Abstract
Background
Chromogranin A (CgA) is a plasma biomarker widely used in the follow-up of patients with neuroendocrine neoplasms (NENs). However, its accuracy as a tumor biomarker is relatively low because plasma CgA can increase also in patients with other diseases or in subjects treated with proton-pump inhibitors (PPIs), a class of widely-used drugs.
Methods
In the attempt to identify a more reliable biomarker for NENs, we investigated, by ELISA, the circulating levels of full-length CgA (CgA1-439) and of various CgA-derived fragments in 17 patients with ileal or pancreatic NENs, 10 healthy controls, and 21 healthy volunteers before and after treatment with PPIs.
Results
Patients with ileal or pancreatic NENs showed increased plasma levels of total-CgA and CgA1-76 fragment (vasostatin-1, VS-1) compared to controls [median (25th-75th-percentiles); total-CgA: 1.85 nM (1.01–4.28) vs 0.75 nM (0.52–0.89), p = 0.004; VS-1: 2.76 nM (1.09–7.10) vs 0.29 nM (0.26–0.32), p<0.001, respectively], but not of CgA1-439 or CgA1-373 fragment. VS-1 positively correlated with total-CgA (r = 0.65, p<0.001). The Receiver Operating Characteristic area under the curve was 0.9935 for VS-1 and 0.8824 for total-CgA (p = 0.067). Treatment of patients with somatostatin analogues decreased both total-CgA and VS-1. In contrast, administration of PPIs increased the plasma levels of total-CgA, but not of VS-1.
Conclusion
These findings suggest that plasma VS-1 is a novel biomarker for ileal and pancreatic NENs. Considering that VS-1 is a well-defined fragment not induced by proton-pump inhibitors, this polypeptide might represent a biomarker for NENs diagnosis and follow-up more accurate and easier to standardize than CgA.
Introduction
Human Chromogranin A (CgA), a 439-residue-long protein present in the secretory granules of many normal and neoplastic neuroendocrine cells, currently represents the main biomarker for neuroendocrine neoplasms (NENs) [1, 2]. CgA is exocytotically released in circulation, to reach approximately 0.5 nM levels in healthy subjects and up to 100–500–fold higher values in NENs patients [3–5]. Elevated levels of circulating CgA have been reported also for sub-populations of patients with non-small-cell lung cancer, prostate or breast cancer, or for patients with heart failure, renal failure, hypertension, rheumatoid arthritis, atrophic gastritis, liver disease, inflammatory bowel disease, sepsis and other inflammatory diseases [4–13]. Elevated levels of circulating CgA are present also in subjects treated with proton-pump inhibitors (PPIs), a class of drugs largely used in patients [14, 15]. Therefore, although plasma CgA is still widely used as a biomarker for NENs, its clinical utility is limited to prognostic stratification of patients with advanced disease [16, 17].
A further complication for the use of CgA as tumor biomarker is that CgA assays are difficult to standardize because this protein is a heterogeneous analyte due to extensive proteolytic processing and differential post-translational modifications [18–22]. The proteolytic processing of CgA can be triggered by intra-granular and/or extracellular proteases including prohormone convertase 1 and 2, furin, cathepsin L, plasmin and thrombin [23]. CgA, upon proteolysis, can give rise to several biologically active peptides, such as vasostatin-1 (VS-1, human CgA1-76), catestatin (human CgA352-372), pancreastatin (human CgA250-301), serpinin (CgA411-436) and other larger polypeptides consisting of CgA molecules lacking part or most of the C-terminal region (e.g. CgA1-373) [10, 24–27]. These peptides have been implicated in the regulation of vascular tension, angiogenesis, endothelial-barrier function, cardiovascular function, inflammation, gastrointestinal motility and glucose and calcium metabolism [3]. Regarding angiogenesis, it has been recently proposed that CgA and its fragments can form a balance of pro- and anti-angiogenic factors. For example, while the full-length CgA (hereinafter CgA1-439) and VS-1 can inhibit angiogenesis, the CgA1-373 fragment can promote angiogenesis [20, 28].
In the attempt to identify a more reliable biomarker for NENs we investigated the circulating levels of CgA1-439 and of the anti- and pro-angiogenic fragments VS-1 and CgA1-373 in patients affected with ileal or pancreatic NENs, before and after therapy with somatostatin analogues (SSAs), and in healthy volunteers, before and after administration of PPIs. Plasma levels of each polypeptide were measured using specific ELISAs. Furthermore, considering that most assays typically used for CgA detection can detect mixtures of full-length CgA and fragments [23], we also used an ELISA with a broader specificity, based on antibodies capable of detecting full-length CgA and fragments lacking the C-terminal region and larger than VS-1 (here defined as “total-CgA”), thus unable to detect VS-1.
Materials and methods
Plasma samples collection
We evaluated blood samples from 17 patients with pancreatic or ileal NENs diagnosed between 1996 and 2012 at IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy. We included only patients with G1/G2 neoplasms since G3 neoplasms are frequently poorly differentiated and therefore may lose the ability to secrete CgA [29, 30]. Blood samples were collected at diagnosis and at follow-up visit after administration of Octreotide LAR.
Blood samples were also collected from 10 healthy donors and 21 healthy volunteers before and after therapy with oral pantoprazole (40 mg/day, 14 days). Of the 21 healthy volunteers, 16 (76.2%) were females and 5 (23.8%) were males. 19 subjects were Caucasians (90.5%) and 2 subjects (9.5%) were Hispanics. Median age was 52.0 years.
Blood was collected in EDTA-containing tubes and plasma was obtained immediately by centrifuging each sample (2000g for 15 min, room temperature). The plasma samples were then stored at < -20°C until assay.
Data collection
Diagnosis of NENs was confirmed by conventional histology and immunohistochemical studies (CgA, synaptophysin, neuron-specific enolase).
All patients were classified according to primary tumor sites (ileus or pancreas), and WHO-2010 Classification [31].
All the 31 healthy subjects underwent a brief interview in order to collect demographic data and relevant clinical information and to enlist only subjects without comorbidities that might affect CgA plasma levels. We excluded subjects with kidney disease (Glomerular Filtration Rate < 60 ml/min), history of neoplasia, liver failure (Child-Pugh score > 4), inflammatory diseases (inflammatory bowel disease, rheumatoid arthritis), chronic atrophic gastritis, PPIs therapy in the last 30 days or uncontrolled hypertension (BP > 140/90 mmHg). Written informed consent was obtained from all participants and the study was approved by the San Raffaele Ethics Committee (NTBK001).
Reagents
Mouse monoclonal antibodies (mAbs) 5A8 and B4E11 (epitope: CgA53-57 and CgA68-71, respectively), rabbit polyclonal antisera αVS-I(76), αCgA(373), αCgA(439), raised against the peptide CgA71-76, CgA369-373, and CgA435-439, respectively and rabbit polyclonal antiserum αCgA(FRs), raised against recombinant human CgA, were produced and characterized as described previously[19, 32, 33].
Sandwich ELISAs
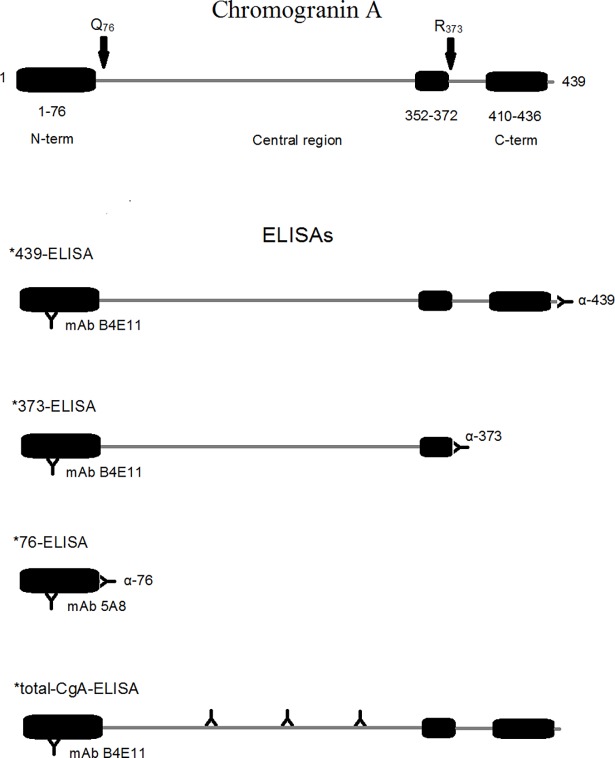
CgA and CgA fragments in plasma samples were detected using four sandwich ELISAs, called *76, *373, *439, and total-CgA ELISA (see Fig 1 for a schematic representation of each assay and the antibodies used) previously described [19].
Fig 1. Schematic representation of the ELISAs used to detect CgA and its fragments.
Upper panel—Schematic representation of human full-length CgA (439-residues long, CgA1-439): Q76 and R373 cleavage sites are indicated; vasostatin-1 (CgA1-76, VS-1), catestatin (CgA352-372) and serpinin regions (CgA 410–436) are boxed. Lower panel—Schematic representation of the *439-, *373-, *76-, and *total-CgA-ELISAs (sandwich ELISA). The epitope location of mAb B4E11 and 5A8 (capturing antibodies), and α439, α373, α76 and αFRs (detecting antibodies) is shown. The rational for using different capturing antibodies (mAb B4E11 and mAb 5A8) in the different assays relies on the fact that, in the case of *76-ELISA, mAb B4E11 and α76 cannot form molecular sandwiches, because of steric hindrance. *439-ELISA can detect molecules with N-terminal domain and intact C-terminal region (i.e. “full-length CgA”), but not fragments; *373-ELISA can selectively detect CgA1-373, but not CgA1-439; *76-ELISA can selectively detect CgA1-76, but not the other fragments; total-CgA-ELISA can detect full-length CgA plus fragments containing the N-terminal and all or part of the central and C-terminal regions (FRs) including CgA1-373, but not CgA1-76.
Assay validation experiments [19, 20] showed that these ELISAs can selectively detect: (1) the N-terminal fragment CgA1-76 (VS-1) (*76−ELISA); (2) the fragment CgA1-373 (*373–ELISA); (3) full-length CgA (*439–ELISA); (4) full-length CgA plus fragments containing the N-terminal and all or part of the central and C-terminal regions, including CgA1-373 (hereinafter called “total-CgA”−ELISA). Of note, this assay cannot detect CgA1-76. Intra- and inter-assay coefficient of variation (CV), limits of detection and quantification and specificity of each ELISA are shown in S1 Table.
Statistical analysis
Statistical analysis was performed using Stata, version 11.1 (StataCorp, College Station, TX, USA). Normality of distribution of analyzed variables was evaluated with visual inspection, Shapiro-Wilk normality test, and Skewness-Kurtosis test. Continuous variables were reported as mean and SD if normally distributed, otherwise they were reported as median and interquartile range (IQR). A p<0.05 was considered significant in all tests performed. In case-control analysis, CgA variables and rates were not normally distributed and were compared with the Wilcoxon rank-sum (Mann-Whitney) test. Descriptive data (median, IQR, min, max) were reported in the original units of measure or as true rates.
Since variables and rates were log-normally distributed, a parallel analysis with correction for age could be run, in which variables were previously transformed into their logarithm (± a constant minimizing skewness) and analyzed with analysis of covariance (ANCOVA).
Differences between post-SSAs therapy and pre-SSAs therapy variables (“deltas”) were not normally distributed and were compared with the Wilcoxon signed-rank test. No convenient transformation of the “delta” variables to make them normally distributed was found.
Results
Sample description
The characteristics of patients and the classification according to WHO-2010 are listed in Table 1. Of note, none of the patients was taking PPIs.
Table 1. Sample description (NENs patient characteristics).
| Site of Origin | Total | ||
|---|---|---|---|
| Ileus | Pancreas | ||
| Age: mean±SD, (years) | 60.95±12.17 | 61.74±14.12 | 61.28±12.58 |
| n (%) | n (%) | n (%) | |
| Sex | |||
| Male | 5 (50) | 0 (0) | 5 (29.4) |
| Female | 5 (50) | 7 (100) | 12 (70.6) |
| Total | 10 (100) | 7 (100) | 17 (100) |
| WHO-2010 | |||
| Grade 1 | 8 (80) | 3 (42.8) | 11 (64.7) |
| Grade 2 | 2 (20) | 4 (57.2) | 6 (35.3) |
| Total | 10 (100) | 7 (100) | 17 (100) |
| Functioning NENs | |||
| Carcinoid syndrome | 9 (90) | 0 (0) | 9 (52.9) |
| Verner-Morrison | 0 (0) | 1 (14.3) | 1 (5.9) |
| Non-functioning NENs | 1 (10) | 6 (85.7) | 7 (41.2) |
| Total | 10 (100) | 7 (100) | 17 (100) |
| Comorbidity | |||
| MEN-1a) | 0 (0) | 0 (0) | 0 (0) |
| Hypertension | 5 (50) | 3 (42.8) | 8 (47) |
| Atrophic Gastritis | 0 (0) | 0 (0) | 0 (0) |
| Liver Failure | 1 (10) | 0 (0) | 1 (5.9) |
| Other neoplasms | 0 (0) | 0 (0) | 0 (0) |
| Chronic Kidney Disease | 0 (0) | 0 (0%) | 0 (0%) |
| No comorbidity | 4 (40) | 4 (57.2) | 8 (47.1) |
| Total | 10 (100) | 7 (100) | 17 (100) |
| Treatment | |||
| Octreotide LARb) | 9 (90) | 5 (71.4) | 14 (82.3) |
a) Multiple Endocrine Neoplasia type 1
b) Somatostatin analogues (SSAs)
The circulating levels of total-CgA and VS-1 are increased in patients with ileal or pancreatic NENs
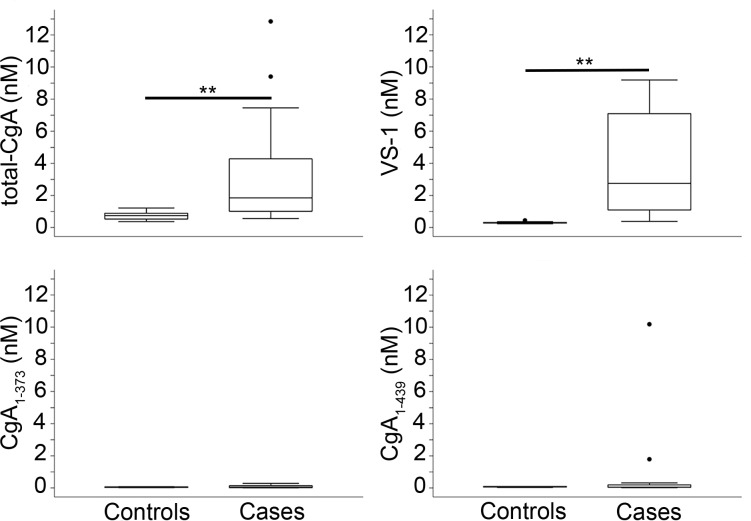
The plasma samples of 17 patients with ileal or pancreatic NENs and 10 healthy subjects (controls) were analyzed using ELISAs specific for CgA1-439, VS-1, and CgA1-373, and an ELISA capable of detecting mixtures of full-length CgA and fragments lacking the C-terminal region but unable to detect VS-1 (total-CgA) [19, 20]. The results (Fig 2) show that the median values of total-CgA and VS-1 were increased in patients compared to controls [median (25th-75th percentiles), total CgA: 1.848 nM (1.010–4.276) vs 0.752 nM (0.522–0.899), p = 0.004; VS-1: 2.757 nM (1.093–7.098) vs 0.288 nM (0.263–0.315), p<0.001, respectively]. In contrast, no difference was found regarding CgA1-439 and CgA1-373. Of note, the fold-increase of VS-1 in patients was markedly higher than that of total-CgA (9.5– vs 2.4–fold, respectively).
Fig 2. Plasma levels of CgA and its fragments in patients with ileal and pancreatic NENs (cases) and healthy subjects (controls).
Box-plots with median (middle line), 75th percentile (top line) and 25th percentile (bottom line). The top and bottom whiskers represent the upper and lower adjacent values, respectively. Values outside the whiskers are plotted individually (circles). Cases (n = 17); controls (n = 10). **(p < 0.01), by analysis of covariance.
The N-terminal proteolytic processing of CgA is increased in patients with ileal or pancreatic NENs
To evaluate whether the increased levels of circulating VS-1 in NENs was the result of increased CgA cleavage at the N-terminal region, or was simply related to increased CgA secretion, we analyzed the ratio of VS-1 and other fragments with total-CgA. Interestingly, we observed an increase of VS-1/total-CgA and a decrease of CgA1-373/total-CgA ratio in patients, compared to controls (p = 0.005 and p = 0.045, respectively) (Fig 3) (S2 Table). These data support the hypothesis that the proteolytic processing of CgA is altered in NENs favoring the production of the N-terminal fragment VS-1.
Fig 3. Levels of VS-1, CgA1-373 and CgA1-439 relative to the total-CgA in the plasma of patients with ileal and pancreatic NENs (cases) and healthy subjects (controls).
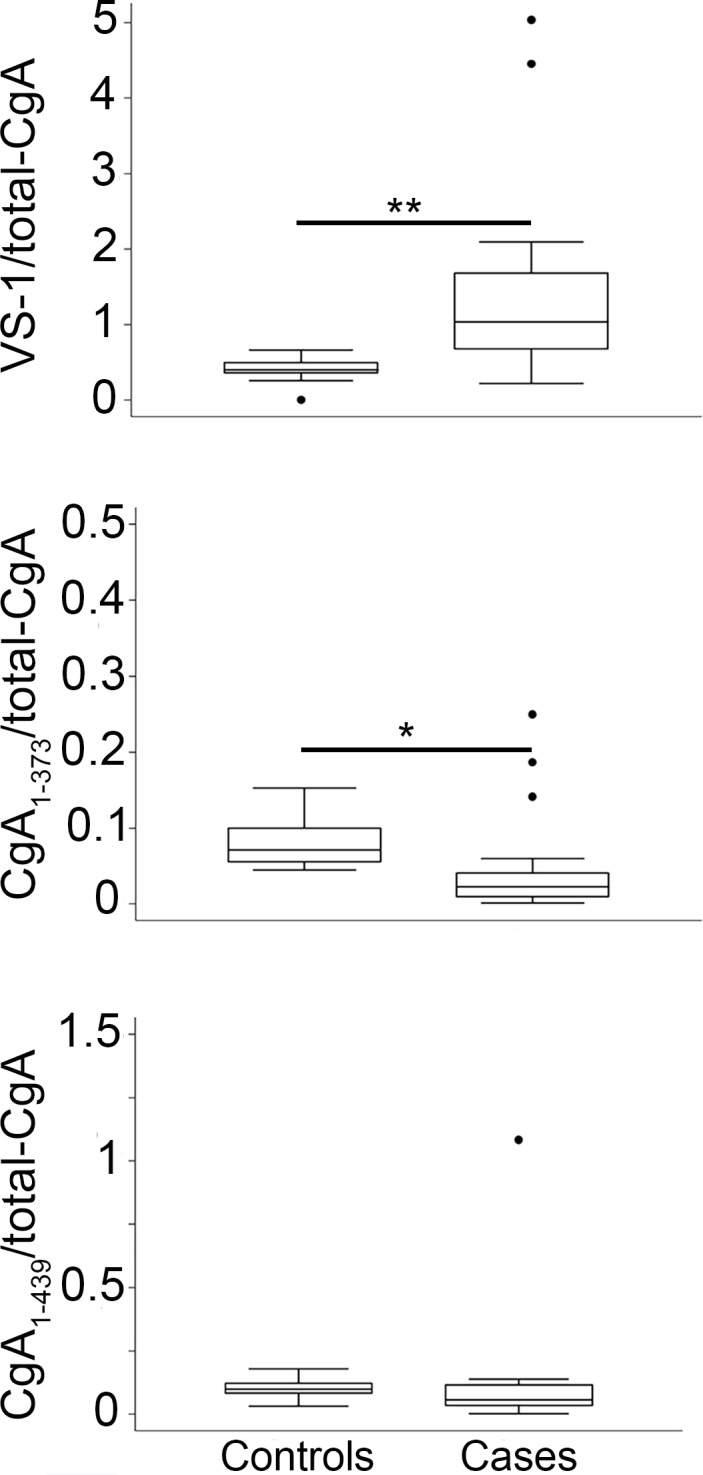
Box-plots with median (middle line), 75th percentile (top line) and 25th percentile (bottom line). The top and bottom whiskers represent the upper and lower adjacent values, respectively. Values outside the whiskers are plotted individually (circles). Cases (n = 17); controls (n = 10). * (p < 0.05), **(p < 0.01), by analysis of covariance. As total-CgA does not include VS-1, VS-1/total-CgA ratio can be also greater than 1.
The CgA processing is similar in ileal and pancreatic NENs
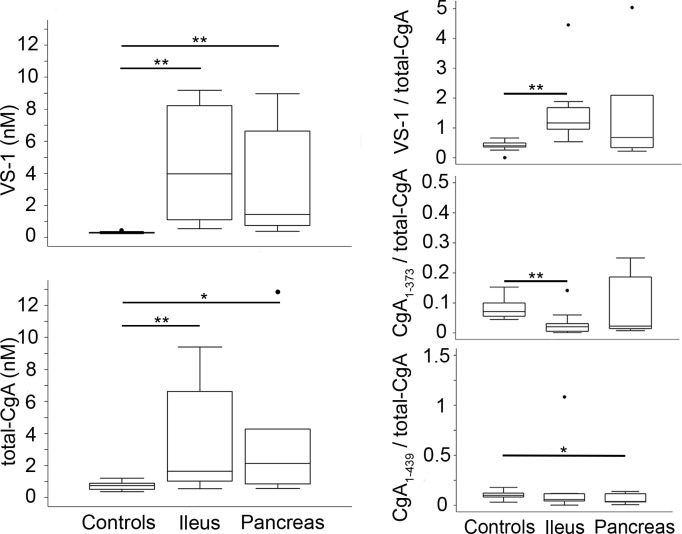
The importance of tumor location on CgA production and cleavage was then investigated. Median total-CgA and VS-1 were significantly higher both in ileal and pancreatic cases compared to controls [ileal vs control, total-CgA: 1.655 nM (1.010–6.624) vs 0.752 nM (0.522–0.899), p = 0.009; VS-1: 3.966 nM (1.113–8.225) vs 0.288 nM (0.263–0.315), p<0.001] [pancreatic vs control, total-CgA: 2.129 nM (0.845–4.277) vs 0.753 nM (0.522–0.899), p = 0.014; VS-1: 1.433 nM (0.751–6.633) vs 0.288 nM (0.263–0.315), p<0.001] (Fig 4). VS-1/total-CgA was significantly increased in ileal but not in pancreatic NENs (p<0.001; p = 0.057, respectively). CgA1-373/total-CgA and CgA1-439/total-CgA were significantly lower in ileal and in pancreatic NENs, respectively (p = 0.007 and p = 0.037) (Fig 4). Furthermore, no differences in total-CgA, CgA1-439, CgA1-373, VS-1, CgA1-439/total-CgA, CgA1-373/total-CgA, VS-1/total-CgA, CgA1-373/CgA1-439 and CgA1-373/CgA1-76 emerged in the two groups (Fig 4, S2 Table). These results suggest that the proteolytic processing of CgA at the N-terminal region is increased in both ileal and pancreatic NENs when compared with healthy subjects.
Fig 4. Plasma levels of VS-1 and total-CgA, VS-1/total-CgA, CgA1-373/total-CgA, and CgA1-439/total-CgA ratios (relative levels) in healthy subjects and patients with ileal and pancreatic NENs (case-control analysis by site).
Box-plots with median (middle line), 75th percentile (top line) and 25th percentile (bottom line). The top and bottom whiskers represent the upper and lower adjacent values, respectively. Values outside the whiskers are plotted individually (circles). Ileal NENs (n = 10); pancreatic NENs (n = 7); controls (n = 10). * (p < 0.05), **(p < 0.01), by analysis of covariance.
Correlation and ROC curve analysis of total-CgA and VS-1
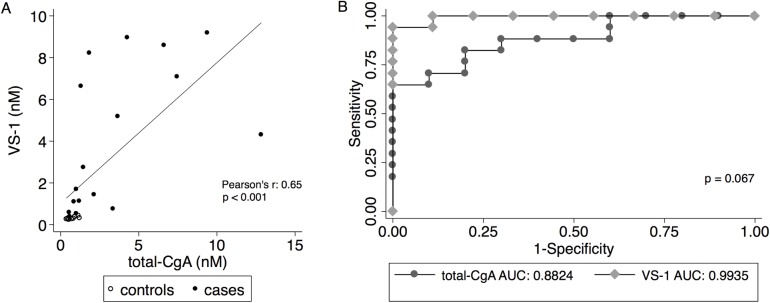
To compare the capability of total-CgA and VS-1 to discriminate between patients and normal subjects we performed correlation and receiver operating characteristic (ROC) analysis. We observed a significant positive correlation between total-CgA and VS-1 (r = 0.65, p<0.001; Fig 5A). ROC analysis showed that the global performance (i.e. area under the curve) of VS-1 was 0.9935, whereas that of total-CgA was 0.8824 (p = 0.067; Fig 5B). The best cut-off for VS-1 was 0.442 nM, which correctly classified 96.1% of our patients, with a sensitivity of 94.1% and a specificity of 100%. The best cut-off for total-CgA was 0.899 nM, which correctly classified 81.5% of patients, with a sensitivity of 82.3% and a specificity of 80%. Notably, some patients had low levels of total-CgA but high levels of VS-1, whereas all healthy subjects had low levels of circulating VS-1 (Fig 5A and 5B). These results, overall, suggest that plasma VS-1 might represent an accurate biomarker for NENs comparable or possibly better than CgA.
Fig 5. Correlation and receiver operating characteristic (ROC) curve of VS-1 and total-CgA.
A) Correlation between the plasma levels of VS-1 and total-CgA in patients with NENs (solid circles) and healthy subjects (hollow circles). B) ROC curve of VS-1 and total-CgA.
Somatostatin analogues affect both CgA and VS-1
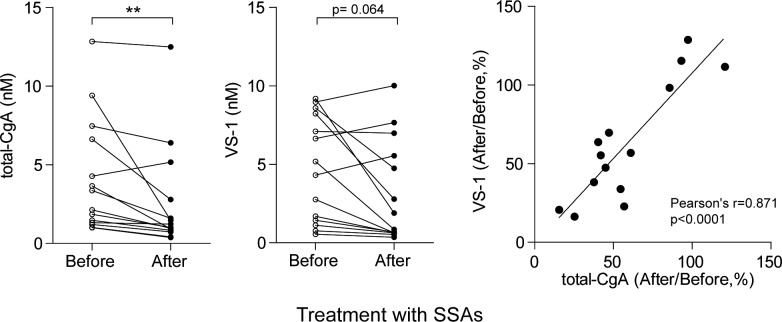
To provide further evidence that VS-1 can be exploited as a marker for pancreatic and ileal NENs follow-up, we then analyzed the effect of therapy with SSAs on VS-1 and total-CgA plasma levels in 14 patients untreated with PPIs. This treatment significantly reduced total-CgA (p<0.01). Furthermore, a not statistically significant (p = 0.064) decrease of VS-1 was also observed (Fig 6A and 6B). No change was observed for CgA1-439 or CgA1-373 (S3 Table). Interestingly, patients showing reduced levels of CgA also showed reduced levels of VS-1, as indicated by a good correlation between the % decrease of the plasma levels of both analytes after therapy (r = 0.871, p<0.0001; Fig 6C). Accordingly, no significant change of VS-1/total-CgA after therapy was observed (S3 Table). Moreover, no difference emerged between ileal and pancreatic NENs.
Fig 6. Effect of therapy with somatostatin analogues (SSAs) on the plasma levels of total-CgA and VS-1 in patients with ileal and pancreatic NENs.
(A, B) Plasma levels of total-CgA and VS-1 in NENs cases (ileal plus pancreatic NENs) before and after the administration of octreotide LAR. ** (p < 0.01), by Wilcoxon signed-rank test. (C) Correlation between the decrease (%) of the plasma levels of VS-1 and total-CgA after therapy.
These data suggest that VS-1, like CgA, responds to therapy with SSAs.
Proton-pump inhibitors increase the plasma levels of total-CgA but not of VS-1
To assess whether PPIs can differentially induce CgA and VS-1 in circulation, we administered pantoprazole (40 mg/day) to 21 healthy volunteers for 14 consecutive days. This treatment caused a marked increase in circulating total-CgA from 0.583 nM (0.466–1.058) to 2.958 nM (2.001–4.576) [median (25th-75th percentiles), p<0.0001]. In contrast, no change in the levels of VS-1 was observed [mean (SD): from 0.244 nM (0.088) to 0.222 nM (0.081), p = 0.4] (Fig 7). These data suggest that PPIs can increase the circulating levels of CgA, in line with current literature, but not those of VS-1, suggesting that VS-1 is a biomarker more accurate and tumor-specific than CgA.
Fig 7. Effect of proton pump inhibitors on the plasma levels of total-CgA, VS-1, CgA1-439 and CgA1-373.
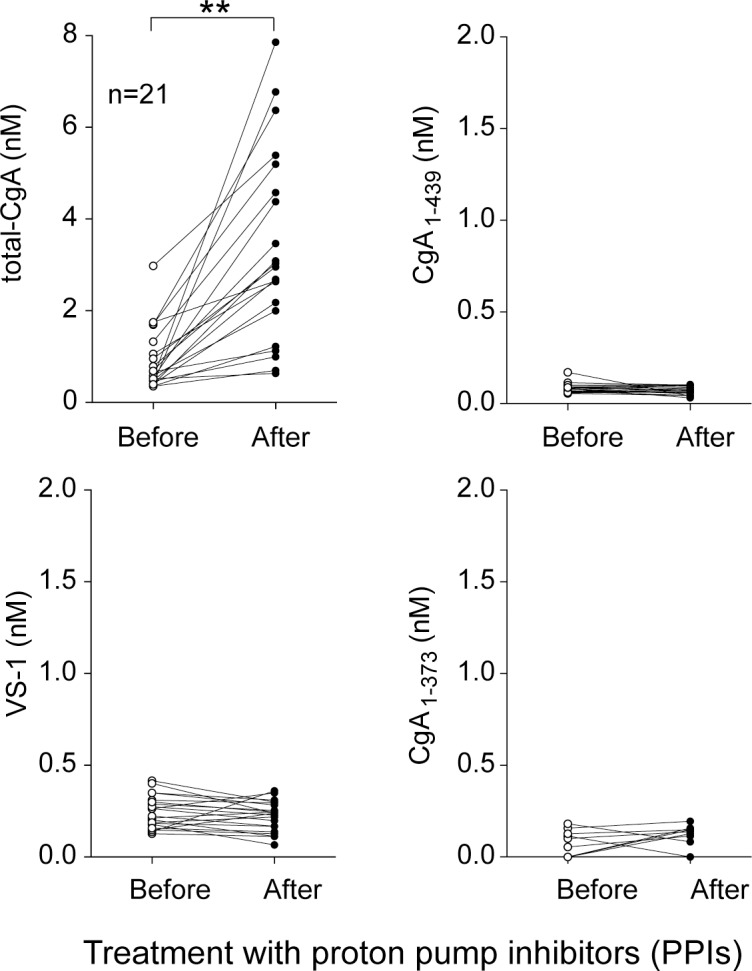
**(p < 0.01), by two-tailed paired t-test.
Discussion
This study shows that plasma VS-1 is significantly increased in patients with pancreatic or ileal NENs compared to healthy subjects (5-fold and 14-fold, respectively). The observation that VS-1, total-CgA, and VS-1/total-CgA ratio (i.e. absolute and relative values of VS-1) were higher in NENs patients suggests that the increase of VS-1 plasma levels reflects an increased production of CgA as well as an increased cleavage at its N-terminal region. No significant difference was observed in patients with ileal or pancreatic NENs, suggesting that VS-1 cleavage occurs in both types of tumor tissues. Remarkably, the results also show that circulating VS-1, unlike total-CgA, did not increase after PPI administration in healthy volunteers. These data suggest that VS-1 represents a novel biomarker for ileal and pancreatic NENs. No significant difference was observed with CgA1-439 and CgA1-373.
It is well-known that the use of CgA as a NENs biomarker has several limitations. Firstly, many other pathologies, such as heart failure, renal failure, hypertension and various inflammatory diseases, can lead to an increase in its concentration [16]. Secondly, circulating CgA is increased after administration of PPIs, as a consequence to the fact that these drugs induce CgA-positive enterochromaffin-like cell hyperplasia [14, 15]. Thirdly, CgA is a highly heterogeneous analyte made by complex mixtures of full-length protein and fragments with different post-translational modifications [3]. Considering that most commercially available tests used for CgA quantification can detect full-length CgA and fragments (like our total-CgA-ELISA), these assays are difficult to standardize and can produce different results depending on antibody epitopes [23].
The observation that VS-1 is increased in NENs patients, but, unlike CgA, not in subjects treated with PPIs, and the notion that VS-1 is a well-defined analyte with no other post-translational modifications (thus easier to standardize than CgA), suggest that this fragment might represent an alternative or complementary biomarker for NENs diagnosis and/or follow up.
Interestingly, some patients with total-CgA close to normal values had abnormal levels of VS-1. Furthermore, the results of ROC analysis showed that VS-1 is as accurate, or even more, than CgA in discriminating patients and controls. These data, overall, suggest that this fragment might be more sensitive and specific than CgA as a NENs biomarker. Further studies on a larger sample of patients are needed to assess this hypothesis. Furthermore, other studies are necessary to assess whether comorbidities known to increase CgA levels can also increase the levels of circulating VS-1 or not. Interestingly, increased circulating levels of VS-1 have been observed so far in critically ill patients [34] and in patients with Takayasu arteritis [35], indicating that abnormal levels of VS-1 are not limited to NENs. However, it is important to note that, in critically ill patients, the VS-1 median value was 1.4-fold higher than that of healthy controls [34], an increase that is much lower than the 9.5-fold increase observed in our cohort of patients with NENs.
The observation that PPIs increase total-CgA levels, but not VS-1 levels, may suggest that enterochromaffin-like cells, which are thought to be the main source of PPI-induced CgA, do not produce the protease(s) responsible for CgA cleavage at its N-terminal domain. In contrast, ileal and pancreatic NENs tissues, which are expected to be the main source of the increased circulating CgA in patients, likely produce these proteases, leading to intra- and/or extra-cellular processing of CgA at its N-terminal domain, thereby increasing VS-1 production.
The fact that VS-1 and total-CgA are increased in patients with NENs raises the question as to whether these polypeptides are simply epiphenomena of the disease or they are somehow involved in the regulation of the tumor biology. Interestingly, previous studies have shown different expression of CgA in primary and metastatic cell lines [36]. Different levels of VS-1 and other CgA fragments have been also observed by immuno-histochemical and biochemical analysis of NENs from different sites [37–39]. Although VS-1 and CgA have been implicated as possible players in the regulation of angiogenesis and cell proliferation [20, 36, 40, 41], it is difficult to speculate whether the enhanced proteolytic processing of CgA in patients is a favorable or detrimental process, considering that the presence of VS-1 likely implicates the presence of other fragments, not tested in the present study, which might contribute to regulate tumor growth in an unpredictable manner. Further assays have to be developed to detect other CgA-derived peptides and to evaluate their implications in tumor biology.
In conclusion, the results of the present study suggest that the plasma levels of VS-1 are markedly increased in patients with ileal and pancreatic NENs and that this polypeptide, not affected by PPI therapy, might represent a novel biochemical marker for these NENs, easier to standardize and more accurate than CgA. Studies with a larger cohort of patients are necessary to assess how comorbidities might interfere with proteolytic processing of CgA, to evaluate the accuracy of VS-1 as an alternative or complementary NENs biomarker, and to assess its prognostic value.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
This work was supported by grants from Associazione Italiana Ricerca sul Cancro (Grant No. AIRC: IG-14338 and IG-9965 to A.C.) and from Italfarmaco SpA.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC, Special Program Molecular Clinical Oncology 5x1000-9965, AIRC: IG-14338, IG-9965, and IG-19220) and from Italfarmaco SpA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Oberg K. Biochemical diagnosis of neuroendocrine GEP tumor. The Yale journal of biology and medicine. 1997;70(5–6):501–8. Epub 1998/11/24. ; PubMed Central PMCID: PMCPMC2589273. [PMC free article] [PubMed] [Google Scholar]
- 2.Tomassetti P, Migliori M, Simoni P, Casadei R, De Iasio R, Corinaldesi R, et al. Diagnostic value of plasma chromogranin A in neuroendocrine tumours. European journal of gastroenterology & hepatology. 2001;13(1):55–8. Epub 2001/02/24. . [DOI] [PubMed] [Google Scholar]
- 3.Helle KB, Corti A, Metz-Boutigue MH, Tota B. The endocrine role for chromogranin A: a prohormone for peptides with regulatory properties. Cellular and molecular life sciences: CMLS. 2007;64(22):2863–86. Epub 2007/08/25. doi: 10.1007/s00018-007-7254-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corti A. Chromogranin A and the tumor microenvironment. Cellular and molecular neurobiology. 2010;30(8):1163–70. Epub 2010/11/17. doi: 10.1007/s10571-010-9587-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Connor DT, Deftos LJ. Secretion of chromogranin A by peptide-producing endocrine neoplasms. The New England journal of medicine. 1986;314(18):1145–51. Epub 1986/05/01. doi: 10.1056/NEJM198605013141803 . [DOI] [PubMed] [Google Scholar]
- 6.Corti A, Gasparri A, Chen FX, Pelagi M, Brandazza A, Sidoli A, et al. Characterisation of circulating chromogranin A in human cancer patients. British journal of cancer. 1996;73(8):924–32. Epub 1996/04/01. ; PubMed Central PMCID: PMCPMC2075816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregorc V, Spreafico A, Floriani I, Colombo B, Ludovini V, Pistola L, et al. Prognostic value of circulating chromogranin A and soluble tumor necrosis factor receptors in advanced nonsmall cell lung cancer. Cancer. 2007;110(4):845–53. Epub 2007/06/30. doi: 10.1002/cncr.22856 . [DOI] [PubMed] [Google Scholar]
- 8.Ceconi C, Ferrari R, Bachetti T, Opasich C, Volterrani M, Colombo B, et al. Chromogranin A in heart failure; a novel neurohumoral factor and a predictor for mortality. European heart journal. 2002;23(12):967–74. Epub 2002/06/19. doi: 10.1053/euhj.2001.2977 . [DOI] [PubMed] [Google Scholar]
- 9.Corti A, Ferrari R, Ceconi C. Chromogranin A and tumor necrosis factor-alpha (TNF) in chronic heart failure. Advances in experimental medicine and biology. 2000;482:351–9. Epub 2001/02/24. doi: 10.1007/0-306-46837-9_28 . [DOI] [PubMed] [Google Scholar]
- 10.Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. The New England journal of medicine. 2003;348(12):1134–49. Epub 2003/03/21. doi: 10.1056/NEJMra021405 . [DOI] [PubMed] [Google Scholar]
- 11.Ziegler MG, Kennedy B, Morrissey E, O'Connor DT. Norepinephrine clearance, chromogranin A and dopamine beta hydroxylase in renal failure. Kidney international. 1990;37(5):1357–62. Epub 1990/05/01. . [DOI] [PubMed] [Google Scholar]
- 12.Sciola V, Massironi S, Conte D, Caprioli F, Ferrero S, Ciafardini C, et al. Plasma chromogranin a in patients with inflammatory bowel disease. Inflammatory bowel diseases. 2009;15(6):867–71. Epub 2008/12/19. doi: 10.1002/ibd.20851 . [DOI] [PubMed] [Google Scholar]
- 13.Massironi S, Fraquelli M, Paggi S, Sangiovanni A, Conte D, Sciola V, et al. Chromogranin A levels in chronic liver disease and hepatocellular carcinoma. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2009;41(1):31–5. Epub 2008/09/03. doi: 10.1016/j.dld.2008.05.002 . [DOI] [PubMed] [Google Scholar]
- 14.Giusti M, Sidoti M, Augeri C, Rabitti C, Minuto F. Effect of short-term treatment with low dosages of the proton-pump inhibitor omeprazole on serum chromogranin A levels in man. European journal of endocrinology. 2004;150(3):299–303. Epub 2004/03/12. . [DOI] [PubMed] [Google Scholar]
- 15.Pregun I, Herszenyi L, Juhasz M, Miheller P, Hritz I, Patocs A, et al. Effect of proton-pump inhibitor therapy on serum chromogranin a level. Digestion. 2011;84(1):22–8. Epub 2011/02/10. doi: 10.1159/000321535 . [DOI] [PubMed] [Google Scholar]
- 16.Gut P, Czarnywojtek A, Fischbach J, Baczyk M, Ziemnicka K, Wrotkowska E, et al. Chromogranin A—unspecific neuroendocrine marker. Clinical utility and potential diagnostic pitfalls. Archives of medical science: AMS. 2016;12(1):1–9. Epub 2016/03/01. doi: 10.5114/aoms.2016.57577 ; PubMed Central PMCID: PMCPMC4754364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marotta V, Zatelli MC, Sciammarella C, Ambrosio MR, Bondanelli M, Colao AAL, et al. Chromogranin A as circulating marker for diagnosis and management of neuroendocrine neoplasms: more flaws than fame. Endocrine-related cancer. 2017. Epub 2017/10/27. doi: 10.1530/ERC-17-0269 . [DOI] [PubMed] [Google Scholar]
- 18.Colombo B, Longhi R, Marinzi C, Magni F, Cattaneo A, Yoo SH, et al. Cleavage of chromogranin A N-terminal domain by plasmin provides a new mechanism for regulating cell adhesion. The Journal of biological chemistry. 2002;277(48):45911–9. Epub 2002/09/26. doi: 10.1074/jbc.M202637200 . [DOI] [PubMed] [Google Scholar]
- 19.Crippa L, Bianco M, Colombo B, Gasparri AM, Ferrero E, Loh YP, et al. A new chromogranin A-dependent angiogenic switch activated by thrombin. Blood. 2013;121(2):392–402. Epub 2012/11/30. doi: 10.1182/blood-2012-05-430314 ; PubMed Central PMCID: PMCPMC3544118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bianco M, Gasparri AM, Colombo B, Curnis F, Girlanda S, Ponzoni M, et al. Chromogranin A Is Preferentially Cleaved into Proangiogenic Peptides in the Bone Marrow of Multiple Myeloma Patients. Cancer research. 2016;76(7):1781–91. Epub 2016/02/13. doi: 10.1158/0008-5472.CAN-15-1637 . [DOI] [PubMed] [Google Scholar]
- 21.Loh YP, Cheng Y, Mahata SK, Corti A, Tota B. Chromogranin A and derived peptides in health and disease. Journal of molecular neuroscience: MN. 2012;48(2):347–56. Epub 2012/03/06. doi: 10.1007/s12031-012-9728-2 ; PubMed Central PMCID: PMCPMC3402615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gadroy P, Stridsberg M, Capon C, Michalski JC, Strub JM, Van Dorsselaer A, et al. Phosphorylation and O-glycosylation sites of human chromogranin A (CGA79-439) from urine of patients with carcinoid tumors. The Journal of biological chemistry. 1998;273(51):34087–97. Epub 1998/12/16. . [DOI] [PubMed] [Google Scholar]
- 23.Corti A, Marcucci F, Bachetti T. Circulating chromogranin A and its fragments as diagnostic and prognostic disease markers. Pflugers Archiv: European journal of physiology. 2017. Epub 2017/10/12. doi: 10.1007/s00424-017-2030-y . [DOI] [PubMed] [Google Scholar]
- 24.Aardal S, Helle KB. The vasoinhibitory activity of bovine chromogranin A fragment (vasostatin) and its independence of extracellular calcium in isolated segments of human blood vessels. Regulatory peptides. 1992;41(1):9–18. Epub 1992/09/03. . [DOI] [PubMed] [Google Scholar]
- 25.Mahata SK, O'Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, et al. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. The Journal of clinical investigation. 1997;100(6):1623–33. Epub 1997/09/18. doi: 10.1172/JCI119686 ; PubMed Central PMCID: PMCPMC508344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tatemoto K, Efendic S, Mutt V, Makk G, Feistner GJ, Barchas JD. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature. 1986;324(6096):476–8. Epub 1986/12/04. doi: 10.1038/324476a0 . [DOI] [PubMed] [Google Scholar]
- 27.Koshimizu H, Cawley NX, Kim T, Yergey AL, Loh YP. Serpinin: a novel chromogranin A-derived, secreted peptide up-regulates protease nexin-1 expression and granule biogenesis in endocrine cells. Mol Endocrinol. 2011;25(5):732–44. Epub 2011/03/26. doi: 10.1210/me.2010-0124 ; PubMed Central PMCID: PMCPMC3082324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theurl M, Schgoer W, Albrecht K, Jeschke J, Egger M, Beer AG, et al. The neuropeptide catestatin acts as a novel angiogenic cytokine via a basic fibroblast growth factor-dependent mechanism. Circulation research. 2010;107(11):1326–35. Epub 2010/10/12. doi: 10.1161/CIRCRESAHA.110.219493 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stridsberg M, Oberg K, Li Q, Engstrom U, Lundqvist G. Measurements of chromogranin A, chromogranin B (secretogranin I), chromogranin C (secretogranin II) and pancreastatin in plasma and urine from patients with carcinoid tumours and endocrine pancreatic tumours. The Journal of endocrinology. 1995;144(1):49–59. Epub 1995/01/01. . [DOI] [PubMed] [Google Scholar]
- 30.Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A—biological function and clinical utility in neuro endocrine tumor disease. Annals of surgical oncology. 2010;17(9):2427–43. Epub 2010/03/11. doi: 10.1245/s10434-010-1006-3 . [DOI] [PubMed] [Google Scholar]
- 31.Bosman FH, R. WHO Classification of Tumours of the Digestive System IARC. Lyon: 2010. IARC Press; 2010. [Google Scholar]
- 32.Corti A, Longhi R, Gasparri A, Chen F, Pelagi M, Siccardi AG. Antigenic regions of human chromogranin A and their topographic relationships with structural/functional domains. European journal of biochemistry. 1996;235(1–2):275–80. Epub 1996/01/15. . [DOI] [PubMed] [Google Scholar]
- 33.Ratti S, Curnis F, Longhi R, Colombo B, Gasparri A, Magni F, et al. Structure-activity relationships of chromogranin A in cell adhesion. Identification of an adhesion site for fibroblasts and smooth muscle cells. The Journal of biological chemistry. 2000;275(38):29257–63. Epub 2000/07/06. doi: 10.1074/jbc.M003796200 . [DOI] [PubMed] [Google Scholar]
- 34.Schneider F, Bach C, Chung H, Crippa L, Lavaux T, Bollaert PE, et al. Vasostatin-I, a chromogranin A-derived peptide, in non-selected critically ill patients: distribution, kinetics, and prognostic significance. Intensive care medicine. 2012;38(9):1514–22. Epub 2012/06/19. doi: 10.1007/s00134-012-2611-3 . [DOI] [PubMed] [Google Scholar]
- 35.Tombetti E, Colombo B, Di Chio MC, Sartorelli S, Papa M, Salerno A, et al. Chromogranin-A production and fragmentation in patients with Takayasu arteritis. Arthritis research & therapy. 2016;18:187 Epub 2016/08/18. doi: 10.1186/s13075-016-1082-2 ; PubMed Central PMCID: PMCPMC4987982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giovinazzo F, Schimmack S, Svejda B, Alaimo D, Pfragner R, Modlin I, et al. Chromogranin A and its fragments as regulators of small intestinal neuroendocrine neoplasm proliferation. PloS one. 2013;8(11):e81111 Epub 2013/11/22. doi: 10.1371/journal.pone.0081111 ; PubMed Central PMCID: PMCPMC3834250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Portela-Gomes GM, Grimelius L, Stridsberg M. Immunohistochemical and biochemical studies with region-specific antibodies to chromogranins A and B and secretogranins II and III in neuroendocrine tumors. Cellular and molecular neurobiology. 2010;30(8):1147–53. Epub 2010/11/04. doi: 10.1007/s10571-010-9585-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stridsberg M, Eriksson B, Oberg K, Janson ET. A panel of 11 region-specific radioimmunoassays for measurements of human chromogranin A. Regulatory peptides. 2004;117(3):219–27. Epub 2004/01/30. . [DOI] [PubMed] [Google Scholar]
- 39.Cunningham RT, Pogue KM, Curry WJ, Johnston CF, Sloan JM, Buchanan KD. Immunostaining for vasostatin I distinguishes between ileal and lung carcinoids. The Journal of pathology. 1999;187(3):321–5. Epub 1999/07/09. doi: 10.1002/(SICI)1096-9896(199902)187:3<321::AID-PATH258>3.0.CO;2-9 . [DOI] [PubMed] [Google Scholar]
- 40.Blois A, Srebro B, Mandala M, Corti A, Helle KB, Serck-Hanssen G. The chromogranin A peptide vasostatin-I inhibits gap formation and signal transduction mediated by inflammatory agents in cultured bovine pulmonary and coronary arterial endothelial cells. Regulatory peptides. 2006;135(1–2):78–84. Epub 2006/05/27. doi: 10.1016/j.regpep.2006.04.007 . [DOI] [PubMed] [Google Scholar]
- 41.Veschini L, Crippa L, Dondossola E, Doglioni C, Corti A, Ferrero E. The vasostatin-1 fragment of chromogranin A preserves a quiescent phenotype in hypoxia-driven endothelial cells and regulates tumor neovascularization. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25(11):3906–14. Epub 2011/08/10. doi: 10.1096/fj.11-182410 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.