Abstract
Dispersed zinnia (Zinnia elegans) mesophyll cells cannot differentiate into tracheary elements (TEs) at low cell density conditions even if auxin and cytokinin are present in the medium, indicating the involvement of intercellular interactions during the initiation and/or subsequent progresses in TE differentiation. When zinnia cells were incubated at a low density (2.5 × 104 cells mL−1) in TE-inductive medium in the presence of various concentrations of phytosulfokine (PSK)-α, which was originally identified as an intercellular signal peptide involved in cell proliferation, TE differentiation was strongly stimulated in a dose-dependent fashion; more than 35% of the living cells differentiated into TEs by 5 d of culture in the presence of 10 nm PSK-α. Enzyme-linked immunosorbent assay and mass spectroscopy confirmed that cultured zinnia cells produce nanomolar levels of PSKs under inductive conditions. These results suggest that PSK-α is a factor responsible for TE differentiation of zinnia mesophyll cells.
TE formation in culture has been used as a model system for the study of cell differentiation of higher plants (for review, see Fukuda, 1992). In mechanically isolated mesophyll cells of zinnia (Zinnia elegans) in liquid culture, differentiation into TEs can be readily induced by phytohormones and clearly distinguished on the basis of morphological features (Fukuda and Komamine, 1980a). Because of the nature of this system, individual cells respond homogeneously to physiological and chemical stimuli, and synchronously differentiate at relatively high frequency. Many researchers have investigated the determining factor(s) of TE differentiation at the cellular level and found that differentiation necessitates at least two exogenous factors, an auxin and a cytokinin (for review, see Fukuda and Komamine, 1985). In particular, cytokinins have been considered an absolute requirement in zinnia mesophyll cells (Fukuda and Komamine, 1980a).
The initial cell density is also a determining factor in TE differentiation in zinnia mesophyll cell cultures (Fukuda and Komamine, 1980a). Differentiation occurs synchronously at high frequency above an initial cell density of 4.2 × 104 cells mL−1, but is significantly suppressed below this threshold, suggesting that intercellular interactions are involved in the initiation and/or subsequent progresses in TE differentiation. An oligosaccharide-like factor in zinnia conditioned medium appear to play an important role in cell expansion and metaxylem-like TE differentiation (Roberts et al., 1997), but the active principle has not been chemically identified.
The relative growth rate of the plant cells in culture also strictly depends on the initial cell density, even if sufficient amounts of auxins and cytokinins are present in the medium, so additional factors must play a role in cell proliferation (Bellincampi and Morpurgo, 1987; Birnberg et al., 1988). In 1996, we first isolated one of these factors from conditioned medium derived from mesophyll culture of asparagus, and determined its structure to be a sulfated pentapeptide,H-Tyr(SO3H)-Ile-Tyr(SO3H)-Thr-Gln-OH (Matsubayashi and Sakagami, 1996). This peptide, named PSK-α, compensates for cell growth suppression in low-density cultures at a concentration as low as 1.0 nm. PSK-α has also been identified in conditioned medium derived from rice and maize cell cultures, apparently promoting cell growth by interacting with specific binding sites distributed upon plasma membranes (Matsubayashi et al., 1997; Matsubayashi and Sakagami, 1999).
Although the generality of PSK-α in plant kingdom has not yet been well clarified, its influence on cell differentiation and proliferation are clearly of interest. In the present study, we investigated the physiological effects of PSK-α on TE differentiation and proliferation of zinnia mesophyll cells, and also determined, using ELISA and MS, whether zinnia cells themselves produce PSK-α.
MATERIALS AND METHODS
Materials
Sep-Pak cartridges were purchased from Millipore and Develosil ODS-5 and Develosil ODS-10 reverse-phase columns were purchased from Nomura Chemicals (Seto, Japan). Twenty four-well microplates were obtained from Iwaki (Chiba, Japan). Polystyrene, 96-well plates for ELISA were from Nunc (Naperville, IL). All of the other inorganic and organic chemicals were obtained from Wako Pure Chemicals (Osaka). PSK-α was prepared by solid-phase synthesis as previously described (Matsubayashi et al., 1996).
Plants
Seeds of zinnia (Zinnia elegans cv Canary bird; Takii Shubyo, Kyoto) were grown on moist sterile soil at 25 ± 2°C with a 16-h light period (approximately 20,000 lux at the plant level).
Isolation of Single Cells from the Mesophyll
Zinnia leaves were sterilized for 10 min in a solution of 0.05% (w/v) NaOCl containing 0.05% (w/v) Tween 20, then rinsed three times with sterile water. Single cells were liberated by homogenization with a glass homogenizer in 0.2 m mannitol solution. The homogenate was filtered through a 37-μm stainless-steel mesh, and the filtrate was centrifuged at 100g for 3 min. The separated single cells were washed with 0.2 m mannitol three times and used in the following experiments.
Cell Culture and Bioassay Methods
The basal medium used for TE differentiation experiments was prepared according to the method of Fukuda and Komamine (1980a), except that NH4Cl was eliminated to improve the cell viability under low-density conditions. The medium was adjusted to pH 5.5 with 1.0 n KOH. Isolated single mesophyll cells were suspended in 0.2 m mannitol, adjusted to twice the final cell density using a hemocytometer, and dispensed into 24-well microplates at a volume of 250 μL per well. Culture medium (125 μL) prepared at a 4-fold concentration and various sample solutions (125 μL) were sterilized by filtration and then added to the cell suspension in each well. The plates were sealed with laboratory film to avoid evaporation of the medium, and were then incubated in the dark at 25°C with continuous rotary shaking at 120 rpm. The TE differentiation frequency was determined by counting the numbers of undifferentiated and differentiated cells under an inverted microscope. The TE differentiation frequency for each well was calculated by dividing the number of TE-differentiated cells by the total number of undifferentiated and differentiated cells. The cell viability for each well was calculated by dividing the number of living cells (determined by bromphenol blue; Suzuki et al., 1992) by the number of total cells.
For the preparation of conditioned medium, single zinnia mesophyll cells were suspended in 200 mL of culture medium at a density of 2.0 × 105 cells mL−1. This suspension was cultured in 500-mL Erlenmeyer flasks in the dark at 25°C with rotary shaking at 120 rpm. After 6 d of culture, conditioned medium was collected by filtration and stored at −20°C until use.
Competition ELISA Procedure
Preparation of anti-PSK-α polyclonal antibodies and the procedure for competitive ELISA were described previously (Matsubayashi et al., 1999). Two different PSK-α conjugated proteins, Tyr(SO3H)-Ile-Tyr(SO3H)-Thr-Gln-(Gly)3-Cys-linker-KLH (antigen A) and Tyr(SO3H)-Ile-Tyr(SO3H)-Thr-Gln-(Ala)3-Lys-linker-BSA (antigen B) were prepared by coupling the peptides with the corresponding proteins. Rabbits were immunized with antigen A, and the obtained antibodies were purified with an immunoaffinity column containing Tyr(SO3H)-Ile-Tyr(SO3H)-Thr-Gln-Cys-linker-resin. For quantification of PSK-α, polystyrene 96-well plates were coated with antigen B and blocked with 0.1% (w/v) BSA in PBS (10 mm phosphate buffer [pH 7.0] containing 8.0 g L−1 NaCl). Purified antibody and samples were then added and the plates were incubated at 37°C for 1.5 h. During this period, competition occurred for the antibody between antigen B bound to the plate and free PSK-α in solution. After washing with PBS containing 0.1% (w/v) Tween 20, plates were incubated for 1.5 h with a solution of horseradish peroxidase-coupled anti-rabbit immunoglobulin. After three washings, orthophenylenediamine solution containing 0.01% hydrogen peroxide was added, and the plates were incubated for 20 min at 37°C. Color development was terminated with sulfuric acid, and optical density was recorded at a wavelength of 490 nm with a plate reader.
Purification of PSK-α from Conditioned Medium
Conditioned medium derived from zinnia suspension cultures (400 mL) was concentrated to one-third volume, adjusted to pH 8.0 with 6.0 n KOH, and then applied to a DEAE Sephadex A-25 column (3.2 × 12 cm, flow rate 100 mL h−1) equilibrated with 20 mm Tris-HCl buffer, pH 8.0. The column was washed with 200 mL of the buffer, and fractions were eluted successively with 200 mL of this buffer containing 400, 800, or 1,200 mm KCl. Fractions of 800 and 1,200 mm were concentrated to one-half volume and TFA was added to this combined fraction at a final concentration of 0.1% (v/v). This solution was applied to C18 cartridges (Sep-Pak Vac, 12 mL × 2, flow rate 150 mL h−1) that had been equilibrated with 0.1% TFA. After washing with 80 mL of 0.1% (v/v) TFA in water, fractions were eluted with 30% (v/v) acetonitrile containing 0.1% (v/v) TFA. After lyophilization, materials were dissolved in 1.0 mL of 20 mm KH2PO4-KOH buffer, pH 5.8, and then applied to a Bio-Gel P-2 extra-fine column (1.7 × 42 cm), previously equilibrated with the same buffer. Five-milliliter fractions were collected and assayed by ELISA. Positive fractions recovered from the Bio-Gel column were lyophilized, dissolved in 200 μL of 10% (v/v) acetonitrile in 0.1% TFA, and chromatographed on a Develosil ODS-5 column (4.6 × 250 mm, Nomura Chemicals) by isocratic elution with 10% (v/v) acetonitrile in 0.1% TFA at a flow rate of 1.0 mL/min with monitoring of the UV A220. Fractions were collected every 1.0 min and assayed by ELISA after lyophilization.
MS
Positive fractions determined by ELISA were dissolved in 20 μL of water and directly analyzed with a vacuum generator platform quadrupole mass spectrometer (Fisons, Cheshire, UK) equipped for electrospray ionization. The source temperature was maintained at 70°C, and the range m/z 50 to 1,000 was scanned over 1.9 s.
RESULTS AND DISCUSSION
Effects of Initial Cell Density on TE Differentiation
Although Fukuda and Komamine's medium (Fukuda and Komamine, 1980a) has been widely used in TE differentiation experiments, zinnia cells cultured at low cell density often show relatively low viability in this medium (approximately 35% at 2.5 × 104 cells mL−1). This phe-nomenon is likely attributed to ammonium ions, because cell growth of mesophyll primary cultures under low cell density conditions is strongly inhibited by ammonium ions even if sufficient amounts of PSK-α are added to the medium (Matsubayashi and Sakagami, 1998). Therefore, we modified Fukuda and Komamine's medium to eliminate NH4Cl.
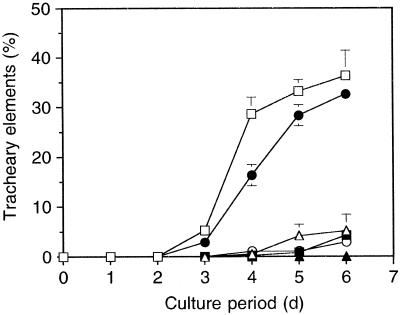
The TE differentiation frequency of single zinnia cells in suspension culture was markedly dependent on the initial cell density (Fig. 1). The TEs formed at relatively high frequency at the initial cell density of 1.0 × 105 and 5.0 × 104 cells mL−1, reaching approximately 30% after 5 d of culture. In contrast, TE differentiation was markedly inhibited at densities below 2.5 × 104 cells mL−1, and the frequencies remained approximately 5% or less of these values even after 5 d of culture. A similar observation was made in a previous report (Fukuda and Komamine, 1980a), suggesting the presence of intercellular communication mediated by chemical, if not physical, signals.
Figure 1.
Effects of the initial cell density on TE differentiation. Single zinnia cells were suspended in liquid medium, dispensed into 24-well culture plates at a final volume of 500 μL per well, and incubated at 25°C with shaking at 120 rpm. The TE differentiation frequency for each well was calculated by dividing the number of TEs by the total number of living cells. Data are mean values of three replicates ± sd. □, 1 × 105 cells/mL; •, 5 × 104 cells/mL; ▵, 2.5 × 104 cells/mL; ▪, 1.3 × 104 cells/mL; ○, 6.2 × 103 cells/mL; ▴, 3.1 × 103 cells/mL.
Effects of PSK-α on TE Differentiation
On the assumption that a chemical factor(s) other than auxin or cytokinin is involved in TE differentiation, we investigated the effects of PSK-α, an endogenous peptide growth factor shown to compensate for growth suppression observed at low cell density, on TE differentiation of zinnia cells.
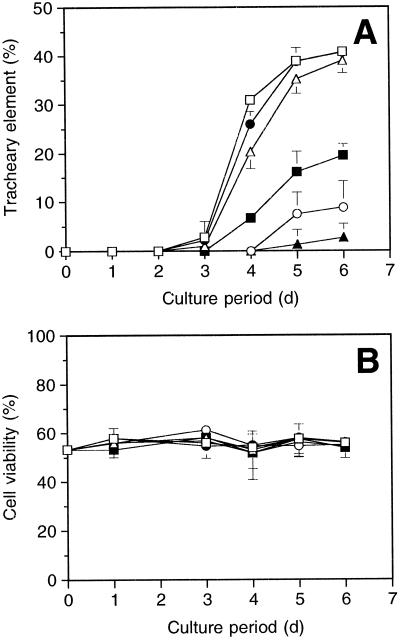
When the cells, cultured at a density of 2.5 × 104 cells mL−1, were treated with PSK-α at a concentration range from 0.1 nm to 1.0 μm, TE differentiation was strongly stimulated in a dose-dependent manner (Fig. 2A). The TE-formation-stimulating activity of PSK-α was detected at concentrations as low as 0.01 μm, where more than 35% of the living cells differentiated into TEs by 5 d of culture in the presence of PSK-α. In contrast, the frequency of TE differentiation in the absence of PSK-α remained at approximately 1% by 5 d, indicating that PSK-α compensates for the suppression of TE differentiation observed at low cell densities.
Figure 2.
Effects of PSK-α on TE differentiation (A) and cell viability (B). Single zinnia cells were incubated in liquid medium at a density of 2.5 × 104 cells mL−1 in the presence of various concentrations of PSK-α. The TE differentiation frequency for each well was calculated by dividing the number of TEs by the total number of living cells, and cell viability was calculated by dividing the number of living cells by the number of total cells. Data are means of three replicates ± sd. □, 1.0 μm; •, 0.1 μm; ▵, 0.01 μm; ▪, 1.0 nm; ○, 0.1 nm; ▴, control.
Zinnia mesophyll cells have an absolute requirement for an auxin and a cytokinin for TE differentiation (Fukuda and Komamine, 1980a). To determine how these two phytohormones and PSK-α are involved in the stimulation of TE differentiation, we cultured mesophyll cells in media that contained 1.0 μm PSK-α and four combinations of plant hormones: NAA and 6-BA, NAA only, 6-BA only, and no hormones. Elimination of NAA and/or BA completely suppressed TE differentiation (0%) even when the culture medium contained a sufficient amount of PSK-α for the stimulation. We conclude that PSK-α requires both auxin and cytokinin to stimulate TE differentiation of zinnia cells.
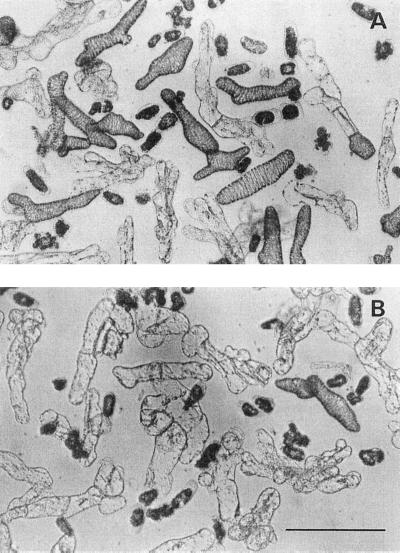
TE differentiation stimulated by PSK-α is not a secondary effect caused by inhibiting cell death, because cell viabilities were not altered by changing the PSK-α concentration (Fig. 2B). It is also unlikely that PSK-α promoted the differentiation by increasing a cell division, because more than 80% of TEs differentiated directly from the dispersed mesophyll cells without intervening cell division (Table I; Fig. 3). Direct TE differentiation without intervening cell division has been reported in many studies of colchicine-treated (Fukuda and Komamine, 1980b) or γ-irradiated cells (Phillips, 1981; Sugiyama et al., 1986), as well as after serial observation of single mesophyll cells (Fukuda and Komamine, 1980b).
Table I.
Percentages of TEs differentiated without intervening cell division
| PSK-α Concentration | TEs | |
|---|---|---|
| % of living cells | % of total TEs | |
| 1.0 μm | 39 ± 7 | 81 ± 7 |
| 0.1 μm | 39 ± 2 | 87 ± 2 |
| 0.01 μm | 35 ± 2 | 82 ± 4 |
| 1.0 nm | 16 ± 5 | 86 ± 3 |
| 0.1 nm | 8 ± 4 | NDa |
| None | 1 ± 2 | ND |
Zinnia mesophyll cells were cultured at an initial density of 2.5 × 104 cells mL−1 in media containing PSK-α at various concentrations. The TE differentiation frequency was determined after 5 d of culture. Data are mean values of three replicates ± sd.
ND, Not determined.
Figure 3.
Micrographs of zinnia mesophyll cells cultured in the presence or absence of PSK-α. Single zinnia cells were incubated for 5 d in medium containing PSK-α at a concentration of 10 nM (A) or in the absence of PSK-α (B). Bar =100 μm.
The fact that the minimum density required for zinnia cell division in this medium was approximately 103 cells mL−1, which is far lower than that required for TE differentiation, further supports the independence of differentiation and proliferation. At a density of 2.5 × 104 cells mL−1, more than 90% of the viable cells that had not differentiated into TEs had divided by 5 d of culture regardless of the concentration of PSK-α. Thus, it may be concluded that PSK-α stimulated TE differentiation by recruiting more cells into the TE developmental pathway without altering cell viability or cell division.
Identification of PSKs in Conditioned Medium Derived from Zinnia Suspension Culture
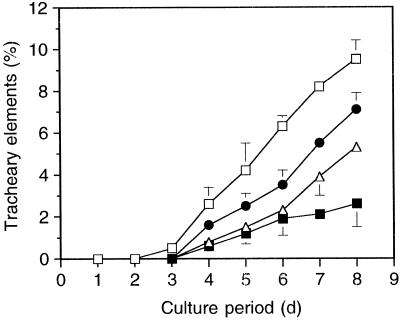
Because PSK-α stimulates TE differentiation of zinnia mesophyll cells, we next investigated whether zinnia mesophyll cells themselves produce PSK-α. As a first step, we tested the effects of crude conditioned medium on TE differentiation by adding aliquots to bioassay media. As shown in Figure 4, TE differentiation was stimulated (compared with control) by conditioned medium; the maximum frequency was about 10% after 8 d of the start of bioassay when the conditioned medium concentration was 12.5% (v/v), indicating that zinnia conditioned medium may contain PSK-α or PSK-α-like compounds. In contrast, the addition of conditioned medium at a concentration above 12.5% resulted in inhibition of TE differentiation (data not shown). Conditioned medium may contain inhibitory factor(s) for TE differentiation as well as stimulatory factor(s).
Figure 4.
Effects of conditioned medium on TE differentiation. Single zinnia cells were incubated in liquid medium at a density of 2.5 × 104 cells mL−1 in the presence of various concentrations of conditioned medium. Data are the means of three replicates ± sd. □, 12.5%; •, 3.2%; ▵, 0.8%; ▪, control.
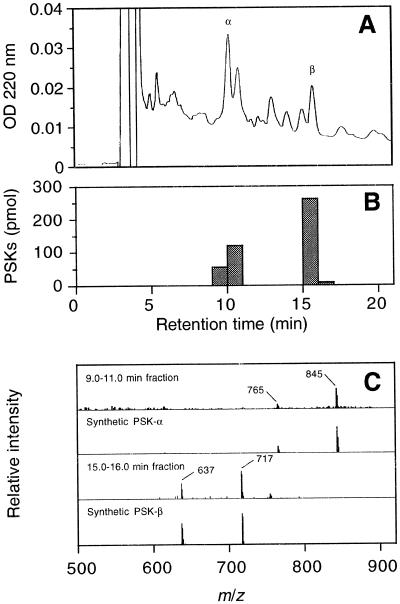
To determine if conditioned medium contains PSK-α, we fractionated zinnia conditioned medium by stepwise elution from a DEAE Sephadex column. Fractions of 800 and 1,200 mm KCl were desalted on a Sep-Pak column and on Bio-Gel P-2 by gel-permeation chromatography. Each eluted fraction (5.0 mL) was assayed by ELISA, and positive fractions (40–55 mL) were pooled and lyophilized. These fractions were further purified by reverse-phase HPLC, with monitoring of UV A220 (Fig. 5A). Fractions (1.0 mL) were collected from 0 to 20 min and assayed by ELISA. Significant amounts of PSKs were detected in the 9.0 to 11.0 min and the 15.0 to 16.0 min fractions (Fig. 5B). A combined fraction of 9.0 to 11.0 min had a pseudomolecular ion of m/z 845 corresponding to [M-H]− and a fragment ion of m/z 765 corresponding to [M-H-80]−, as shown by MS, confirming that this fraction contains PSK-α (Fig. 5C). By comparing the retention time of eluted peak and that of synthetic PSK-α (data not shown), a peak eluting at 10.2 min was determined to be PSK-α. A similar procedure showed that a peak eluting at 15.7 min was PSK-β, a C-terminal truncated peptide of PSK-α. A control experiment revealed that the total recovery of PSK-α by this purification procedure was 15%. Therefore, the total amount of PSKs contained in 400 mL of conditioned medium was estimated to be 3.0 nmol equivalent to PSK-α.
Figure 5.
Identification of PSKs in conditioned medium derived from zinnia mesophyll cell culture. A, HPLC profile of purified conditioned medium. Conditioned medium derived from TE-inductive culture was separated by two steps of open-column chromatography and reverse-phase HPLC. Fractions were collected every minute. B, Results of competitive ELISA. The amount of PSKs contained in each fraction was determined by competitive ELISA based on anti-PSK-α antibodies. Peaks eluting at 10.2 and 15.7 min were estimated to be PSK-α and PSK-β, respectively. C, Comparison of mass spectrum of natural sample and synthetic PSK-α. A combined fraction (9.0–11.0 min) was concentrated and analyzed by MS (1st row). A pseudomolecular ion of m/z 845 corresponds to [M-H]− and a fragment ion of m/z 765 corresponds to [M-H-80]− of PSK-α, coinciding well with the spectrum of synthetic PSK-α (2nd row). A fraction (15.0–16.0 min) was concentrated and analyzed by MS (3rd row). A pseudomolecular ion of m/z 717 corresponds to [M-H]−, and a fragment ion of m/z 637 corresponds to [M-H-80]− of PSK-β, coinciding well with the spectrum of synthetic PSK-β (4th row).
We have shown that PSK-α stimulates TE differentiation of dispersed zinnia mesophyll cells without intervening cell division in the presence of auxin and cytokinin. We also confirmed that zinnia cells in culture produce considerable amounts of PSK-α. Although this peptide was originally isolated as a mitogenic factor from conditioned medium derived from an asparagus mesophyll culture (Matsubayashi and Sakagami, 1996), current results indicate that PSK-α stimulates cell differentiation instead of cell division under the specified conditions.
What is the fundamental function of PSK-α? One possibility is that PSK-α makes cells receptive to signals that ultimately determine the cell destiny, i.e. cell division or cell differentiation. Research into PSK-α receptors transmitting secondary messages that activate a specific set of genes appears to be warranted.
ACKNOWLEDGMENTS
We thank Dr. Hiroo Fukuda and Hiroyasu Motose (Graduate School of Sciences, University of Tokyo) for useful discussions.
Abbreviations:
- PSK
phytosulfokine
- TE
tracheary element
- TFA
trifluoroacetic acid
Footnotes
This research was supported by the Program for Promotion of Basic Research Activities for Innovative Biosciences.
LITERATURE CITED
- Bellincampi D, Morpurgo G. Conditioning factor affecting growth in plant cells in culture. Plant Sci. 1987;51:83–91. [Google Scholar]
- Birnberg PR, Somers DA, Brenner ML. Characterization of conditioning factors that increase colony formation from black Mexican sweet corn protoplasts. J Plant Physiol. 1988;132:316–321. [Google Scholar]
- Fukuda H. Tracheary element formation as a model system of cell differentiation. Int Rev Cytol. 1992;136:289–332. [Google Scholar]
- Fukuda H, Komamine A. Establishment of an experimental system for the study of tracheary element differentiation from single cells isolated from the mesophyll cells of Zinnia elegans. Plant Physiol. 1980a;65:57–60. doi: 10.1104/pp.65.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H, Komamine A. Direct evidence for cytodifferentiation to tracheary elements without intervening mitosis in a culture of single cells isolated from the mesophyll of Zinnia elegans. Plant Physiol. 1980b;65:61–64. doi: 10.1104/pp.65.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H, Komamine A (1985) Cytodifferentiation. In IK Vasil, ed, Cell Culture and Somatic Cell Genetics of Plants, Vol 2. Academic Press, New York, pp 149–212
- Matsubayashi Y, Hanai H, Hara O, Sakagami Y. Active fragments and analogs of the plant growth factor, phytosulfokine: structure-activity relationships. Biochem Biophys Res Commun. 1996;225:209–214. doi: 10.1006/bbrc.1996.1155. [DOI] [PubMed] [Google Scholar]
- Matsubayashi Y, Morita A, Matsunaga E, Furuya A, Hanai N, Sakagami Y. Physiological relationships between auxin, cytokinin, and a peptide growth factor, phytosulfokine-α, in stimulation of asparagus cell proliferation. Planta. 1999;207:559–565. [Google Scholar]
- Matsubayashi Y, Sakagami Y. Phytosulfokine, sulfated peptides that induce the proliferation of single mesophyll cells of Asparagus officinalis L. Proc Natl Acad Sci USA. 1996;93:7623–7627. doi: 10.1073/pnas.93.15.7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi Y, Sakagami Y. Effects of the medium ammonium-nitrate ratio on competence for asparagus cell division induced by phytosulfokine-α. Plant Cell Rep. 1998;17:368–372. doi: 10.1007/s002990050408. [DOI] [PubMed] [Google Scholar]
- Matsubayashi Y, Sakagami Y (1999) Characterization of specific binding sites for a mitogenic sulfated peptide, phytosulfokine-α, in the plasma membrane fraction derived from Oryza sativa L. Eur J Biochem (in press) [DOI] [PubMed]
- Matsubayashi Y, Takagi L, Sakagami Y. Phytosulfokine-α, a sulfated pentapeptide, stimulates the proliferation of rice cells by means of specific high- and low-affinity binding sites. Proc Natl Acad Sci USA. 1997;94:13357–13362. doi: 10.1073/pnas.94.24.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R. Direct differentiation of tracheary elements in cultured explants of gamma-irradiated tubers of Helianthus tuberosus. Planta. 1981;153:262–266. doi: 10.1007/BF00383897. [DOI] [PubMed] [Google Scholar]
- Roberts AW, Donovan SG, Haigler CH. A secreted factor induces cell expansion and formation of metaxylem-like tracheary elements in xylogenic suspension cultures of zinnia. Plant Physiol. 1997;115:683–692. doi: 10.1104/pp.115.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama M, Fukuda H, Komamine A. Effects of nutrient limitation and γ-irradiation on tracheary element differentiation and cell division in single mesophyll cells of Zinnia elegans. Plant Cell Physiol. 1986;27:601–606. [Google Scholar]
- Suzuki K, Ingold E, Sugiyama M, Fukuda H, Komamine A. Effects of 2,6-dichlorobenzonitrile on differentiation to tracheary elements of isolated mesophyll cells of Zinnia elegans and formation of secondary cell walls. Physiol Plant. 1992;86:43–48. [Google Scholar]