PubMed] [Google Scholar]
- Kass D. (2005) Ventricular arterial stiffening: integrating the pathophysiology. Hypertension 46: 185–193. [DOI] [PubMed] [Google Scholar]
- Krzesinski P., Uzieblo-Zyczkowska B., Gielerak G., Stanczyk A., Kurpaska M., Piotrowicz K. (2015) Global longitudinal two-dimensional systolic strain is associated with hemodynamic alterations in arterial hypertension. J Am Soc Hypertens 9: 680–689. [DOI] [PubMed] [Google Scholar]
- Lantelme P., Laurent S., Besnard C., Bricca G., Vincent M., Legedz L., et al. (2008) Arterial stiffness is associated with left atrial size in hypertensive patients. Arch Cardiovasc Dis 101: 35–40. [DOI] [PubMed] [Google Scholar]
- Mitchell G., Izzo J., Jr., Lacourciere Y., Ouellet J., Neutel J., Qian C., et al. (2002) Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the conduit hemodynamics of omapatrilat international research study. Circulation 105: 2955–2961. [DOI] [PubMed] [Google Scholar]
- Mitchell G., Van Buchem M., Sigurdsson S., Gotal J., Jonsdottir M., Kjartansson O., et al. (2011) Arterial stiffness, pressure and flow pulsatility and brain structure and function: the age, gene/environment susceptibility – Reykjavik study. Brain 134: 3398–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita Y., Saiki A., Endo K., Ban N., Yamaguchi T., Kawana H., et al. (2009) Effects of Olmesartan, an Angiotensin Ii Receptor Blocker, and Amlodipine, a Calcium Channel Blocker, on Cardio-Ankle Vascular Index (Cavi) in Type 2 Diabetic Patients with Hypertension. J Atheroscler Thromb 16: 621–626. [DOI] [PubMed] [Google Scholar]
- Morgan T., Lauri J., Bertram D., Anderson A. (2004) Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens 17: 118–123. [DOI] [PubMed] [Google Scholar]
- Redheuil A., Yu W., Wu C., Mousseaux E., De Cesare A., Yan R., et al. (2010) Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension 55: 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruilope L., Dukat A., Bohm M., Lacourciere Y., Gong J., Lefkowitz M. (2010) Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet 375: 1255–1266. [DOI] [PubMed] [Google Scholar]
- Williams B. (2009) The aorta and resistant hypertension. J Am Coll Cardiol 53: 452–454. [DOI] [PubMed] [Google Scholar]
- Williams B., Cockcroft J., Kario K., Zappe D., Cardenas P., Hester A., et al. (2014) Rationale and study design of the prospective comparison of angiotensin receptor neprilysin inhibitor with angiotensin receptor blocker measuring arterial stiffness in the elderly (PARAMETER) study. BMJ Open 4: e004254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B., Lacy P., Thom S., Cruickshank K., Stanton A., Collier D., et al. (2006) Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the conduit artery function evaluation (CAFE) study. Circulation 113: 1213–1225. [DOI] [PubMed] [Google Scholar]
- Williams B., Lindholm L., Sever P. (2008) Systolic pressure is all that matters. Lancet 371: 2219–2221. [DOI] [PubMed] [Google Scholar]
- Wilson W., Herbert K., Mistry Y., Stevens S., Patel H., Hastings R., et al. (2008) Blood leucocyte telomere DNA content predicts vascular telomere DNA content in humans with and without vascular disease. Eur Heart J 29: 2689–2694. [DOI] [PubMed] [Google Scholar]