Abstract
Objectives:
The present study was conducted to assess the efficacy, safety and tolerability of fluticasone propionate/formoterol fumarate combination therapy (FP/FORM; Flutiform®) compared with fluticasone propionate/salmeterol xinafoate (FP/SAL; Seretide® Evohaler®) in children with asthma.
Methods:
This was an open-label, randomized, controlled, phase III trial and extension. Patients aged 4–12 years with reversible asthma [% predicted forced expiratory volume in 1 second (FEV1) 60–100%; documented reversibility of ⩾15% in FEV1] were randomized to receive FP/FORM (100/10 µg b.i.d.) or FP/SAL (100/50 µg b.i.d.) for 12 weeks. Eligible patients completing the 12-week core phase entered a 24-week extension phase with FP/FORM (100/10 µg b.i.d.). The primary efficacy endpoint was the change in predose FEV1 from day 0 to day 84. Secondary efficacy endpoints included change in predose to 2-hours postdose FEV1 from day 0 to day 84, peak expiratory flow rate (PEFR), patient-reported outcomes, rescue-medication use and asthma exacerbations.
Results:
In total, 211 patients were randomized and 210 completed the core phase; of these patients, 208 entered and 205 completed the extension phase of the study. Predose FEV1 increased from day 0 to day 84 [FP/FORM, 182 ml; 95% confidence interval (CI), 127, 236; FP/SAL, 212 ml, 95% CI, 160, 265] and FP/FORM was noninferior to FP/SAL: least squares (LS) mean treatment difference: –0.031 (95% CI, –0.093, 0.031; p = 0.026). Secondary efficacy analyses indicated similar efficacy with both therapies. There were no notable differences observed in the safety and tolerability profile between treatments. No safety concerns were identified with long-term FP/FORM therapy, and there was no evidence of an effect of FP/FORM on plasma cortisol.
Conclusions:
FP/FORM improved lung function and measures of asthma control with comparable efficacy to FP/SAL, and demonstrated a favourable safety and tolerability profile in children aged 4–12 years.
Keywords: asthma, children, combination therapy, fluticasone propionate, formoterol fumarate, pMDI
Introduction
Asthma is one of the most common chronic medical conditions and affects around 300 million people globally. Its prevalence is on the increase, particularly among children [GINA, 2015].
Underlying inflammation plays a critical role in the pathophysiology of asthma, and may lead to bronchial hyper-responsiveness, airway obstruction, and respiratory symptoms, that contribute to the chronic nature of the disease [Murphy and O’Byrne, 2010]. Chronic inflammation and subsequent structural changes can result in persistent symptoms and reduced lung function [Reddel et al. 2009], especially in children whose symptoms begin before 3 years of age [National Asthma Education and Prevention Program, Expert Report 3, 2007].
Asthma can be effectively controlled with pharmacotherapy. A stepwise-treatment approach is recommended for the control of asthma symptoms in children aged 6–11 years, with alterations of ongoing therapy decided based on a cycle of assessment, treatment adjustment, and review of therapeutic response [GINA, 2015]. Low-dose inhaled corticosteroids (ICSs) are highly effective in reducing symptoms and the risk of asthma exacerbations, and initiation of ICS treatment [and an as-needed short-acting β2-agonist (SABA)] early on in the disease course is recommended in patients at risk of exacerbations in order to avoid long-term decline in lung function [GINA, 2015]. However, a substantial proportion of children do not achieve asthma control on low-dose ICS treatment [Sorkness et al. 2007]. The GINA guideline recommends increasing ICS dose as a preferred step-up therapy for children uncontrolled on low-dose ICS or switching to a low-dose combination treatment with an ICS and a long-acting β2-agonist (LABA) as another option. Both strategies have been shown to be equally effective [Vaessen-Verberne et al. 2010], although a study by Lemanske Jr. and colleagues showed that addition of LABA to low-dose ICS was more likely to result in best response than increasing the dose of ICS [Lemanske Jr. et al. 2010]. Concerns have also been raised regarding doubling the dose of ICS monotherapy in children due to possible effects on adrenal function and short-term growth suppression [Allen, 2006; Pedersen, 2001; Robinson et al. 2002].
Treatment with ICS/LABA combinations has been shown to significantly improve lung function, reduce symptoms, and decrease the need for rescue medication with SABAs compared with ICS alone [Ni Chroinin et al. 2009]. Furthermore, the use of single-inhaler ICS/LABA combinations has been shown to increase patients’ treatment adherence compared with separate inhalers [Murphy and Bender, 2009], which may improve outcomes.
Although a number of ICS/LABA formulations are available for the treatment of asthma in adolescent and adult patients, there are only a few approved for use in children aged 4–12 years. In Europe, fluticasone propionate/salmeterol (FP/SAL) 100/50 μg b.i.d. is available as a dry-powder inhaler (DPI) or a pressurized metered-dose inhaler (pMDI) for patients aged 4 years and over. Budesonide/formoterol 200/12 μg b.i.d. is the only other approved ICS/LABA combination for children, and is available as a DPI for patients aged 6 years and over.
Fluticasone propionate/formoterol fumarate [(FP/FORM) Flutiform®; Napp Pharmaceuticals Ltd, UK] combination administered via a hydrofluoroalkane (HFA) pMDI is approved in 35 countries for the maintenance treatment of asthma in patients aged no less than 12 years. We report findings from a 12-week, phase III, randomized, open-label, active-controlled multicenter trial and 24-week extension [ClinicalTrials.gov identifier: NCT00475813; EudraCT number: 2006-005928-16] that compared the efficacy, safety and tolerability of FP/FORM with fluticasone propionate/salmeterol pMDI (FP/SAL; Seretide® Evohaler®; Glaxo Wellcome UK Ltd, UK) in asthmatic children between 4 and 12 years of age.
Methods and materials
Patients
Patients had to have had asthma for at least 6 months before screening. At screening, eligible patients had a forced expiratory volume in 1 second (FEV1) between at least 60% and up to and including 100% of predicted normal levels [Zapletal et al. 1977] following appropriate withholding of asthma medication and documented FEV1 reversibility of at least 15%. Patients were required to demonstrate satisfactory use of both the inhaler and spacer devices and to be able to substitute study medication for their prestudy prescribed asthma treatment.
Patients were excluded if they had experienced near-fatal or life-threatening asthma (including intubation) within the past year, required hospitalization or an emergency visit due to asthma in the previous 4 weeks, had a history of systemic (injectable) corticosteroid use within 1 month before, or leukotriene receptor antagonist use (e.g. montelukast) within 1 week before screening. Patients with any clinically significant disease or abnormality, a clinically relevant upper or lower respiratory infection within 4 weeks prior to screening or significant nonreversible pulmonary disease were also excluded.
Study design
The primary objective of the study was to demonstrate the noninferiority of FP/FORM versus FP/SAL based on change in predose FEV1 from day 0 to day 84. The objective of the extension phase was to collect long-term safety data for FP/FORM in children.
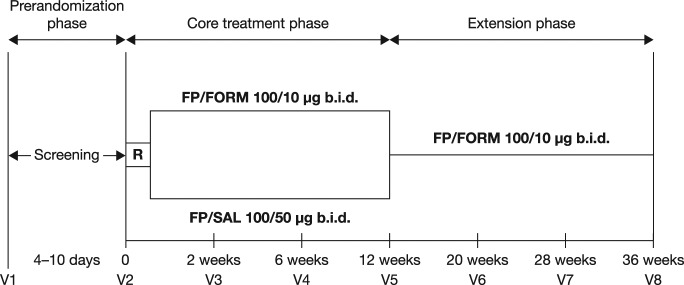
The study consisted of a 4–10-day screening phase, after which patients discontinued their prestudy asthma medication. Patients were randomized 1:1 to receive either FP/FORM (100/10 μg b.i.d.) or FP/SAL (100/50 μg b.i.d.) during the 12-week treatment phase (core trial) in an open-label fashion (Figure 1).
Figure 1.
Study design.
R, randomization; FP/FORM, fluticasone propionate/formoterol fumarate; FP/SAL, fluticasone propionate/salmeterol xinafoate; b.i.d., twice daily; V, visit.
Randomization was stratified for age groups (4–6 years and 7–12 years of age) to ensure balance across treatment groups. All patients completing the core phase were eligible to enter a 24-week extension period, during which they received FP/FORM 100/10 μg b.i.d.
During both the core trial and the extension, patients were permitted to take salbutamol (one 100 µg puff) up to four times daily as rescue therapy. Both study and rescue medication was administered using an Aerochamber® Plus spacer device (GlaxoSmithKline, UK). Oral steroids up to a dose of 4 mg/day prednisolone equivalent, and theophylline, were permitted provided that doses were stable and were continued from prestudy therapy. Leukotriene modifiers; β blockers; monoamine oxidase inhibitors; tricyclic antidepressants; quinidine-type antiarrhythmics; oral, injectable or topical steroids (for conditions other than asthma); nasal corticosteroids and mucolytics; potent CYP 3A4 inhibitors such as ketoconazole; LABAs other than that included in the study medication, and SABAs other than rescue, were not permitted during the study.
The study was approved by independent ethics committees and conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization (ICH) guidelines on good clinical practice (GCP), and European Union (EU) Clinical Trials Directive 2001/20/EC. Written informed consent was obtained from the parent(s)/legal representative(s) of all participants.
Assessments
During the core trial, the primary efficacy endpoint was the change in predose FEV1 from day 0 to day 84. Change in predose FEV1 at day 0 to 2-hours postdose at day 84 was a secondary efficacy endpoint. FEV1 is recommended as one of the fundamental measures of asthma control [Reddel et al. 2009] and therefore its use as a primary efficacy variable was appropriate. Other efficacy endpoints included discontinuation due to lack of efficacy, time to onset of action (first time-point postdose when FEV1 was at least 12% greater than the predose value), peak expiratory flow rate (PEFR), forced vital capacity (FVC) and asthma exacerbations. Lung function tests were performed within 30 minutes before study medication (predose) and then at 5, 10, 60 and 120 minutes after treatment at baseline and at each site visit (weeks 2, 6 and 12) during the core trial. Before the lung functions tests, LABAs were withheld for 12 hours during screening and SABAs were withheld for 6 hours throughout the study. Patients who displayed worsening of asthma symptoms were required to be withdrawn from the study.
Patients (with the help of a parent or guardian) completed an electronic diary daily to record morning and evening PEFR, rescue medication use, asthma symptom scores and sleep disturbance scores. Asthma symptoms were measured daily using a 6-point scale, from 0 (no symptoms) to 5 (asthma resulting in inability to perform daily activities). Sleep disturbance, also recorded daily, was rated on a 5-point scale, from 0 (slept through the night, no asthma) to 4 (unable to sleep at all due to asthma). Mild or moderate exacerbations were predefined as predose morning PEFR greater than 30% below baseline on at least 2 consecutive days, or awakening at night due to asthma on at least 2 consecutive days, that is, sleep disturbance scores increased by 2 or more points, or the use of salbutamol rescue medication more than 4 times per day for at least 2 consecutive days. Severe asthma exacerbations were defined as deterioration in asthma requiring additional therapy (systemic glucocorticosteroids), an emergency visit, or hospitalization due to asthma. During the extension phase, predose FEV1, FVC and PEFR were measured at weeks 8, 16 and 24 after the start of the extension as efficacy parameters.
To assess adherence to treatment, patients were requested to bring their study medication with them at visits 3 (week 2), 4 (week 6) and 5 (week 12). Treatment adherence was calculated based on the number of actuations of study medication actually taken as a percentage of the number of actuations that should have been taken. Safety was evaluated based on adverse events (AEs), standard clinical and laboratory tests (hematology biochemistry and urinalysis), vital signs, and 12-lead electrocardiograms (ECGs) during the core study and extension phase. Hematology analyses included red blood cell count, hemoglobin, platelets, and white blood cell count (total and differential). Biochemistry tests included serum electrolytes, liver function tests, renal function tests, and others (glucose, calcium, albumin, cholesterol, triglycerides, phosphorus, lactate dehydrogenase, total protein, globulin and uric acid). In addition, plasma cortisol was measured at the beginning and end of the extension phase.
Data analyses
Sample size was based on the difference between the treatment groups in the change from baseline in predose FEV1 at day 84. Eighty-six patients were required per group, assuming a standard deviation (SD) 0.2 l, with a noninferiority margin of −0.1 l and 90% power (α = 0.05). Noninferiority efficacy analyses were based on the per-protocol set [(PPS); all patients who completed the study without major protocol violations]. All other efficacy analyses were based on the full-analysis set [(FAS); all randomized patients who received study treatment and had at least one postdose primary efficacy measurement]. As supportive analysis, the primary endpoint was also performed on the FAS, using the last observation carried forward (LOCF) approach to impute missing data.
The primary endpoint was analyzed with an analysis of covariance (ANCOVA) using treatment and age group as factors, baseline predose FEV1 value as a covariate, and center as a random effect. The secondary endpoints 2-hours postdose FEV1, discontinuations due to lack of efficacy and time to onset of action were tested in a hierarchical manner using a gate-keeping strategy and were to have confirmatory significance only if the primary endpoint was statistically significant. Two-hours postdose FEV1 was analyzed using an ANCOVA similar to the primary endpoint. For discontinuation due to lack of efficacy, odds ratio and the 95% confidence interval (CI) were calculated between the treatment groups, and time to onset of action was analyzed using the multiple-failures time model [Wei et al. 1989]. Rescue-medication use, asthma symptoms and sleep disturbance, and patient assessment of study medication were assessed using the Wilcoxon rank sum test, linear regression and a proportional odds model, respectively. All statistical testing was conducted with α = 0.05 with the exception of the primary endpoint, where α was 0.0465 due to a preplanned interim analysis. Only exploratory efficacy analyses were conducted for the extension phase.
Safety parameters were summarized using descriptive statistics based on data from the safety set (all patients who received treatment and had at least one postdose safety assessment). A post hoc analysis was conducted to evaluate any effect of FP/FORM on patient growth based on height and weight data between day 84 (extension start) and day 252 (extension end), with reference to standardized height and weight tables [National Center for Health Statistics – CDC, 2000].
Results
Patients
In total, 235 patients were enrolled into the core trial, of which 211 were randomized and 210 (99.5%) completed 12 weeks of randomized therapy (Figure 2).
Figure 2.
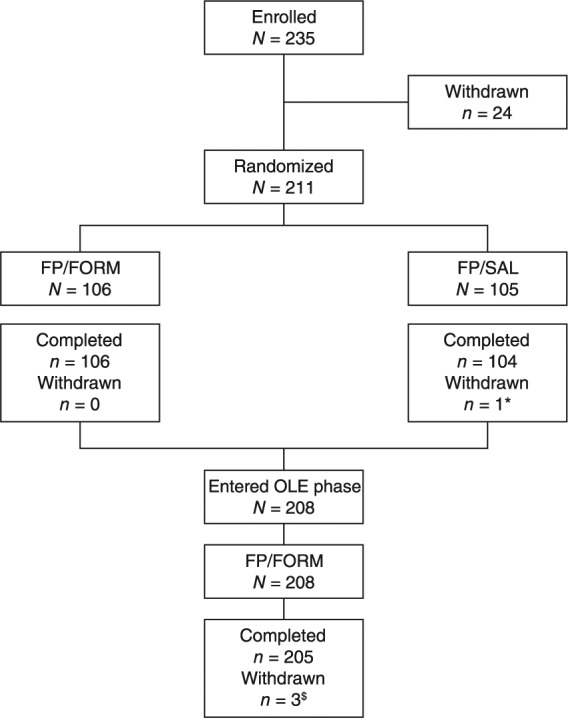
Patient disposition during the core trial and extension phase.
FP/FORM, fluticasone propionate/formoterol fumarate; FP/SAL, fluticasone propionate/salmeterol xinafoate; OLE, open-label extension phase; *patient withdrew by choice; $two patients withdrew by choice; one patient withdrew for administrative reasons.
One patient, randomized to the FP/SAL treatment group, withdrew during the core trial by choice. Subsequently, 208 patients entered and 205 (98.6%) completed the 24-week extension phase. Three patients withdrew during the extension: two by choice and one for administrative reasons. The PPS for the core trial comprised 201 patients and the FAS comprised 211 patients.
Demographic and disease characteristics were comparable across treatment groups (Table 1). Before study start, ICSs were taken by 86.8% of FP/FORM and 83.8% of FP/SAL patients (median daily dose 200 μg fluticasone in both groups).
Table 1.
Patient demographics and asthma characteristics at baseline of core trial (full-analysis set).
| FP/FORM (N = 106) | FP/SAL (N = 105) | ||
|---|---|---|---|
| Demographics | |||
| Age (years) | Mean (SD) | 8.8 (2.1) | 8.5 (2.2) |
| Median (range) | 9.0 (4–12) | 9.0 (4–12) | |
| Age groups: | |||
| 4–6 years | n (%) | 16 (15.1) | 20 (19.0) |
| 7–12 years | n (%) | 90 (84.9) | 85 (81.0) |
| Gender | |||
| Male | n (%) | 72 (67.9) | 73 (69.5) |
| Female | n (%) | 34 (32.1) | 32 (30.5) |
| Race | |||
| Caucasian | n (%) | 106 (100) | 105 (100) |
| Weight (kg) | Mean (SD) | 33.9 (9.7) | 35.6 (13.0) |
| Height (cm) | Mean (SD) | 137.1 (12.5) | 136.4 (14.0) |
| BMI (kg/m2) | Mean (SD) | 17.7 (3.1) | 18.6 (3.7) |
| Asthma characteristics | |||
| FEV1 (presalbutamol; l) | Mean (SD) | 1.53 (0.34) | 1.54 (0.44) |
| FEV1 (postsalbutamol; l) | Mean (SD) | 1.89 (0.42) | 1.92 (0.53) |
| Predicted FEV1 (l) | Mean (SD) | 1.90 (0.49) | 1.88 (0.54) |
| FEV1 (% predicted) | Mean (SD) | 82.0 (9.5) | 82.5 (9.5) |
| FEV1 reversibility (%) | Mean (SD) | 23.7 (9.4) | 25.5 (9.9) |
| Asthma therapy at screening | |||
| Taking ICS | n (%) | 92 (86.8) | 88 (83.8) |
| Taking LABA | n (%) | 68 (64.2) | 50 (47.6) |
BMI, body mass index; FEV1, forced expiratory volume in 1 second; FP/FORM, fluticasone propionate/formoterol fumarate; FP/SAL, fluticasone propionate/salmeterol xinafoate; ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; SD, standard deviation.
Treatment
Treatment adherence was over 75% in 98.1% of patients in the FP/FORM group and 99.0% in the FP/SAL group during the core trial. Adherence greater than 75% was recorded for 88.4% of all patients in the extension phase.
Efficacy
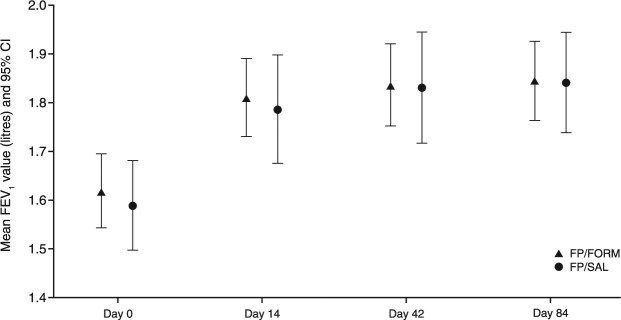
Predose FEV1 values over the course of the core trial are summarized in Figure 3.
Figure 3.
Mean predose forced expiratory volume in 1 second (FEV1) (with 95% confidence interval) during the core study (per-protocol set).
FP/FORM, fluticasone propionate/formoterol fumarate; FP/SAL, fluticasone propionate/salmeterol xinafoate. Core study: day 0 to day 84.
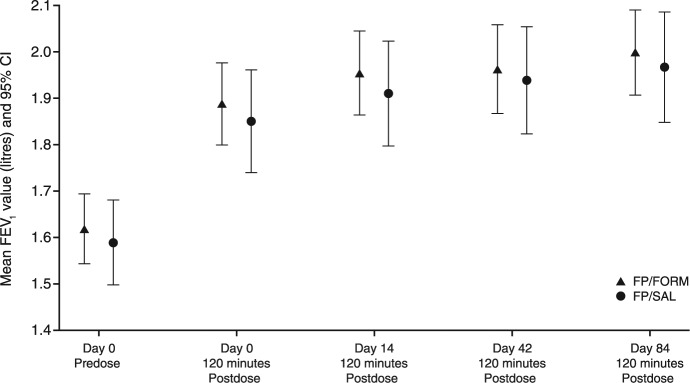
The primary efficacy analysis based on the change in predose FEV1 from day 0 to day 84 demonstrated that FP/FORM was noninferior to FP/SAL (Table 2). The lower limit of the 95.35% CI for the between-treatment difference was within the noninferiority margin (–0.1 l): least squares (LS) mean treatment difference was −0.031 (95.35% CI, −0.093, 0.031; p = 0.026). The LOCF analysis based on the FAS provided similar findings. Analysis of the change in predose FEV1 on day 0, to 2-hours postdose on day 84 also supported noninferiority of FP/FORM; LS mean treatment difference −0.017 (95% CI, −0.089, 0.055; p = 0.025) (Figure 4).
Table 2.
Change in primary and secondary forced expiratory volume in 1 second (FEV1) endpoints between days 0 and 84 of randomized therapy (per-protocol set).
|
Parameter
|
Change (L)
|
Treatment difference*
|
||||
|---|---|---|---|---|---|---|
| Treatment | N | LS mean | 95% CI | LS mean | 95% CI | p value$ |
| Change in predose FEV1 from day 0 to day 84 (l) | ||||||
| FP/FORM | 102 | 0.182 | 0.127, 0.236 | −0.031 | −0.093, 0.031 | 0.026 |
| FP/SAL | 99 | 0.212 | 0.160, 0.265 | – | – | – |
| Change in FEV1 from day 0 (predose) to day 84 (2-hours postdose; l) | ||||||
| FP/FORM | 102 | 0.308 | 0.243, 0.373 | −0.017 | −0.089, 0.055 | 0.025 |
| FP/SAL | 99 | 0.325 | 0.263, 0.387 | – | – | – |
CI, confidence interval; FEV1, forced expiratory volume in 1 second; FP/FORM, fluticasone propionate/formoterol fumarate; FP/SAL, fluticasone propionate/salmeterol xinafoate; LS mean, least squares mean from ANCOVA with treatment and age group as factors, predose FEV1 value at day 0 as a covariate and center as a random effect.
Difference between FP/FORM and FP/SAL; $p value for noninferiority [shown if the lower limit of the 95% CI (95.35% CI for change in predose FEV1) from ANCOVA was ⩾ –0.1 l].
Figure 4.
Mean forced expiratory volume in 1 second (FEV1) (with 95% confidence interval) on day 0 predose and at 2-hours postdose thereafter during the core study (per-protocol set).
FP/FORM, fluticasone propionate/formoterol fumarate; FP/SAL, fluticasone propionate/salmeterol xinafoate. Core study: day 0 to day 84.
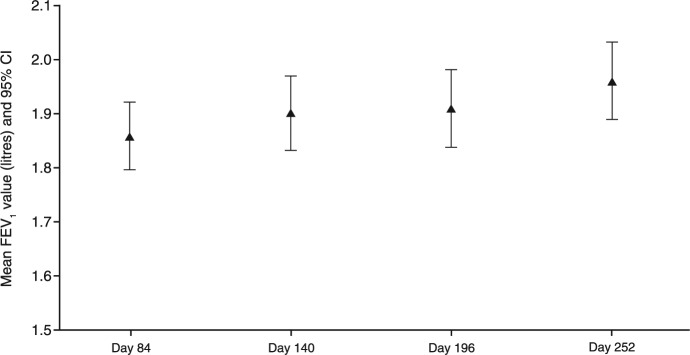
At day 84 (end of core trial), the mean predose FEV1 was similar in both treatment groups (1.85 l and 1.84 l for FP/FORM and FP/SAL, respectively), and subsequently increased by 0.105 l during the FP/FORM extension phase between day 84 and day 252 (Figure 5).
Figure 5.
Mean predose forced expiratory volume in 1 second (FEV1) (with 95% confidence interval) during the extension phase (full-analysis set).
Extension phase: day 84 to day 252.
No patients discontinued either FP/FORM or FP/SAL during the core trial due to lack of efficacy.
Overall, FEV1 increased from the predose value to each consecutive postdose value on day 0 (at 5, 10, 60, and 120 minutes) in both treatment groups. Evaluation of time to onset of action showed that the proportion of patients who achieved at least a 12% increase in FEV1 was marginally higher in the FP/FORM group compared to FP/SAL by 5 minutes postdose (38% versus 30%), and by 10 minutes postdose (51% versus 40%), but was similar at 60 (58% versus 60%) and 120 minutes (68% versus 68%). However, multiple-failures time model did not show a statistically significant difference between the two treatments.
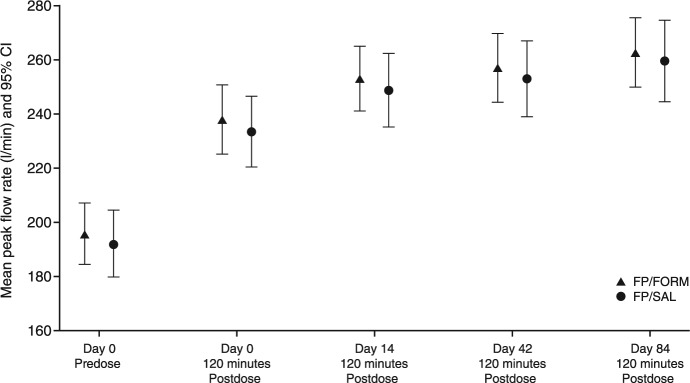
Predose and 2-hours postdose PEFR values were greater on days 0, 14, 42 and 84 compared with predose values on day 0 in both treatment groups during the core trial (Figure 6).
Figure 6.
Mean peak expiratory flow rate measurements (with 95% confidence interval) on day 0 predose and at 2-hours postdose thereafter during the core study (full-analysis set).
FP/FORM, fluticasone propionate/formoterol fumarate; FP/SAL, fluticasone propionate/salmeterol xinafoate. Core study: day 0 to day 84.
Mean morning and evening PEFR collected daily in the electronic diary improved over the period of core trial and were comparable in both FP/FORM and FP/SAL groups. At the beginning of the extension phase, mean predose PEFRs were very similar in patients who received FP/FORM and FP/SAL during the core trial (242 l/min and 243 l/min, respectively). Subsequently, mean predose PEFR among all patients increased by 13.7 l/min between day 84 and day 252.
Mean increases in FVC and MEF25, MEF50 and MEF75 were comparable in the FP/FORM and FP/SAL groups during the core trial (Table 3).
Table 3.
Summary of secondary endpoint data between days 0 and 84 of randomized therapy (full-analysis set).
|
Parameter
|
Treatment difference
|
||||
|---|---|---|---|---|---|
| Treatment | N | Mean* (95% CI) | LS Mean | 95% CI | p value $ |
| Change in FVC (l) from predose on day 0 to 2-hours postdose on day 84 | |||||
| FP/FORM | 106 | 0.223 (0.150, 0.295) | −0.005 | −0.086, 0.077 | 0.911 |
| FP/SAL | 105 | 0.227 (0.157, 0.298) | – | – | – |
| Change in MEF25 (%) from predose on day 0 to 2-hours postdose on day 84 | |||||
| FP/FORM | 106 | 16.1 (10.7, 21.6) | −5.4 | −11.8, 1.0 | 0.099 |
| FP/SAL | 105 | 21.5 (16.3, 26.8) | – | – | – |
| Change in MEF50 (%) from predose on day 0 to 2-hours postdose on day 84 | |||||
| FP/FORM | 106 | 37.1 (28.1, 46.0) | −4.2 | −14.6, 6.3 | 0.433 |
| FP/SAL | 105 | 41.2 (32.5, 49.9) | – | – | – |
| Change in MEF75 (%) from predose on day 0 to 2-hours postdose on day 84 | |||||
| FP/FORM | 106 | 52.5 (39.8, 65.3) | 4.9 | −10.0, 19.8 | 0.517 |
| FP/SAL | 105 | 47.6 (35.3, 60.0) | – | – | – |
| Treatment | N | Mean§ ± SE | Mean | 95% CI | p value^ |
| Asthma symptom scores | |||||
| FP/FORM | 106 | 0.11 ± 0.03 | −0.03 | −0.11, 0.05 | 0.440 |
| FP/SAL | 105 | 0.14 ± 0.03 | – | – | – |
| Sleep disturbance scores | |||||
| FP/FORM | 106 | 0.04 ± 0.02 | −0.06 | −0.11, 0.00 | 0.064 |
| FP/SAL | 105 | 0.10 ± 0.02 | – | – | – |
FVC, forced vital capacity; MEF25, MEF50, MEF75, maximum expiratory flow rate at 25%, 50%, and 75% of the volume to exhale, respectively; CI, confidence interval; LS mean, least squares mean; SE, standard error; FP/FORM, fluticasone propionate/formoterol fumarate; FP/SAL, fluticasone propionate/salmeterol xinafoate.
Least squares mean from ANCOVA with treatment and age group as factors, predose value at day 0 as a covariate, and center as a random effect; §adjusted mean over the course of study treatment based on linear model on mean score with treatment group as a factor; $two-sided p value, significance level 5% (ANCOVA); ^two-sided p value, significance level 5% (linear model).
Overall, asthma symptom scores were low and comparable between treatment groups (Table 3), and sleep disturbance scores were marginally higher in the FP/SAL group than in the FP/FORM group (Table 3). The use of rescue medication was very low throughout the study, and there were no significant between-treatment differences. Very few patients experienced mild or moderate asthma exacerbations in the core study [4 (3.8%) FP/FORM and 3 (2.9%) FP/SAL patients]: there were no cases of severe exacerbations throughout either the core trial or the extension phase. Over 95% of patients in each group assessed study medication as ‘very good’ or ‘good’.
Safety
AEs occurring in at least 2% of patients during the core and extension phases are listed in Table 4.
Table 4.
Adverse events occurring in at least 2% of patients (safety set).
|
Incidence, n (%)
|
|||
|---|---|---|---|
| FP/FORM (N = 106) | FP/SAL (N = 105) | FP/FORM EXT* (N = 208) | |
| Patients with ⩾1 AE | 31 (29.2) | 28 (26.7) | 91 (43.8) |
| Infections and infestations | 24 (22.6) | 22 (21.0) | 79 (38.0) |
| Nasopharyngitis | 3 (2.8) | 5 (4.8) | 17 (8.2) |
| Pharyngitis | 4 (3.8) | 4 (3.8) | 15 (7.2) |
| Bronchitis | 4 (3.8) | 3 (2.9) | 11 (5.3) |
| Respiratory, thoracic and mediastinal disorders | 5 (4.7) | 3 (2.9) | 9 (4.3) |
| Cough | 3 (2.8) | 1 (1.0) | 3 (1.4) |
AE, adverse event; EXT, extension treatment; N, number of patients in a treatment group; n, number of patients with specified AEs; SAE, serious adverse event; %, percentage calculated relative to n; FP/FORM, fluticasone propionate/formoterol fumarate; FP/SAL, fluticasone propionate/salmeterol xinafoate.
Comprises patients who received 12-weeks of randomized FP/FORM then 24-weeks of extension FP/FORM treatment, and those who received 12-weeks of randomized FP/SAL then 24-weeks of extension FP/FORM treatment.
In the core trial, 59/211 patients (28.0%) experienced at least one AE: 29.2% in the FP/FORM group and 26.7% in the FP/SAL group. The most commonly recorded AEs in both groups were nasopharyngitis (in 2.8% and 4.8% of patients, respectively), pharyngitis (3.8% in both groups) and bronchitis (3.8% and 2.9%, respectively). All AEs were of mild or moderate severity. One case of possibly treatment-related mild dizziness was recorded in the FP/FORM group; no treatment-related AEs were recorded in the FP/SAL group. Serious AEs (SAEs) were reported in two FP/FORM-treated patients (two cases of appendicitis considered not related to study medication), and one FP/SAL-treated patient (pneumonia, considered not related to study treatment). All SAEs resolved without clinical sequelae.
During the extension phase, the profile of AEs was similar to that in the core trial: nasopharyngitis, pharyngitis and bronchitis were most frequent. Two cases of oral candidiasis were considered related to study medication, and resolved by the end of extension phase. SAEs were reported in three patients: one case each of appendicitis, epilepsy and pneumococcal pneumonia, none of which were considered related to study medication. No patients discontinued therapy due to AEs and there were no deaths during the study.
There were no trends for clinically relevant changes in vital signs or ECGs. General hematology values remained within the normal range in the majority of patients who received FP/FORM. Mean (SD) and median plasma cortisol values were within normal range at the beginning of the extension phase [296.2 (145.9) and 278.7 nmol/l, respectively] and remained stable up to study end [290.2 (135.7) and 270.4 nmol/l, respectively]. A low number of patients (n = 7; 3%) showed shifts from within-normal range at week 12 to below-normal at study end, and a similar number (n = 8; 4%) shifted from low to within-normal range. Most patients who were on ICS at the beginning of the extension phase had plasma cortisol within the normal reference range: only 11 (5%) patients had cortisol levels below the lower limit of normal.
The post hoc growth evaluation did not indicate any treatment effects on height over the 24-week FP/FORM extension. Patients’ mean (SD) height increased 2.8 cm (1.7), as expected in children aged 4–12 years. The mean (SD) height percentile was 0.609 (0.272) at day 84 (end of core phase) and 0.608 (0.275) at day 252 (extension end).
Discussion
This study demonstrated the noninferiority of FP/FORM compared with FP/SAL in children over 12 weeks based on comparable improvements in predose FEV1 from day 0 to day 84, change in FEV1 predose at day 0 to 2-hours postdose at day 84, and lack of discontinuations due to lack of efficacy. Predose and 2-hour postdose PEFR also improved at all the visits with both FP/FORM and FP/SAL. The improvements from baseline in predose FEV1 with both treatments is comparable to previous studies assessing the efficacy of ICS/LABA combination therapies in a broadly similar patient population, albeit the present study did not require the patients to have impaired lung function or to be symptomatic before randomization [Pohunek et al. 2006; Morice et al. 2008]. These efficacy findings are also in agreement with published data from previous studies in adolescents and adults that have demonstrated the noninferiority of FP/FORM compared with other ICS/LABA combinations in asthma [Bodzenta-Lukaszyk et al. 2011a, 2012; Papi et al. 2015]. The increase in the lung function in the core study was sustained throughout the 24-week extension phase. Although there was a modest increase in mean FEV1 of 105 ml during the extension phase, FEV1 expressed as a percentage of predicted FEV1 (calculated using patients’ age and height recorded at each visit) remained very stable between day 84 and day 252 (data not shown), indicating that the increase in FEV1 was mainly due to the growth of children during the 24-week extension phase. Nevertheless, these findings are in concordance with a number of previous studies that have assessed the efficacy of FP/FORM and other ICS/LABA combinations in adult patient populations over the medium-to-long term. No attenuation of treatment effects was observed in an open-label study that assessed the efficacy and safety of FP/FORM in 472 adult and adolescent patients with mild-to-moderate to severe asthma over 6–12 months [Mansur and Kaiser, 2013]. Previous studies have also reported sustained long-term beneficial effects on lung function parameters in asthma patients treated with other ICS/LABA combinations, including a 1-year randomized study with budesonide/formoterol in 2760 patients, among whom 341 (12%) were aged 4–11 years [O’Byrne et al. 2005], and a 1-year randomized controlled trial and extension study with budesonide/formoterol in 321 adults [Rosenhall et al. 2003].
As expected based on previous data from adolescents and adults [Papi et al. 2013, 2015], a low number of patients experienced asthma exacerbations during either FP/FORM or FP/SAL treatment in the core trial [4 (3.8%) FP/FORM patients and 3 (2.9%) FP/SAL patients]. This finding supports data from the previous pooled analysis of studies with FP/FORM in patients aged 12 years and over that showed a lower incidence of any exacerbation type compared with FP monotherapy [Papi et al. 2015]. It is also notable that there were no severe exacerbations (requiring oral or parenteral steroid use, emergency treatment or hospitalization) in either treatment arm in the core phase or with FP/FORM throughout the extension phase in the current study. This is in line with findings from a previous analysis that assessed the occurrence of severe exacerbations (requiring oral corticosteroids) during two long-term studies with FP/FORM compared with data for other ICS/LABA combinations from published Cochrane analyses [Papi et al. 2013; Lasserson et al. 2008; Ducharme et al. 2010]. A low incidence of corticosteroid-requiring exacerbations was observed during 6–12 months of treatment with FP/FORM in this pooled analysis among a total of 752 patients aged 12 years and above which compared favourably with the incidence observed in long-term studies with single-inhaler FP/SAL and budesonide/formoterol, and with free combinations of individual ICS and LABA formulations [Papi et al. 2013].
A marginally higher percentage of patients achieved at least a 12% increase in FEV1 at 5 and 10 minutes postdose on day 0 with FP/FORM compared with FP/SAL in this study, however, the difference in the time to onset of action between the two treatments was not statistically different. The latter is rather surprising, as FP/FORM has previously been shown to have a faster onset of action compared with FP/SAL in adult patients, due to the rapid bronchodilatory effects of formoterol [Bodzenta-Lukaszyk et al. 2011a; Palmqvist et al. 1997; Politiek et al. 1999]. In a previous 12-week randomized, open-label, active-controlled study in 202 adults with mild-to-moderately severe persistent asthma, over twice as many patients on FP/FORM had a bronchodilatory response that met the same onset of action criterion (⩾12% increase in FEV1) in the first 5 minutes postdose compared with FP/SAL [Bodzenta-Lukaszyk et al. 2011a]. Further, logistic regression and odds ratio analysis of this previous adult study showed that the likelihood of a patient achieving bronchodilation within 5 minutes of dosing was almost four-times higher with FP/FORM than with FP/SAL on day 0 [Aalbers et al. 2012]. The reasons for the disparity between these studies are not clear, but the younger age of the patients in the current study, and the fact that patients continued their ongoing asthma treatment during screening and were therefore stable and asymptomatic at commencement of randomized therapy, could be confounding factors.
FP/FORM had a favourable safety and tolerability profile during the 12 weeks of randomized therapy and throughout the 24-week extension phase in this pediatric population which was consistent with previous published medium-to-long term data from patients aged 12 years and older [Mansur and Kaiser, 2013; Papi et al. 2013]. Analyses of AEs, laboratory values, and vital signs did not reveal any safety signals relating to the use of FP/FORM in children. Of particular note, FP/FORM did not affect normal growth. Data from a survey-based epidemiological study in the UK has indicated an increased possibility of adrenal crisis and/or growth retardation in children treated with high doses of fluticasone (500–2000 µg/day) [Todd et al. 2002]. In the current study, patients received relatively low total daily doses of fluticasone from combination treatment FP/FORM (200 µg/day). Observed plasma cortisol levels were in line with normal values for this patient age group (85.5–618 nmol/L) throughout the 24-week extension phase, indicating no clinically relevant hypothalamic–pituitary–adrenal axis suppression, and there were no AEs suggestive of adrenal hypofunction. The proportion of patients showing shifts in plasma cortisol from within-normal to below-normal levels (3%) was similar to that of patients with shifts from below-normal to within-normal (4%), indicating that outside-normal values more than likely resulted from natural variability: the vast majority of patients had stable plasma cortisol levels throughout the 24-week extension. Furthermore, the post hoc analysis of patient growth rates indicated no effect of FP/FORM on patient growth over the 6-month extension period, based on comparison with standard height percentiles for children of this age. A similar lack of any measureable effect of FP/FORM on growth in children has been reported elsewhere [Wolthers et al. 2015].
Increased rates of pneumonia have been observed in patients with chronic obstructive pulmonary disease receiving treatment with FP/SAL [Wedzicha et al. 2008; Halpin et al. 2011; Nannini et al. 2012; Janson et al. 2013], and it has been questioned whether ICS therapy might be associated with a similarly increased risk in asthma [Pedersen, 2001; Ernst et al. 2007]. The current study does not provide any evidence for an increased risk of pneumonia in children treated with FP/FORM. Two patients developed pneumonia: one case was a patient on FP/SAL during the core trial and one was a patient on FP/FORM during the extension phase. In both cases, pneumonia was reported as an SAE and resolved with treatment. Both were also considered by the treating physicians to be unrelated to study medication. These findings support data from previous studies with FP/FORM in adolescents and adults. In the pooled analysis of FP/FORM randomized controlled studies that included 528 patients treated with FP/FORM and 527 patients on FP monotherapy, only one patient on FP/FORM had pneumonia [Papi et al. 2015]. In addition, a previous, large-scale retrospective analysis of the incidence of pneumonia in 14,993 patients with asthma who participated in randomized, placebo-controlled trials with budesonide or FP, the calculated incidence of pneumonia events with either drug did not support any association between inhaled corticosteroid use and risk of pneumonia [O’Byrne et al. 2011].
As has been observed in a number of other studies with FP/FORM [Papi et al. 2015; Bodzenta-Lukaszyk et al. 2011b; Pertseva et al. 2013; Corren et al. 2012; Pearlman et al. 2013; Nathan et al. 2012], patient retention in this study was high, with only one patient choosing to withdraw during the 12-week core phase (completion rate 99.5%) and two patients choosing to do so during the 6-month extension phase (completion rate 98.6%). This may be related to the patient perceptions of the good tolerability and efficacy of FP/FORM. Real-world data from a number of observational studies have shown high levels of treatment persistence (88–92%) after patients have switched to FP/FORM [Hamill and Spyridon, 2014; Lim et al. 2014; Roe and Junor, 2014].
The current data should be interpreted in view of a number of study limitations. This study was not blinded during the core, randomized phase. However, it was not anticipated that this would be detrimental to the results as the primary efficacy measure was a physical endpoint rather than a subjective measure. FP/SAL was selected as a comparator because it is a marketed ICS/LABA combination pMDI and is licenced for use in children aged older than 4 years. Noninferiority studies should ideally have a third comparator arm to compare the efficacy of FP/FORM and FP/SAL with patient responses to FP monotherapy for assay sensitivity as recommended by ICH E10 [ICH, 2010]. However, a second study was undertaken with a third FP arm that confirmed noninferiority of FP/FORM compared with FP/SAL for efficacy in children between 5- and 12-years old [Ploszczuk et al. 2014]. Finally, the study design did not include a run-in period before commencement of study medication. Instead, patients were effectively run in on current, ongoing treatment, and were therefore not destabilized or symptomatic, which could have influenced the magnitude of observed postrandomization treatment effects.
Conclusion
This study demonstrated the noninferiority of FP/FORM to FP/SAL in terms of predose and postdose FEV1, and discontinuations due to lack of efficacy in children with asthma. Analysis of the other efficacy parameters including patient-reported outcomes, rescue-medication use, and asthma exacerbations yielded comparable results in the two treatment groups. Long-term treatment with FP/FORM appeared to be well tolerated.
Acknowledgments
The authors would like to thank all study participants. Medical writing assistance was provided by Matthew Reilly PhD at InTouch Medical Ltd, funded by Mundipharma Research Limited.
(R) FLUTIFORM is a registered trade mark of Jagotec AG and is used under licence.
(R) SERETIDE and EVOHALER are registered trade marks of Glaxo Group Limited.
(R) AEROCHAMBER PLUS is a registered trade mark of Trudell Medical International.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Mundipharma Research Limited.
Conflict of interests statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Professor Emeryk was the coordinating investigator for this study; Dr Klink was an investigator in this study; Mrs McIver was an employee of Mundipharma Research Limited at the time this study was conducted, and Dr Prashant Dalvi is an employee of Mundipharma Research Limited. All authors reviewed each draft of the manuscript and approved the final version for submission.
Contributor Information
Andrzej Emeryk, Department of Paediatric Lung Diseases and Rheumatology, Medical University, Lublin, Poland.
Rabih Klink, Cabinet de Pédiatrie et de Pneumo Allergologie Pédiatriques, Laon, France.
Tammy McIver, Mundipharma Research Limited, Cambridge, UK.
Prashant Dalvi, Mundipharma Research Limited, Cambridge, UK.
References
- Aalbers R., Brusselle G., McIver T., Grothe B., Bodzenta-Lukaszyk A. (2012) Onset of bronchodilation with fluticasone/formoterol combination versus fluticasone/salmeterol in an open-label, randomized study. Adv Ther 29: 958–969. [DOI] [PubMed] [Google Scholar]
- Allen D. (2006) Effects of inhaled steroids on growth, bone metabolism, and adrenal function. Adv Pediatr 53: 101–110. [DOI] [PubMed] [Google Scholar]
- Bodzenta-Lukaszyk A., Buhl R., Balint B., Lomax M., Spooner K. (2012) Fluticasone/formoterol combination therapy versus budesonide/formoterol for the treatment of asthma: a randomised, controlled, non-inferiority trial of efficacy and safety. J Asthma 49: 1060–1070. [DOI] [PubMed] [Google Scholar]
- Bodzenta-Lukaszyk A., Dymek A., McAulay K., Mansikka H. (2011a) Fluticasone/formoterol combination therapy is as effective as fluticasone/salmeterol in the treatment of asthma, but has a more rapid onset of action: an open-label, randomized study. BMC Pulm Med 11: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodzenta-Lukaszyk A., Pulka G., Dymek A., Bumbacea D., McIver T., Schwab B., et al. (2011b) Efficacy and safety of fluticasone and formoterol in a single pressurized metered dose inhaler. Respir Med 105: 674–682. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics – CDC (2000) United States Growth Charts. Available at: http://www.cdc.gov (accessed 25 March 2015).
- Corren J., Mansfield L., Pertseva T., Blahzko V., Kaiser K. (2012) Efficacy and safety of fluticasone/formoterol combination therapy in patients with moderate-to-severe asthma. Respir Med 107: 180–195. [DOI] [PubMed] [Google Scholar]
- Ducharme F., Ni Chroinin M., Greenstone I., Lasserson T. (2010) Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database Syst Rev 5: CD005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst P., Gonzalez A., Brassard P., Suissa S. (2007) Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med 176: 162–166. [DOI] [PubMed] [Google Scholar]
- Global Initiative for Asthma (GINA). (2015) Global strategy for asthma management and prevention. Available from: www.ginasthma.org. Accessed 25 March 2015.
- Halpin D., Gray J., Edwards S., Morais J., Singh D. (2011) Budesonide/formoterol versus salmeterol/fluticasone in COPD: a systematic review and adjusted indirect comparison of pneumonia in randomised controlled trials. Int J Clin Pract 65: 764–774. [DOI] [PubMed] [Google Scholar]
- Hamill J., Spyridon M. (2014) Real world evidence on asthma review and change from fluticasone propionate/salmeterol to fluticasone propionate/formoterol. Prim Care Respir Med 24: 14110 (abstract 11). [Google Scholar]
- ICH (2010) International conference on harmonization of technical requirements for registration of pharmaceuticals for human use: choice of control group and related issues in clinical trials. Efficacy Guideline ICH E10. Available at: http://www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.html (accessed 11 April 2016).
- Janson C., Larsson K., Lisspers K., Ställberg B., Stratelis G., Goike H., et al. (2013) Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting beta2 agonist: observational matched cohort study (PATHOS). BMJ 346: f3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasserson T., Ferrara G., Casali L. (2008) Combination fluticasone and salmeterol versus fixed dose combination budesonide and formoterol for chronic asthma in adults and children. Cochrane Database Syst Rev 3: CD004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemanske Jr. R., Mauger D., Sorkness C., Jackson D., Boehmer S., Martines F., et al. (2010) Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med 362: 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D., Small I., Wolfe S., Hamill J., Gruffydd-Jones K., Daly C., et al. (2014) Real world effectiveness of changing fixed-dose combination therapy from Seretide® MDI to Flutiform® in UK asthma patients. 7th World Conference of the International Primary Care Respiratory Group (IPCRG). Athens, Greece. [Google Scholar]
- Mansur A., Kaiser K. (2013) Long-term safety and efficacy of fluticasone/formoterol combination therapy in asthma. J Aerosol Med Pulm Drug Deliv 26: 190–199. [DOI] [PubMed] [Google Scholar]
- Morice A., Peterson S., Beckman O., Kukova Z. (2008) Efficacy and safety of a new pressurised metered-dose inhaler formulation of budesonide/formoterol in children with asthma: a superiority and therapeutic equivalence study. Pulm Pharmacol Ther 21: 152–159. [DOI] [PubMed] [Google Scholar]
- Murphy K., Bender B. (2009) Treatment of moderate to severe asthma: patient perspectives on combination inhaler therapy and implications for adherence. J Asthma Allergy 2: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D., O’Byrne P. (2010) Recent advances in the pathophysiology of asthma. Chest 137:1417–1426. [DOI] [PubMed] [Google Scholar]
- Nannini L., Lasserson T., Poole P. (2012) Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 9: CD006829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan R., D’Urzo A., Blazhko V., Kaiser K. (2012) Safety and efficacy of fluticasone/formoterol combination therapy in adolescent and adult patients with mild-to-moderate asthma: a randomised controlled trial. BMC Pulm Med 12: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute (2007) Expert panel report 3: guidelines for the diagnosis and management of asthma. National Asthma Education and Prevention Program, third expert panel on the diagnosis and management of asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; Available at: http://www.ncbi.nlm.nih.gov/books/NBK7232/ (accessed 25 March 2015). [Google Scholar]
- Ni Chroinin M., Greenstone I., Lasserson T., Ducharme F. (2009) Addition of long-acting beta2-agonists to inhaled steroids as first line therapy for persistent asthma in steroid-naïve adults and children (review). Cochrane Database Syst Rev 4: CD005307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Byrne P., Bisgaard H., Godard P., Pistolesi M., Palmqvist M., Zhu Y., et al. (2005) Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med 171: 129–136. [DOI] [PubMed] [Google Scholar]
- O’Byrne P., Pedersen S., Carlsson L., Radner F., Thorén A., Peterson S., et al. (2011) Risks of pneumonia in patients with asthma taking inhaled corticosteroids. Am J Respir Crit Care Med 183: 589–595. [DOI] [PubMed] [Google Scholar]
- Palmqvist M., Persson G., Lazer L., Rosenborg J., Larsson P., Lotvall J. (1997) Inhaled dry-powder formoterol and salmeterol in asthmatic patients: onset of action, duration of effect and potency. Eur Respir J 10: 2484–2489. [DOI] [PubMed] [Google Scholar]
- Papi A., Mansur A., Dissanayake S., Pertseva T., McIver T., Kaiser K. (2013) Long-term fluticasone propionate/formoterol fumarate combination therapy is associated with a low incidence of severe asthma exacerbations. Respirology 18: 111–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papi A., Price D., Sastre J., Kaiser K., Lomax M., McIver T., et al. (2015) Efficacy of fluticasone propionate/formoterol fumarate in the treatment of asthma: A pooled analysis. Respir Med 109: 208–217. [DOI] [PubMed] [Google Scholar]
- Pearlman D., LaForce C., Kaiser K. (2013) Fluticasone/Formoterol combination therapy compared with monotherapy in adolescent and adult patients with mild to moderate asthma. Clin Ther 35: 950–966. [DOI] [PubMed] [Google Scholar]
- Pedersen S. (2001) Do inhaled corticosteroids inhibit growth in children? Am J Respir Crit Care Med 164: 521–535. [DOI] [PubMed] [Google Scholar]
- Pertseva T., Dissanayake S., Kaiser K. (2013) Superiority of fluticasone propionate/formoterol fumarate versus fluticasone propionate alone in patients with moderate-to-severe asthma: a randomised controlled trial. Curr Med Res Opin 29: 1357–1369. [DOI] [PubMed] [Google Scholar]
- Ploszczuk A., Bosheva M., Spooner K., McIver T., Dissanayake S. (2014) Efficacy and safety of fluticasone propionate/formoterol in paediatric patients with asthma. Eur Respir J 44: Abstract 1167. [Google Scholar]
- Pohunek P., Kuna P., Jorup C., De Boeck K. (2006) Budesonide/formoterol improves lung function compared with budesonide alone in children with asthma. Pediatr Allergy Immunol 17: 458–465. [DOI] [PubMed] [Google Scholar]
- Politiek M., Boorsma M., Aalbers R. (1999) Comparison of formoterol, salbutamol and salmeterol in methacholine-induced severe bronchoconstriction. Eur Respir J 13: 988–992. [DOI] [PubMed] [Google Scholar]
- Reddel H., Taylor D., Bateman E., Boulet L., Boushey H., Busse W., et al. (2009) An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 180: 59–99. [DOI] [PubMed] [Google Scholar]
- Robinson J., Angelini B., Krahnke J., Skoner D. (2002) Inhaled steroids and the risk of adrenal suppression in children. Expert Opin Drug Saf 1: 237–244. [DOI] [PubMed] [Google Scholar]
- Roe R., Junor R. (2014) Real-world insights into treatment persistence with fluticasone propionate/ formoterol (FP/FORM) combination inhaler. npj Prim Care Respir Med 24: 14110 (abstract 27). [Google Scholar]
- Rosenhall L., Elvstrand A., Tilling B., Vinge I, Jemsby P., Ståhl E., et al. (2003) One-year safety and efficacy of budesonide/formoterol in a single inhaler (Symbicort Turbuhaler) for the treatment of asthma. Respir Med 97: 702–708. [DOI] [PubMed] [Google Scholar]
- Sorkness C., Lemanske R., Jr., Mauger D., Boehmer S., Chinchilli V., Martines F., et al. (2007) Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol 119: 64–72. [DOI] [PubMed] [Google Scholar]
- Todd G., Acerini C., Ross-Russell R., Zahra S., Warner J., McCance D. (2002) Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom. Arch Dis Child 87: 457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaessen-Verberne A., van den Berg N., van Nierop J., Brackel H., Gerrits G., Hop W., et al. (2010) Combination therapy salmeterol/fluticasone versus doubling dose of fluticasone in children with asthma. Am J Respir Crit Care Med 182: 1221–1227. [DOI] [PubMed] [Google Scholar]
- Wedzicha J., Calverley P., Seemungal T., Hagan G., Ansari Z., Stockley R. (2008) The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med 177: 19–26. [DOI] [PubMed] [Google Scholar]
- Wei L., Lin D., Weissfeld L. (1989) Regression analysis of multivariate incomplete failure time data by modelling marginal distributions. J Am Stat Assoc 84: 1065–1073. [Google Scholar]
- Wolthers O., Moore A., Mersmann S., Dissanayake S. (2015) Knemometry assessment of short-term lower leg growth in children with asthma treated with fluticasone propionate/formoterol combination therapy. Eur Respir J 46: PA1292. [Google Scholar]
- Zapletal A., Paul T., Samanek M. (1977) [Significance of contemporary methods of lung function testing for the detection of airway obstruction in children and adolescents (author’s transl)]. Z Erkr Atmungsorgane 149: 343–371. [PubMed] [Google Scholar]