Abstract
Purpose
To assess if there are differences in the structure-function associations between healthy and glaucomatous eyes.
Methods
Structure-function associations were assessed in healthy and glaucomatous eyes in three datasets, globally and in the six sectors of the optic nerve head. Structural parameters included rim area (RA) and retinal nerve fiber layer thickness (RNFLT). Functional parameters included unweighted mean of sensitivity thresholds (MS) and unweighted mean of total deviation values (MD), assessed with standard automated perimetry, short-wavelength automated perimetry, frequency-doubling technology perimetry, or contrast sensitivity perimetry. All structural and functional parameters were expressed as percent of mean normal. SF associations were assessed with correlation analyses (Pearson, Spearman and Kendall). We also assessed the SF associations with linear regression analyses: the generalized estimating equation (GEE) was used to adjust for inter-eye correlations and ordinary least squares (OLS) linear models were used when these adjustments were not necessary. We applied Bonferroni corrections to adjust for the impact of multiple comparisons.
Results
Overall, none of the Pearson correlations tested in healthy eyes were significant (correlations ranged from -0.17 to 0.37), whereas 77% of the correlations tested in glaucomatous eyes were significant (correlations ranged from 0.01 to 0.79). Similarly, none of the slopes obtained with GEE and OLS were significant in healthy eyes (slopes ranged from -0.30 to 0.87), whereas 82% of the slopes obtained in glaucomatous eyes were significant (slopes ranged from 0.02 to 1.38).
Conclusions
Significant associations between structure and function were consistently observed in glaucomatous eyes, but not in healthy eyes. These differences in association should be considered in the design of structure-function models for progression.
Introduction
Primary open-angle glaucoma (POAG) is a chronic optic neuropathy characterized by the progressive degeneration of the retinal ganglion cells and their axons.[1] Ganglion cell degeneration results in morphological changes to the optic nerve head and retinal nerve fiber layer, and also in the loss of visual function. Some degree of association is therefore expected between structural damage and loss of visual function in glaucomatous eyes.[2] Both histological[3–7] and clinical[8–13] studies have shown the existence of correlations between structure and function in glaucomatous eyes. In healthy eyes, however, the relationship between structure and function remains unclear.
Models that characterize the structure-function (SF) relationship in glaucoma make different assumptions about whether SF associations are different or not in healthy and glaucomatous eyes. Some models assume a linear (or monotonic) association between structure and function when data from both healthy and glaucomatous eyes are pooled together.[14–16] Other models assume that there is no association between structure and function in healthy eyes.[17–19] These assumptions have important implications because they can lead to different conclusions about the form (linear or curvilinear) and the strength of the SF relationship. Consequently, the assumptions may impact models that use structural and functional data jointly for the detection of glaucoma and its progression.[14, 18]
While the observed SF relationship is influenced by several factors,[20] the inconsistent results observed between studies[21] regarding the relationship between structural and functional parameters may be due in part to the assumptions made about the associations in healthy eyes. The aim of this study was to determine whether differences exist in the SF associations between healthy and glaucomatous eyes.
Methods
This secondary data analysis adheres to the tenets of the Declaration of Helsinki. Institutional review board (IRB) approval to perform this study was initially obtained from Indiana University, and then from the University of Alabama at Birmingham following the relocation of the laboratory of the corresponding author. IRB approval was also obtained at each of the institutions at which the original datasets were collected. The SF associations in healthy and glaucomatous eyes were assessed using three different datasets to ensure that our findings are not due to the intricacies of one specific dataset. We used the Diagnostic Innovations in Glaucoma Study and the African Descent and Glaucoma Evaluation Study (DIGS-ADAGES) dataset[22] and a dataset from the State University of New York and Indiana University (SUNY-IU)[23] that each had healthy and glaucomatous eyes and a dataset from the University of Iowa with only healthy eyes.[19] Written informed consent was obtained from each participant. All studies were approved by the appropriate Institutional Review Boards, adhered to the tenets of the declaration of Helsinki for research involving human subjects, and were performed in conformity with the Health Insurance Portability and Accountability Act (HIPAA).
Datasets
The DIGS-ADAGES dataset[22]
These two, ongoing, longitudinal multi-center studies were prospectively designed to assess structure and function in glaucoma. They were conducted at the University of California San Diego (UCSD), the New York Eye and Ear Infirmary and the University of Alabama. Participants with healthy and glaucomatous eyes were selected based on the definition of healthy and glaucomatous eyes outlined in Table 1 of Sample et al.[22] In brief, healthy eyes were those in the normal diagnostic category, while glaucomatous eyes were those that had both abnormal visual fields test result and glaucomatous optic neuropathy.
Table 1. Number of observations and power for analysis for the critical value of 0.2 of Pearson correlation.
| Healthy eyes | Glaucomatous eyes | |||
|---|---|---|---|---|
| Subjects (eyes) | Power | Subjects (eyes) | Power | |
| DIGS/ADAGES | ||||
| RA vs SAP MS and MD | 694 (1212) | 1.00 | 271 (362) | 0.91 |
| RA vs SWAP MS and MD | 472 (802) | 0.99 | 247 (328) | 0.89 |
| RA vs FDP MS | 685 (1207) | 1.00 | 266 (356) | 0.91 |
| RNFLT vs SAP MS and MD | 204 (336) | 0.82 | 180 (238) | 0.77 |
| RNFLT vs FDP MS | 181 (295) | 0.77 | 151 (200) | 0.70 |
| IOWA | ||||
| RNFLT vs SAP MD | 76 (76) | 0.41 | ||
| SUNY-IU | ||||
| RNFLT vs SAP, CSP, FDP MS | 62 (62) | 0.35 | 51 (51) | 0.29 |
In this dataset, rim area (RA) was collected using the Heidelberg Retina Tomograph (Heidelberg Engineering, Heidelberg, Germany), and retinal nerve fiber layer thickness (RNFLT) was collected with optical coherence tomography (OCT) using the Stratus OCT (Carl Zeiss Meditec, Dublin, CA). Standard automated perimetry (SAP) (Carl Zeiss Meditec, Dublin, CA), short-wavelength automated perimetry (SWAP) (Carl Zeiss Meditec, Dublin, CA), and frequency-doubling perimetry (FDP) (Carl Zeiss Meditec, Dublin, CA) were also collected. Healthy and glaucomatous eyes from this dataset were included in the present retrospective cross-sectional study if they had at least one pair of SF data taken within a 30-day period. When several SF pairs were available, the first SF pair was selected for all subjects. This minimized the risk of including eyes that may have developed early glaucoma in the healthy eyes group. While this could have increased the risk of including visual fields with learning effects, the protocol of these studies required that all subjects be given practice tests if they were new to perimetry.[24] In addition, we only included visual fields that were determined to have no learning effect by the Visual Field Assessment Center (VisFACT) reading center at UCSD[24]. We also required the presence of at least 50 eyes for each SF pair. When available, eligible data from both eyes were included.
The IOWA dataset[19]
The IOWA dataset consisted of subjects with healthy eyes obtained from two sources. The first group, referred to as the long-term repeat group,[19] consisted of 60 healthy eyes from 60 subjects that had 2 visits each. The second group, referred to as the short-term repeat group,[19] consisted of 22 healthy eyes from 22 subjects with 4 or 5 visits each all taken within a 60-day window (two subjects had only 4 visits[19]). RNFLT was measured with OCT3; Stratus fast RNFL circular scan; Carl Zeiss Meditec) and SAP was obtained with the Humphrey Field Analyzer, program 24–2 SITA standard (Carl Zeiss Meditec, Dublin, CA). Learning effects were not directly assessed in the IOWA dataset. We therefore discarded the first visit to minimize the impact of possible learning effects and averaged the remaining visits. While we report the results of the analyses in which the first visit was excluded, it should be noted that all results were similar when we included all visits in the analyses. For the present study, we pooled the data from these two sources and used a sample of 76 healthy eyes from 76 subjects. Data from glaucomatous eyes were not available in this dataset and it therefore could not be used to directly determine whether differences in SF associations between healthy and glaucomatous eyes exist. We nonetheless included this dataset because it allows us to assess the SF associations in healthy eyes and to compare them to those obtained in the other datasets used here.
The SUNY-IU dataset[23]
Participants included in this dataset were enrolled in a multicenter longitudinal study at three different university clinics. One center was at the State University of New York (SUNY) and the other two centers were affiliated with Indiana University, one in Indianapolis and one in Bloomington. In brief, 62 participants with healthy eyes and 51 patients with glaucoma were included. Patients with glaucoma had visual field loss and glaucomatous optic neuropathy. RNFLT was measured with Stratus OCT3, Model 3000(Carl Zeiss Meditec, Inc), SAP was obtained with the HFAII, 24–2 SITA standard (Carl Zeiss Meditec, Dublin, CA), FDP was performed with the Humphrey Matrix (Carl Zeiss Meditec, Dublin, CA) using the 24–2 test pattern, and contrast sensitivity perimetry (CSP) testing was performed using custom testing stations based on 21-inch cathode-ray tube displays driven by a visual stimulus generator (ViSaGe; Cambridge Research Systems, Ltd, Rochester, Kent, UK). One eye of each participant was tested on a single visit. As for the DIGS-ADAGES dataset, all subjects had previous experience with perimetry, so no learning effect was expected.[23]
Structural and functional tests and parameters
We assessed the SF associations in several combinations of structural and functional parameters to characterize the SF relationship as comprehensively as possible. All parameters included in this study are the ones available in the DIGS-ADAGES, IOWA and SUNY-IU dataset. The structural parameters included RA and RNFLT. Functional parameters included unweighted mean of the 52 sensitivity thresholds (MS) of the 24−2 test (the two locations above and below the blind spot were excluded) and the unweighted mean of total deviation values (MD). We used this definition of MD[18] instead of the instrument-generated MD because we assessed the SF association globally and in each of six sectors.
These parameters were obtained for SAP, SWAP, FDP, and CSP when available. The detailed description of each of the structural and functional tests and parameters can be found in the papers cited above for each study.[19, 22, 23]
Structure-function pairs
In the DIGS-ADAGES dataset, the following eight combinations of parameters for SF pairs were assessed: RA-MS (SAP, SWAP and FDP), RA-MD (SAP and SWAP), RNFLT-MS (SAP and FDP), RNFLT-MD (SAP). The SF associations were assessed globally (G), and in the following sectors: temporal (T), supero-temporal (ST), infero-temporal (IT), nasal (N), supero-nasal (SN), and infero-nasal (IN) based on the Garway-Heath map.[8] In the SUNY-IU dataset, the RNFLT-MD pair was assessed using SAP, FDP and CSP in the ST and IT sectors. Finally, in the IOWA dataset, SF associations were assessed using the RNFLT-MD parameters globally and in the ST and IT sectors.
Statistical analysis
To assess the associations between structural and functional measurements, all data were expressed in percent of mean normal. For MS, this was done after converting the sensitivities at each location from a logarithmic scale (dB) to a linear scale (1/Lambert).[25] The values were then averaged across locations to obtain the MS value. For MD, we first converted the total deviation (TD) values from the logarithmic units to linear units, and then averaged them across the 52 locations.[18] All values were then expressed as percent of mean normal (the average obtained for healthy eyes for each dataset included in the study).
We conducted two types of analyses: 1) correlation analyses after selecting one eye at random from subjects for which both eyes were included in the study and 2) linear regression analyses with all eyes available to accommodate for the fact that some subjects had both eyes included in the study. All the statistical analyses were made with R.[26]
Correlation analyses
For all three datasets, we assessed the SF associations using Pearson, Spearman, and Kendall correlations with a subset of data that met the underlying assumption of independence, that is, all pairs of measurements are independent from each other. For the DIGS-ADAGES dataset in which both eyes of some subjects were included, one eye was selected at random and the contralateral eye was discarded from the analysis. While Pearson correlation assumes a linear association between the data, both Spearman and Kendall correlations assume a monotonic association. The calculations of Spearman correlation are based on rank correlations, whereas those of Kendall correlation are based on concordant versus discordant pairs. For all correlation analyses, the p-values were calculated for the significance test of zero correlation. In addition, we computed the statistical power for the Pearson correlation significance test using the alternative hypothesis of correlation of 0.2, a correlation regarded to be small enough for any practical uses and often labeled as “weak” or negligible.[27]
Linear regression analyses
In this approach, the functional measure was treated as the response variable and the structural measure as the explanatory variable, as has traditionally been done. The unit effect from the structural on the functional measure indicates how much function changes per one unit of change on structure. In the DIGS-ADAGES dataset, data were available for two eyes for some of the participants. The SF associations were therefore assessed using the generalized estimating equation (GEE), which adjusts for potential correlations between the two eyes of the same participant. GEE also allows to compensate for age effects in MS and MD (even though MD is already age-corrected). The unit effect in GEE has a similar interpretation as in ordinary least square (OLS) regression: change in response per unit change in explanatory variable. The unit effect in GEE estimates the average effect over the population and was assumed with an exchangeable working correlation to account for within-subject correlations. An additional analysis using a linear mixed effect model (LMM) was also performed that, as GEE, accounts for the presence of both eyes in some subjects in the DIGS-ADAGES dataset. For LMM, the unit effect estimates the marginal effect as the fixed effect since the within-subject correlations are accounted through the random effect term in LMM. For the IOWA and the SUNY-IU datasets, we used OLS which was sufficient to analyze the data obtained from one eye per subject. For all linear regression analyses, the p-values were calculated for the significance test of zero slope.
We applied Bonferroni corrections to adjust for the impact of multiple comparisons. Thus, significance level was set at 0.05 divided by the respective number of tests performed in each analysis. Normality and linearity of associations were assumed for GEE, LMM, and OLS, as well as for the significance test for Pearson correlation.
Results
Correlation analyses
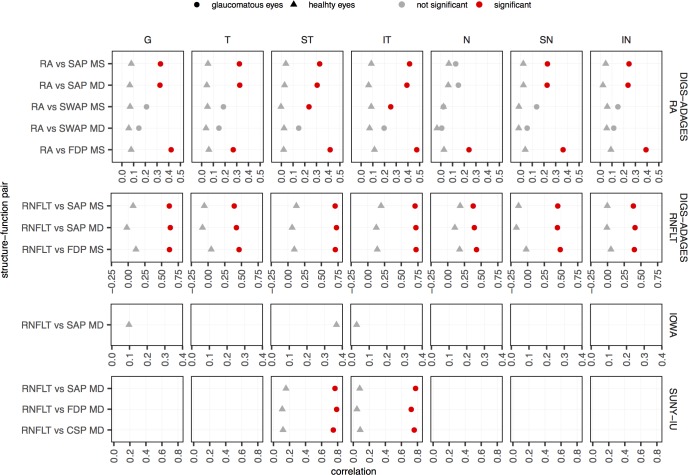
Fig 1 summarizes the Pearson correlations for the SF pairs in all sectors in the three datasets. The results for Spearman and Kendall correlations were similar to the ones obtained for Pearson correlation and are presented as supporting information in S1 and S2 Figs, respectively. Table 1 shows the sample size and statistical power of the significance tests of zero correlation computed against the alternative hypothesis of a 0.2 correlation.
Fig 1. Pearson correlation for each of the structure-function pairs.
Results are presented globally and in all sectors for healthy (triangles) and glaucomatous (circles) eyes. Correlations that were found to be significantly different from zero after Bonferroni correction are shown in red. Note that the range of the x-axes are different for the different datasets; we plotted the graph using the range observed in each dataset to highlight the differences between healthy and glaucomatous eyes in each dataset.
Overall, no significant correlations were found for healthy eyes (correlations ranged from -0.17 to 0.37), whereas 48 out of 62 correlations (77%) were significant for glaucomatous eyes (correlations ranged from 0.01 to 0.79). In the DIGS-ADAGES dataset, similar results were observed in glaucomatous eyes when RA and RNFLT were used as the structural test. All correlations were significant in glaucomatous eyes, except when SWAP data was used. For the SUNY-IU dataset, all 6 correlations for glaucomatous eyes and none for healthy eyes were significantly different from zero.
Linear regression analyses
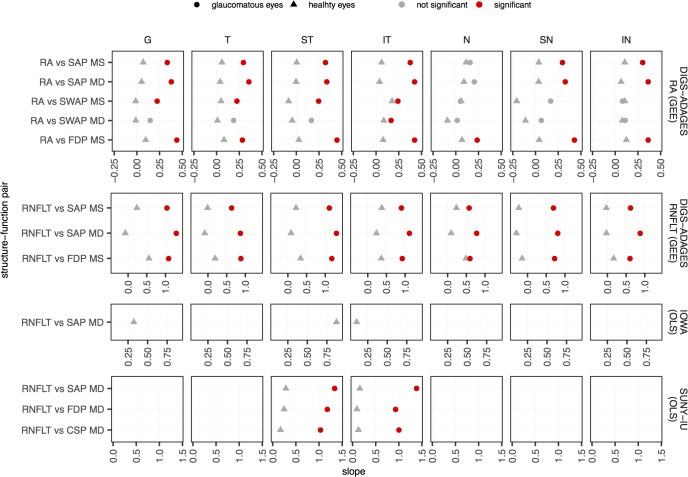
Fig 2 summarizes the unit effects estimated with GEE and OLS for function on structure for the SF pairs globally and in all sectors available in the three datasets. Similar results were obtained when these analyses were performed for structure on function (see supporting information in S3 Fig) and for the LMM analysis (see supporting information in S4 Fig).
Fig 2. Unit effect (slopes) estimated with GEE or OLS regression of function on structure for each of the structure-function pairs.
Results are presented globally and in all sectors for healthy (triangles) and glaucomatous (circles) eyes. Slopes that were found to be significantly different from zero after Bonferroni correction are shown in red. Note that the range of the x-axes are different for the different datasets; we plotted the graph using the range observed in each dataset to highlight the differences between healthy and glaucomatous eyes in each dataset.
Overall, no significant slopes were found for healthy eyes (slopes ranged from -0.30 to 0.87), whereas 51 out of 62 correlations (82%) were significant for glaucomatous eyes (correlations ranged from 0.02 to 1.38). Except for the pairs that included SWAP, all slopes were significantly different from zero for glaucomatous eyes. For the SUNY-IU dataset, all 6 OLS slopes for glaucomatous eyes and none for healthy eyes were found significantly different from zero.
Discussion
In the present study, we consistently found that the associations between structure and function are different in healthy eyes compared to glaucomatous eyes. In healthy eyes, no associations were found between structure and function, while significant associations were found in glaucomatous eyes for several of the comparisons we performed. This robust finding was observed globally and in each of the sectors we assessed, in each of the analyses we performed, as well as across all datasets.
The different associations between structure and function in healthy and glaucomatous eyes need to be taken into account when studying the SF relationship in glaucoma. These differences also have implications for the development of models that use both structural and functional parameters to monitor glaucoma progression. Our results support the assumptions made by the Hood and Kardon model,[18, 19] that there is negligible or no correlation between structural and functional measurements in healthy eyes. Our results, however, are in disagreement with previous results[15] and the assumptions made in other models, such as the Bayesian linear regression[14] and sANSWERS[28] that the associations between structure and function are linear with the same slope for healthy and glaucomatous eyes. Pooling healthy and glaucomatous eyes together leads to models that are not representative of either group. For instance, if we fit a line to the observed structure and function data (with e.g. OLS, GEE, or LMM), the slope will underestimate the results for glaucomatous eyes while overestimating the results for healthy eyes. The biases generated in both healthy and glaucomatous eyes that occur when pooling the data from both groups are bound to reduce the sensitivity of glaucomatous progression of models that use structural and functional information. In this study, we only applied statistical methods that describe linear or monotonic associations because non-monotonic associations (e.g. sinusoidal or other where, for example, RA passes from increasing to decreasing with MS) are biologically implausible.
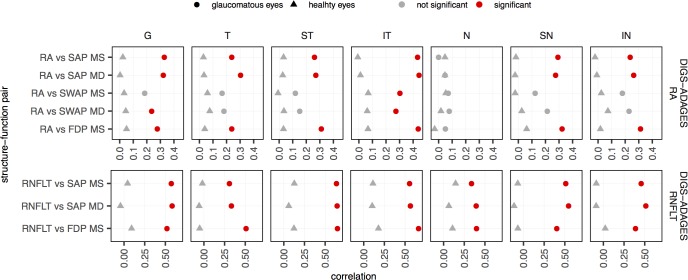
While glaucomatous eyes can range from very mild visual loss to blindness, healthy eyes have a narrower range of possible measurements. Our finding may therefore be an artifact of the restricted-range problem,[29] in which correlations decrease with restricted ranges. To quantify the effect of the restricted-range problem, we performed Pearson correlation on a subset of the DIGS/ADAGES dataset in which we matched the measurement range across groups. We used the DIGS/ADAGES dataset to perform this analysis because it is large enough to allow range restriction while maintaining reasonable levels of statistical power overall (see Table 2). The ranges were matched by generating intervals for the 95% central distribution for MS, MD, RA, and RNFLT for the healthy eyes. We then centered this range onto the median value obtained for the glaucomatous eyes and excluded all values that fell outside that range. In this way, we restricted the range in both healthy and glaucomatous groups of eyes, so that the range in structure and function in any pair was equal to or smaller in the glaucomatous group than in the healthy group. Fig 3 shows the Pearson correlation after matching the ranges. We obtained similar results as those shown in Fig 1, with significant correlations in glaucomatous eyes and no correlations in healthy eyes (Fig 3). Table 2 shows the sample size and statistical power for this analysis. Differences in Pearson correlations cannot be explained by the smaller range of healthy eyes with respect to glaucomatous eyes. It is more likely that the results are due to differences in the SF associations between healthy and glaucomatous eyes.
Table 2. Number of observations and power for analysis for the critical value of 0.2 of Pearson correlation after restricting the range for glaucomatous eyes.
Since range restriction yields different number of eyes and subjects in each sector, the values reported here are the minimum sample sizes and power over all sectors.
| Healthy eyes | Glaucomatous eyes | |||
|---|---|---|---|---|
| Number of eyes | Power | Number of eyes | Power | |
| RA vs SAP MS | 623 | 1.00 | 212 | 0.84 |
| RA vs SAP MD | 623 | 1.00 | 201 | 0.82 |
| RA vs SWAP MS | 426 | 0.99 | 197 | 0.81 |
| RA vs SWAP MD | 428 | 0.99 | 189 | 0.79 |
| RA vs FDP MS | 616 | 1.00 | 213 | 0.84 |
| RNFLT vs SAP MS and MD | 181 | 0.77 | 129 | 0.63 |
| RNFLT vs SAP MD | 180 | 0.77 | 127 | 0.62 |
| RNFLT vs FDP MS | 161 | 0.72 | 118 | 0.59 |
Fig 3. Pearson correlation for each of the structure-function pairs for DIGS-ADAGES dataset after restricting the range for glaucomatous eyes.
Results are presented globally and in all sectors for healthy (triangles) and glaucomatous (circles) eyes. Correlations that were found to be significantly different from zero after Bonferroni correction are shown in red. Note that the range of the x-axes are different for the different datasets; we plotted the graph using the range observed in each dataset to highlight the differences between healthy and glaucomatous eyes in each dataset.
Although it is commonly assumed that structural defects precede functional defects in glaucoma,[1] large population-based studies have reported that functional defects can be detected before structural defects.[30, 31] There is therefore no consensus that our decision to use structure as the explanatory variable was justified for the linear regression analyses. Furthermore, GEE, LMM, and OLS univariate models assume that the explanatory variable can be not only controlled, but measured without error, two assumptions that are not met by our data. Thus, even though the methods are appropriate to test for an association between structure and function, [32] they lead to biased slope estimates. If we swapped the explanatory variable (structure) and the response variable (function), the fitted line would be different.[21] We therefore performed two additional analyses.
First, we reran the GEE and OLS analyses using function as the explanatory variable. We obtained similar results (supporting information in S3 Fig): stronger associations were systematically observed in glaucomatous but not in healthy eyes. This suggests that our finding is likely not due to our choice of an explanatory variable.
Second, to directly assess the limitation of a lack of an error-free, independent explanatory variable, we used an error-in-variables model. The standardized major axis (SMA), which assumes equal signal-to-noise ratios for both variables, was therefore applied after randomly selecting one eye per subject when both eyes were included in the study. SMA accounts for the measurement errors in both structure and function.[32] P-values were calculated to test for differences between SMA slopes for healthy and glaucomatous eyes.[33] We found that the estimated slope for healthy eyes were significantly different from the estimated slope for glaucomatous eyes after Bonferroni correction in 50 out of 62 (81%) of all SF pairs globally and in each sector. This is consistent with the results we obtained using Pearson, Spearman, and Kendall correlations, as well as the GEE, LMM and OLS fits that all showed that the SF relationship is different in healthy and glaucomatous eyes.
In healthy subjects, we systematically found no association between structure and function in both age-corrected (MD) and non-age-corrected (MS) indices. Yet, non-pathologic, age-related loss of retinal ganglion cells occurs.[34–37] This loss has been associated with a decrease in RNFLT of about 2.5 μm per decade [38, 39] and a decrease in RA of about 0.09 mm2 per decade,[39] and with a decrease of visual sensitivity of about 0.7dB per decade.[40] The structural parameters included in this study were not corrected for aging effects. We therefore expected some correlation between structure and the non-age-corrected MS index in healthy subjects. Yet, we did not find any significant correlation after Bonferroni correction because the age-related losses are very small compared with inter-individual differences and test-retest variability. After removing the effects of age, i.e. with the MD index, correlations for healthy eyes should be smaller. The Pearson correlation showed this trend, with correlations between structure and MS being slightly greater by about 0.03 in general for all sectors than those observed between structure and MD. Likewise, slopes for the GEE and OLS linear regression models that removed the effect of age by including it as a covariate are smaller than without correction by 0.6% of mean normal function per % of mean normal structure (see supporting information in S5 Fig).
In summary, across all sectors, stronger associations were consistently found in glaucomatous eyes than in healthy eyes using three different datasets, different structure and function pairs, and different statistical approaches. The associations between structure and function are different for healthy and glaucomatous eyes, suggesting that data from these two groups should not be pooled together when assessing the structure-function relationship in glaucoma. Doing so would result in weaker structure-function associations in glaucoma patients. Similarly, approaches using structure and function to model glaucoma progression should consider these differences in associations between structure and function between healthy and glaucomatous eyes.
Supporting information
Results are presented globally and in all sectors for healthy (triangles) and glaucomatous (circles) eyes. Correlations that were found to be significantly different from zero after Bonferroni correction are shown in red. Note that the range of the x-axes are different for the different datasets; we plotted the graph using the range observed in each dataset to highlight the differences between healthy and glaucomatous eyes in each dataset.
(TIFF)
Results are presented globally and in all sectors for healthy (triangles) and glaucomatous (circles) eyes. Correlations that were found to be significantly different from zero after Bonferroni correction are shown in red. Note that the range of the x-axes are different for the different datasets; we plotted the graph using the range observed in each dataset to highlight the differences between healthy and glaucomatous eyes in each dataset.
(TIFF)
Results are presented globally and in all sectors for healthy (triangles) and glaucomatous (circles) eyes. Slopes that were found to be significantly different from zero after Bonferroni correction are shown in red. Note that the range of the x-axes are different for the different datasets; we plotted the graph using the range observed in each dataset to highlight the differences between healthy and glaucomatous eyes in each dataset.
(TIFF)
Results are presented globally and in all sectors for healthy (triangles) and glaucomatous (circles) eyes. Slopes that were found to be significantly different from zero after Bonferroni correction are shown in red. Note that the range of the x-axes are different for the different datasets; we plotted the graph using the range observed in each dataset to highlight the differences between healthy and glaucomatous eyes in each dataset.
(TIFF)
Results are presented globally and in all sectors for healthy (triangles) and glaucomatous (circles) eyes. Slopes that were found to be significantly different from zero after Bonferroni correction are shown in red. Note that the range of the x-axes are different for the different datasets; we plotted the graph using the range observed in each dataset to highlight the differences between healthy and glaucomatous eyes in each dataset.
(TIFF)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
Acknowledgment is made to the donors of the National Glaucoma Research, a program of BrightFocus Foundation, for support of this research. This work was also supported in part by NIH grant EY025756, by an Indiana University—Purdue University Indianapolis DRIVE award, by an unrestricted grant from the EyeSight Foundation of Alabama, Birmingham, AL, and by an unrestricted grant from Research to Prevent Blindness.
We thank Linda M. Zangwill, Jeffrey M. Liebmann, Christopher A. Girkin and Felipe A. Medeiros (DIGS-ADAGES dataset), William H. Swanson and Mitchell W. Dul (SUNY-IU dataset), and Michael Wall and Randy H. Kardon (IOWA dataset) for providing the datasets used in this study. The DIGS and ADAGES studies were supported by NIH grants P30EY022589, EY021818, EY11008, U10EY14267, EY019869; Eyesight Foundation of Alabama; Edith C. Blum Research Fund of the New York Glaucoma Research Institute, New York, NY; unrestricted grant from Research to Prevent Blindness, New York, New York.
The SUNY-IU dataset was supported by National Institutes of Health Grants R01EY007716 (William H. Swanson) and 5P30EY019008 (Indiana University School of Optometry) and partial funding (Rizwan Malik) from the Department of Health’s NIHR Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. David F. Garway-Heath served as member of the executive committee that defined the diagnostic criteria.
The IOWA datasets were supported by National Eye Institute Grants R01-EY-09076 and R01-EY-02115, Iowa City Veteran Administration (VA) Center for the Prevention and Treatment of Visual Loss VA RR&D Division grant C9251-C (Randy H. Kardon and Michael Wall), grant C1821-R (Michael Wall) from the Veterans Administration (Rehabilitation Division and Merit Review), an unrestricted grant from Research to Prevent Blindness, New York, New York, a VA Merit Review Grant and by an unrestricted grant to the Department of Ophthalmology, University of Iowa College of Medicine from Research to Prevent Blindness, New York, New York.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors acknowledge the donors of the National Glaucoma Research, a program of BrightFocus Foundation, for support of this research (G2014096) (LR) (https://urldefense.proofpoint.com/v2/url?u=https-3A__www.brightfocus.org_research_national-2Dglaucoma-2Dresearch-2Dprogram&d=DwIF-g&c=o3PTkfaYAd6-No7SurnLtwPssd47t-De9Do23lQNz7U&r=HR114ge2epP7E1kzaXbJNhspuecE7A-UvYSBMitAaPg&m=V4J62rak7qMOKQx-TrNXCQeuwHcCnoZ6XC5L5wFk9Pc&s=px_8cdeckXSQhtvAgvHaGqQGctTzj505G84fGEb4LaQ&e=); National Institutes of Health (National Eye Institute) grant EY025756 (LR) EY025756 (https://nei.nih.gov); Indiana University - Purdue University Indianapolis DRIVE award (LR); an unrestricted grant from the EyeSight Foundation of Alabama, Birmingham, AL; and an unrestricted grant from Research to Prevent Blindness. The DIGS and ADAGES studies were supported by NIH grants P30EY022589, EY021818, EY11008, U10EY14267, EY019869; Eyesight Foundation of Alabama; Edith C. Blum Research Fund of the New York Glaucoma Research Institute, New York, NY; unrestricted grant from Research to Prevent Blindness, New York, New York. The SUNY-IU dataset was supported by National Institutes of Health Grants R01EY007716 (William H. Swanson) and 5P30EY019008 (Indiana University School of Optometry) and partial funding (Rizwan Malik) from the Department of Health’s NIHR Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. The IOWA datasets were supported by National Eye Institute Grants R01-EY-09076 and R01-EY-02115, Iowa City Veteran Administration (VA) Center for the Prevention and Treatment of Visual Loss VA RR&D Division grant C9251-C (Randy H. Kardon and Michael Wall), grant C1821-R (Michael Wall) from the Veterans Administration (Rehabilitation Division and Merit Review), an unrestricted grant from Research to Prevent Blindness, New York, New York, a VA Merit Review Grant and by an unrestricted grant to the Department of Ophthalmology, University of Iowa College of Medicine from Research to Prevent Blindness, New York, New York. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–20. doi: 10.1016/S0140-6736(04)16257-0 . [DOI] [PubMed] [Google Scholar]
- 2.Malik R, Swanson WH, Garway-Heath DF. 'Structure-function relationship' in glaucoma: past thinking and current concepts. Clin Exp Ophthalmol. 2012;40(4):369–80. doi: 10.1111/j.1442-9071.2012.02770.x ; PubMed Central PMCID: PMC3693944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harwerth RS, Carter-Dawson L, Shen F, Smith EL 3rd, Crawford ML. Ganglion cell losses underlying visual field defects from experimental glaucoma. Investigative ophthalmology & visual science. 1999;40(10):2242–50. . [PubMed] [Google Scholar]
- 4.Harwerth RS, Carter-Dawson L, Smith EL 3rd, Barnes G, Holt WF, Crawford ML. Neural losses correlated with visual losses in clinical perimetry. Investigative ophthalmology & visual science. 2004;45(9):3152–60. doi: 10.1167/iovs.04-0227 . [DOI] [PubMed] [Google Scholar]
- 5.Harwerth RS, Crawford ML, Frishman LJ, Viswanathan S, Smith EL 3rd, Carter-Dawson L. Visual field defects and neural losses from experimental glaucoma. Progress in retinal and eye research. 2002;21(1):91–125. . [DOI] [PubMed] [Google Scholar]
- 6.Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Investigative ophthalmology & visual science. 2000;41(3):741–8. Epub 2000/03/11. . [PubMed] [Google Scholar]
- 7.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. American journal of ophthalmology. 1989;107(5):453–64. Epub 1989/05/15. . [DOI] [PubMed] [Google Scholar]
- 8.Garway-Heath DF, Holder GE, Fitzke FW, Hitchings RA. Relationship between electrophysiological, psychophysical, and anatomical measurements in glaucoma. Investigative ophthalmology & visual science. 2002;43(7):2213–20. . [PubMed] [Google Scholar]
- 9.Kanamori A, Nakamura M, Tomioka M, Kawaka Y, Yamada Y, Negi A. Structure-function relationship among three types of spectral-domain optical coherent tomography instruments in measuring parapapillary retinal nerve fibre layer thickness. Acta ophthalmologica. 2013;91(3):e196–202. Epub 2013/04/18. doi: 10.1111/aos.12028 . [DOI] [PubMed] [Google Scholar]
- 10.Leung CK, Chan WM, Chong KK, Yung WH, Tang KT, Woo J, et al. Comparative study of retinal nerve fiber layer measurement by StratusOCT and GDx VCC, I: correlation analysis in glaucoma. Investigative ophthalmology & visual science. 2005;46(9):3214–20. doi: 10.1167/iovs.05-0294 . [DOI] [PubMed] [Google Scholar]
- 11.Rao HL, Zangwill LM, Weinreb RN, Leite MT, Sample PA, Medeiros FA. Structure-function relationship in glaucoma using spectral-domain optical coherence tomography. Archives of ophthalmology. 2011;129(7):864–71. doi: 10.1001/archophthalmol.2011.145 ; PubMed Central PMCID: PMC3855237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reus NJ, Lemij HG. Relationships between standard automated perimetry, HRT confocal scanning laser ophthalmoscopy, and GDx VCC scanning laser polarimetry. Investigative ophthalmology & visual science. 2005;46(11):4182–8. doi: 10.1167/iovs.04-1029 . [DOI] [PubMed] [Google Scholar]
- 13.Schlottmann PG, De Cilla S, Greenfield DS, Caprioli J, Garway-Heath DF. Relationship between visual field sensitivity and retinal nerve fiber layer thickness as measured by scanning laser polarimetry. Investigative ophthalmology & visual science. 2004;45(6):1823–9. . [DOI] [PubMed] [Google Scholar]
- 14.Russell RA, Malik R, Chauhan BC, Crabb DP, Garway-Heath DF. Improved estimates of visual field progression using bayesian linear regression to integrate structural information in patients with ocular hypertension. Investigative ophthalmology & visual science. 2012;53(6):2760–9. doi: 10.1167/iovs.11-7976 ; PubMed Central PMCID: PMC4632869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redmond T, Anderson RS, Russell RA, Garway-Heath DF. Relating retinal nerve fiber layer thickness and functional estimates of ganglion cell sampling density in healthy eyes and in early glaucoma. Investigative ophthalmology & visual science. 2013;54(3):2153–62. doi: 10.1167/iovs.12-10342 . [DOI] [PubMed] [Google Scholar]
- 16.Aptel F, Sayous R, Fortoul V, Beccat S, Denis P. Structure-function relationships using spectral-domain optical coherence tomography: comparison with scanning laser polarimetry. American journal of ophthalmology. 2010;150(6):825–33. doi: 10.1016/j.ajo.2010.06.011 . [DOI] [PubMed] [Google Scholar]
- 17.Wollstein G, Kagemann L, Bilonick RA, Ishikawa H, Folio LS, Gabriele ML, et al. Retinal nerve fibre layer and visual function loss in glaucoma: the tipping point. The British journal of ophthalmology. 2012;96(1):47–52. doi: 10.1136/bjo.2010.196907 ; PubMed Central PMCID: PMC3193885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Progress in retinal and eye research. 2007;26(6):688–710. doi: 10.1016/j.preteyeres.2007.08.001 ; PubMed Central PMCID: PMC2110881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hood DC, Anderson SC, Wall M, Raza AS, Kardon RH. A test of a linear model of glaucomatous structure-function loss reveals sources of variability in retinal nerve fiber and visual field measurements. Investigative ophthalmology & visual science. 2009;50(9):4254–66. doi: 10.1167/iovs.08-2697 ; PubMed Central PMCID: PMC3003847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Racette L, Medeiros FA, Bowd C, Zangwill LM, Weinreb RN, Sample PA. The impact of the perimetric measurement scale, sample composition, and statistical method on the structure-function relationship in glaucoma. Journal of glaucoma. 2007;16(8):676–84. doi: 10.1097/IJG.0b013e31804d23c2 . [DOI] [PubMed] [Google Scholar]
- 21.Marin-Franch I, Malik R, Crabb DP, Swanson WH. Choice of statistical method influences apparent association between structure and function in glaucoma. Investigative ophthalmology & visual science. 2013;54(6):4189–96. doi: 10.1167/iovs.12-10377 ; PubMed Central PMCID: PMC3687963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sample PA, Girkin CA, Zangwill LM, Jain S, Racette L, Becerra LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Archives of ophthalmology. 2009;127(9):1136–45. doi: 10.1001/archophthalmol.2009.187 ; PubMed Central PMCID: PMC2761830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swanson WH, Malinovsky VE, Dul MW, Malik R, Torbit JK, Sutton BM, et al. Contrast sensitivity perimetry and clinical measures of glaucomatous damage. Optometry and vision science: official publication of the American Academy of Optometry. 2014;91(11):1302–11. doi: 10.1097/OPX.0000000000000395 ; PubMed Central PMCID: PMC4243800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Racette L, Liebmann JM, Girkin CA, Zangwill LM, Jain S, Becerra LM, et al. African Descent and Glaucoma Evaluation Study (ADAGES): III. Ancestry differences in visual function in healthy eyes. Archives of ophthalmology. 2010;128(5):551–9. doi: 10.1001/archophthalmol.2010.58 ; PubMed Central PMCID: PMC2907156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garway-Heath DF, Caprioli J, Fitzke FW, Hitchings RA. Scaling the hill of vision: the physiological relationship between light sensitivity and ganglion cell numbers. Investigative ophthalmology & visual science. 2000;41(7):1774–82. . [PubMed] [Google Scholar]
- 26.R Development Core Team. R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 27.Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi medical journal: the journal of Medical Association of Malawi. 2012;24(3):69–71. ; PubMed Central PMCID: PMC3576830. [PMC free article] [PubMed] [Google Scholar]
- 28.Garway-Heath DF, Quartilho A, Prah P, Crabb DP, Cheng Q, Zhu H. Evaluation of Visual Field and Imaging Outcomes for Glaucoma Clinical Trials (An American Ophthalomological Society Thesis). Trans Am Ophthalmol Soc. 2017;115:T4 ; PubMed Central PMCID: PMCPMC5652981. [PMC free article] [PubMed] [Google Scholar]
- 29.Moore GPM D. S., and Craig B. A. Introduction to the Practice of Statistics. 7 ed. New York, NY: W.H. Freeman & Company; 2010. [Google Scholar]
- 30.Miglior S, Zeyen T, Pfeiffer N, Cunha-Vaz J, Torri V, Adamsons I, et al. Results of the European Glaucoma Prevention Study. Ophthalmology. 2005;112(3):366–75. doi: 10.1016/j.ophtha.2004.11.030 . [DOI] [PubMed] [Google Scholar]
- 31.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Archives of ophthalmology. 2002;120(6):701–13; discussion 829–30. . [DOI] [PubMed] [Google Scholar]
- 32.Warton DI, Wright IJ, Falster DS, Westoby M. Bivariate line-fitting methods for allometry. Biological reviews of the Cambridge Philosophical Society. 2006;81(2):259–91. doi: 10.1017/S1464793106007007 . [DOI] [PubMed] [Google Scholar]
- 33.Taskinen S, Warton DI. Robust tests for one or more allometric lines. J Theor Biol. 2013;333:38–46. doi: 10.1016/j.jtbi.2013.05.010 . [DOI] [PubMed] [Google Scholar]
- 34.Harwerth RS, Wheat JL, Rangaswamy NV. Age-related losses of retinal ganglion cells and axons. Investigative ophthalmology & visual science. 2008;49(10):4437–43. doi: 10.1167/iovs.08-1753 . [DOI] [PubMed] [Google Scholar]
- 35.Balazsi AG, Rootman J, Drance SM, Schulzer M, Douglas GR. The effect of age on the nerve fiber population of the human optic nerve. American journal of ophthalmology. 1984;97(6):760–6. . [DOI] [PubMed] [Google Scholar]
- 36.Blanks JC, Torigoe Y, Hinton DR, Blanks RH. Retinal pathology in Alzheimer's disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiology of aging. 1996;17(3):377–84. . [DOI] [PubMed] [Google Scholar]
- 37.Alamouti B, Funk J. Retinal thickness decreases with age: an OCT study. The British journal of ophthalmology. 2003;87(7):899–901. ; PubMed Central PMCID: PMC1771773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel NB, Lim M, Gajjar A, Evans KB, Harwerth RS. Age-associated changes in the retinal nerve fiber layer and optic nerve head. Investigative ophthalmology & visual science. 2014;55(8):5134–43. doi: 10.1167/iovs.14-14303 ; PubMed Central PMCID: PMC4137486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sung KR, Wollstein G, Bilonick RA, Townsend KA, Ishikawa H, Kagemann L, et al. Effects of age on optical coherence tomography measurements of healthy retinal nerve fiber layer, macula, and optic nerve head. Ophthalmology. 2009;116(6):1119–24. doi: 10.1016/j.ophtha.2009.01.004 ; PubMed Central PMCID: PMC2747246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Archives of ophthalmology. 2002;120(10):1268–79. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results are presented globally and in all sectors for healthy (triangles) and glaucomatous (circles) eyes. Correlations that were found to be significantly different from zero after Bonferroni correction are shown in red. Note that the range of the x-axes are different for the different datasets; we plotted the graph using the range observed in each dataset to highlight the differences between healthy and glaucomatous eyes in each dataset.
(TIFF)
Results are presented globally and in all sectors for healthy (triangles) and glaucomatous (circles) eyes. Correlations that were found to be significantly different from zero after Bonferroni correction are shown in red. Note that the range of the x-axes are different for the different datasets; we plotted the graph using the range observed in each dataset to highlight the differences between healthy and glaucomatous eyes in each dataset.
(TIFF)
Results are presented globally and in all sectors for healthy (triangles) and glaucomatous (circles) eyes. Slopes that were found to be significantly different from zero after Bonferroni correction are shown in red. Note that the range of the x-axes are different for the different datasets; we plotted the graph using the range observed in each dataset to highlight the differences between healthy and glaucomatous eyes in each dataset.
(TIFF)
Results are presented globally and in all sectors for healthy (triangles) and glaucomatous (circles) eyes. Slopes that were found to be significantly different from zero after Bonferroni correction are shown in red. Note that the range of the x-axes are different for the different datasets; we plotted the graph using the range observed in each dataset to highlight the differences between healthy and glaucomatous eyes in each dataset.
(TIFF)
Results are presented globally and in all sectors for healthy (triangles) and glaucomatous (circles) eyes. Slopes that were found to be significantly different from zero after Bonferroni correction are shown in red. Note that the range of the x-axes are different for the different datasets; we plotted the graph using the range observed in each dataset to highlight the differences between healthy and glaucomatous eyes in each dataset.
(TIFF)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.