Pathogens that expand host range by shifting to a novel host taxon are a key factor for diversification and evolution of host–pathogen associations [1]. Such shifts are often also the initial spark for new emerging infectious diseases [2]. As a result, pathogen host shifts are of considerable concern for humans, wildlife, and agriculture, with obvious economic and public health impacts that threaten food biosecurity and human health [2,3]. Shifting to a new host may have a large impact on the evolution and genetic organization of the pathogen [4]. Indeed, many recent studies have investigated past and ongoing pathogen host shifts using genomic and population genetic methods [5–11], with much emphasis placed on characterizing the mutations, hybridizations, chromosomal reorganizations, or horizontal gene transfer events involved in host-shift genetics [12–14]. The rationale behind these studies is that such genomic changes often represent pathogen adaptation in response to the new environment of a new host. Because of the usually slow accumulation of mutational nucleotide changes (Fig 1), these genomic changes do not necessarily represent the factors responsible for facilitating the host shift in the first place. Instead, the extent to which a pathogen is able to adjust and produce a phenotype that can survive in the novel host, either via phenotypic plasticity [15,16] or cryptic genetic variation in the pathogen population [17–19], is increasingly recognized as an important driver for evolutionary innovation that can lead to niche expansion and pathogen host shifts [20–22].
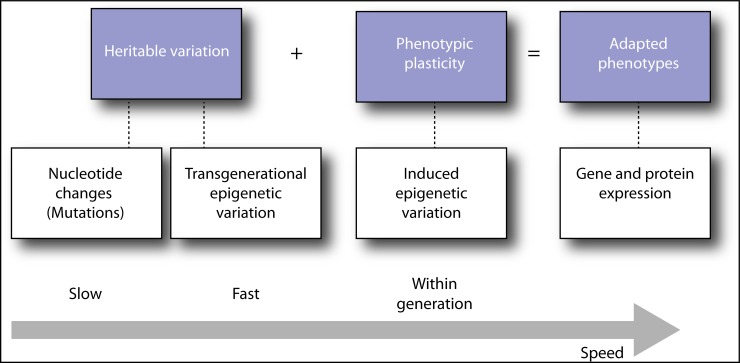
Fig 1. Heritable variation and phenotypic plasticity in combination shape–adapted phenotypes.
Heritable variation consists of genetic variation and transgenerational epigenetic variation that differ in the rate at which changes occur. The most rapid changes occur in epigenetic variation that results in phenotypic plasticity within an organism’s lifetime, which generally are exempt from natural selection that only acts on heritable variation.
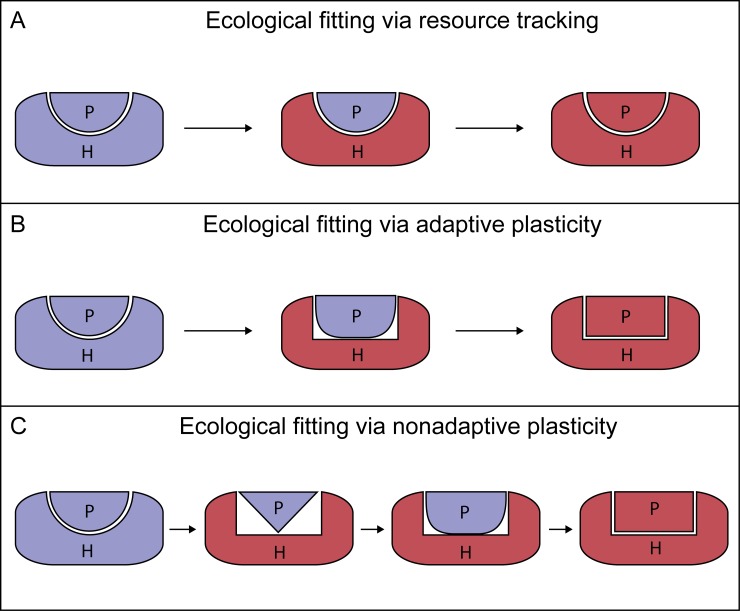
The process of host shifting involves several stages that each represent different ecological and evolutionary barriers for a new host–pathogen association to become established (Box 1). When ecological and spatial hindrances are overcome, pathogens are generally considered to shift hosts in either of two ways [21,23]. First, pathogens may colonize new hosts that represent a very similar resource to the ancestral host, i.e., ecological fitting via resource tracking (Fig 2A). This can occur if the original and new hosts are closely related or if the pathogen is exploiting traits that are evolutionarily conserved between the two host species. Hosts may be genetically diverse [24] or variably express the traits targeted by pathogens [25] so that only part of the new host population is susceptible at any given point in time. Second, pathogens may colonize new hosts that represent previously unencountered resources, i.e., ecological fitting via adaptive plasticity to host traits outside the range of conditions in which the pathogen evolved (Fig 2B). The completely novel and potentially stressful environment that a new host represents is, under this scenario, considered to “release” cryptic genetic variation in plasticity [26]. This variation in plastic responses leads to greater variation in pathogen phenotypes that provide the raw material for natural selection to shape the evolution of pathogenically relevant traits [20,27,28]. In cases where such plasticity produces an adaptive phenotype with improved fitness on the new host, it seems unproblematic to envisage how these novel and apparently “preadapted” pathogen phenotypes can eventually lead to a host shift (Fig 2B).
Box 1. The biology of pathogen host shifts.
The process of host shifts incorporates several steps. First, the pathogen must have the opportunity to shift hosts by exposure of the new host species to the pathogen. Many ecological barriers to transmission are breached by global trade, modern agricultural practices, and climate change, which facilitate more pathogen encounters and opportunities for infecting new potential hosts [68]. Second, the pathogen must be able to infect the new host. For example, viral pathogens use phylogenetically conserved receptors to infect host cells, and only hosts with appropriate cell receptors are compatible hosts [69]. The third and final step is for the pathogen to be sufficiently able to spread between individuals in the new host population. Between-host transmission is necessary for establishing the long-term associations characterizing a successful host shift in contrast to occasional spillover pathogen infections in the new host [70,71].
Fig 2. Scenarios of ecological fitting leading to pathogen host shifts.
(A) The pathogen (blue P) is adapted to the native host (blue H), drawn as compatible pathogen and host shapes. During pathogen colonization of a new host (red H), the pathogen is readily able to infect the new host because of similarity in traits between the old and new host (compatible shapes between blue P and red H), which facilitates subsequent pathogen adaptation to the new host (red P and H). (B) When there is no similarity in traits between old (blue H) and new (red H) hosts, the pathogen cannot readily infect the new host. Instead, given that the pathogen changes phenotype via adaptive plasticity, it becomes more compatible with the new host (a close but not perfect match between shapes of blue P and red H). Such adaptive pathogen plasticity to previously unencountered host traits can thus facilitate subsequent pathogen adaptation to the new host (red P and H). (C) Similar to scenario B, there is no similarity in traits between old (blue H) and new (red H) hosts, and the pathogen cannot readily infect the new host. In this scenario, the pathogen changes phenotype via nonadaptive plasticity, which initially results in low compatibility between the pathogen (blue P) and the new host (red H). Such plastic responses with initially negative pathogen fitness on the novel host often expose hidden genetic variation to new regimes of natural selection. This genetic variation, previously shielded from natural selection, may then facilitate subsequent pathogen adaptation to the new host.
Here, I propose a third route for pathogenic host shifts that occur when the induced pathogen phenotypes on the new host are the result of nonadaptive plasticity (Fig 2C). Nonadaptive plasticity leads to an induced pathogen phenotype in the new host that, on average, has reduced fitness [26]. Nonadaptive plasticity may reflect a breakdown in an organism’s ability to maintain homeostasis and proper function, and this is usually considered to prevent pathogen host shifts from occurring [21,23,29]. However, similar to adaptive plastic responses, nonadaptive plasticity also exposes standing genetic variation to new regimes of natural selection. Evidence gathered to date, mainly from studies on interactions between nonpathogenic organisms, suggests that nonadaptive plasticity can have a major evolutionary impact and potentiate rapid adaptive evolution [28,30]. For example, in an experiment under natural conditions, wild guppy populations (Poecilia reticulata) were experimentally transplanted between streams with or without natural cichlid predators. After only 3 to 4 generations in the new environments, patterns of brain gene expression were shifted further away from the local optimum—i.e., nonadaptive plasticity—and potentiated adaptive evolution by increasing the strength of directional selection [30]. Nonadaptive plastic responses of pathogens undergoing a host shift therefore have the ability to further enhance the strong directional selection from the new host. Such pathogens will, however, initially have reduced fitness on the new hosts, which requires that the new host niche is initially not very competitive or that the new host compensates a lower rate of host exploitation by enhancing transmission. Furthermore, it is assumed that pathogen populations can persist for extended periods on suboptimal hosts, which is supported as a likely scenario in recent theoretical studies [29]. Finally, the new host population is assumed to vary in susceptibility to pathogen infection [24,31], which is characteristic of many host–pathogen systems and also supported by theoretical models [25].
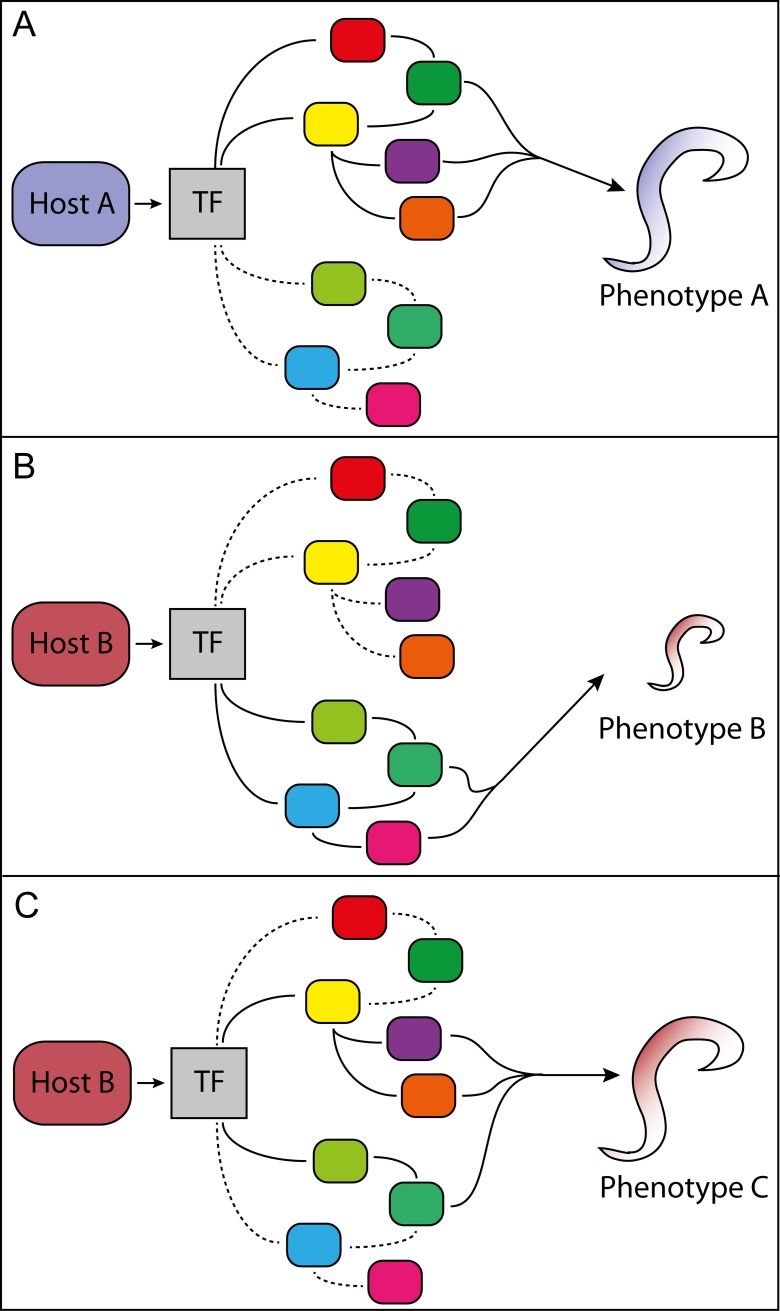
Pathogens often display considerable phenotypic plasticity in response to changing environmental conditions in the host [32]. The expression of virulence traits may, for example, be contingent upon whether the host is infected with a single or multiple pathogens [33], and growth and size of pathogenic nematodes and trematodes can vary more than 10-fold, depending on infection intensity and host environment [34–37]. Pathogen plasticity may also be present as discrete phenotypes (polyphenisms), such as the lancet liver fluke Dicrocoelium dendriticum, in which a single cercaria usually positions itself against the subesophageal ganglion in the brain of the intermediate Formica spp. ant host, whereas the remaining cercariae develop into metacercariae in the gaster [38,39]. Plastic gene expression underlies phenotypic plasticity [40], which provides a way to measure subtle changes in phenotypic plasticity [41]. Recent methodological advances in dual-RNA sequencing (dual-RNAseq) analysis [42–44] allow changes in gene expression during host shifts to be monitored in many pathogen–host systems. Although transcriptome-wide datasets of gene expression are notoriously difficult to interpret, measuring changes in pathogen gene expression following host shifts provides a method to experimentally explore the role of nonadaptive plasticity for pathogen host shifts. This could, for example, be achieved by designing experiments that serially passage pathogens on novel hosts for multiple generations. Identifying genes that initially are differentially expressed on the new host but that, after passaging, change expression in the opposite direction would indicate nonadaptive expression. There is ample evidence that pathogens, such as the fungal human pathogens Aspergillus fumigatus and Candida albicans [45–49], adapt and change their gene repertoire in response to novel hosts or treatments. Similarly, the opportunistic human bacterial pathogen Pseudomonas aeruginosa employs plastic gene expression in response to variable infection conditions [50,51]. Even slight alterations of pathogen genes or transcription factors connected in gene regulatory networks (GRNs) may have a large evolutionary impact [15,52–54]. Such alteration of existing GRNs could be mediated by heritable epigenetic changes [55], and only need to involve partial or modular co-option of GRNs into new GRNs [56,57]. This would create an evolutionarily novel GRN combination that is exposed to strong directional selection in the new host and may eventually lead to a host shift (Fig 3).
Fig 3. Illustration of how genetic assimilation of a nonadaptive plastic trait may be altered and fixed via partial co-option of GRNs.
(A) The host-induced transcription factor (TF) controls a GRN of genes (different colored boxes), which leads to pathogen phenotype A (solid lines). Stippled lines connect genes in a GRN that is unused in this host and contains cryptic genetic variation. (B) A new host environment induces the TF to elicit previously unused modules of the GRN, which result in a nonadaptive plastic response (smaller size of phenotype B). (C) Provided phenotype B can survive long enough in host B, the old and new GRN modules may be co-opted into a new GRN, resulting in an adaptive phenotype C. GRN, gene regulatory network; TF, transcription factor.
Genome evolution differs fundamentally between eukaryotes on one hand and bacteria and viruses on the other, in which small gene-dense genomes, short generation times, and frequent horizontal gene exchange provide ample opportunity for new mutations to arise [10]. Populations of RNA viruses often contain extensive genetic diversity because of high mutation rates during RNA viral replication coupled with limited proofreading capacity [19]. The presence and generation of cryptic genetic diversity has been shown to be important in many RNA viral host shifts [13,58–60], for example, in avian influenza viruses by providing adaptive mutations in specific polymerase subunits that increase RNA polymerase activity in mammalian cells [61], or mutations that alter receptor binding to sialic acids or glycan linkages in mammalian cells [14]. Nonadaptive plasticity is considered to be of limited importance for RNA virus host shifts that are constrained and principally governed by genetic mutations [9,62] but is likely more important for host shifts influenced by variation in the conformation of RNA virus secondary structures that modulate interaction with the host immune system and increase persistence [63,64]. Eukaryotes contain larger and more plastic genomes, longer generation times, and sexual reproduction with recombination, which implies that rapid evolutionary adaptation is often governed by changes in gene expression and epigenetic markers instead of mutations that tend to emerge later [65] (Fig 1). Therefore, the importance of nonadaptive plasticity for mediating host shifts is likely higher in eukaryote pathogens such as pathogenic fungi, infectious worms, and trypanosome and malaria parasites than for bacterial and viral pathogens. Nonadaptive pathogen plasticity could help explain instances of extreme interkingdom host shifting [66] and the wide host range of some eukaryotic pathogens [67], which are not always easily explained by current host-shift models. However, more empirical work on how transcriptional, protein, and developmental networks in pathogens change in response to different host environments is required to understand the relative importance of adaptive versus nonadaptive plasticity for facilitating pathogen host shifts.
Funding Statement
This research was supported by a Villum Foundation Young Investigator Grant to HHDFL (grant number 10122, www.http://veluxfoundations.dk/en). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Schmid-Hempel P. Evolutionary parasitology: The integrated study of infections, immunology, ecology and genetics Oxford, UK: Oxdord University Press; 2011. [Google Scholar]
- 2.Woolhouse MEJ, Haydon DT, Antia R. Emerging pathogens: The epidemiology and evolution of species jumps. Trends Ecol Evol. 2005;20(5): 238–244. doi: 10.1016/j.tree.2005.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, et al. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484(7393): 186–194. doi: 10.1038/nature10947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambrechts L. Dissecting the genetic architecture of host-pathogen specificity. PLoS Pathog. 2010;6(8): 9–10. doi: 10.1371/journal.ppat.1001019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stukenbrock EH. Evolution, selection and isolation: a genomic view of speciation in fungal plant pathogens. New Phytol. 2013;199(4): 895–907. doi: 10.1111/nph.12374 [DOI] [PubMed] [Google Scholar]
- 6.Friesen TL, Stukenbrock EH, Liu Z, Meinhardt S, Ling H, Faris JD, et al. Emergence of a new disease as a result of interspecific virulence gene transfer. Nat Genet. 2006;38(8): 953–956. doi: 10.1038/ng1839 [DOI] [PubMed] [Google Scholar]
- 7.Richards TA, Soanes DM, Jones MDM, Vasieva O, Leonard G, Paszkiewicz K, et al. Horizontal gene transfer facilitated the evolution of plant parasitic mechanisms in the oomycetes. Proc Natl Acad Sci U S A. 2011;108(37): 15258–15263. doi: 10.1073/pnas.1105100108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menardo F, Praz CR, Wyder S, Ben-David R, Bourras S, Matsumae H, et al. Hybridization of powdery mildew strains gives rise to pathogens on novel agricultural crop species. Nat Genet. Nature Research; 2016;48(2): 201–205. doi: 10.1038/ng.3485 [DOI] [PubMed] [Google Scholar]
- 9.Longdon B, Brockhurst MA, Russell CA, Welch JJ, Jiggins FM. The evolution and genetics of virus host shifts. PLoS Pathog. 2014;10(11): e1004395 doi: 10.1371/journal.ppat.1004395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koonin E V, Wolf YI. Constraints and plasticity in genome and molecular-phenome evolution. Nat Rev Genet. 2010;11(7): 487–498. doi: 10.1038/nrg2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plissonneau C, Benevenuto J, Mohd-assaad N, Fouché S, Hartmann FE, Croll D. Using population and comparative genomics to understand the genetic basis of effector-driven fungal pathogen evolution. Front Plant Sci. 2017;8: 119 doi: 10.3389/fpls.2017.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stukenbrock EH, Bataillon T. A population genomics perspective on the emergence and adaptation of new plant pathogens in agro-ecosystems. PLoS Pathog. 2012;8(9): e1002893 doi: 10.1371/journal.ppat.1002893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pepin KM, Lass S, Pulliam JRC, Read AF, Lloyd-Smith JO. Identifying genetic markers of adaptation for surveillance of viral host jumps. Nat Rev Microbiol. 2010;8(11): 802–813. doi: 10.1038/nrmicro2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parrish CR, Holmes EC, Morens DM, Park E-C, Burke DS, Calisher CH, et al. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol Mol Biol Rev. 2008;72(3): 457–70. doi: 10.1128/MMBR.00004-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West-Eberhard MJ. Developmental plasticity and evolution Oxford, UK: Oxford University Press; 2003. doi: 10.2002/ajpa.20219 [Google Scholar]
- 16.Hu J, Barrett RDH. Epigenetics in natural animal populations. J Evol Biol. 2017;30(9): 1612–1632. doi: 10.1111/jeb.13130 [DOI] [PubMed] [Google Scholar]
- 17.Bay RA, Rose N, Barrett R, Bernatchez L, Ghalambor CK, Lasky JR, et al. Predicting responses to contemporary environmental change using evolutionary response architectures. Am Nat. 2017;189(5): 463–473. doi: 10.1086/691233 [DOI] [PubMed] [Google Scholar]
- 18.Grant BR, Grant PR. Watching speciation in action. Science. 2017;355(6328): 910–912. doi: 10.1126/science.aam6411 [DOI] [PubMed] [Google Scholar]
- 19.Vignuzzi M, Stone JK, Arnold JJ, Cameron CE, Andino R. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature. 2006;439(7074): 344–348. doi: 10.1038/nature04388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfennig DW, Wund MA, Snell-Rood EC, Cruickshank T, Schlichting CD, Moczek AP. Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol Evol. 2010;25(8): 459–467. doi: 10.1016/j.tree.2010.05.006 [DOI] [PubMed] [Google Scholar]
- 21.Agosta SJ, Klemens JA. Ecological fitting by phenotypically flexible genotypes: implications for species associations, community assembly and evolution. Ecol Lett. 2008;11(11): 1123–1134. doi: 10.1111/j.1461-0248.2008.01237.x [DOI] [PubMed] [Google Scholar]
- 22.Sexton JP, Montiel J, Shay JE, Stephens MR, Slatyer RA. Evolution of ecological niche breadth. Annu Rev Ecol Evol Syst. 2017;48: 183–206. doi: 10.1146/annurev-ecolsys-110316-023003 [Google Scholar]
- 23.Agosta SJ, Janz N, Brooks DR. How specialists can be generalists: resolving the “parasite paradox” and implications for emerging infectious disease. Zoologia. 2010;27(2): 151–162. doi: 10.1590/S1984-46702010000200001 [Google Scholar]
- 24.Fellous S, Duncan AB, Quillery E, Vale PF, Kaltz O. Genetic influence on disease spread following arrival of infected carriers. Ecol Lett. 2012;15(3): 186–192. doi: 10.1111/j.1461-0248.2011.01723.x [DOI] [PubMed] [Google Scholar]
- 25.Mason PA. On the role of host phenotypic plasticity in host shifting by parasites. Ecol Lett. 2016;19(2): 121–132. doi: 10.1111/ele.12555 [DOI] [PubMed] [Google Scholar]
- 26.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol. 2007;21(3): 394–407. doi: 10.1111/j.1365-2435.2007.01283.x [Google Scholar]
- 27.Schlichting CD, Wund MA. Phenotypic plasticity and epigenetic marking: An assessment of evidence for genetic accommodation. Evolution. 2014;68(3): 656–672. doi: 10.1111/evo.12348 [DOI] [PubMed] [Google Scholar]
- 28.Levis NA, Pfennig DW. Evaluating “plasticity-first” evolution in nature: Key criteria and empirical approaches. Trends Ecol Evol. 2016;31(7): 563–574. doi: 10.1016/j.tree.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 29.Araujo SBL, Braga MP, Brooks DR, Agosta SJ, Hoberg EP, von Hartenthal FW, et al. Understanding host-switching by ecological fitting. PLoS ONE. 2015;10(10): e0139225 doi: 10.1371/journal.pone.0139225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghalambor CK, Hoke KL, Ruell EW, Fischer EK, Reznick DN, Hughes K a. Non-adaptive plasticity potentiates rapid adaptive evolution of gene expression in nature. Nature. 2015;525: 372–375. doi: 10.1038/nature15256 [DOI] [PubMed] [Google Scholar]
- 31.Lambrechts L, Fellous S, Koella JC. Coevolutionary interactions between host and parasite genotypes. Trends Parasitol. 2006;22(1): 12–16. doi: 10.1016/j.pt.2005.11.008 [DOI] [PubMed] [Google Scholar]
- 32.Poulin R. The evolution of life history strategies in parasitic animals. Adv Parasitol. 1996;37: 107–134. doi: 10.1016/S0065-308X(08)60220-1 [DOI] [PubMed] [Google Scholar]
- 33.Alizon S, de Roode JC, Michalakis Y. Multiple infections and the evolution of virulence. Ecol Lett. 2013;16(4): 556–567. doi: 10.1111/ele.12076 [DOI] [PubMed] [Google Scholar]
- 34.Saldanha I, Leung TLF, Poulin R. Causes of intraspecific variation in body size among trematode metacercariae. J Helminthol. 2009;83(3): 289–293. doi: 10.1017/S0022149X09224175 [DOI] [PubMed] [Google Scholar]
- 35.Stear MJ, Bairden K, Duncan JL, Holmes PH, McKellar QA, Park M, et al. How hosts control worms. Nature. 1997;389(6646): 27–27. doi: 10.1038/37895 [DOI] [PubMed] [Google Scholar]
- 36.Davies SJ, McKerrow JH. Developmental plasticity in schistosomes and other helminths. Int J Parasitol. 2003;33(11): 1277–1284. doi: 10.1016/S0020-7519(03)00161-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vignoles P, Ménard A, Rondelaud D, Agoulon A, Dreyfuss G. Fasciola hepatica: The growth and larval productivity of redial generations in Galba truncatula subjected to miracidia differing in their mammalian origin. J Parasitol. 2004;90(2): 430–433. doi: 10.1645/GE-2682RN [DOI] [PubMed] [Google Scholar]
- 38.Botnevik CF, Malagocka J, Jensen AB, Fredensborg BL. Relative Effects of Temperature, Light, and Humidity on Clinging Behavior of Metacercariae-Infected Ants. J Parasitol. 2016;102(5): 495–500. doi: 10.1645/16-53 [DOI] [PubMed] [Google Scholar]
- 39.Krull W, Mapes C. Studies on the biology of Dicrocoelium dendriticum (Rudolphi, 1819) Looss, 1899 (Trematoda: Dicrocoeliidae), including its relation to the intermediate host, Cionella lubrica (Müller). V. Notes on infections of Dicrocoelium dendriticum. Cornell Vet. 1952;42(3): 339–351. [PubMed] [Google Scholar]
- 40.Schrader L, Helanterä H, Oettler J. Accelerated evolution of developmentally biased genes in the tetraphenic ant Cardiocondyla obscurior. Mol Biol Evol. 2017;34(3): 535–544. doi: 10.1093/molbev/msw240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer EK, Ghalambor CK, Hoke KL. Can a network approach resolve how adaptive vs nonadaptive plasticity impacts evolutionary trajectories? Integr Comp Biol. 2016;56(5): 877–888. doi: 10.1093/icb/icw087 [DOI] [PubMed] [Google Scholar]
- 42.Westermann AJ, Förstner KU, Amman F, Barquist L, Chao Y, Schulte LN, et al. Dual RNA-seq unveils noncoding RNA functions in host–pathogen interactions. Nature. 2016;529(7587): 496–501. doi: 10.1038/nature16547 [DOI] [PubMed] [Google Scholar]
- 43.Westermann AJ, Barquist L, Vogel J. Resolving host–pathogen interactions by dual RNA-seq. PLoS Pathog. 2017;13(2): e1006033 doi: 10.1371/journal.ppat.1006033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulze S, Henkel SG, Driesch D, Guthke R, Linde J. Computational prediction of molecular pathogen-host interactions based on dual transcriptome data. Front Microbiol. 2015;6: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van de Veerdonk FL, Gresnigt MS, Romani L, Netea MG, Latgé J-P. Aspergillus fumigatus morphology and dynamic host interactions. Nat Rev Microbiol. 2017;15(11): 661–674. doi: 10.1038/nrmicro.2017.90 [DOI] [PubMed] [Google Scholar]
- 46.Hampe IAI, Friedman J, Edgerton M, Morschhäuser J. An acquired mechanism of antifungal drug resistance simultaneously enables Candida albicans to escape from intrinsic host defenses. PLOS Pathog. 2017;13(9): e1006655 doi: 10.1371/journal.ppat.1006655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown AJP, Odds FC, Gow NAR. Infection-related gene expression in Candida albicans. Curr Opin Microbiol. 2007;10(4): 307–313. doi: 10.1016/j.mib.2007.04.001 [DOI] [PubMed] [Google Scholar]
- 48.Raffaele S, Farrer R a, Cano LM, Studholme DJ, MacLean D, Thines M, et al. Genome evolution following host jumps in the Irish potato famine pathogen lineage. Science. 2010;330(6010): 1540–1543. doi: 10.1126/science.1193070 [DOI] [PubMed] [Google Scholar]
- 49.Kumamoto CA. Niche-specific gene expression during C. albicans infection. Curr Opin Microbiol. 2008;11(4): 325–330. doi: 10.1016/j.mib.2008.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bielecki P, Puchałka J, Wos-Oxley ML, Loessner H, Glik J, Kawecki M, et al. In-vivo expression profiling of pseudomonas aeruginosa infections reveals niche-specific and strain-independent transcriptional programs. PLoS ONE. 2011;6(9): e24235 doi: 10.1371/journal.pone.0024235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kümmerli R, Jiricny N, Clarke LS, West SA, Griffin AS. Phenotypic plasticity of a cooperative behaviour in bacteria. J Evol Biol. 2009;22(3): 589–598. doi: 10.1111/j.1420-9101.2008.01666.x [DOI] [PubMed] [Google Scholar]
- 52.West-Eberhard MJ. Developmental plasticity and the origin of species differences. Proc Natl Acad Sci U S A. 2005;102 Suppl 1: 6543–6549. doi: 10.1073/pnas.0501844102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Espinosa-Soto C, Martin OC, Wagner A. Phenotypic plasticity can facilitate adaptive evolution in gene regulatory circuits. BMC Evol Biol. 2011;11: 5 doi: 10.1186/1471-2148-11-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schneider RF, Li Y, Meyer A, Gunter HM. Regulatory gene networks that shape the development of adaptive phenotypic plasticity in a cichlid fish. Mol Ecol. 2014;23(18): 4511–4526. doi: 10.1111/mec.12851 [DOI] [PubMed] [Google Scholar]
- 55.Kasuga T, Gijzen M. Epigenetics and the evolution of virulence. Trends Microbiol. 2013;21(11): 575–582. doi: 10.1016/j.tim.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 56.Pfennig DW, Ehrenreich IM. Towards a gene regulatory network perspective on phenotypic plasticity, genetic accommodation and genetic assimilation. Mol Ecol. 2014;23(18): 4438–4440. doi: 10.1111/mec.12887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneider RF, Meyer A. How plasticity, genetic assimilation and cryptic genetic variation may contribute to adaptive radiations. Mol Ecol. 2017;26(1): 330–350. doi: 10.1111/mec.13880 [DOI] [PubMed] [Google Scholar]
- 58.Mollentze N, Biek R, Streicker DG. The role of viral evolution in rabies host shifts and emergence. Curr Opin Virol. Elsevier B.V.; 2014;8: 68–72. doi: 10.1016/j.coviro.2014.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Streicker DG, Altizer SM, Velasco-Villa A, Rupprecht CE. Variable evolutionary routes to host establishment across repeated rabies virus host shifts among bats. Proc Natl Acad Sci USA. 2012;109(48): 19715–20. doi: 10.1073/pnas.1203456109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Webby R, Hoffmann E, Webster R. Molecular constraints to interspecies transmission of viral pathogens. Nat Med. Nature Publishing Group; 2004;10(Suppl 12): S77–S81. doi: 10.1038/nm1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mänz B, Schwemmle M, Brunotte L. Adaptation of avian influenza A virus polymerase in mammals to overcome the host species barrier. J Virol. American Society for Microbiology; 2013;87(13): 7200–9. doi: 10.1128/JVI.00980-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Longdon B, Hadfield JD, Day JP, Smith SCL, McGonigle JE, Cogni R, et al. The causes and consequences of changes in virulence following pathogen host shifts. PLoS Pathog. 2015;11(3): e1004728 doi: 10.1371/journal.ppat.1004728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wagner A. Mutational robustness accelerates the origin of novel RNA phenotypes through phenotypic plasticity. Biophys J. 2014;106(4): 955–965. doi: 10.1016/j.bpj.2014.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Witteveldt J, Blundell R, Maarleveld JJ, Mcfadden N, Evans DJ, Simmonds P. The influence of viral RNA secondary structure on interactions with innate host cell defences. Nucleic Acids Res. 2014;42(5): 3314–3329. doi: 10.1093/nar/gkt1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klironomos FD, Berg J, Collins S. How epigenetic mutations can affect genetic evolution: Model and mechanism. BioEssays. 2013;35(6): 571–578. doi: 10.1002/bies.201200169 [DOI] [PubMed] [Google Scholar]
- 66.Nikoh N, Fukatsu T. Interkingdom host jumping underground: phylogenetic analysis of entomoparasitic fungi of the genus Cordyceps. Mol Biol Evol. 2000;17(4): 629–638. doi: 10.1093/oxfordjournals.molbev.a026341 [DOI] [PubMed] [Google Scholar]
- 67.Hellgren O, Pérez-Tris J, Bensch S. A jack-of-all-trades and still a master of some: Prevalence and host range in avian malaria and related blood parasites. Ecology. 2009;90(10): 2840–2849. doi: 10.1890/08-1059.1 [DOI] [PubMed] [Google Scholar]
- 68.Hoberg EP, Brooks DR, Brooks DR. Evolution in action: climate change, biodiversity dynamics and emerging infectious disease. Philos Trans R Soc B Biol Sci. 2015;370(1665): 20130553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woolhouse MEJ. Population biology of emerging and re-emerging pathogens. Trends Microbiol. 2002;10(Suppl 10): 3–7. doi: 10.1016/S0966-842X(02)02428-9 [DOI] [PubMed] [Google Scholar]
- 70.Woolhouse M, Antia R. Emergence of new infectious diseases In: Stearns SC, Koella JC, editors. Evolution in Health and Disease. Oxford, UK: Oxford University Press; 2007. pp. 215–228. [Google Scholar]
- 71.Antia R, Regoes RR, Koella JC, Bergstrom CT. The role of evolution in the emergence of infectious diseases. Nature. 2003;426(6967): 658–661. doi: 10.1038/nature02104 [DOI] [PMC free article] [PubMed] [Google Scholar]