Abstract
Peroxisome proliferator-activated receptor-γ (PPARγ), is a transcription factor that governs pathways, such as lipid metabolism and immune response, that have been implicated in the etiology of LOAD. Previously, we established HepG2-derived cell-lines with stable knockdown of PPARγ gene, and showed an increase in mRNA levels of genes mapped in the APOE linkage disequilibrium (LD) region on chromosome 19q13.32, with the greatest effect observed for APOE-mRNA. Here, we extended the analysis using our PPARγ knockdown model system and investigated the broader effect on expression changes of genes implicated in LOAD via genome wide association studies (GWAS). We applied the nCounter gene expression assay (NanoString) using a panel of twenty-four LOAD-associated genes inferred by proximity to the top significantly associated SNPs. Two independent PPARγ knockdown cell-lines showed changes in mRNA levels of a total of seven genes compared to a control HepG2 cell-line; six of which, ABCA7, APOE, CASS4, CELF1, PTK2B, and ZCWPW1, were upregulated and one, DSG2, was downregulated upon PPARγ knockdown. Our results propose that PPARγ may act as a master regulator of the transcription of several genes involved in LOAD pathogenesis. Our study provided the premise for further analyses including a larger set of genes positioned within a wider range of linkage disequilibrium (LD) regions tagged by all LOAD significantly associated SNPs.
Introduction
Large multi-center genome-wide association studies (GWAS) found associations between late-onset Alzheimer’s disease (LOAD) and over twenty genomic loci [1–6]. Subsequent studies have mapped pathways on which the genes within LOAD-associated regions participate, and identified diverse biological pathways, including lipid metabolism, immune and inflammatory response, and endocytosis [7–9]. The involvement of various pathways supports the concept of LOAD as a system-wide disorder. However, the molecular mechanisms through which the LOAD-associated loci exert their pathogenic effects remain to be fully elucidated.
It has been suggested that alteration in the levels of normal (wild-type) genes that are important in maintaining normal brain function can lead to neurodegenerative diseases, including LOAD [10–13]. Furthermore, expression quantitative trait loci (eQTLs) within LOAD-associated regions were described in brain regions vulnerable to LOAD [14, 15]. These studies strengthened the important role of the regulation of gene expression in LOAD etiology. Thus, it is imperative to better understand the mechanisms such as transcription regulation, that mediate the expression levels of the LOAD-associated genes.
The ligand-activated nuclear transcription factor, peroxisome proliferator-activated receptor-γ (PPARγ), has been shown to regulate the transcription of numerous genes playing key roles in adipocyte differentiation, inflammation and immune response, insulin sensitivity, and lipid and glucose metabolism [16–18]. Intriguingly, PPARγ governed pathways overlap, to some extent, with the biological pathways implicated in LOAD pathogenesis via GWAS, epidemiological studies, and other evidence [7–9, 19].
The APOE linkage disequilibrium (LD) region on 19q13.32 is the strongest genetic risk factor for LOAD [20–31]. Recently, using the short hairpin RNA (shRNA) method in HepG2 cells, we measured the effects of PPARγ knockdown on mRNA expression of genes within the chr19q13.32 region, and demonstrated increases in the levels of TOMM40-, APOE-, and APOC1-mRNAs; APOE-mRNA was the most responsive, showing a 50% increase in expression relative to control [32]. As a complementary approach, we applied PPARγ agonists and demonstrated that PPARγ activation decreased the levels of all three transcripts, with the strongest effect on APOE-mRNA as well [32]. These observations further established a role for PPARγ in the transcriptional regulation of the most significant LOAD genetic risk factor. The intersection between biological processes regulated by PPARγ and those involved in LOAD pathogenesis may be mediated through a master role of PPARγ in the transcriptional modulation of additional LOAD-associated genes. Here, we extended our previous analysis to other genes implicated in LOAD, and focused on the genes inferred by proximity to the LOAD-associated SNPs (Table 1). We applied NanoString technology to characterize the effect of PPARγ knockdown on the expression of twenty-four LOAD-GWAS genes involved in various physiological pathways.
Table 1. LOAD-risk genes located ± 100 kb of the top SNP.
| Closest gene | Top SNP* | Chr. | Position** | OR (95% CI) | P value | mRNA expression HepG2 |
|---|---|---|---|---|---|---|
| CR1 | rs6656401 | 1 | 207,692,049 | 1.18 (1.14–1.22) | 5.7×10−24 | n.a. |
| BIN1 | rs6733839 | 2 | 127,892,810 | 1.22 (1.18–1.25) | 6.9×10−44 | + |
| INPP5D | rs35349669 | 2 | 234,068,476 | 1.08 (1.05–1.11) | 3.2×10−8 | ++ |
| MEF2C | rs190982 | 5 | 88,223,420 | 0.93 (0.90–0.95) | 3.2×10−8 | n.a. |
| CD2AP | rs10948363 | 6 | 47,487,762 | 1.10 (1.07–1.13) | 5.2×10−11 | +++ |
| TREM2/TREML2 | rs9381040 | 6 | 41,154,650 | 0.93 (0.91–0.96) | 6.3×10−7 | n.a. |
| HLA-DRB5–HLA-DRB1 | rs9271192 | 6 | 32,578,530 | 1.11 (1.08–1.15) | 2.9×10−12 | n.a. |
| NME8 | rs2718058 | 7 | 37,841,534 | 0.93 (0.90–0.95) | 4.8×10−9 | n.a. |
| ZCWPW1 | rs1476679 | 7 | 100,004,446 | 0.91 (0.89–0.94) | 5.6×10−10 | + |
| EPHA1 | rs11771145 | 7 | 143,110,762 | 0.90 (0.88–0.93) | 1.1×10−13 | + |
| PTK2B | rs28834970 | 8 | 27,195,121 | 1.10 (1.08–1.13) | 7.4×10−14 | + |
| CLU | rs9331896 | 8 | 27,467,686 | 0.86 (0.84–0.89) | 2.8×10−25 | n.a. |
| CELF1 | rs10838725 | 11 | 47,557,871 | 1.08 (1.05–1.11) | 1.1×10−8 | ++ |
| MS4A6A-MS4A1 | rs983392 | 11 | 59,923,508 | 0.90 (0.87–0.92) | 6.1×10−16 | n.a. |
| PICALM | rs10792832 | 11 | 85,867,875 | 0.87 (0.85–0.89) | 9.3×10−26 | ++ |
| SORL1 | rs11218343 | 11 | 121,435,587 | 0.77 (0.72–0.82) | 9.7×10−15 | ++ |
| FERMT2 | rs17125944 | 14 | 53,400,629 | 1.14 (1.09–1.19) | 7.9×10−9 | ++ |
| SLC24A4-RIN3 | rs10498633 | 14 | 92,926,952 | 0.91 (0.88–0.94) | 5.5×10−9 | n.a. |
| DSG2 | rs8093731 | 18 | 29,088,958 | 0.73 (0.62–0.86) | 1.0×10−4 | ++ |
| ABCA7 | rs4147929 | 19 | 1,063,443 | 1.15 (1.11–1.19) | 1.1×10−15 | + |
| APOE# | rs4420638 | 19 | 45,422,945 | 3.58 (3.37–3.80) | 1.1×10−300 | +++ |
| CD33 | rs3865444 | 19 | 51,727,962 | 0.94 (0.91–0.96) | 3.0×10−6 | n.a. |
| CASS4 | rs7274581 | 20 | 55,018,260 | 0.88 (0.84–0.92) | 2.5×10−8 | + |
Adapted from the largest meta analysis (Lambert et al, 2013).
*SNPs showing the best level of association after meta-analysis of stages 1 and 2;
**Build 37/ hg19;
#Naj et al (2011); relative mRNA expression levels in HepG2 derived cell-lines: n.a.’ not detected; +, low; ++, intermediate; +++, high
Materials and methods
Cell lines
HepG2 derived cell-lines with stable PPARγ knockdown were generated by lentiviral shRNA transduction as previously described [32]. Two clones ID TRCN0000001673 (PPARγ 1673) [32] and ID TRCN0000001674 (PPARγ 1674), were selected for further analysis. The PPARγ knockdown cell-lines are referred as ‘PPARγ-KD1’ and ‘PPARγ-KD2’, respectively. To control for the effect of viral transduction and general shRNA expression, we used a control HepG2 derived cell line expressing shRNA targeting green fluorescent protein (GFP) as previously described [32], hereafter referred as ‘GFP’. In addition, we used the control un-transduced HepG2 cell-line, hereafter referred as ‘U’. The efficiency of PPARγ knockdown at the RNA level was previously validated by quantitative reverse transcription PCR (qRT-PCR) using the TaqMan system [32].
Cell culture
The transduced HepG2 cell-lines were cultured in Eagle's minimum essential medium (MEM), supplemented with 10% fetal bovine serum, 2 mM GlutaMAX, 1 mM sodium pyruvate, 0.1 mM non-essential amino acids, penicillin streptomycin (10,000 units/mL penicillin and 10,000 μg/mL streptomycin), and puromycin dihydrochloride (2 μg/mL). Cells were maintained in a humidified incubator at 37 °C and 5% CO2.
Four HepG2 derived cell-lines were studied: PPARγ-KD1, PPARγ-KD2, GFP, and un-transduced. For each HepG2 derived cell-line 1.5 × 105 cells were plated onto each well of a 6-well plate and were cultured for thirty-six hours in puromycin-free media prior to harvesting for molecular evaluations. We repeated this experiment four times for each HepG2 derived cell-line, i.e., cells were cultured for thirty-six hours and harvested in four independent experiments.
RNA extraction and sample preparation
Total RNA was extracted from cells using TRIzol reagent (Invitrogen, Carlsbad, CA), and purified using RNeasy Mini Kits (QIAGEN, Valencia, CA) according to the manufacturer's protocol. The concentration of RNA samples was determined spectrophotometrically by NanoDrop, and the quality of the RNA and lack of significant degradation was confirmed utilizing an Agilent Bioanalyzer. For all samples used, the RNA Integrity Number (RIN) was greater than eight, considered high quality RNA.
For each HepG2 derived cell-line, RNA was extracted from four independent experiments, and then was pooled such that each RNA sample represents four independent experiments (i.e., four biological replicates) for a particular HepG2 derived cell-line.
NanoString nCounter gene expression analysis
Gene expression was quantified digitally using the nCounter Gene Expression Assay (NanoString Technologies, Seattle, WA). We developed a custom probe set termed CodeSet containing reporter and capture probes for twenty-five target genes and three housekeeping genes (GAPDH, B2M, and LDHA) (S1 Table). The CodeSet was designed and validated by NanoString such that each target-specific probe would cover all known transcript isoforms of a particular gene and (each probe sequence provided in S1 Table). Assay was performed using the NanoString protocols according to the manufacturer’s instructions. Briefly, for each pooled RNA sample 100ng was hybridized to the CodeSet overnight at 65°C. The hybridized samples underwent automated processing on the nCounter Prep Station, nCounter Master Kit reagents were added to remove the excess probes, and the purified target/probe complexes were immobilized in the nCounter cartridge for data collection. Data collection for digital quantification was carried out in the nCounter Digital Analyzer by processing the digital images of the color-coded barcodes on the surface of the cartridge, and tabulating the barcode counts for each target mRNA in each sample. The raw counts expression data was analyzed using nSolver Analysis Software (NanoString). Briefly, we normalized samples according to six positive and eight negative control probes and the geometric mean of the three housekeeping genes. For each sample, the background threshold was set using the geometric mean of the eight negative control probes plus two standard deviations, and the background subtraction was performed. Next, data was normalized using the geometric mean of the six positive control probes and three housekeeping genes as follows: The normalization factor for each sample was calculated using the geometric mean of the six positive control probes, specifically by dividing the arithmetic mean of the geometric mean by the geometric mean value. This was followed by a technical normalization using the geometric mean of the three housekeeping genes included in each run (GAPDH, B2M, and LDHA). The normalized data was log2 transformed and fold expression changes were calculated.
Results
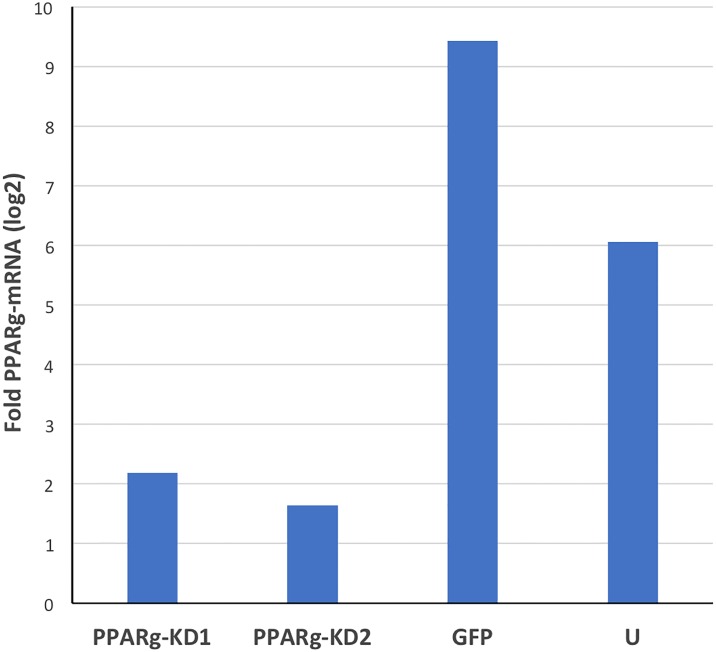
We analyzed the levels of PPARγ-mRNA in both PPARγ knockdown cell-lines, PPARγ-KD1 and PPARγ-KD2 (four biological replicates each), by the NanoString nCounter method. We evaluated the reduction in PPARγ expression, and found that the levels of PPARγ-mRNA in PPARγ-KD1 were reduced to 23% and 36% compared to the GFP and the untransduced control HepG2 cell-lines, respectively (Fig 1). These results reproduced our previous data using TaqMan qRT-PCR that demonstrated >2-fold lower PPARγ-mRNA levels upon PPARγ knockdown in this cell-line [32]. Similarly, the levels of PPARγ-mRNA in PPARγ-KD2 cell-line were decreased to 17% and 26% relative to the GFP and the untransduced controls (Fig 1). For validation we repeated PPARγ-mRNA expression analysis using qRT-PCR (S1 Fig).
Fig 1. Validation of the reduction in PPARγ-mRNA expression in HepG2-derived PPARγ-KD1 and -KD2 cells.
RNA was extracted from four HepG2 derived cell-lines: PPARγ-KD1, PPARγ-KD2, GFP, and untransduced (U). For each cell-line, RNA samples from four biological replicates were pooled. The levels of PPARγ-mRNA relative to the geometric mean of GAPDH, B2M, and LDHA -mRNAs was assessed by nCounter NanoString technology. The different HepG2 derived cell-lines are indicated on the X-axis, and the fold change of mRNA (log2 transformed) is indicated on the Y-axis. PPARγ-mRNA were decreased in both PPARγ-KD cell-lines compared to the GFP cells (by ~4–5 fold), and compared to ‘U’ cells (~3–4 fold).
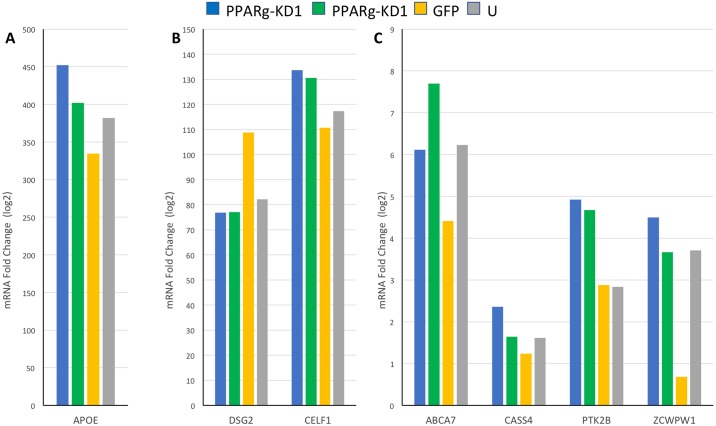
Next, we examined the expression of twenty-four LOAD susceptibility genes inferred by the proximity to the most significant LOAD-associated SNPs (Table 1). Ten genes resulted in counts below the background threshold for all four HepG2 derived cell-lines and were considered as not expressed in our system (Table 1). We assessed the fold-change in mRNA levels for all fourteen remaining genes expressed in our HepG2 cells, by comparing the mRNA levels in PPARγ-KD1 and PPARγ-KD2 cell-lines relative to the GFP cell-line, using a pool of 4 biological replicates for each cell-line. A total of seven genes were found to be affected by PPARγ knockdown and demonstrated a consistent direction of the effect on mRNA levels, reproducible in both PPARγ-KD1 and PPARγ-KD2 cell-lines, that was 10% or more (Table 2, Fig 2). We observed that knockdown of PPARγ led to either increase or decrease in mRNA expression levels. Six genes were upregulated in PPARγ-KD cells: ATP binding cassette subfamily A member 7 (ABCA7), APOE (validated by qRT-PCR, S1 Fig, and consistent with our previous results [32]), Cas scaffolding protein family member 4 (CASS4), CUGBP Elav-like family member 1 (CELF1), protein tyrosine kinase 2 beta (PTK2B), and zinc finger CW-type and PWWP domain containing 1 (ZWPW1) (Table 2, Fig 2). Only one gene, desmoglein 2 (DSG2), was downregulated in the PPARγ-KD cell-lines compared to GFP cell-line (Table 2, Fig 2). These results reinforced the broad role of PPARγ as transcriptional activator and repressor [33], and demonstrated the effects on down- and up-regulation for LOAD-associated genes. Two genes, BIN1 and EPHA1, showed opposites effects in PPARγ-KD1 compared to PPARγ-KD2. While BIN1 and EPHA1 mRNA levels were higher in PPARγ-KD1 relative to the GFP cell-line, they were lower in PPARγ-KD2. Five genes, CD2AP, FERMT2, INPP5D, PICALM and SORL1, showed no effect (<10%) on expression levels upon PPARγ knockdown.
Table 2. PPARγ knockdown effects on mRNA levels of LOAD-genes.
| Gene | PPARγ-KD1 mRNA fold change |
PPARγ-KD2 mRNA fold change |
GO terms: Biological Process (GO ID) |
|---|---|---|---|
| ABCA7 | 1.39 | 1.75 | transport (GO:0006810) phagocytosis (GO:0006909) aminophospholipid transport (GO:0015917) |
| APOE | 1.35 | 1.20 | response to dietary excess (GO:0002021) lipid metabolic process (GO:0006629) transport (GO:0006810) lipid transport (GO:0006869) cellular calcium ion homeostasis (GO:0006874) response to oxidative stress (GO:0006979) steroid metabolic process (GO:0008202) regulation of gene expression (GO:0010468) low-density lipoprotein particle remodeling (GO:0034374) lipoprotein metabolic process (GO:0042157) lipoprotein biosynthetic process (GO:0042158) vasodilation (GO:0042311) positive regulation of catalytic activity (GO:0043085) artery morphogenesis (GO:0048844) negative regulation of inflammatory response (GO:0050728) maintenance of location in cell (GO:0051651) lipid homeostasis (GO:0055088) cardiovascular system development (GO:0072358) negative regulation of triglyceride metabolic process (GO:0090209) regulation of plasma lipoprotein particle levels (GO:0097006) cellular oxidant detoxification (GO:0098869) |
| CASS4 | 1.91 | 1.33 | cell adhesion (GO:0007155) |
| CELF1 | 1.21 | 1.18 | spermatid development (GO:0007286) RNA splicing (GO:0008380) mRNA processing (GO:0006397) negative regulation of translation (GO:0017148) positive regulation of multicellular organism growth (GO:0040018) |
| DSG2 | 0.71 | 0.71 | cell adhesion (GO:0007155) homophilic cell adhesion via plasma membrane adhesion molecules (GO:0007156) response to progesterone (GO:0032570) maternal process involved in female pregnancy (GO:0060135) |
| PTK2B | 1.71 | 1.62 |
*MAPK cascade (GO:0000165) response to reactive oxygen species (GO:0000302) oocyte maturation (GO:0001556) response to hypoxia (GO:0001666) adaptive immune response (GO:0002250) immune system process (GO:0002376) regulation of leukocyte chemotaxis (GO:0002688) response to osmotic stress (GO:0006970) actin filament organization (GO:0007015) cell adhesion (GO:0007155) |
| ZCWPW1 | 6.56 | 5.34 | None |
Fold change in mRNA levels for each PPARγ knockdown cell-line, PPARγ-KD1 and PPARγ-KD2, were calculated relative to the control GFP cell-line. GO terms listed here include the subset using the filter for evidence used in automatic assertion (Inferred from Electronic Annotation (IEA)).
*Showing the first 10 out of 54 GO IDs filtered using IEA evidence.
Fig 2. The effect of PPARγ knockdown in HepG2 cells on mRNA levels of LOAD-GWAS genes.
RNA was extracted from four HepG2 derived cell-lines: PPARγ-KD1, PPARγ-KD2, GFP, and untransduced (U). For each cell-line, RNA samples from four biological replicates were pooled. The fold levels of (A) APOE-mRNA—represents highly expressed genes, (B) DSG2, and CELF -mRNA—represent medium expressed genes, and (C) ABCA7, CASS4, PTK2B, and ZCWPW -mRNA—represent lower expressed genes, compared to the geometric mean of GAPDH, B2M, and LDHA -mRNAs were assessed by nCounter NanoString technology. The different HepG2 derived cell-lines are indicated on the X-axis, and the fold change of mRNA (log2 transformed) is indicated on the Y-axis. The expression levels of ABCA7, APOE, CASS4, CELF1, PTK2B, and ZCWPW1 -mRNAs were increased (A, B, C), and that of DSG2-mRNA (B) was decreased in PPARγ-KD1 and -KD2 cells compared to GFP and U cells.
Discussion
Using NanoString technology, we analyzed the effect of PPARγ knockdown on expression of twenty-four LOAD-associated genes in human hepatocyte-derived cell-lines and demonstrated that PPARγ regulates the expression of seven LOAD-associated genes, including APOE. It has been demonstrated that PPARγ can both activate and repress transcription, in ligand-dependent or independent manners, via binding to coactivators or corepressors, respectively [34]. Here we showed that PPARγ knockdown resulted in upregulation of six genes (ABCA7, APOE, CASS4, CELF1, PTK2B, and ZCWPW1) and downregulation of one gene (DSG2). Annotation of these genes using GO terms for biological processes (Table 2) indicated the involvement of these genes in diverse biological processes including pathways related to lipid metabolism, immune function, and cellular stress response, suggesting a role for PPARγ in these aspects of LOAD etiology.
PPARγ is the most extensively studied member of the PPARs family; it is well known for its role in peripheral metabolism, and has been implicated in the pathology of numerous diseases including diabetes, stroke, cancer, and obesity [18]. Accumulating evidence has also suggested the involvement of PPARγ in the pathogenesis of various disorders of the central nervous system (CNS) and several brain neuropathologies including LOAD [16, 17, 19, 35]. PPARγ exhibits a wide range of activities related to Alzheimer’s pathology. Studies using animal models of Alzheimer’s suggested that PPARγ exerts direct and indirect effects on Aβ metabolism (reviewed in [35]). Furthermore, the Pro12Ala mutation in PPARγ was associated with LOAD risk and age-of-onset, however, other studies failed to detect any significant association between the Ala12 variant and the genetic risk of LOAD [36]. Nonetheless, it was reported that Ala12 carriers showed an increased risk of cognitive decline than non-carriers among diabetic patients [36, 37]. Here we found that out of fourteen LOAD-associated genes expressed in our cellular system, seven are regulated by PPARγ. Our study further strengthened the link between PPARγ and neurodegeneration, in particular the development of LOAD. Collectively, our research supports the potential beneficial impact of PPARγ agonists for ameliorating LOAD-related phenotypes, reinforcing the concept that PPARγ agonists may represent an attractive class of drugs for preventing or delaying the onset of LOAD. However, it is important to note that one limitation of this study is the cellular system, as it derived from hepatocytes and does not represent brain cell types relevant to LOAD. Therefore, further investigations using the three major brain cell-types implicated in LOAD, neurons, astrocytes, and microglia, are warranted. These follow up studies will provide insight into the regulatory impact of PPARγ on the complete repertoire of LOAD-associated genes, in the context of the intracellular environments of cell-types involved in LOAD pathogenesis.
PPARγ governs metabolism, immune response, and other biological processes that are also critical for the resilience of human tissues and organs through life-span and aging ‘physiological failure’. We hypothesize that the possible master role of PPARγ in tissue resilience underlies its involvement in distinct diseases from cancer to LOAD. Here we found the effect of PPARγ on genes that were implicated in both LOAD and cancer. The LOAD-associated genes, cas scaffolding protein family member 4 (CASS4) and protein tyrosine kinase 2 beta (PTK2B), have been studied primarily for their roles in the directly cancer-relevant processes of migration and survival signaling. CASS4 and PTK2B act as interacting partners regulating oncogenesis and metastasis, and are known to be active in the brain during development and in cancer [38]. In addition, CELF1 has been associated with certain types of cancer [39, 40], and expression of ABCA7 significantly increased in ovarian carcinoma [41]. It has been reported that cancer survivors are at reduced risk for LOAD, and that people with LOAD may be at a reduced risk of developing cancer [42, 43]. The identification of genetic associations of cancer genes with LOAD risk suggested the intriguing hypothesis of mechanistic overlap between cancer and Alzheimer’s disease; moreover, our findings that four of these common genes are co-regulated by PPARγ suggest a possible role for PPARγ in the interplay between LOAD and cancer.
GWAS identified over twenty tagging SNPs associated with LOAD—however, the exact target genes that contribute directly to the disease within each of the LOAD associated genomic regions have yet to be identified. For example, there are about ten genes in the region defined by the LOAD associated-SNPs that has been inferred to as ZCWPW1. Here, we described the expression analysis of LOAD GWAS genes inferred by the proximity to the most significantly associated SNPs. In-depth exploration of the regulatory role of PPARγ in the context of LOAD warrants further investigations evaluating an inclusive and unbiased list of genes that are positioned within +/-1Mb (a range proposed as a range of LD for mapping disease genes [44, 45]) surrounding the genome-wide significant associated-SNPs.
Supporting information
RNA was extracted from four HepG2 derived cell-lines: PPARγ-KD1, PPARγ-KD2, GFP, and untransduced (U). The levels of (A) PPARγ-mRNA, and (B) APOE-mRNA, relative to the geometric mean of GAPDH- and PPIA -–mRNAs, were assessed by real-time PCR and analyzed by the 2-ΔΔCt method. The different HepG2 derived cell-lines are indicated on the X-axis, and the fold change of mRNA (log2 transformed) is indicated on the Y-axis. The values presented here are means levels±SEM of 4 replicates. Student’s t-test analysis was used to determine significant differences. (A) PPARγ-mRNA were significantly decreased (p<0.0001) in PPARγ-KD1 and PPARγ-KD2 cells compared to GFP cells and U cells. (B) APOE-mRNA were significantly increased in PPARγ-KD1 (p = 0.0003) and PPARγ-KD2 (p = 0.03) cells compared to GFP cells.
(DOCX)
Genes analyzed using NanoString technology, including twenty-four LOAD-associated genes, PPARγ, and the housekeeping genes B2M, GAPDH, and LDHA. Gene Accession numbers and the region and sequence targeted by each probe are described.
(XLSX)
Acknowledgments
This work was funded by the National Institute on Aging (NIA) [R01AG040370 and R01AG057522 to O.C.].
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by the National Institute on Aging (NIA) [R01AG040370 and R01AG057522 to O.C.]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
References
- 1.Lambert J-C, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;45(12):1452–8. doi: 10.1038/ng.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet.43(5):436–41. Epub 2011/04/05. doi: 10.1038/ng.801 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet.43(5):429–35. Epub 2011/04/05. doi: 10.1038/ng.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nature genetics. 2007;39(1):17–23. doi: 10.1038/ng1934 . [DOI] [PubMed] [Google Scholar]
- 5.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nature genetics. 2009;41(10):1088–93. doi: 10.1038/ng.440 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nature genetics. 2009;41(10):1094–9. doi: 10.1038/ng.439 . [DOI] [PubMed] [Google Scholar]
- 7.Jones L, Holmans PA, Hamshere ML, Harold D, Moskvina V, Ivanov D, et al. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer's disease. PloS one. 2010;5(11):e13950 Epub 2010/11/19. doi: 10.1371/journal.pone.0013950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones L, Harold D, Williams J. Genetic evidence for the involvement of lipid metabolism in Alzheimer's disease. Biochimica et biophysica acta. 2010;1801(8):754–61. Epub 2010/04/28. doi: 10.1016/j.bbalip.2010.04.005 . [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal SL, Kamboh MI. Late-Onset Alzheimer's Disease Genes and the Potentially Implicated Pathways. Curr Genet Med Rep. 2014;2:85–101. Epub 2014/05/16. doi: 10.1007/s40142-014-0034-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singleton A, Myers A, Hardy J. The law of mass action applied to neurodegenerative disease: a hypothesis concerning the etiology and pathogenesis of complex diseases. Human molecular genetics. 2004;13 Spec No 1:R123–6. doi: 10.1093/hmg/ddh093 . [DOI] [PubMed] [Google Scholar]
- 11.Linnertz C, Anderson L, Gottschalk W, Crenshaw D, Lutz MW, Allen J, et al. The cis-regulatory effect of an Alzheimer's disease-associated poly-T locus on expression of TOMM40 and apolipoprotein E genes. Alzheimers Dement. 2014;10(5):541–51. doi: 10.1016/j.jalz.2013.08.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsui T, Ingelsson M, Fukumoto H, Ramasamy K, Kowa H, Frosch MP, et al. Expression of APP pathway mRNAs and proteins in Alzheimer's disease. Brain Res. 2007;1161:116–23. Epub 2007/06/26. doi: 10.1016/j.brainres.2007.05.050 . [DOI] [PubMed] [Google Scholar]
- 13.Zarow C, Victoroff J. Increased apolipoprotein E mRNA in the hippocampus in Alzheimer disease and in rats after entorhinal cortex lesioning. Experimental neurology. 1998;149(1):79–86. Epub 1998/02/10. doi: 10.1006/exnr.1997.6709 . [DOI] [PubMed] [Google Scholar]
- 14.Allen M, Zou F, Chai HS, Younkin CS, Crook J, Pankratz VS, et al. Novel late-onset Alzheimer disease loci variants associate with brain gene expression. Neurology. 2012;79(3):221–8. doi: 10.1212/WNL.0b013e3182605801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou F, Chai HS, Younkin CS, Allen M, Crook J, Pankratz VS, et al. Brain expression genome-wide association study (eGWAS) identifies human disease-associated variants. PLoS genetics. 2012;8(6):e1002707 doi: 10.1371/journal.pgen.1002707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villapol S. Roles of Peroxisome Proliferator-Activated Receptor Gamma on Brain and Peripheral Inflammation. Cell Mol Neurobiol. 2017. Epub 2017/10/05. doi: 10.1007/s10571-017-0554-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai W, Yang T, Liu H, Han L, Zhang K, Hu X, et al. Peroxisome proliferator-activated receptor gamma (PPARgamma): A master gatekeeper in CNS injury and repair. Prog Neurobiol. 2017. Epub 2017/10/17. doi: 10.1016/j.pneurobio.2017.10.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janani C, Ranjitha Kumari BD. PPAR gamma gene—a review. Diabetes Metab Syndr. 2015;9(1):46–50. Epub 2014/12/03. doi: 10.1016/j.dsx.2014.09.015 . [DOI] [PubMed] [Google Scholar]
- 19.Chen YC, Wu JS, Tsai HD, Huang CY, Chen JJ, Sun GY, et al. Peroxisome proliferator-activated receptor gamma (PPAR-gamma) and neurodegenerative disorders. Mol Neurobiol. 2012;46(1):114–24. Epub 2012/03/22. doi: 10.1007/s12035-012-8259-8 . [DOI] [PubMed] [Google Scholar]
- 20.Coon KD, Myers AJ, Craig DW, Webster JA, Pearson JV, Lince DH, et al. A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer’s disease. Journal of Clinical Psychiatry. 2007;68:613–8. [DOI] [PubMed] [Google Scholar]
- 21.Grupe A, Abraham R, Li Y, Rowland C, Hollingworth P, Morgan A, et al. Evidence for novel susceptibility genes for late-onset Alzheimer’s disease from a genome-wide association study of putative functional variants. Human Molecular Genetics. 2007;16:865–73. doi: 10.1093/hmg/ddm031 [DOI] [PubMed] [Google Scholar]
- 22.Abraham R, Moskvina V, Sims R, Hollingworth P, Morgan A, Georgieva L, et al. A genome-wide association study for late-onset Alzheimer’s disease using DNA pooling. BMC Medical Genomics. 2008;1:44 doi: 10.1186/1755-8794-1-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertram L, Lange C, Mullin K, Parkinson M, Hsiao M., Hogan MF, et al. Genome-wide association analysis reveals putative Alzheimer’s disease susceptibility loci in addition to APOE. American Journal of Human Genetics. 2008;83:623–32. doi: 10.1016/j.ajhg.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beecham GW, Martin ER, Li YG, Slifer MA, Gilbert JR, Haines JL, et al. Genome-wide association study implicates a chromosome 12 risk locus for late-onset Alzheimer disease. American Journal of Human Genetics. 2009;84:35–43. doi: 10.1016/j.ajhg.2008.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrasquillo MM, Zou F, Pankratz VS, Wilcox SL, Ma L, Walker LP, et al. Genetic variation in PCDH11X is associated with susceptibility to late-onset Alzheimer’s disease. Nature Genetics. 2009;41:192–8. doi: 10.1038/ng.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nature Genetics. 2009;41:1088–93. doi: 10.1038/ng.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nature Genetics. 2009;41:1094–9. doi: 10.1038/ng.439 [DOI] [PubMed] [Google Scholar]
- 28.Potkin SG, Guffanti G, Lakatos A, Turner JA, Kruggel F, Fallon JH, et al. Hippocampal atrophy as a quantitative trait in a genome-wide association study identifying novel susceptibility genes for Alzheimer’s disease. PLoS One 4. 2009;4:e6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinzen EL, Need AC, Hayden KM, Chiba-Falek O, Roses AD, Strittmatter WJ, et al. Genome-wide scan of copy number variation in late-onset Alzheimer’s disease. Journal of Alzheimer’s Disease. Journal of Alzheimer’s Disease. 2010;19:69–77. doi: 10.3233/JAD-2010-1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303:1832–40. doi: 10.1001/jama.2010.574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamboh MI, Barmada MM, Demirci FY, Minster RL, Carrasquillo MM, Pankratz VS, et al. Genome-wide association analysis of age-of-onset in Alzheimer’s disease. Molecular Psychiatry. 2012;17:1340–6. doi: 10.1038/mp.2011.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subramanian S, Gottschalk WK, Kim SY, Roses AD, Chiba-Falek O. The effects of PPARgamma on the regulation of the TOMM40-APOE-C1 genes cluster. Biochim Biophys Acta. 2017;1863(3):810–6. Epub 2017/01/10. doi: 10.1016/j.bbadis.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su Y, Shen X, Chen J, Isales CM, Zhao J, Shi XM. Differentially expressed genes in PPARgamma-deficient MSCs. Molecular and cellular endocrinology. 2017. Epub 2017/08/05. doi: 10.1016/j.mce.2017.07.037 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochimica et biophysica acta. 2007;1771(8):926–35. Epub 2007/04/17. doi: 10.1016/j.bbalip.2007.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heneka MT, Reyes-Irisarri E, Hull M, Kummer MP. Impact and Therapeutic Potential of PPARs in Alzheimer's Disease. Curr Neuropharmacol. 2011;9(4):643–50. Epub 2012/06/02. doi: 10.2174/157015911798376325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He W. PPARgamma2 polymorphism and human health. PPAR Res. 2009;2009:849538 Epub 2009/04/25. doi: 10.1155/2009/849538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson W, Harris SE, Starr JM, Whalley LJ, Deary IJ. PPARG Pro12Ala genotype and risk of cognitive decline in elders? Maybe with diabetes. Neuroscience Letters. 2008;434:50–5. doi: 10.1016/j.neulet.2008.01.027 [DOI] [PubMed] [Google Scholar]
- 38.Beck TN, Nicolas E, Kopp MC, Golemis EA. Adaptors for disorders of the brain? The cancer signaling proteins NEDD9, CASS4, and PTK2B in Alzheimer's disease. Oncoscience. 2014;1(7):486–503. Epub 2015/01/17. doi: 10.18632/oncoscience.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cifdaloz M, Osterloh L, Grana O, Riveiro-Falkenbach E, Ximenez-Embun P, Munoz J, et al. Systems analysis identifies melanoma-enriched pro-oncogenic networks controlled by the RNA binding protein CELF1. Nat Commun. 2017;8(1):2249 Epub 2017/12/23. doi: 10.1038/s41467-017-02353-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis K, Valanejad L, Cast A, Wright M, Wei C, Iakova P, et al. RNA Binding Protein CUGBP1 Inhibits Liver Cancer in a Phosphorylation-Dependent Manner. Molecular and cellular biology. 2017;37(16). Epub 2017/06/01. doi: 10.1128/MCB.00128-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elsnerova K, Bartakova A, Tihlarik J, Bouda J, Rob L, Skapa P, et al. Gene Expression Profiling Reveals Novel Candidate Markers of Ovarian Carcinoma Intraperitoneal Metastasis. J Cancer. 2017;8(17):3598–606. Epub 2017/11/21. doi: 10.7150/jca.20766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Musicco M, Adorni F, Di Santo S, Prinelli F, Pettenati C, Caltagirone C, et al. Inverse occurrence of cancer and Alzheimer disease: a population-based incidence study. Neurology. 2013;81(4):322–8. Epub 2013/07/12. doi: 10.1212/WNL.0b013e31829c5ec1 . [DOI] [PubMed] [Google Scholar]
- 43.Yarchoan M, James BD, Shah RC, Arvanitakis Z, Wilson RS, Schneider J, et al. Association of Cancer History with Alzheimer's Disease Dementia and Neuropathology. J Alzheimers Dis. 2017;56(2):699–706. Epub 2016/12/31. doi: 10.3233/JAD-160977 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ott J. Predicting the range of linkage disequilibrium. Proc Natl Acad Sci U S A. 2000;97(1):2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickson SP, Wang K, Krantz I, Hakonarson H, Goldstein DB. Rare variants create synthetic genome-wide associations. PLoS Biol. 2010;8(1):e1000294 doi: 10.1371/journal.pbio.1000294 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RNA was extracted from four HepG2 derived cell-lines: PPARγ-KD1, PPARγ-KD2, GFP, and untransduced (U). The levels of (A) PPARγ-mRNA, and (B) APOE-mRNA, relative to the geometric mean of GAPDH- and PPIA -–mRNAs, were assessed by real-time PCR and analyzed by the 2-ΔΔCt method. The different HepG2 derived cell-lines are indicated on the X-axis, and the fold change of mRNA (log2 transformed) is indicated on the Y-axis. The values presented here are means levels±SEM of 4 replicates. Student’s t-test analysis was used to determine significant differences. (A) PPARγ-mRNA were significantly decreased (p<0.0001) in PPARγ-KD1 and PPARγ-KD2 cells compared to GFP cells and U cells. (B) APOE-mRNA were significantly increased in PPARγ-KD1 (p = 0.0003) and PPARγ-KD2 (p = 0.03) cells compared to GFP cells.
(DOCX)
Genes analyzed using NanoString technology, including twenty-four LOAD-associated genes, PPARγ, and the housekeeping genes B2M, GAPDH, and LDHA. Gene Accession numbers and the region and sequence targeted by each probe are described.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.