Abstract
Hypertension is recognized as an important risk factor for cardiovascular morbidity and mortality. Lowering of blood pressure has been shown to minimize the risk of cardiovascular events, with the majority of antihypertensives demonstrating a similar ability to reduce coronary events and stroke for a given reduction in blood pressure. Agents that modify the activity of the renin–angiotensin system (RAS) have been proposed to exhibit additional effects that might go beyond simple blood pressure lowering. The RAS is a crucial system that regulates extracellular fluid volume and blood pressure. Proposed potential benefits of RAS blockade that go beyond blood pressure lowering include a reduction in platelet aggregation and thrombosis, blunting of cardiac and vascular remodeling, favorable metabolic effects and reno- and cerebro-protection. However, factors such as treatment adherence, duration of action of antihypertensive agents and differences in effects on central versus brachial blood pressure may also result in apparent differences in efficacy of different antihypertensives. The aim of this review article is to examine the available data from clinical studies of antihypertensive drugs for evidence of effects that might legitimately be claimed to go beyond simple blood pressure lowering.
Keywords: ACE inhibitors, angiotensin II, blood pressure, RAS, renin–angiotensin system
Introduction
Hypertension is recognized as an important risk factor for cardiovascular morbidity and mortality [Gu et al. 2012]. Numerous interventional studies, the earliest of which were published almost 50 years ago, convincingly show that lowering of blood pressure (BP) with drug treatment improves morbidity and mortality in such patients [Veterans Administration Cooperative Study Group on Antihypertensive Agents, 1967, 1970]. Since then, the main aim of antihypertensive treatments has been to ensure adequate BP control to minimize the risk of cardiovascular events [Gu et al. 2012]. This concept is supported by meta-analyses of hypertension intervention trials, which have demonstrated that all classes of BP-lowering drugs, with the exception of β-blockers, have a similar ability to reduce coronary events and stroke for a given reduction in BP [Carlberg et al. 2004; Bangalore et al. 2008; Law et al. 2009].
While the predominant role of BP lowering as the mediator of cardiovascular protection through the use of antihypertensive therapy has been widely accepted, experimental and clinical studies have claimed additional effects of certain BP-lowering strategies. In this context, agents modifying the activity of the renin–angiotensin system (RAS), such as angiotensin-converting enzyme inhibitors (ACE-Is) and angiotensin receptor blockers (ARBs), may exhibit effects beyond BP lowering.
One aspect of this hypothesis relies on data from different sources suggesting that high plasma renin activity may itself be an independent predictor of risk for major vascular events and mortality in both hypertensive patients, and in patients with high cardiovascular risk [Verma et al. 2011]. However, it remains unclear how much of this observation may be related to confounding circumstances such as pre-existing therapies (e.g. diuretics), or other conditions such as volume depletion or undiagnosed heart failure in the patients investigated.
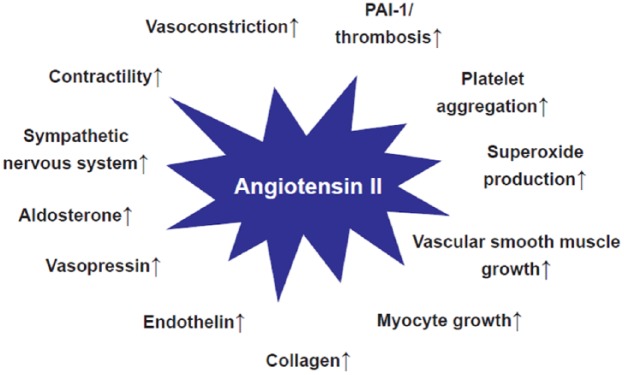
In addition to these clinical data, support for effects beyond BP by ACE-Is and ARBs has been derived from experimental studies in which effects of the RAS on various regulatory functions capable of modifying cardiovascular disease mechanisms have been described (Figure 1) [Burnier and Brunner, 2000].
Figure 1.
Proposed (patho)physiological effects of angiotensin II via angiotensin II type 1 (AT1)-receptor stimulation (Adapted from [Burnier and Brunner, 2000]).
Finally, it has been claimed that certain ARBs or their metabolites may exhibit a glitazone-like partial agonistic activity on the peroxisome proliferator-activated receptor-gamma (PPARγ) in vitro, with telmisartan being the only ARB to show an effect at physiologically achievable plasma concentrations [Kintscher and Unger, 2005]. Such a PPARγ modification may contribute to the low rate of new onset diabetes observed in most interventional trials of certain RAS blockers [Elliott and Meyer, 2007]. However, clinical evidence from large interventional studies did not demonstrate the superiority of telmisartan with respect to new onset diabetes when compared with ramipril in the Ongoing Telmisartan Alone or in Combination with Ramipril Global Endpoint Trial (ONTARGET), or compared to placebo in the Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (TRANSCEND) trial [Yusuf et al. 2008]. Therefore, the clinical relevance of the proposed effect of certain ARBs on PPARγ, at least with respect to carbohydrate metabolism, remains questionable.
In view of the ongoing controversy about many of the effects beyond BP lowering that have been proposed for both ACE-Is and ARBs, this short review examines the clinical evidence for such effects in an attempt to identify those which have proven clinically relevant.
Clinical efficacy of ACE-I and ARB independent of BP lowering
Convincing support for cardiovascular protection by ACE-Is and ARBs independent of an effect on BP was provided by studies in patients with heart failure and post-myocardial infarction (MI), in which such treatment provided marked prognostic improvement in the presence of minor or no effects on BP [SOLVD Investigators, 1991; Pfeffer et al. 1991, 2003; Cohn and Tognoni, 2001; Granger et al. 2003; Shamshad et al. 2010]. In order to understand the effects of both ACE-Is and ARBs in these particular clinical indications, one has to appreciate the complex interplay of the RAS with the sympathetic nervous system. In contrast to other predominantly arterial vasodilatory substances such as hydralazine, reflex tachycardia is not observed with such interventions [Royster et al. 1990]. Vasodilation resulting in afterload reduction without reflex sympathetic activation and volume retention may underlie, at least in part, the marked effects seen with both ACE-I and ARB both in patients with congestive heart failure and post-MI [De Leeuw and Kroon, 2008].
As an example, the first published clinical trial to examine the benefits of RAS intervention on morbidity and mortality was a relatively small study conducted in 253 patients with congestive heart failure (New York Heart Association [NYHA] functional class IV) and published in 1987 by the CONSENSUS Trial Study Group. This study examined the effects of the addition of the ACE-I enalapril, dosed at 2.5–40 mg/day, to conventional vasodilator therapy (including hydralazine, prazosin, and nitrates). At the end of 6 months, the crude mortality rate in the enalapril arm was 26%, compared with 44% in the placebo group, a relative reduction of 40% (p = 0.002) [CONSENSUS Trial Study Group, 1987]. In addition, mortality was reduced by 31% at 1 year (p = 0.001), with a 27% reduction in death rate at the end of the study (p = 0.003) [CONSENSUS Trial Study Group, 1987].
In addition, numerous studies in patients with renal disease, mostly those with diabetic nephropathy, have demonstrated renal protection by ACE-Is and ARBs that cannot be ascribed to an effect on arterial BP [Düsing, 2016]. Renal physiological studies have demonstrated that angiotensin II exerts a vasoconstrictor effect preferentially in the postglomerular (efferent) arterioles [Arima and Ito, 2000]. Consequently, by decreasing efferent arteriolar tone, RAS inhibition reduces filtration pressure and may thus act as a means of renoprotection [Van Der Meer et al. 2010].
The RAS: friend or foe?
The RAS is the crucial system that regulates extracellular fluid volume and BP through renal sodium chloride (NaCl) retention and vasoconstriction [MacGregor et al. 1981; Burnier and Brunner, 2000]. In the presence of volume depletion (e.g. low NaCl intake, acute or chronic hemorrhage, diarrhea, or excessive vomiting) activation of this system will serve to maintain, not to increase BP. However, in subjects on high-salt diets in whom BP is more often high than low, and vascular death more common than hemorrhage or dehydration, this system is likely to participate in the pathogenesis of hypertension and the resulting organ damage [Brown, 2007]. Under these circumstances, pharmacological interventions to reduce the activity of the RAS (e.g. with ACE-Is or ARBs) have proven beneficial in numerous interventional studies.
Multiple lines of evidence demonstrate that the increased peripheral resistance in hypertension is mediated not only by vasoconstriction, but that structural changes within the resistance vessels may play an important role [Mulvany, 2012; Renna et al. 2013]. Furthermore, experimental and clinical data suggest that the RAS may play a ‘growth factor-like’ role in this remodeling process within the small resistance vessels [Campbell-Boswell and Robertson, 1981; Geisterfer et al. 1988; Gibbons et al. 1992]. In agreement with this concept, clinical studies have shown a more effective ‘reverse remodeling’ of resistance arteries with ACE-Is and ARBs versus β-blockers [Mulvany, 1996; Schiffrin, 2002]. However, more recent data have suggested that vasoconstriction itself may represent an unspecific mechanism underlying this structural remodeling, and that this can be prevented by vasodilator therapy, which includes ACE-Is and ARBs [Mulvany, 2012]. This would also explain the poor performance of nonvasodilator β-blockers used in most of the comparator studies in terms of regression of vascular structural changes in hypertension.
Similarly, left ventricular hypertrophy (LVH) in the heart predominantly represents structural adaptation to increased pressure (like in aortic stenosis). Again, various lines of evidence suggest a role for the RAS in this adaptive process. This appears to be supported by the observation that the effectiveness of different classes of antihypertensive drugs in reducing LVH varies, with ACE-Is, ARBs and calcium channel blockers (CCBs) being more effective than β-blockers [Klingbeil et al. 2003]. In this context it should be considered that antihypertensives may affect BP in the central aorta differently from that measured over the brachial artery [Williams et al. 2006]. This was demonstrated in a subgroup analysis of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) [Dahlof et al. 2005], the Conduit Artery Function Evaluation (CAFE) study [Williams et al. 2006]. The CAFE study recruited 2199 patients from five ASCOT centers. Following treatment with either atenolol ± thiazide-based therapy or amlodipine ± perindopril-based therapy, aortic pressure and brachial systolic BP were assessed. Despite similar brachial systolic BP between treatment groups during and at the end of the study, substantial reductions in central aortic pressure were observed in those patients who received the amlodipine-based therapy as compared to the atenolol-based regimen [Williams et al. 2006]. Other studies have also demonstrated that atenolol is less effective than other antihypertensive agents in reducing central aortic pressure [Mackenzie et al. 2009]. Such substantially dissimilar effects on aortic pressures compared with brachial BP may explain the variation in clinical outcomes seen for the different antihypertensive treatments, and may also underlie the poor efficacy of atenolol in regressing LVH [Hashimoto et al. 2007].
When considering effects beyond BP for the RAS and consecutively RAS blockers, it is also interesting to note that this system is stimulated during diuretic treatment. The RAS is also markedly activated in Bartter’s/Gitelman’s syndrome in which tubular reabsorption of NaCl is impaired, mimicking chronic diuretic treatment. In spite of marked RAS activation in these syndromes, patients do not develop hypertension and cardiovascular remodeling [Calo and Maiolino, 2015].
Yet another mechanism by which the RAS may confer effects beyond BP lowering is related to the presence of different angiotensin II receptors. Plasma renin converts angiotensinogen released by the liver into angiotensin I. Angiotensin I is subsequently converted to angiotensin II, predominantly by ACE. The principal effects of the RAS are then mediated via the binding of angiotensin II to type 1 (AT1) receptors [Paul et al. 2006], which then induces a range of (patho)physiological effects. In this context, it is important to note that ACE-Is and ARBs act at different points in the RAS. Thus, ARBs specifically block the binding of angiotensin II to the AT1 receptor [Esteras et al. 2015] Blockade of AT1 receptors by an ARB results in increased angiotensin II levels and consequently increased stimulation of unblocked AT2 receptors [Fournier et al. 2004]. In contrast, ACE-Is block the hydrolysis of angiotensin I to angiotensin II, resulting in lower angiotensin II levels and consequently reduced stimulation of both AT1 and AT2 receptors. Questions, however, remain as to whether any effects beyond BP lowering occur through differential actions of these drugs on AT1 and AT2 receptors.
Claiming effects beyond BP lowering: what factors should be considered?
Although it is possible that differences in clinical efficacy observed with RAS interventions indicate effects beyond BP lowering, the variation seen could also be due to other factors, which should seriously be considered before claiming such an effect.
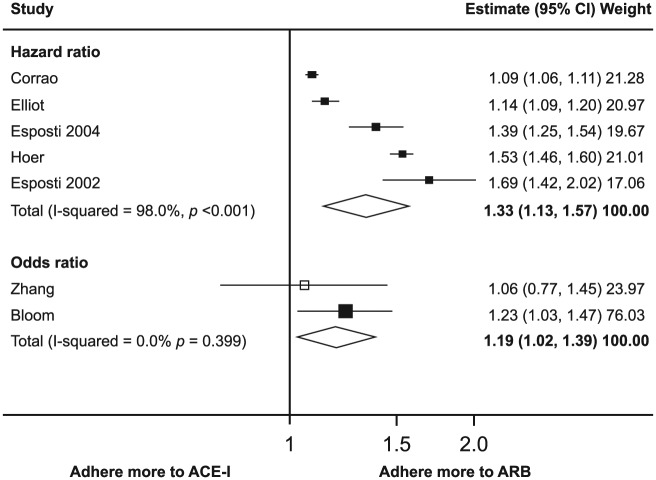
Adherence to prescribed antihypertensive medication is one such factor that should be taken into account. In this context, it should be noted that adherence in clinical trials may be generally higher than in the routine clinical setting [Megometschnigg, 1999]. However, marked nonadherence is also regularly observed in clinical trial settings. An early study had suggested that persistence with antihypertensive therapy is lowest with diuretics and highest with ARBs slightly ahead of ACE-Is [Bloom, 1998]. This principal finding has recently been supported by a meta-analysis demonstrating similar differences in adherence for different classes of antihypertensives [Kronish et al. 2011]. Lowest adherence to antihypertensive therapy was observed with diuretics and β-blockers, while highest adherence was seen with ACE-Is and ARBs [Kronish et al. 2011]. Even between ACE-Is and ARBs, differences in adherence and persistence could be demonstrated with ARBs being slightly superior to ACE-Is (Figure 2). Therefore, long-term adherence to antihypertensive therapy, together with other factors, may depend on the class of antihypertensive agent prescribed. These differences may, in part, be due to the adverse events associated with some drugs [Kronish et al. 2011].
Figure 2.
Adherence to ARBs compared with ACE-Is: meta-analysis results. Hazard ratios and odds ratios with 95% CI on a logarithmic scale for individual or pooled study data for relative risk of adherence. Black boxes indicate studies in which adherence was measured as persistence; white box, study in which adherence was measured as compliance. Various adjustments were performed (Adapted from [Kronish et al. 2011]).
ACE-I, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; CI, confidence interval.
It is important to note that patient adherence has been shown to be high at the time of a doctor’s visit, a phenomenon named white coat compliance [Urquhart, 1994; Düsing et al. 2001]. Thus, differences in adherence with prescribed treatment in the period between two doctor’s visits may result in poor overall control of BP. This may be particularly prevalent in patients receiving drugs that are associated with low levels of patient adherence. This lack of control of BP may not be evident to the physician, however, as white coat compliance often ensures adequate BP control is achieved at the time of the doctor’s visit. Within randomized clinical trials, it is possible that poor adherence with treatment administered in one arm of the study may result in a perceived greater benefit for the other treatment arm. Such bias may, in time, lead to suggestions of additional benefits beyond a simple antihypertensive action.
Low treatment adherence may be particularly problematic in patients who are prescribed drugs with a relatively short duration of action. In such patients, a missed dose is more likely to result in a period without therapeutic coverage. For example, the β-blockers betaxolol and atenolol when taken consistently as a monotherapy are equally effective in controlling BP. However, owing to the relatively short-term duration of action of atenolol, the BP and heart rate response to betaxalol is significantly superior in the 24 hours following a missed dose, as demonstrated in a double-blind, 6-week study comparing once-daily oral betaxolol and atenolol in 114 patients with mild-to-moderate hypertension [Johnson and Whelton, 1994]. Similar BP and heart rate responses were seen in these patients. However, when patients randomly received placebo in either the fifth or sixth week of the study to simulate the effect of missing doses, the magnitude and duration of the BP lowering effect was significantly greater for betaxolol than for atenolol as calculated from ambulatory BP monitoring data [Johnson and Whelton, 1994].
These data should be considered against the background of recent studies using electronic medication event monitoring showing that on any given day, antihypertensive medication is not taken within the respective time frame by approximately 8% of patients [Burnier et al. 2013]. In such subjects, drugs with long (and more ‘forgiving’) duration of action may compensate for an irregular intake of medication when the dosing interval is prolonged beyond 24 hours. In contrast, drugs with a short duration of action will not offer this protection, with considerable variation in BP.
As an example, in the Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial [Jamerson et al. 2008] combination therapy with benazepril-amlodipine was superior to benazepril-hydrochlorothiazide in reducing cardiovascular events in patients with hypertension, in spite of similar BP lowering in the two treatment arms. Effects beyond BP have been widely claimed to explain the clinical outcome of the trial. Alternatively, it should be noted that amlodipine is a long-acting and thus ‘forgiving’ drug with an elimination half-life of 40–60 hours [Abernethy, 1992]. In contrast, the comparator drug hydrochlorothiazide is short-acting with an elimination half-life of approximately 9–10 hours just permitting effective once daily dosing [Welling, 1986; Ernst and Moser, 2009]. Therefore, the clinical outcome in ACCOMPLISH could simply be due to differences in therapeutic coverage resulting from the comparison of a long-acting with a short-acting drug [Meredith, 1999].
Thus, before concluding effects beyond BP lowering for any antihypertensive, it is important to verify the apparent superior efficacy observed is not merely a consequence of other factors such as better adherence, differences in the duration of action, or in reducing central aortic pressure not mirrored by differences in brachial BP.
Is there evidence of effects beyond BP lowering?
In the presence of conflicting data from experimental and small clinical studies, a key challenge is to examine the outcomes of large clinical trials of RAS inhibitors for evidence of clear effects beyond BP lowering. As discussed earlier in this article, interventional trials in congestive heart failure, post-MI and in patients with chronic renal disease have provided clear evidence of improvements in surrogate as well as hard morbidity and mortality endpoints, largely independent of BP lowering.
In other trials often cited, the evidence for such an effect remains controversial. For example, in the Heart Outcomes Prevention Evaluation (HOPE) trial, over 9000 high-risk patients with vascular disease or diabetes (including 47% with hypertension) were randomized to receive the ACE-I ramipril or placebo over a 5-year period [Yusuf et al. 2000]. Death, MI and stroke were significantly reduced in ramipril-treated patients, but only minor changes in office BP were observed (-3/-2 mmHg). However, these results must be interpreted cautiously as further analysis from a small HOPE substudy, in which ambulatory BP was monitored over a 24-hour period, found significant differences in systolic and diastolic BP throughout the day [Svensson et al. 2001]. Thus, 24-hour ambulatory BP was significantly reduced in ramipril-treated patients (-10/-4 mmHg, p = 0.03), mainly because of a more pronounced BP-lowering effect at night (-17/-8 mmHg, p < 0.001). As the study drugs were taken at night in the HOPE trial, the effects on cardiovascular morbidity and mortality seen with ramipril in this patient group may, to a larger extent than initially ascribed, relate to effects on BP patterns over the 24 hour period [Yusuf et al. 2000; Svensson et al. 2001; Düsing, 2016].
The Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) study compared the benefits of the ARB losartan with those of the β-blocker atenolol [Dahlof et al. 2002]. LIFE claimed that losartan confers additional benefits beyond BP lowering, as despite a similar reduction in BP with both drugs, losartan was associated with a greater reduction in the combined primary endpoint of cardiovascular death, stroke and MI than atenolol [Dahlof et al. 2002]. However, a number of questions arise regarding the choice of the comparator in this study. As previously noted, β-blockers such as atenolol are associated with poorer treatment adherence compared with an ARB such as losartan [Kronish et al. 2011] and are less effective than other antihypertensive agents in reducing central aortic pressure [Mackenzie et al. 2009]. Consequently, some meta-analyses have shown β-blockers to be less effective in the prevention of cardiovascular complications, especially stroke, than other antihypertensive agents [Carlberg et al. 2004; Bangalore et al. 2008].
Several proposed effects of angiotensin II mediated by AT1 receptors claimed on the basis of experimental or small clinical studies (Figure 1) have not been supported by data from larger clinical trials. This especially applies to the proposed effects on platelet aggregation and fibrinolysis.
Physiologically, angiotensin II induces platelet activation and promotes platelet aggregation [Brown and Vaughan, 2000; Larsson et al. 2000]. Therefore, RAS blockade with either ACE-Is or ARB should be associated with reduced platelet function. However, there are marked discrepancies between the clinical and laboratory effects of different ACE-Is and ARBs studied in this respect [Blann et al. 2003]. In addition, no relevant difference in platelet function compatible with a favorable effect due to RAS blockade compared with other agents has ever been observed in either ACE-I or ARB intervention trials [Düsing, 2016]. Also, in some studies, most classes of antihypertensive agents exhibit some degree of antiplatelet activity, but this is likely due to an improvement in endothelial dysfunction seen with BP lowering [Blann et al. 2003].
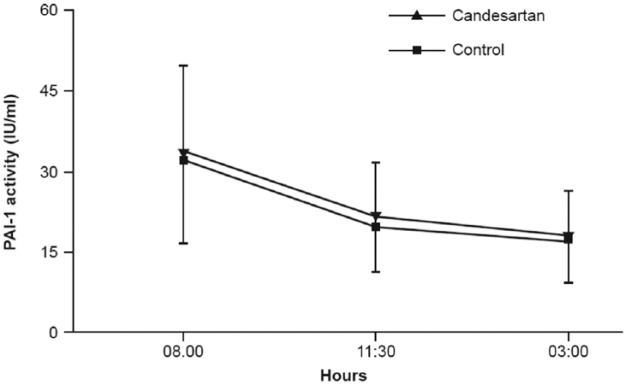
Whether blockade of the RAS results in clinically relevant changes in fibrinolytic activity has also been questioned. Fibrinolysis occurs physiologically through a complex regulation and interplay of fibrinolytic factors with a continual dissolu-tion of microscopic clots in the circulation by plasmin. Through effects on plasminogen activator-inhibitor-1 (PAI-1), angiotensin II has been proposed to prevent the conversion of plasminogen to plasmin, thus preventing the breakdown of fibrin. Accordingly, in vitro studies imply that angiotensin II induces PAI-1 expression in endothelial cell cultures [Vaughan et al. 1995]. A first study in vivo, in which four normotensive subjects and six hypertensive patients received an intravenous infusion of angiotensin II demonstrated a rapid increase in circulating levels of PAI-1 [Ridker et al. 1993]. However, other study groups were unable to confirm an increase in PAI-1 levels in response to angiotensin II infusion or following ARB treatment (Figure 3) [Lottermoser et al. 2000, 2004; Skurk et al. 2004].
Figure 3.
PAI-1 activity in 74 patients randomly assigned to a 7-day treatment period with either 16 mg candesartan or placebo (Control). (Adapted from [Skurk et al. 2004]).
PAI-1, plasminogen activator-inhibitor-1; IU, international units.
ACE-Is and ARBs may be associated with a reduced rate of new-onset diabetes compared with other antihypertensives. A network meta-analysis of 22 clinical trials involving 143,153 patients showed that the lowest incidence of new-onset diabetes occurs in those who are treated with an ARB or ACE-I [Elliott and Meyer, 2007]. The mechanisms for this metabolic effect remain unclear. Peripheral vasodilation without reflex activation of the sympathetic nervous system by ACE-I and ARB may improve the microcirculation in the musculature and could thereby improve insulin sensitivity [Düsing, 2007]. In addition to this simple concept, various cellular mechanisms have been speculated to participate in this effect [Düsing, 2007; Hershon, 2011; Sauter et al. 2015]. This metabolic effect of RAS blockers could indeed represent a relevant clinical effect beyond BP lowering since the clinical consequences of their modest effect on glucose metabolism may take long time periods to translate into clinical benefits not covered, and therefore not detected, by clinical trials.
Interestingly, an opposite effect on glucose metabolism has recently been demonstrated for statins. Thus, a population-based study of 8749 nondiabetic patients indicated that statin treatment is associated with a 46% increase in new-onset diabetes [Cederberg et al. 2015]. It is interesting to speculate that the combination of a RAS inhibitor with a statin may reduce hypercholesterolemia and BP with less or no increased risk of new-onset diabetes.
It has been proposed that drugs that activate AT2 receptors via increased angiotensin II levels, such as diuretics, calcium antagonists, and ARBs, are associated with trends for more beneficial stroke reduction than drugs devoid of such activation, such as β-blockers and ACE-Is despite an equal fall in arterial pressure [Fournier et al. 2004]. Inhibition of AT1 receptor stimulation following ARB administration results in enhanced angiotensin II binding to and stimulation of AT2 receptors [Siragy, 1999]. Activation of the AT2 receptor has been shown to mediate several potentially beneficial effects in the cardiovascular system, including vasodilation, antiproliferation, and apoptosis. Also, cerebroprotective effects of ARBs have been demonstrated in vivo in experimental stroke models [Fernandez et al. 1986; Dalmay et al. 2001]. In addition, while ARBs have been shown to be as effective as ACE-I in terms of reducing the risk of MI and cardiovascular mortality, head-to-head comparison of ACE-Is and ARBs in six trials with a total of 49,924 patients showed a slightly greater degree of stroke protection for ARB [Reboldi et al. 2008]. Further studies are required to show whether this cerebroprotective effect of ARB represents a true benefit that goes beyond simple BP lowering.
Conclusion
Before concluding that agents modulating the RAS might have actions that go beyond BP lowering, several factors should be taken into consideration. One crucial aspect in this regard is patient adherence with the prescribed treatment regimen. Among the many factors involved, the class of medication prescribed can have a significant impact on patient adherence. In addition, it is important to consider the duration of action of the drug and thus any potential period of noncoverage that might arise if doses are missed. Observed differences in central BP compared with brachial BP have also been observed and may impact the apparent efficacy of a treatment regimen. To date, experimental and clinical studies have failed to provide definitive evidence of specific effects of RAS blockade beyond BP lowering in terms of regression of vascular or myocardial remodeling, fibrinolysis and platelet function. In contrast, there is still inconclusive evidence suggesting that ARBs may exert cerebroprotective effects, perhaps via stimulation of AT2 receptors. Furthermore, both ACE-Is and ARBs have positive effects on glucose metabolism. However, the mechanism and the clinical relevance of this effect remain unclear.
Acknowledgments
The author has received honoraria for scientific lectures and financial support for conducting clinical studies from Novartis, Servier, Berlin Chemie, and UCB Pharma. Editorial assistance was provided by Sarah Birch of Novartis Ireland Ltd., Dublin, Ireland.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author declares that there is no conflict of interest.
References
- Abernethy D. (1992) pharmacokinetics and pharmacodynamics of amlodipine. Cardiology 80: 31–36. [DOI] [PubMed] [Google Scholar]
- Arima S., Ito S. (2000) Angiotensin II type 2 receptors in the kidney: evidence for endothelial-cell-mediated renal vasodilation. Nephrol Dial Transplant 15: 448–451. [DOI] [PubMed] [Google Scholar]
- Bangalore S., Sawhney S., Messerli F. (2008) Relation of beta-blocker-induced heart rate lowering and cardioprotection in hypertension. J Am Coll Cardiol 52: 1482–1489. [DOI] [PubMed] [Google Scholar]
- Blann A., Nadar S., Lip G. (2003) Pharmacological modulation of platelet function in hypertension. Hypertension 42: 1–7. [DOI] [PubMed] [Google Scholar]
- Bloom B. (1998) Continuation of initial antihypertensive medication after 1 year of therapy. Clin Ther 20: 671–681. [DOI] [PubMed] [Google Scholar]
- Brown M. (2007) Renin: friend or foe? Heart 93: 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N., Vaughan D. (2000) Prothrombotic effects of angiotensin. Adv Intern Med 45: 419–429. [PubMed] [Google Scholar]
- Burnier M., Brunner H. (2000) Angiotensin II receptor antagonists. The Lancet 355: 637–645. [DOI] [PubMed] [Google Scholar]
- Burnier M., Wuerzner G., Struijker-Boudier H., Urquhart J. (2013) Measuring, analyzing and managing drug adherence in resistant hypertension. Hypertension 62: 218–225. [DOI] [PubMed] [Google Scholar]
- Calo L., Maiolino G. (2015) Mechanistic approach to the pathophysiology of target organ damage in hypertension from studies in a human model with characteristics opposite to hypertension: Bartter’s and Gitelman’s syndromes. J Endocrinol Invest 38: 711–716. [DOI] [PubMed] [Google Scholar]
- Campbell-Boswell M., Robertson A. (1981) Effects of angiotensin II and vasopressin on human smooth muscle cells in vitro. Exp Mol Pathol 35: 265–276. [DOI] [PubMed] [Google Scholar]
- Carlberg B., Samuelsson O., Lindholm L. (2004) Atenolol in hypertension:is it a wise choice? Lancet 364: 1684–1689. [DOI] [PubMed] [Google Scholar]
- Cederberg H., Stancakova A., Yaluri N., Modi S., Kuusisto J., Laakso M. (2015) Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: a 6 year follow-up study of the METSIM cohort. Diabetologia 58: 1109–1117. [DOI] [PubMed] [Google Scholar]
- Cohn J., Tognoni G. (2001) A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 345: 1667–1675. [DOI] [PubMed] [Google Scholar]
- CONSENSUS Trial Study Group (1987) Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med 316: 1429–1435. [DOI] [PubMed] [Google Scholar]
- Dahlof B., Devereux R., Kjeldsen S., Julius S., Beevers G., De Faire U., et al. (2002) Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 359: 995–1003. [DOI] [PubMed] [Google Scholar]
- Dahlof B., Sever P., Poulter N., Wedel H., Beevers D., Caulfield M., et al. (2005) Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 366: 895–906. [DOI] [PubMed] [Google Scholar]
- Dalmay F., Mazouz H., Allard J., Pesteil F., Achard J., Fournier A. (2001) Non-AT(1)-receptor-mediated protective effect of angiotensin against acute ischaemic stroke in the gerbil. J Renin Angiotensin Aldosterone Syst 2: 103–106. [DOI] [PubMed] [Google Scholar]
- De Leeuw P., Kroon A. (2008) Interaction of antihypertensive drugs with mechanisms of blood pressure regulation. In: McInnes G. (ed), Handbook of Hypertension, Volume 25: Clinical Pharmacology and Therapeutics of Hypertension. Elsevier, pp. 19–34. [Google Scholar]
- Düsing R. (2007) New-onset diabetes mellitus during antihypertensive treatment. Dtsch Med Wochenschr 132: 689–695. [DOI] [PubMed] [Google Scholar]
- Düsing R. (2016) Mega clinical trials which have shaped the RAS intervention clinical practice. Ther Adv Cardiovasc Dis 10: 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Düsing R., Lottermoser K., Mengden T. (2001) Compliance with drug therapy-new answers to an old question. Nephrol Dial Transplant 16: 1317–1321. [DOI] [PubMed] [Google Scholar]
- Elliott W., Meyer P. (2007) Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet 369: 201–207. [DOI] [PubMed] [Google Scholar]
- Ernst M., Moser M. (2009) Use of diuretics in patients with hypertension. N Engl J Med 361: 2153–2164. [DOI] [PubMed] [Google Scholar]
- Esteras R., Perez-Gomez M., Rodriguez-Osorio L., Ortiz A., Fernandez-Fernandez B. (2015) Combination use of medicines from two classes of renin–angiotensin system blocking agents: risk of hyperkalemia, hypotension, and impaired renal function. Ther Adv Drug Saf 6: 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez L., Spencer D., Kaczmar T. (1986) Angiotensin II decreases mortality rate in gerbils with unilateral carotid ligature. Stroke 17: 82–85. [DOI] [PubMed] [Google Scholar]
- Fournier A., Messerli F., Achard J., Fernandez L. (2004) Cerebroprotection mediated by angiotensin II: a hypothesis supported by recent randomized clinical trials. J Am Coll Cardiol 43: 1343–1347. [DOI] [PubMed] [Google Scholar]
- Geisterfer A., Peach M., Owens G. (1988) Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res 62: 749–756. [DOI] [PubMed] [Google Scholar]
- Gibbons G., Pratt R., Dzau V. (1992) Vascular smooth muscle cell hypertrophy vs. hyperplasia. Autocrine transforming growth factor-β1 expression determines growth response to angiotensin II. J Clin Invest 90: 456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger C., McMurray J., Yusuf S., Held P., Michelson E., Olofsson B., et al. (2003) Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors:the CHARM-alternative trial. Lancet 362: 772–776. [DOI] [PubMed] [Google Scholar]
- Gu Q., Burt V., Dillon C., Yoon S. (2012) Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the national health and nutrition examination survey, 2001 to 2010. Circulation 126: 2105–2114. [DOI] [PubMed] [Google Scholar]
- Hashimoto J., Imai Y., O’Rourke M. (2007) Monitoring of antihypertensive therapy for reduction in left ventricular mass. Am J Hypertens 20: 1229–1233. [DOI] [PubMed] [Google Scholar]
- Hershon K. (2011) Mechanistic and clinical aspects of renin–angiotensin-aldosterone system blockade. Endocr Pract 17: 430–440. [DOI] [PubMed] [Google Scholar]
- Jamerson K., Weber M., Bakris G., Dahlof B., Pitt B., Shi V., et al. (2008) Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 359: 2417–2428. [DOI] [PubMed] [Google Scholar]
- Johnson B., Whelton A. (1994) A study design for comparing the effects of missing daily doses of antihypertensive drugs. Am J Ther 1: 260–267. [DOI] [PubMed] [Google Scholar]
- Kintscher U., Unger T. (2005) Vascular protection in diabetes: a pharmacological view of angiotensin type 1 receptor blockers. Acta Diabetol 42: S26–32. [DOI] [PubMed] [Google Scholar]
- Klingbeil A., Schneider M., Martus P., Messerli F., Schmieder R. (2003) A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med 115: 41–46. [DOI] [PubMed] [Google Scholar]
- Kronish I., Woodward M., Sergie Z., Ogedegbe G., Falzon L., Mann D. (2011) Meta-analysis: impact of drug class on adherence to antihypertensives. Circulation 123: 1611–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson P., Schwieler J., Wallen N. (2000) Platelet activation during angiotensin II infusion in healthy volunteers. Blood Coagul Fibrinolysis 11: 61–69. [PubMed] [Google Scholar]
- Law M., Morris J., Wald N. (2009) Use of blood pressure lowering drugs in the prevention of cardiovascular disease. BMJ 338: b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottermoser K., Hertfelder H., Gohlke P., Vetter H., Dusing R. (2004) Short-term effects of exogenous angiotensin II on plasma fibrinolytic balance in normal subjects. Clin Exp Hypertens 26: 13–26. [DOI] [PubMed] [Google Scholar]
- Lottermoser K., Hertfelder H., Wehling M., Schiermeyer B., Vetter H., Dusing R. (2000) Effects of the mineralocorticoid fludrocortisone on fibrinolytic function in healthy subjects. J Renin Angiotensin Aldosterone Syst 1: 357–360. [DOI] [PubMed] [Google Scholar]
- MacGregor G., Markandu N., Roulston J., Jones J., Morton J. (1981) Maintenance of blood pressure by the renin–angiotensin system in normal man. Nature 291: 329–331. [DOI] [PubMed] [Google Scholar]
- Mackenzie I., McEniery C., Dhakam Z., Brown M., Cockcroft J., Wilkinson I. (2009) Comparison of the effects of antihypertensive agents on central blood pressure. Hypertension 54: 409–413. [DOI] [PubMed] [Google Scholar]
- Megometschnigg D. (1999) The role of compliance in clinical care. In: Metry J., Meyer U. (eds), Drug Regimen Compliance. Issues in Clinical Trials and Patient Management. Wiley, pp. 155–162. [Google Scholar]
- Meredith P. (1999) Achieving and assessing therapeutic coverage. In: Metry J., Meyer P. (eds), Drug Regimen Compliance. Issues in Clinical Trials and Patient Management. Wiley, pp. 41–60. [Google Scholar]
- Mulvany M. (1996) Effects of angiotensin converting enzyme inhibition on vascular remodelling of resistance vessels in hypertensive patients. J Hypertens Suppl 14: S21–24. [PubMed] [Google Scholar]
- Mulvany M. (2012) Small artery remodelling in hypertension. Basic Clin Pharmacol Toxicol 110: 49–55. [DOI] [PubMed] [Google Scholar]
- Paul M., Poyan Mehr A., Kreutz R. (2006) Physiology of local renin–angiotensin systems. Physiol Rev 86: 747–803. [DOI] [PubMed] [Google Scholar]
- Pfeffer M., Moyé L., Braunwald E., Basta L., Brown E., Cuddy T., et al. (1991) Selection bias in the use of thrombolytic therapy in acute myocardial infarction. JAMA 266: 528–532. [PubMed] [Google Scholar]
- Pfeffer M., Swedberg K., Granger C., Held P., Mcmurray J., Michelson E., et al. (2003) Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-overall programme. The Lancet 362: 759–766. [DOI] [PubMed] [Google Scholar]
- Reboldi G., Angeli F., Cavallini C., Gentile G., Mancia G., Verdecchia P. (2008) Comparison between angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on the risk of myocardial infarction, stroke and death: a meta-analysis. J Hypertens 26: 1282–1289. [DOI] [PubMed] [Google Scholar]
- Renna N., De Las Heras N., Miatello R. (2013) Pathophysiology of vascular remodeling in hypertension. Int J Hypertens 2013: 808353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker P., Gaboury C., Conlin P., Seely E., Williams G., Vaughan D. (1993) Stimulation of plasminogen activator inhibitor in vivo by infusion of angiotensin. Circulation 87: 1969–1973. [DOI] [PubMed] [Google Scholar]
- Royster R., Butterworth J., Groban L., Slaughter T., Zvara D. (1990) Cardiovascular pharmacology. J Cardiothroac Anesth 6: 17. [Google Scholar]
- Sauter N., Thienel C., Plutino Y., Kampe K., Dror E., Traub S., et al. (2015) Angiotensin II induces interleukin-1β-mediated islet inflammation and ß-cell dysfunction independently of vasoconstrictive effects. Diabetes 64: 1273–1283. [DOI] [PubMed] [Google Scholar]
- Schiffrin E. (2002) Vascular changes in hypertension in response to drug treatment:effects of angiotensin receptor blockers. Can J Cardiol 18: 15A–18A. [PubMed] [Google Scholar]
- Shamshad F., Kenchaiah S., Finn P., Soler-Soler J., Mcmurray J., Velazquez E., et al. (2010) Fatal myocardial rupture after acute myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: the valsartan in acute myocardial infarction trial (VALIANT). Am Heart J 160: 145–151. [DOI] [PubMed] [Google Scholar]
- Siragy H. (1999) Angiotensin II receptor blockers:review of the binding characteristics. Am J Cardiol 84: 3S–8S. [DOI] [PubMed] [Google Scholar]
- Skurk T., Lee Y., Nicuta-Rolfs T., Haastert B., Wirth A., Hauner H. (2004) Effect of the angiotensin II receptor blocker candesartan on fibrinolysis in patients with mild hypertension. Diabetes Obes Metab 6: 56–62. [DOI] [PubMed] [Google Scholar]
- SOLVD Investigators (1991) Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 325: 293–302. [DOI] [PubMed] [Google Scholar]
- Svensson P., De Faire U., Sleight P., Yusuf S., Ostergren J. (2001) Comparative effects of ramipril on ambulatory and office blood pressures: a HOPE substudy. Hypertension 38: E28–32. [DOI] [PubMed] [Google Scholar]
- Urquhart J. (1994) Role of patient compliance in clinical pharmacokinetics. A review of recent research. Clin Pharmacokinet 27: 202–215. [DOI] [PubMed] [Google Scholar]
- Van Der Meer I., Cravedi P., Remuzzi G. (2010) The role of renin angiotensin system inhibition in kidney repair. Fibrogenesis Tissue Repair 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan D., Lazos S., Tong K. (1995) Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin–angiotensin system and thrombosis. J Clin Invest 95: 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S., Gupta M., Holmes D., Xu L., Teoh H., Gupta S., et al. (2011) Plasma renin activity predicts cardiovascular mortality in the heart outcomes prevention evaluation (HOPE) study. Eur Heart J 32: 2135–2142. [DOI] [PubMed] [Google Scholar]
- Veterans Administration Cooperative Study Group on Antihypertensive Agents (1967) Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. J Am Med Assoc 202: 1028–1034. [PubMed] [Google Scholar]
- Veterans Administration Cooperative Study Group on Antihypertensive Agents (1970) Effects of treatment on morbidity in hypertension II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. J Am Med Assoc 213: 1143–1152. [PubMed] [Google Scholar]
- Welling P. (1986) Pharmacokinetics of the thiazide diuretics. Biopharm Drug Dispos 7: 501–535. [DOI] [PubMed] [Google Scholar]
- Williams B., Lacy P., Thom S., Cruickshank K., Stanton A., Collier D., et al. (2006) Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes:principal results of the conduit artery function evaluation (CAFE) study. Circulation 113: 1213–1225. [DOI] [PubMed] [Google Scholar]
- Yusuf S., Sleight P, Pogue J., Bosch J., Davies R., Dagenais G. (2000) Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 342: 145–153. [DOI] [PubMed] [Google Scholar]
- Yusuf S., Teo K., Anderson C., Pogue J., Dyal L., Copland I., et al. (2008) Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events. Lancet 372: 1174–1183. [DOI] [PubMed] [Google Scholar]