Abstract
Background
We aimed to clarify the factors associated with the presentation of erosive esophagitis (EE) symptoms in subjects undergoing health checkups.
Methods
We utilized baseline data from 7,552 subjects who underwent upper endoscopy for health screening in a prospective, multicenter cohort study. The subjects were asked to complete a questionnaire detailing their upper abdominal symptoms and lifestyle. Based on the heartburn and/or acid regurgitation frequency, the EE subjects were stratified into the following three groups: (1) at least one day a week (symptomatic EE [sEE]), (2) less than one day a week (mild symptomatic EE [msEE]), and (3) never (asymptomatic EE [aEE]). Postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS) were defined according to the Rome III criteria.
Results
Of the 1,262 (16.7%) subjects (male 83.8%, mean age 52.6 years) with EE, the proportions of sEE, msEE and aEE were 15.0%, 37.2% and 47.9%, respectively. The sEE group showed significant associations with overlapping EPS (OR: 58.4, 95% CI: 25.2–160.0), overlapping PDS (OR: 9.96, 95% CI: 3.91–26.8), severe hiatal hernia (OR: 2.43, 95% CI: 1.43–4.05), experiencing high levels of stress (OR: 2.20, 95% CI: 1.43–3.40), atrophic gastritis (OR: 1.57, 95% CI: 1.03–2.36) and Los Angeles (LA) grade B or worse (OR: 1.72, 95% CI: 1.12–2.60) in the multivariate analysis.
Conclusions
Approximately one-sixth of EE subjects were symptomatic. A multifactorial etiology, including factors unrelated to gastric acid secretion, was associated with the symptom presentation of EE subjects.
Introduction
Gastroesophageal reflux disease (GERD) is a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications [1]. GERD is prevalent worldwide [2], and the prevalence of GERD in Japan has been increasing since the end of the 1990s [3,4].
GERD is divided into two groups according to endoscopic findings: (1) erosive esophagitis (EE) and (2) nonerosive gastroesophageal reflux disease (NERD). The EE prevalence is 15.5% in the Swedish adult population [5] and 12.4% in Spanish patients who have undergone upper gastrointestinal endoscopy [6]. In Asia, including Japan, the prevalence of EE ranges from 4.5% to 15.7% [3, 7]. GERD symptoms are not always associated with the presence or absence of esophagitis, and asymptomatic EE is common. In a study of the adult Swedish population, 37% of EE cases were asymptomatic [5]. The nationwide Japanese GERD survey reported that 210 of 600 (35%) patients with EE were asymptomatic [8]. Similarly, recent studies from other Asian countries conducted in subjects undergoing health checkups have reported that approximately half or more of EE patients are asymptomatic [9–11]. The symptom presentation of EE patients is clinically important because GERD symptoms reduce patient quality of life (QOL) [12,13]. However, the factors associated with the presentation of EE symptoms have not been fully elucidated.
The present study aimed to prospectively investigate the prevalence of EE in a large population undergoing health checkups at 7 facilities and to determine the factors associated with the symptom manifestation of EE, which lowers patient QOL.
Materials and methods
Subjects
This study was based on baseline data (n = 8,889) from the Upper Gastro Intestinal Disease (UGID) study currently being conducted in Japan. The UGID study is a prospective, multicenter cohort study that aims to investigate the prevalence and natural history of EE, NERD and functional dyspepsia (FD). In the UGID study, subjects 18 years of age or older who underwent upper endoscopy for health screenings at 7 facilities will be followed for up to 5 years. This study population includes 7,552 subjects who were enrolled between April 2013 and March 2015. The following exclusion criteria were applied: treatment with a proton pump inhibitor (PPI) or histamine type 2-receptor antagonist (H2RA) (n = 597), incomplete data for endoscopic findings (n = 59), reflux symptoms or the dyspeptic symptom questionnaire (n = 79), smoking (n = 25), consumption of alcohol (n = 24), kyphosis (n = 112), and State-Trait Anxiety Inventory (STAI) traits (n = 441). This study was conducted in accordance with the Declaration of Helsinki and its amendments (UMIN-CTR ID: 000022504). The study protocol was approved by the ethics committee at each institution (the ethics committees of Kyoto Second Red Cross Hospital, Yodogawa Christian Hospital, Fukui Red Cross Hospital, Kakogawa Central City Hospital, Kita-harima Medical Center, Saiseikai Nakatsu Hospital and Hotel Okura Kobe Clinic). Written informed consent was obtained from all study subjects. All authors had access to the study data and reviewed and approved the final manuscript.
Questionnaire
The subjects were asked to complete the questionnaire about their upper abdominal symptoms (reflux symptoms, dyspeptic symptoms), height, body weight, smoking (never, ex-smoker, current smoker), alcohol consumption (none, ≤20 g/day, 20–60 g/day or >60 g/day), sleep shortage (yes, no), exercise shortage (yes, no), irregular meal times (yes, no), experiencing high levels of stress (yes, no), feeling depressed (yes, no), and kyphosis. For reflux symptoms, the frequency of heartburn and/or acid regurgitation in past 3 months was investigated (i.e., never, less than one day a month, one day a month, two to three days a month, one day a week, more than one day a week, or every day). For dyspeptic symptoms of postprandial distress syndrome (PDS), the frequency of bothersome postprandial fullness and/or early satiation in the past 3 months was investigated (i.e., never, less than one day a month, one day a month, two to three days a month, one day a week, more than one day a week, or every day). For dyspeptic symptoms of epigastric pain syndrome (EPS), the frequency of epigastric pain and/or epigastric burning experienced in the past 3 months was investigated (i.e., never, less than one day a month, one day a month, two to three days a month, one day a week, more than one day a week, or every day). In addition, the subjects were asked whether the onset of dyspeptic symptoms occurred more than 6 months ago. The presence or absence of "sleep shortage", "exercise shortage", "irregular meal times", "experience of high levels of stress" and "feeling depressed" was investigated without the inclusion of objective definitions in this self-reported questionnaire survey. Kyphosis was assessed with the following question. “Does the back of your head touch the wall when you stand with your heels touching the wall?” The possible answers were “Yes, easily”, “Yes but not easily”, or “No”. The subjects who answered “No” were defined as positive for kyphosis, as diagnosed through the questionnaire.
Anxiety was assessed based on the STAI score [14]. The STAI (trait) inventory consists of twenty questions, and higher scores indicate stronger anxiety. A high STAI score was defined as ≥44 in male subjects and ≥45 in female subjects.
The medications that the subjects were prescribed were investigated by the health screening questionnaire used at each institution. In this study, the use of PPI (presence, absence), H2RA (presence, absence), other gastromucoprotective agents (presence, absence), non-steroidal anti-inflammatory drugs (NSAIDs) (presence, absence), low-dose aspirin (presence, absence), calcium (Ca) antagonists (presence, absence), angiotensin II receptor blockers (ARBs) (presence, absence), statins (presence, absence), oral antidiabetic agents (presence, absence), and bisphosphonate (presence, absence) was investigated.
Endoscopic findings
EE was diagnosed based on the presence of endoscopically detectable mucosal breaks and was graded according to the Los Angeles (LA) classification. The presence of endoscopic Barrett’s mucosa was defined by a greater than 10-mm length of the endoscopic columnar-lined epithelium, which was diagnosed using the palisade vessels as a landmark for the esophagogastric junction [15]. Hiatal hernia was diagnosed based on proximal dislocation of the esophagogastric junction of more than 2 cm above the diaphragmatic hiatus. Hiatal hernia severity was classified by the length of dislocation of the esophagogastric junction; 2–4 cm was considered mild, while >4 cm was considered severe. Atrophic gastritis was endoscopically diagnosed, and the endoscopic extent of atrophic mucosa was graded according to the Kimura–Takemoto classification from C-1 to O-3 [16]. Subjects with atrophic mucosa (graded as C-2, C-3, O-1, O-2, and O-3) were defined as positive for atrophic gastritis.
Definitions of symptomatic EE, mild symptomatic EE, asymptomatic EE, NERD, and the control group
The EE subjects who had neither peptic ulcers nor any history of upper gastrointestinal tract surgery were classified into one of three groups according to the frequency of reflux symptoms: the symptomatic EE (sEE) group (subjects with heartburn and/or acid regurgitation occurring at least one day a week), mild symptomatic EE (msEE) group (subjects with heartburn and/or acid regurgitation occurring less than one day a week), or asymptomatic EE (aEE) group (subjects with neither heartburn nor acid regurgitation). Subjects with heartburn and/or acid regurgitation occurring at least one day a week, without EE, peptic ulcers or any history of upper gastrointestinal tract surgery, were defined as having NERD. Subjects with no heartburn, acid regurgitation, bothersome postprandial fullness, early satiation, epigastric pain or epigastric burning, and without EE, peptic ulcer, upper gastrointestinal tract malignancy, or a history of upper gastrointestinal tract surgery were defined as the control group.
Definitions of FD, PDS and EPS
The FD group was defined as those subjects who experienced PDS and/or EPS, according to the Rome III criteria [17]. PDS was defined as "bothersome postprandial fullness" and/or "early satiation" occurring at least two days a week in the past 3 months, with symptom onset occurring 6 months ago, and EPS was defined as "epigastric pain" or "epigastric burning " occurring at least one day a week in the past 3 months, with an onset of symptoms occurring 6 months ago.
In this study, the GERD subjects were not excluded from the FD group.
Statistical analyses
All statistical analyses were conducted using JMP version 10 (SAS Institute, Cary, NC, USA). Comparisons between the subjects with EE and the control group as well as between the subjects with sEE and the subjects with msEE plus aEE were performed with the χ2 test (or the Fisher exact test, if appropriate) for categorical variables and Student’s t-test for continuous variables. Categorical variables, including age, gender, current smoking status, alcohol consumption ≥20 g/day, and variables with P values less than 0.2 (according to the χ2 test) were used for the multiple logistic regression analyses, and P<0.05 was considered statistically significant.
Results
Prevalence and factors associated with EE
Of the 7,552 study subjects, 1,262 subjects (16.7%) (1,058 males and 204 females, mean age±SD: 52.6±9.4 years) were found to have EE. Most cases of EE were of mild severity (LA grade A, 79.7%, and LA grade B, 18.0%) (Table 1).
Table 1. Characteristics of the study participants.
| Total (n = 7,552) | |
|---|---|
| Age, mean±SD (years) | 52.4±10.0 |
| Age group | |
| ≤39 years | 715 (9.5%) |
| 40–59 years | 4,997 (66.2%) |
| ≥60 years | 1,840 (24.4%) |
| Gender | |
| Men | 4,766 (63.1%) |
| Women | 2,786 (36.9%) |
| Body mass index, mean±SD (kg/m2) | 22.9±3.3 |
| Body mass index ≥25 kg/m2 | 1,747 (23.1%) |
| Current smoking | 1,247 (16.5%) |
| Alcohol consumption ≥20 g/day | 2,027 (26.8%) |
| Self-assessment on the daily life questionnaire | |
| Sleep shortage | 2,480 (32.8%) |
| Exercise shortage | 4,814 (63.7%) |
| Irregular meal time | 1,597 (21.1%) |
| Experiencing high levels of stress | 2,099 (27.8%) |
| Feeling depressed | 671 (8.9%) |
| Kyphosis diagnosed by questionnaire | 39 (0.52%) |
| STAI score, mean±SD | 41.6±9.9 |
| High STAI score | 2,867 (38.0%) |
| Endoscopic findings | |
| Atrophic gastritis | 2,949 (39.0%) |
| Hiatal hernia | 2,212 (29.3%) |
| Mild | 1,934/2,212 (87.4%) |
| Severe | 278/2,212 (12.6%) |
| Endoscopic Barret’s mucosa ≥10 mm | 160 (2.1%) |
| Erosive esophagitis (EE) | 1,262 (16.7%) |
| LA grade A | 1,006/1,262 (79.7%) |
| LA grade B | 227/1,262 (18.0%) |
| LA grade C | 27/1,262 (2.1%) |
| LA grade D | 2/1,262 (0.16%) |
| Symptomatic EE | 189/1,262 (15.0%) |
| Mild symptomatic EE | 469/1,262 (37.2%) |
| Asymptomatic EE | 604/1,262 (47.9%) |
| NERD | 363 (4.8%) |
| FD | 299 (4.0%) |
| PDS | 170 (2.3%) |
| EPS | 183 (2.4%) |
| Control group | 3,254 (43.1%) |
| Current medication | |
| NSAIDs | 122 (1.6%) |
| Low-dose aspirin | 89 (1.2%) |
| Ca antagonists | 702 (9.3%) |
| ARB | 602 (8.0%) |
| Statins | 717 (9.5%) |
| Oral hypoglycemic agents | 251 (3.3%) |
| Bisphosphonate | 34 (0.45%) |
| Gastromucoprotective agents | 176 (2.3%) |
SD, standard deviation; STAI, State-Trait Anxiety Inventory; NERD, nonerosive reflux disease; FD, functional dyspepsia; PDS, postprandial distress syndrome; EPS, epigastric pain syndrome; NSAIDs, non-steroidal anti-inflammatory drugs; Ca, calcium; ARB, angiotensin II receptor blocker.
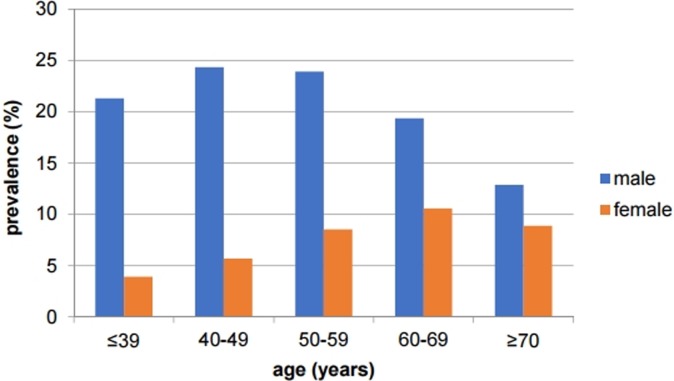
Fig 1 shows the age and gender distribution for the prevalence of EE. At all ages, male subjects were diagnosed with EE more frequently than female subjects (22.2% in males vs. 7.3% in females, P<0.0001), although the difference was less pronounced in the elderly (12.9% in males over 70 years old vs. 8.9% in females over 70 years old, P = 0.2497).
Fig 1. Age and gender distributions for the prevalence of erosive esophagitis.
At all ages, male subjects were diagnosed with erosive esophagitis more frequently than female subjects.
Comparisons of the clinical characteristics of the subjects with EE and the control group are shown in Table 2. Gender, body mass index (BMI), current smoking, alcohol consumption ≥20 g/day, exercise shortage, irregular meal time, experiencing high levels of stress, feeling depressed, STAI score, hiatal hernia, endoscopic Barrett’s mucosa ≥10 mm, atrophic gastritis, and the use of Ca antagonists, ARB, and oral hypoglycemic agents differed significantly between the EE group and the control group.
Table 2. Comparison of clinical characteristics between the subjects with erosive esophagitis and the control group.
| EE (n = 1,262) |
Control (n = 3,254) | P value | |
|---|---|---|---|
| Age, mean±SD (years) | 52.6±9.4 | 52.7±10.3 | 0.5524 |
| Age group | 0.0037 | ||
| ≤39 years (%) | 7.8 | 9.5 | |
| 40–59 years (%) | 69.3 | 64.0 | |
| ≥60 years (%) | 22.9 | 26.5 | |
| Male (%) | 83.8 | 61.7 | <0.0001 |
| BMI, mean±SD (kg/m2) | 24.3±3.6 | 22.7±3.1 | <0.0001 |
| BMI ≥25 kg/m2 (%) | 37.0 | 20.3 | <0.0001 |
| Current smoking (%) | 24.9 | 14.8 | <0.0001 |
| Alcohol consumption ≥20 g /day (%) | 38.9 | 23.7 | <0.0001 |
| Sleep shortage (%) | 30.0 | 28.4 | 0.3001 |
| Exercise shortage (%) | 65.1 | 60.8 | 0.0076 |
| Irregular meal time (%) | 22.7 | 19.2 | 0.0074 |
| Experiencing high levels of stress (%) | 28.0 | 20.6 | <0.0001 |
| Feeling depressed (%) | 7.1 | 5.5 | 0.0419 |
| Kyphosis diagnosed by questionnaire (%) | 0.71 | 0.34 | 0.0885 |
| STAI score, mean±SD | 41.1±9.9 | 40.0±9.5 | 0.0004 |
| High STAI score (%) | 37.4 | 31.3 | <0.0001 |
| Hiatal hernia (%) | 48.3 | 24.4 | <0.0001 |
| Endoscopic Barret’s mucosa ≥10 mm (%) | 4.5 | 1.4 | <0.0001 |
| Atrophic gastritis (%) | 24.5 | 40.8 | <0.0001 |
| Use of NSAIDs (%) | 1.7 | 1.2 | 0.2561 |
| Use of low-dose aspirin (%) | 0.87 | 1.4 | 0.1636 |
| Use of Ca antagonists (%) | 12.5 | 9.0 | 0.0005 |
| Use of ARB (%) | 10.1 | 7.3 | 0.0018 |
| Use of statins (%) | 10.7 | 9.3 | 0.1678 |
| Use of oral hypoglycemic agents (%) | 5.1 | 3.0 | 0.0010 |
| Use of bisphosphonate (%) | 0.16 | 0.40 | 0.2606 |
| Use of gastromucoprotective agents (%) | 2.6 | 2.0 | 0.2270 |
EE, erosive esophagitis; SD, standard deviation; BMI, body mass index; STAI, State-Trait Anxiety Inventory; NSAIDs, non-steroidal anti-inflammatory drugs; Ca, calcium; ARB, angiotensin II receptor blocker.
The results of the multivariate analysis of factors associated with EE indicated that an age of 40–59 years (reference: ≤39 years) (OR 1.44, 95% CI 1.12–1.88), age of ≥60 years (reference: ≤39 years) (OR 1.49, 95% CI 1.10–2.03), male gender (OR 2.32, 95% CI 1.93–2.80), BMI ≥25 kg/m2 (OR 1.87, 95% CI 1.60–2.19), current smoking status (OR 1.34, 95% CI 1.12–1.61), alcohol consumption ≥20 g/day (OR 1.57, 95% CI 1.34–1.84), experiencing high levels of stress (OR 1.40, 95% CI 1.17–1.68), hiatal hernia (OR 2.41, 95% CI 2.08–2.79), endoscopic Barret’s mucosa ≥10 mm (OR 2.62, 95% CI 1.71–4.04), atrophic gastritis (OR 0.40, 95% CI 0.34–0.47), and use of low-dose aspirin (OR 0.38, 95% CI 0.17–0.76) were significantly different between the EE group and the control group (Table 3).
Table 3. Multivariate analysis of the factors associated with erosive esophagitis compared to the control group.
| OR | 95% CI | P value | |
|---|---|---|---|
| Age | |||
| 40–59 years (reference: ≤39 years) | 1.44 | 1.12–1.88 | 0.0050 |
| ≥60 years (reference: ≤39 years) | 1.49 | 1.10–2.03 | 0.0096 |
| Gender (male/female) | 2.32 | 1.93–2.80 | <0.0001 |
| BMI ≥25 kg/m2 (yes/no) | 1.87 | 1.60–2.19 | <0.0001 |
| Current smoking (yes/no) | 1.34 | 1.12–1.61 | 0.0012 |
| Alcohol consumption ≥20 g /day (yes/no) | 1.57 | 1.34–1.84 | <0.0001 |
| Experiencing high levels of stress (yes/no) | 1.40 | 1.17–1.68 | 0.0003 |
| Hiatal hernia (yes/no) | 2.41 | 2.08–2.79 | <0.0001 |
| Endoscopic Barret’s mucosa ≥10 mm (yes/no) | 2.62 | 1.71–4.04 | <0.0001 |
| Atrophic gastritis (yes/no) | 0.40 | 0.34–0.47 | <0.0001 |
| Use of low-dose aspirin (yes/no) | 0.38 | 0.17–0.76 | 0.0055 |
OR, odds ratio; CI, confidence interval; BMI, body mass index.
Since a gender difference was observed in the age distribution of the erosive esophagitis prevalence, we performed a multivariate analysis of factors associated with EE stratified by gender. As shown in S1 Table, the odds ratio for EE increased as the age group increased in the female subjects. Conversely, this relationship between age and EE was not observed in the male subjects, although the odds ratio was significantly higher for the 40- to 59- year-old group than for the ≤39-year-old group.
In addition, we performed a multivariate analysis of factors associated with EE in the subgroup subjects stratified by the presence of a hiatal hernia and/or endoscopic Barret’s mucosa. As shown in S2 Table, the factors associated with EE were similar regardless of the stratification of the study subjects.
Furthermore, we compared the clinical characteristics of the EE subjects and the control group, including subjects with missing STAI values, by bivariate analysis. As shown in S3 Table, the same factors that were significant in Table 2 differed significantly between the two groups, and the proportion of subjects with missing STAI values among the EE subjects was not significantly different from that among control individuals (4.4% (58/1320) vs. 5.5% (189/3443), P = 0.1270). Additionally, a multiple logistic regression analysis of the factors associated with EE compared to the control group was performed using three categories of STAI score (high STAI score, normal and low STAI score, and missing STAI score). As shown in S4 Table, the factors associated with EE were similar regardless of the inclusion of subjects with missing STAI values, and a missing STAI score was not a significant factor.
Factors associated with symptomatic EE compared to mild symptomatic and asymptomatic EE
Among the 1,262 EE subjects, the proportions of subjects with sEE, msEE, and aEE were 15.0% (n = 189), 37.2% (n = 469) and 47.9% (n = 604), respectively. Table 4 shows comparisons of the clinical characteristics among the three EE groups. Alcohol consumption ≥20 g/day, sleep shortage, experiencing high levels of stress, feeling depressed, STAI score, EE ≥LA grade B, hiatal hernia of a severe grade, atrophic gastritis, and overlapping FD (PDS and EPS) differed significantly between the sEE group and the msEE plus aEE group (Tables 4 and S5).
Table 4. Comparison of the clinical characteristics of the three erosive esophagitis groups according to the frequency of reflux symptoms.
| sEE (n = 189) | msEE (n = 469) | aEE (n = 604) | |
|---|---|---|---|
| Age, mean±SD (years) | 52.2±9.3 | 52.3±9.3 | 52.9±9.6 |
| Age group | |||
| ≤39 years (%) | 7.4 | 8.7 | 7.3 |
| 40–59 years (%) | 69.8 | 70.4 | 68.2 |
| ≥60 years (%) | 22.8 | 20.9 | 24.5 |
| Male (%) | 86.8 | 84.7 | 82.3 |
| BMI, mean±SD (kg/m2) | 24.6±3.6 | 24.4±3.4 | 24.2±3.6 |
| BMI ≥25 kg/m2 (%) | 42.9 | 38.2 | 34.3 |
| Current smoking (%) | 26.5 | 24.7 | 24.5 |
| Alcohol consumption ≥20 g /day (%) | 45.5a | 40.9 | 35.3 |
| Sleep shortage (%) | 37.6a | 29.9 | 27.7 |
| Exercise shortage (%) | 68.3 | 70.8 | 59.6 |
| Irregular meal time (%) | 22.8 | 26.0 | 20.2 |
| Experiencing high levels of stress (%) | 45.0b | 30.9 | 20.4 |
| Feeling depressed (%) | 14.3b | 7.9 | 4.3 |
| Kyphosis diagnosed by questionnaire (%) | 1.1 | 0.64 | 0.66 |
| STAI score, mean±SD | 44.9±10.6b | 41.5±10.1 | 39.7±9.1 |
| High STAI score (%) | 51.3b | 37.7 | 32.8 |
| Erosive esophagitis ≥LA grade B (%) | 35.5b | 22.0 | 14.2 |
| Hiatal hernia severe grade (%) | 18.0b | 10.9 | 5.6 |
| Endoscopic Barret’s mucosa ≥10 mm (%) | 5.8 | 5.3 | 3.5 |
| Atrophic gastritis (%) | 31.8a | 23.2 | 23.2 |
| Use of NSAIDs (%) | 1.6 | 1.7 | 1.7 |
| Use of low-dose aspirin (%) | 0.0 | 0.85 | 1.2 |
| Use of Ca antagonists (%) | 14.3 | 12.8 | 11.8 |
| Use of ARB (%) | 11.1 | 11.5 | 8.8 |
| Use of statins (%) | 10.1 | 11.3 | 10.4 |
| Use of oral hypoglycemic agents (%) | 4.8 | 4.3 | 5.8 |
| Use of bisphosphonate (%) | 0.0 | 0.21 | 0.17 |
| Use of gastromucoprotective agents (%) | 2.7 | 2.1 | 3.0 |
| Overlapping with FD (%) | 33.3b | 1.5 | 1.2 |
| PDS (%) | 14.3b | 0.64 | 0.83 |
| EPS (%) | 27.5b | 0.85 | 0.33 |
sEE, symptomatic erosive esophagitis; msEE, mild symptomatic erosive esophagitis; aEE, asymptomatic erosive esophagitis; SD, standard deviation; BMI, body mass index; STAI, State-Trait Anxiety Inventory; NSAIDs, non-steroidal anti-inflammatory drugs; Ca, calcium; ARB, angiotensin II receptor blocker; FD, functional dyspepsia; PDS, postprandial distress syndrome; EPS, epigastric pain syndrome.
a P<0.05 versus msEE plus aEE
b P<0.0001 versus msEE plus aEE
In the multivariate analysis of factors associated with sEE compared to msEE plus aEE, overlapping EPS (OR 58.4, 95% CI 25.2–160.0), overlapping PDS (OR 9.96, 95% CI 3.91–26.8), hiatal hernia of a severe grade (OR 2.43, 95% CI 1.43–4.05), experiencing high levels of stress (OR 2.20, 95% CI 1.43–3.40), EE ≥LA grade B (OR 1.72, 95% CI 1.12–2.60), and atrophic gastritis (OR 1.57, 95% CI 1.03–2.36) were identified as significant factors (Table 5).
Table 5. Multivariate analysis of the factors associated with symptomatic erosive esophagitis compared to mild symptomatic plus asymptomatic erosive esophagitis.
| OR | 95% CI | P value | |
|---|---|---|---|
| Erosive esophagitis ≥LA grade B (yes/no) | 1.72 | 1.12–2.60 | 0.0135 |
| Hiatal hernia severe (yes/no) | 2.43 | 1.43–4.05 | 0.0013 |
| Atrophic gastritis (yes/no) | 1.57 | 1.03–2.36 | 0.0353 |
| Experiencing high levels of stress (yes/no) | 2.20 | 1.43–3.40 | 0.0004 |
| Overlapping with PDS (yes/no) | 9.96 | 3.91–26.8 | <0.0001 |
| Overlapping with EPS (yes/no) | 58.4 | 25.2–160.0 | <0.0001 |
OR, odds ratio; CI, confidence interval; BMI, body mass index; PDS, postprandial distress syndrome; EPS, epigastric pain syndrome.
Discussion
The results of the present study demonstrated that the prevalence of EE in subjects undergoing health checkups was 16.7%, and most EE cases (97.7%) were mild—grade A or B. In Japan, the prevalence of GERD has been increasing since the end of the 1990s [3,4]. One possible reason for this change is an increase in gastric acid secretion in the Japanese population, irrespective of Helicobacter pylori infection [18]. Since dietary fat intake has been reported to stimulate gastric acid secretion in mice [19], the chronological increase in gastric acid secretion in Japanese people may be related to an increase in fat intake [20]. In addition, because H. pylori infection is inversely related to the prevalence of GERD [3], a decrease in the H. pylori infection rate in the Japanese population is considered to be one explanation for this change.
Other than the absence of H. pylori infection, multiple risk factors for EE have been identified, including advanced age, male gender, BMI, smoking, alcohol consumption, the absence of gastric atrophy, endoscopic Barret’s mucosa, or hiatal hernia [6,21–26]. Consistent with previous studies, these factors were also significantly associated with EE compared to the control group in this study. In addition, in this study, experiencing high levels of stress was a significant risk factor associated with EE, which was consistent with the reported association between psychological factors and GERD [27]. Use of low-dose aspirin was a significant preventive factor associated with EE in the multivariate analysis, although it was not identified as a significant factor in the bivariate analysis. Because few low-dose aspirin users participated in this study, a coincidental statistical bias might exist in the analysis. An association between diabetes mellitus and GERD has been reported [28]. Unfortunately, because the fasting blood glucose and hemoglobin A1c values were not investigated in this study, we did not perform statistical analyses using diabetes mellitus as an explanatory variable. The use of oral hypoglycemic agents was a significant risk factor associated with EE in the bivariate analysis but was not a significant factor in the multivariate analysis.
Because it reduces patient QOL, the symptom presentation of EE is clinically important. Although the pathogenesis of reflux symptom manifestation in GERD is not fully understood, sensory nerve stimulation through direct contact with refluxed gastric acid is a primary factor. In addition, other factors, including esophageal visceral hypersensitivity, abnormal tissue resistance, or sustained esophageal contraction, might be involved in the presentation of reflux symptoms [29]. This study showed that the prevalence of sEE in EE subjects was 15.0%, and approximately half (47.9%) of the EE subjects were asymptomatic. In previous studies, the prevalence of sEE in EE subjects has ranged from 11.3% to 66.4% [9–11, 30], which is likely dependent upon the differences in definitions of sEE and study populations.
The significant independent predictors of sEE in this study included higher-grade EE (≥LA grade B), severe hiatal hernia, atrophic gastritis, experiencing high levels of stress, overlapping EPS, and overlapping PDS. The severities of esophagitis and hiatal hernia were associated with esophageal exposure to refluxed gastric acid, which suggests that the inhibition of acid is important for reflux symptom management.
Experiencing high levels of stress is considered a psychological factor. In the bivariate analysis in this study, other psychological stress-related factors, including sleep shortage, feeling depressed and STAI scores, were statistically significant risk factors associated with sEE. Previous studies have also shown that psychological factors, such as anxiety, depression, and somatization symptoms, were risk factors associated with sEE [9–11].
Dyspeptic symptoms are more common in patients with frequent GERD compared with intermittent GERD, and they impact patient QOL [31]. In agreement with previous studies [10,11], FD was a significant risk factor associated with sEE in this study. EPS showed a stronger association with sEE than PDS, which was also consistent with a previous study [10]. Patients with dyspepsia are known to be at risk to develop GERD [31]. In addition, specific dyspeptic symptoms are reportedly associated with poor responses to PPIs in GERD patients [32]. To better manage the reflux symptoms of EE, further pathophysiological studies of the overlap between GERD and FD are needed.
Atrophic gastritis, which is mainly induced by H. pylori infection, leads to hypoacidity of gastric juice and is negatively associated with EE. This negative association was also observed in this study. Interestingly, however, the presence of atrophic gastritis was also a risk factor associated with sEE in this study. A positive association between H. pylori infection and sEE has been reported in a previous study from China [30]. Chronic mucosal inflammation in atrophic gastritis might lead to abnormalities in gastroduodenal motility and sensitivity [33] as well as reflux symptom presentation.
One strength of this study was the use of a large catalog of data from a prospective multicenter cohort study. The size and comprehensiveness of this database enabled us to determine the predictors of EE and the factors associated with symptom presentation in EE while adjusting for potential confounders. Nevertheless, this study has several limitations. First, most of the EE subjects in this study were individuals who underwent health screenings, not patients. In addition, the severity of reflux symptoms was not measured, although symptom frequency was measured in detail. The questionnaire for self-assessment of daily life without an objective definition was simple, and therefore it might be insufficient for evaluating the data or for comparisons to other studies on the same topic. Subjects who were receiving antidepressants or tranquilizers were not excluded from this study, although their perceptions of their stress levels might be influenced by those drugs. Furthermore, the status of H. pylori infection was not evaluated biologically, although endoscopic atrophic gastritis associated with H. pylori infection was evaluated.
In conclusion, approximately one-sixth of the EE subjects in our population were symptomatic. The factors associated with the symptomatic presentation of EE included overlapping EPS, overlapping PDS, experiencing high levels of stress, severe hiatal hernia, atrophic gastritis, and LA grade B or worse. A multifactorial etiology, including factors unrelated to gastric acid secretion, was associated with the symptomatic presentation of EE. The natural history of EE symptom presentation will be investigated in our follow-up studies.
Supporting information
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We are grateful to Ms. Haru Hamanaka for her dedicated secretarial assistance. We thank Mr. Masaki Ashida, Mr. Takahide Kudo, and Mr. Takashi Okamoto for their assistance with the statistical analysis. We thank Ms. Junko Otani for her assistance with the database creation. We thank Mr. Takahiro Hanasaki for his assistance. We thank Dr. Kiyoshi Ashida for providing helpful suggestions for this study. We thank Dr. Kenichi Nishioji, Dr. Mai Kamaguchi, Dr. Hidekazu Mukai, Dr. Atsushi Sugahara, Dr. Akihiko Watanabe, Dr. Saori Matsui, and Dr. Nobuyuki Matsuki for their assistance with data collection.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by grants provided by the NPO-Corporation, Gastrointestinal Medical Care Research and Education Center, and Japan Society of Health Evaluation and Promotion (to T.F.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vakil N, van Zanten SV, Kahriras P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871–880. doi: 10.1136/gutjnl-2012-304269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujiwara Y, Arakawa T. Epidemiology and clinical characteristics of GERD in the Japanese population. J Gastroenterol. 2009;44:518–534. doi: 10.1007/s00535-009-0047-5 [DOI] [PubMed] [Google Scholar]
- 4.Iwakiri K, Kinoshita Y, Habu Y, Oshima T, Manabe N, Fujiwara Y, et al. Gastroesophageal reflux disease 2015. J Gastroenterol. 2016;51:751–767. doi: 10.1007/s00535-016-1227-8 [DOI] [PubMed] [Google Scholar]
- 5.Ronkainen J, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling-Sternevald E, et al. High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: a Kalixanda study report. Scand J Gastroenterol. 2005;40:275–285. [DOI] [PubMed] [Google Scholar]
- 6.Piqué N, Ponce M, Garrigues V, Rodrigo L, Calvo F, de Argila CM, et al. Prevalence of severe esophagitis in Spain. Results of the PRESS study (Prevalence and Risk factors for Esophagitis in Spain: A cross-sectional study). United European Gastroenterol J. 2016;4:229–235. doi: 10.1177/2050640615595916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung HK. Epidemiology of gastroesophageal reflux disease in Asia: a systematic review. J Neurogastroenterol Motil. 2011;17:14–27. doi: 10.5056/jnm.2011.17.1.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohara S, Kouzu T, Kawano T, Kusano M. Nationwide epidemiological survey regarding heartburn and reflux esophagitis in Japanese (in Japanese). Nihon Shokakibyo Gakkai Zasshi. 2005;102:1010–1024. [PubMed] [Google Scholar]
- 9.Lei WY, Yu HC, Wen SH, Liu TT, Yi CH, Wang CC, et al. Predictive factors of silent reflux in subjects with erosive esophagitis. Dig Liver Dis. 2015;47:24–29. doi: 10.1016/j.dld.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 10.Lee D, Lee KJ, Kim KM, Lim SK. Prevalence of asymptomatic erosive esophagitis and factors associated with symptom presentation of erosive esophagitis. Scand J Gastroenterol. 2013;48:906–912 doi: 10.3109/00365521.2013.812236 [DOI] [PubMed] [Google Scholar]
- 11.Choi JY, Jung HK, Song EM, Shim KN, Jung SA. Determinants of symptoms in gastroesophageal reflux disease: nonerosive reflux disease, symptomatic, and silent erosive reflux disease. Eur J Gastroenterol Hepatol. 2013;25:764–771. doi: 10.1097/MEG.0b013e32835f594c [DOI] [PubMed] [Google Scholar]
- 12.Ronkainen J, Aro P, Storskrubb T, Lind T, Bolling-Sternevald E, Junghard O, et al. Gastro-oesophageal reflux symptoms and health-related quality of life in the adult general population—the Kalixanda study. Aliment Pharmacol Ther. 2006;23:1725–1733 doi: 10.1111/j.1365-2036.2006.02952.x [DOI] [PubMed] [Google Scholar]
- 13.Hongo M, Kinoshita Y, Shimozuma K, Kumagai Y, Sawada M, Nii M. Psychometric validation of the Japanese translation of the Quality of Life in Reflux and Dyspepsia questionnaire in patients with heartburn. J Gastroenterol. 2007;42:807–815 doi: 10.1007/s00535-007-2098-9 [DOI] [PubMed] [Google Scholar]
- 14.Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care and Research (Hoboken). 2011;63(Suppl.11):S467–S472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuzaki J, Suzuki H, Asakura K, Saito Y, Hirata K, Takebayashi T, et al. Etiological difference between ultrashort- and short-segment Barrett’s esophagus. J Gastroenterol. 2011;46:332–338. doi: 10.1007/s00535-010-0353-y [DOI] [PubMed] [Google Scholar]
- 16.Kimura K, Takemoto T. An Endoscopic Recognition of the Atrophic Border and its Significance in Chronic Gastritis. Endoscopy 1969;1:87–97 [Google Scholar]
- 17.Tack J, Tally NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479 doi: 10.1053/j.gastro.2005.11.059 [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita Y, Kawanami C, Kishi K, Nakata H, Seino Y, Chiba T. Helicobacter pylori independent chronological change in gastric acid secretion in the Japanese. Gut. 1997;41:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saqui-Salces M, Dowdle WE, Reiter JF, Merchant JL. A high-fat diet regulates gastrin and acid secretion through primary cilia. FASEB J. 2012; 26:3127–3139. doi: 10.1096/fj.11-197426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iijima K, Koike T, Abe Y, Ohara S, Shimosegawa T. A Chronological Increase in Gastric Acid Secretion from 1995 to 2014 in Young Japanese Healthy Volunteers under the Age of 40 Years Old. Tohoku J Exp Med. 2016;239:237–241. doi: 10.1620/tjem.239.237 [DOI] [PubMed] [Google Scholar]
- 21.Mishima I, Adachi K, Arima N, Amano K, Takashima T, Moritani M, et al. Prevalence of endoscopically negative and positive gastroesophageal reflux disease in the Japanese. Scand J Gastroenterol. 2005;40:1005–1009. [DOI] [PubMed] [Google Scholar]
- 22.Kim N, Lee SW, Cho SI, Park CG, Yang CH, Kim HS, et al. The prevalence of and risk factors for erosive oesophagitis and non-erosive reflux disease: a nationwide multicentre prospective study in Korea. Aliment Pharmacol Ther. 2008;27:173–185. doi: 10.1111/j.1365-2036.2007.03561.x [DOI] [PubMed] [Google Scholar]
- 23.Menon S, Jayasena H, Nightingale P, Trudgill NJ. Influence of age and sex on endoscopic findings of gastrooesophageal reflux disease: an endoscopy database study. Eur J Gastroenterol Hepatol. 2011;23:389–395. doi: 10.1097/MEG.0b013e328345d429 [DOI] [PubMed] [Google Scholar]
- 24.Gunji T, Sato H, Iijima K, Fujibayashi K, Okumura M, Sasabe N, et al. Risk factors for erosive esophagitis: a cross-sectional study of a large number of Japanese males. J Gastroenterol. 2011;46:448–455. doi: 10.1007/s00535-010-0359-5 [DOI] [PubMed] [Google Scholar]
- 25.Minatsuki C, Yamamichi N, Shimamoto T, Kakimoto H, Takahashi Y, Fujishiro M, et al. Background factors of reflux esophagitis and non-erosive reflux disease: a cross-sectional study of 10,837 subjects in Japan. PLoS One. 2013;8:e69891 doi: 10.1371/journal.pone.0069891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuzaki J, Suzuki H, Kobayakawa M, Inadomi JM, Takayama M, Makino K, et al. Association of Visceral Fat Area, Smoking, and Alcohol Consumption with Reflux Esophagitis and Barrett's Esophagus in Japan. PLoS One. 2015;10:e0133865 doi: 10.1371/journal.pone.0133865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710–717. doi: 10.1136/gut.2004.051821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun XM, Tan JC, Zhu Y, Lin L. Association between diabetes mellitus and gastroesophageal reflux disease: A meta-analysis. World J Gastroenterol. 2015;21:3085–3092. doi: 10.3748/wjg.v21.i10.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moayyedi P, Talley NJ. Gastro-oesophageal reflux disease. Lancet. 2006;367:2086–2100. doi: 10.1016/S0140-6736(06)68932-0 [DOI] [PubMed] [Google Scholar]
- 30.Peng S, Cui Y, Xiao YL, Xiong LS, Hu PJ, Li CJ, et al. Prevalence of erosive esophagitis and Barrett's esophagus in the adult Chinese population. Endoscopy. 2009;41:1011–1017. doi: 10.1055/s-0029-1215291 [DOI] [PubMed] [Google Scholar]
- 31.Gerson LB, Kahrilas PJ, Fass R. Insights into gastroesophageal reflux disease-associated dyspeptic symptoms. Clin Gastroenterol Hepatol. 2011;9:824–833. doi: 10.1016/j.cgh.2011.05.015 [DOI] [PubMed] [Google Scholar]
- 32.D'Alessandro A, Zito FP, Pesce M, Andreozzi P, Efficie E, Cargiolli M, et al. Specific dyspeptic symptoms are associated with poor response to therapy in patients with gastroesophageal reflux disease. United European Gastroenterol J. 2017;5:54–59. doi: 10.1177/2050640616650061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki H, Moayyedi P. Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10:168–174. doi: 10.1038/nrgastro.2013.9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.